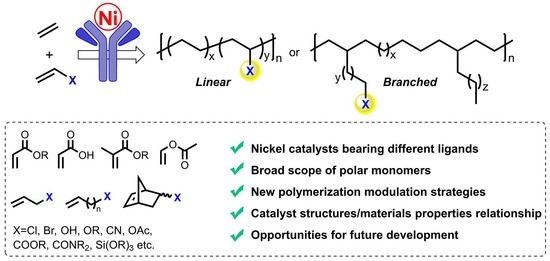
Recent Advances in the Copolymerization of Ethylene with Polar Comonomers by Nickel Catalysts
Abstract
:1. Introduction
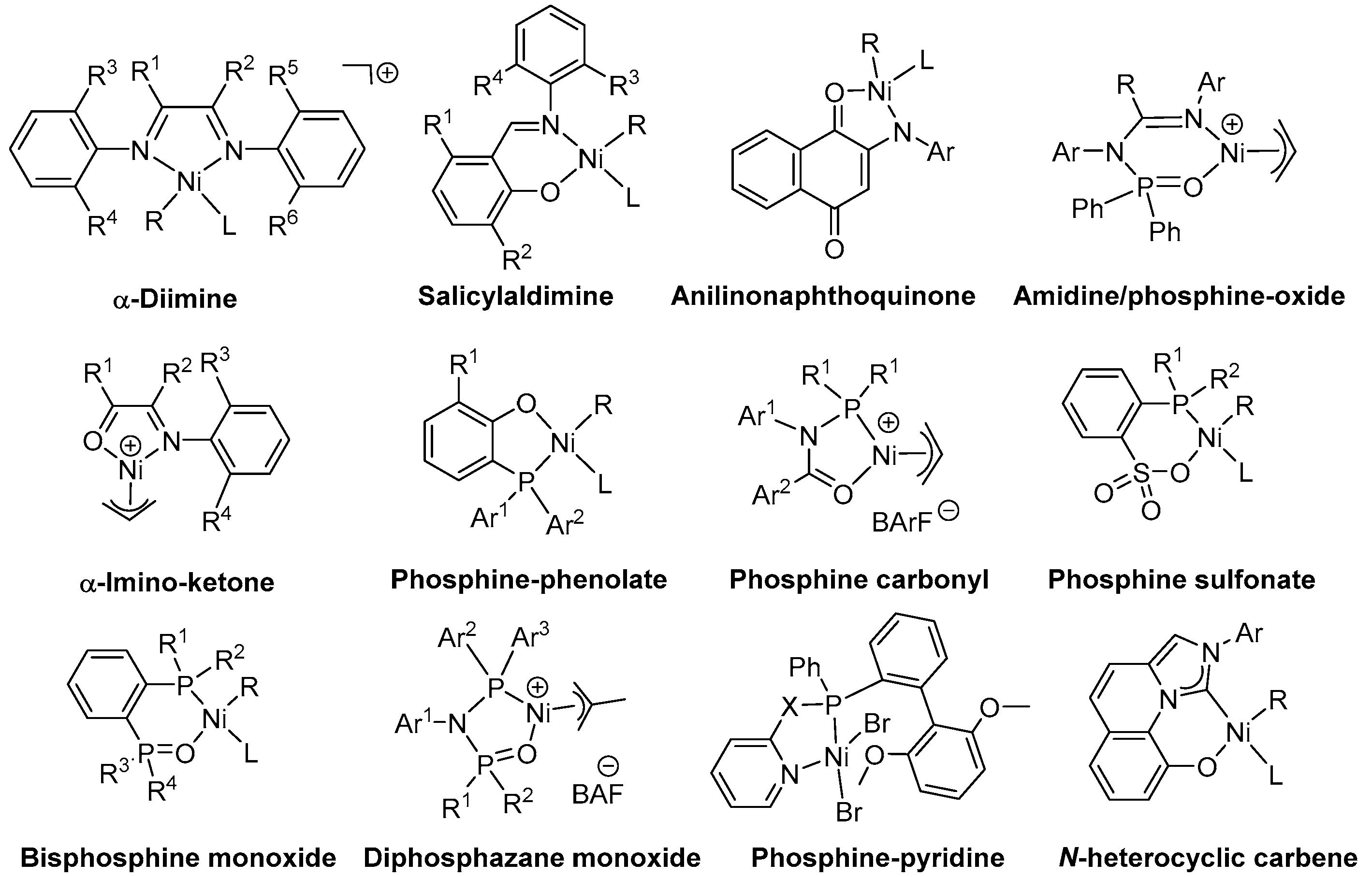
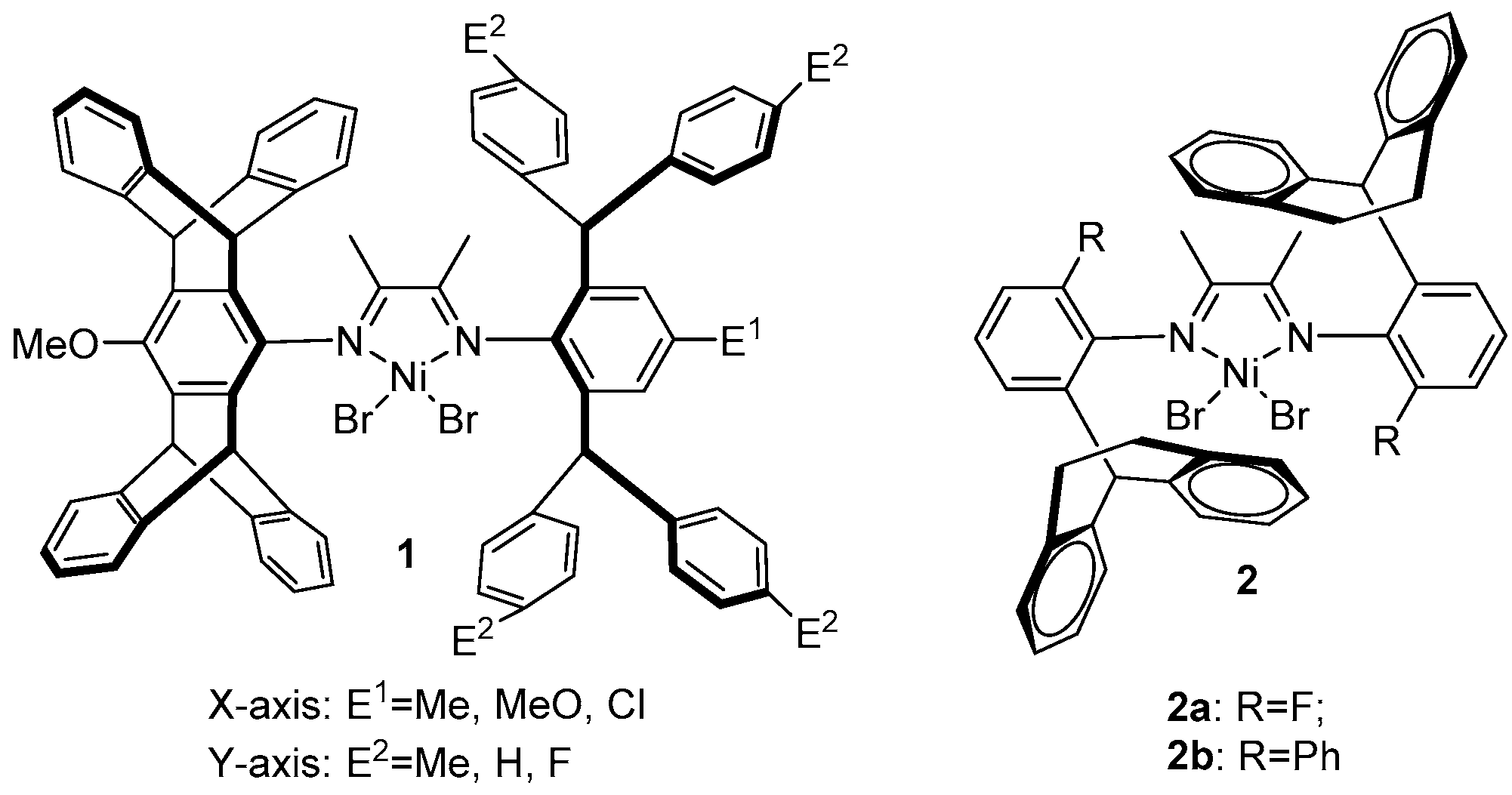
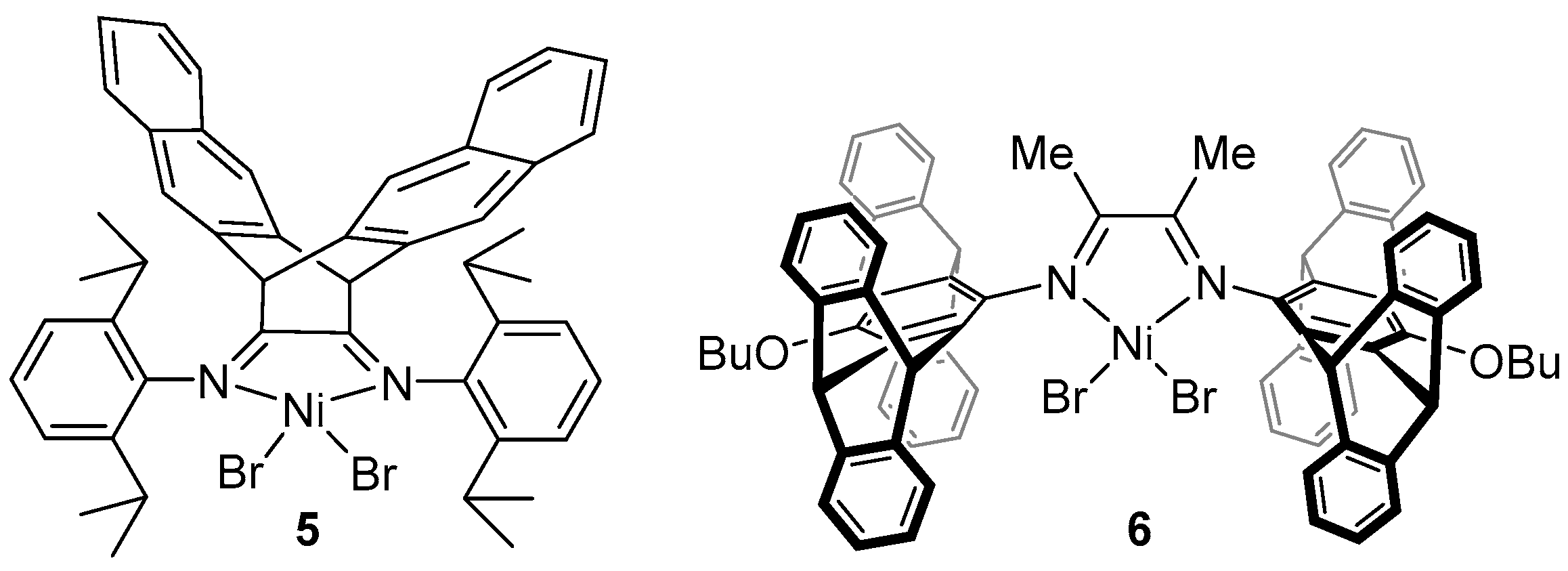
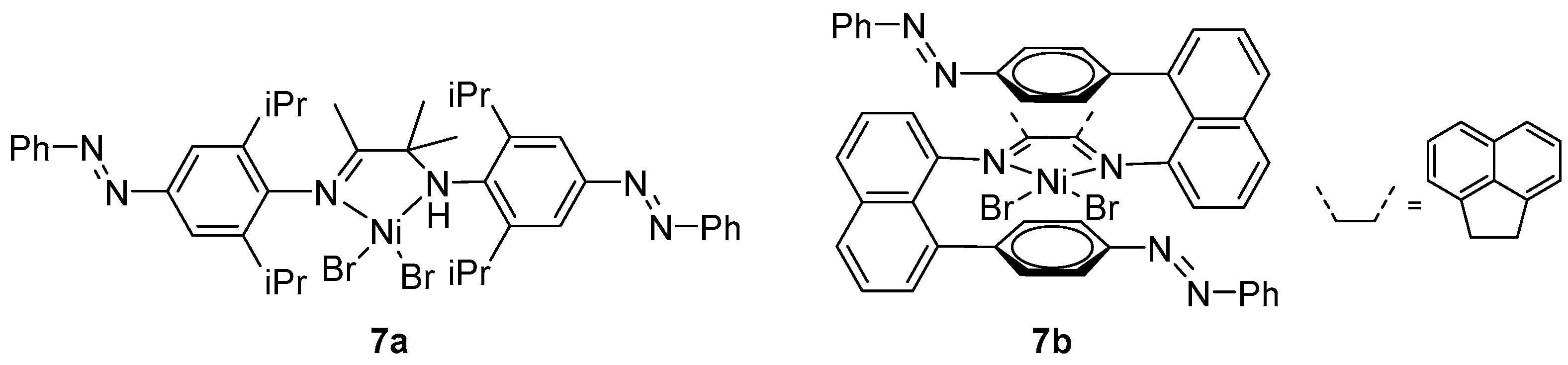
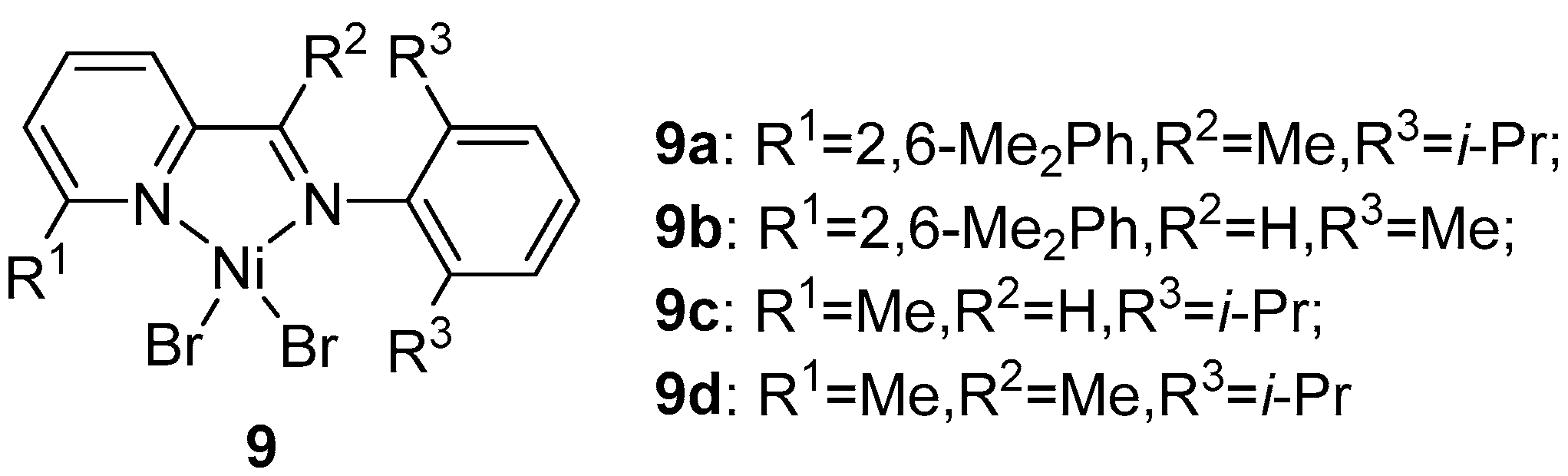
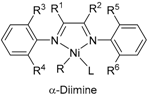
2. α-Diimine Nickel Catalysts
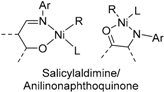
3. Salicylaldimine/Ketoiminato Nickel Catalysts
4. Phosphine–Phenolate Nickel Catalysts
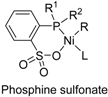
5. Phosphine Sulfonate Nickel Catalysts
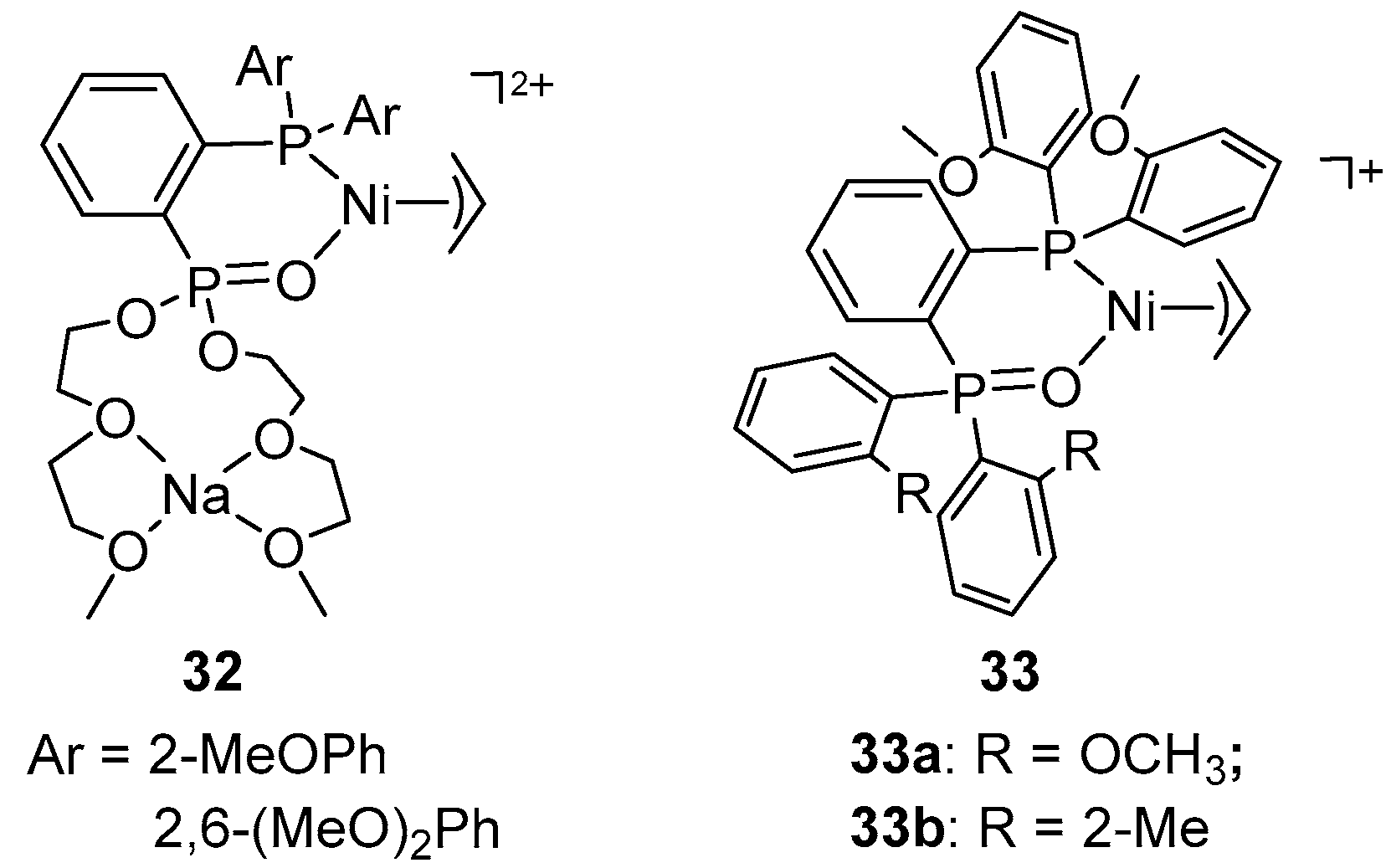
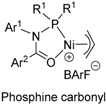
6. Bisphosphine Monoxide Nickel Catalysts
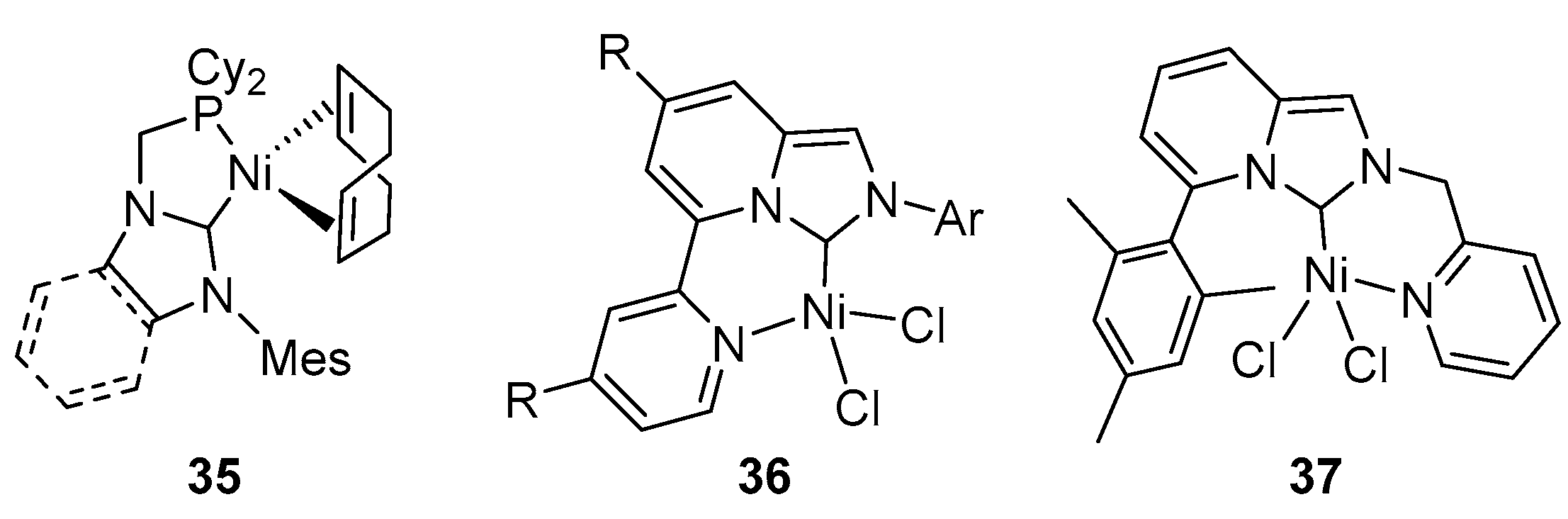
7. N-heterocyclic Carbenes (NHCs) Nickel Catalysts
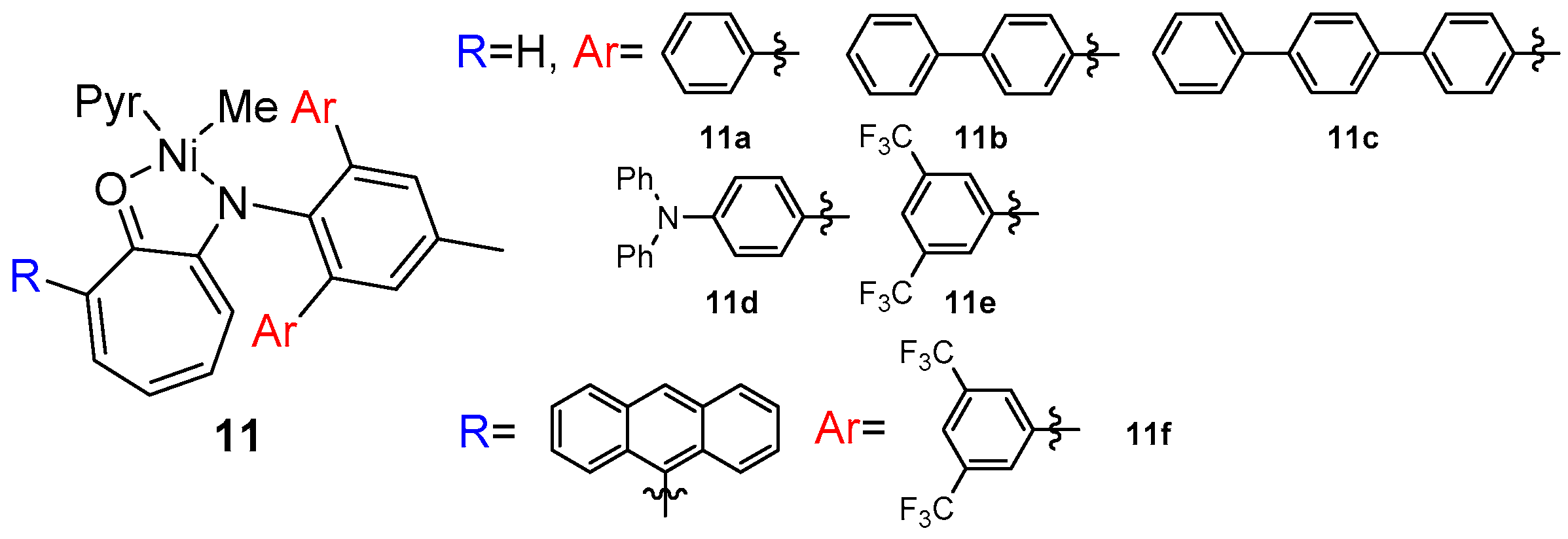
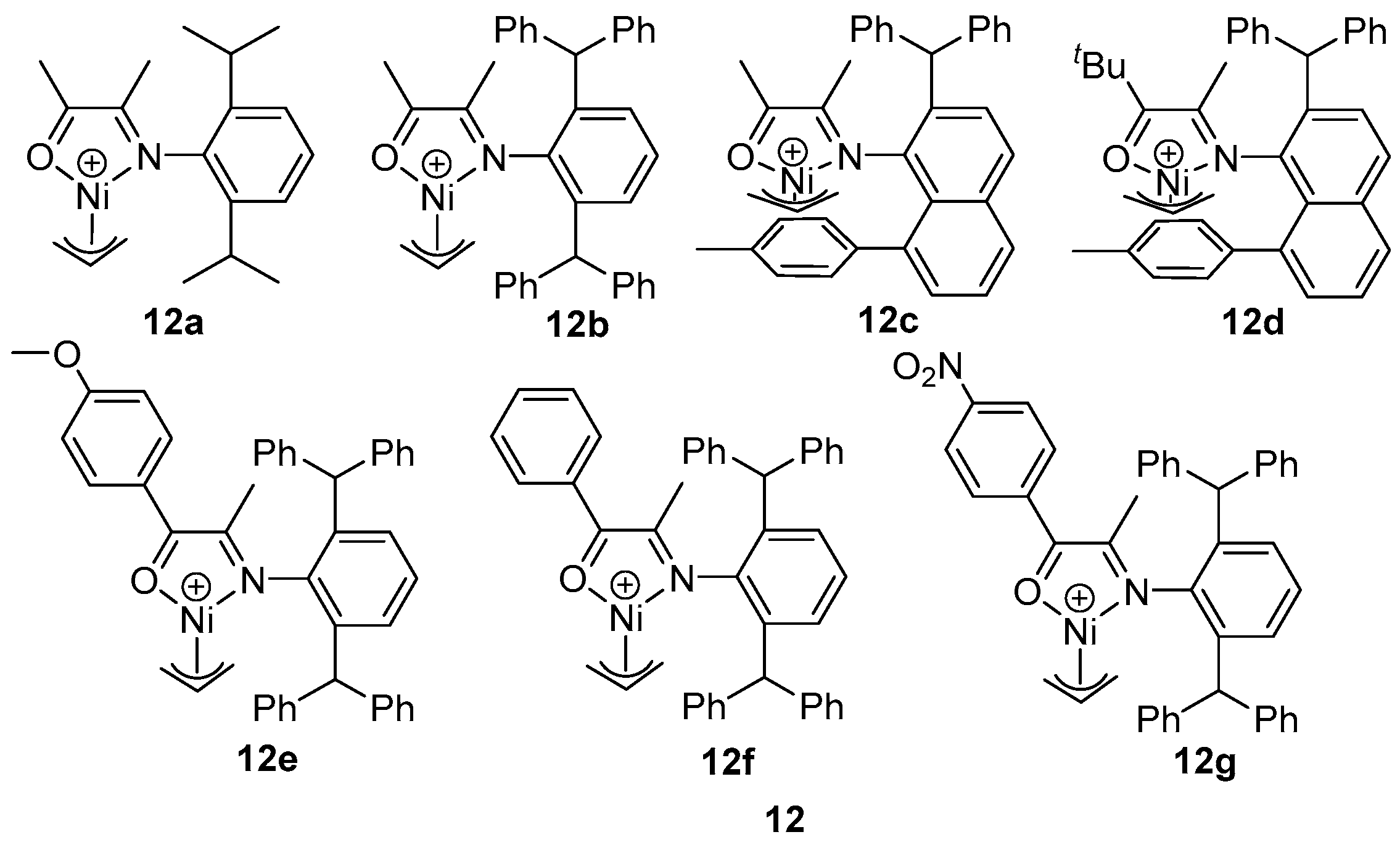
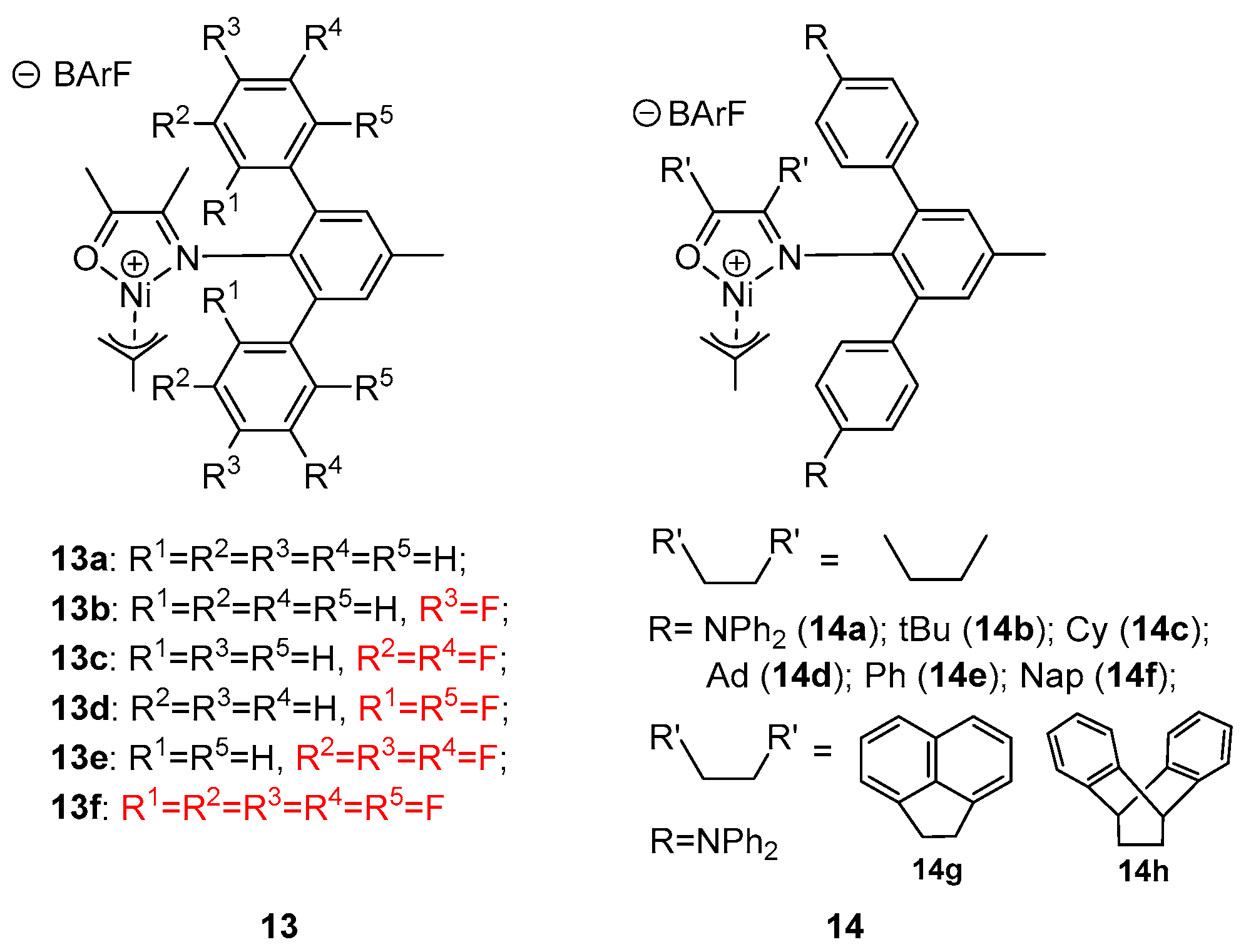
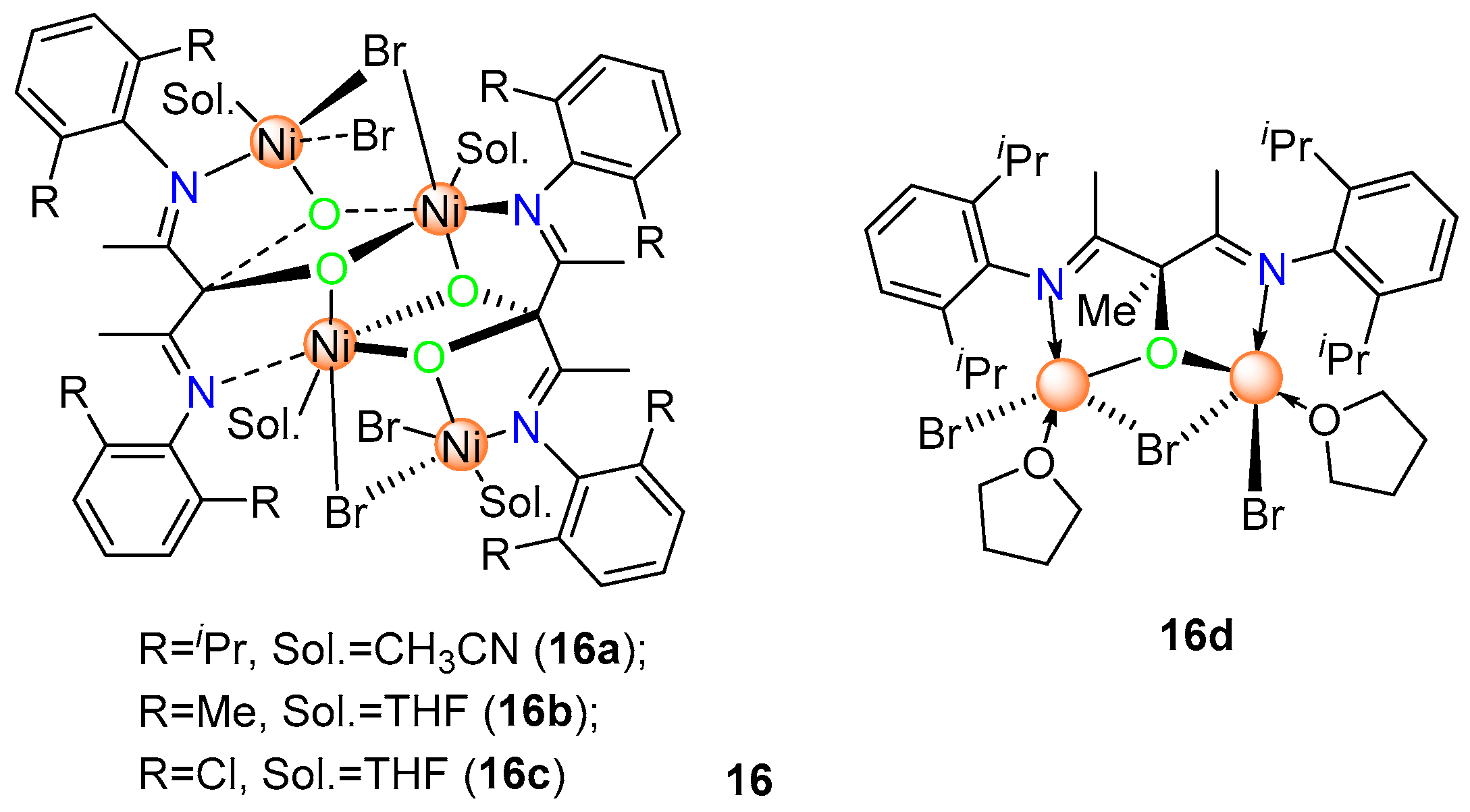
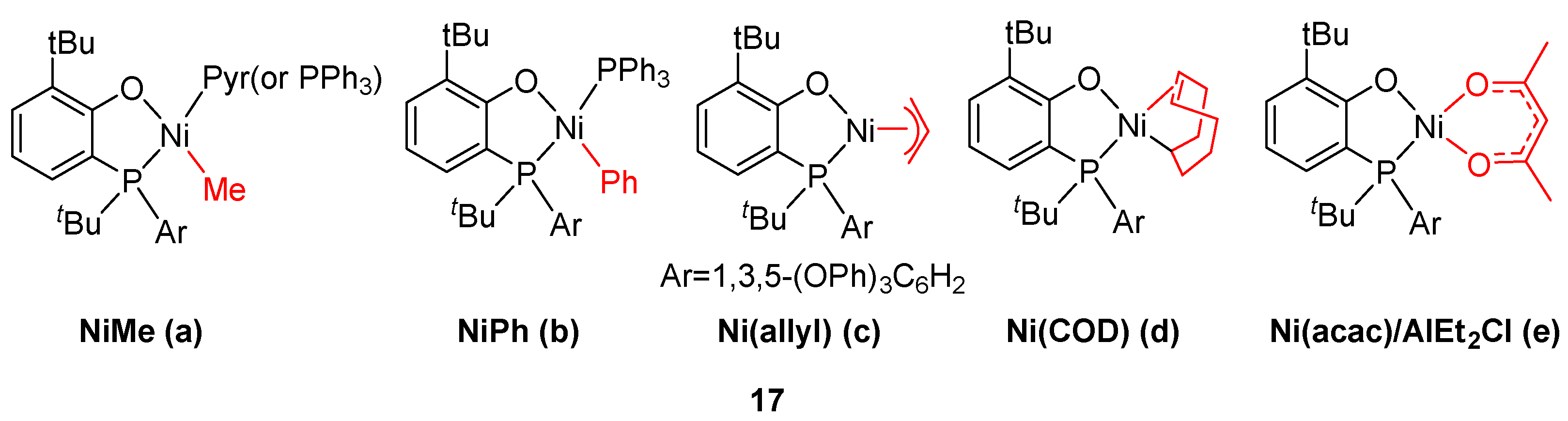
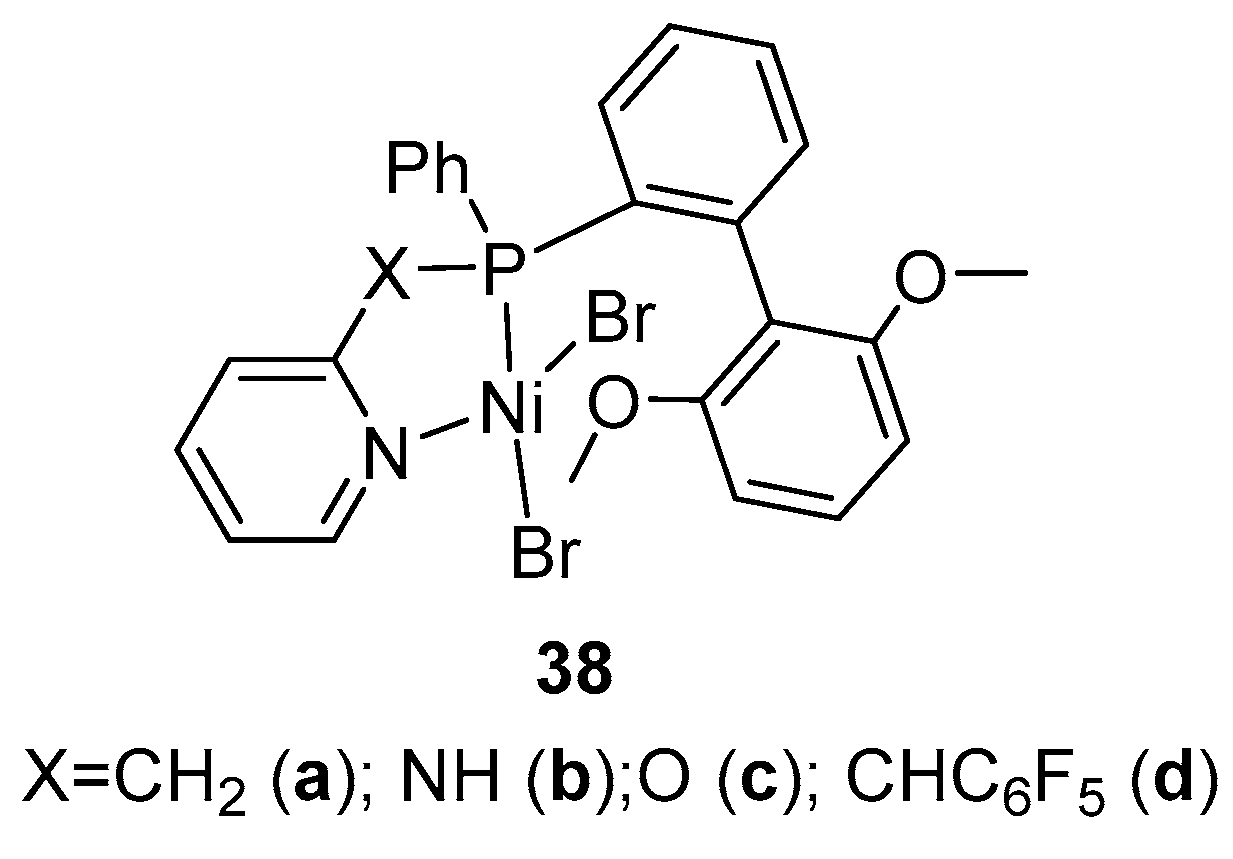
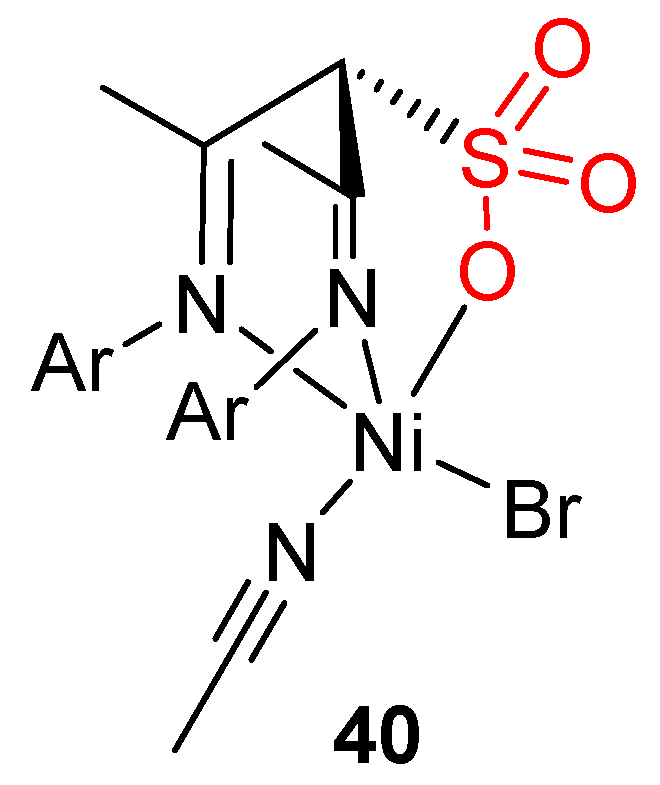
8. Other Unclassified Nickel Catalysts
9. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Baier, M.C.; Zuideveld, M.A.; Mecking, S. Post-metallocenes in the industrial production of polyolefins. Angew. Chem. Int. Ed. 2014, 53, 9722–9744. [Google Scholar] [CrossRef]
- Nakamura, A.; Ito, S.; Nozaki, K. Coordination-Insertion Copolymerization of Fundamental Polar Monomers. Chem. Rev. 2009, 109, 5215–5244. [Google Scholar] [CrossRef]
- Chen, C. Designing catalysts for olefin polymerization and copolymerization: Beyond electronic and steric tuning. Nat. Rev. Chem. 2018, 2, 6–14. [Google Scholar] [CrossRef]
- Sauter, D.W.; Taoufik, M.; Boisson, C. Polyolefins, a Success Story. Polymers 2017, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Sturzel, M.; Mihan, S.; Mulhaupt, R. From Multisite Polymerization Catalysis to Sustainable Materials and All-Polyolefin Composites. Chem. Rev. 2016, 116, 1398–1433. [Google Scholar] [CrossRef]
- Dong, J.-Y.; Hu, Y. Design and synthesis of structurally well-defined functional polyolefins via transition metal-mediated olefin polymerization chemistry. Coord. Chem. Rev. 2006, 250, 47–65. [Google Scholar] [CrossRef]
- Tan, C.; Zou, C.; Chen, C. Material Properties of Functional Polyethylenes from Transition Metal-Catalyzed Ethylene–Polar Monomer Copolymerization. Macromolecules 2022, 55, 1910–1922. [Google Scholar] [CrossRef]
- Boaena, N.K.; Hillmyer, M.A. Post-polymerization functionalization of polyolefins. Chem. Soc. Rev. 2005, 34, 267–275. [Google Scholar] [CrossRef]
- Franssen, N.M.G.; Reek, J.N.H.; Bruin, B. Synthesis of functional ‘polyolefins’: State of the art and remaining challenges. Chem. Soc. Rev. 2013, 42, 5809–5832. [Google Scholar] [CrossRef]
- Mua, H.; Zhou, G.; Hu, X.; Jian, Z. Recent advances in nickel mediated copolymerization of olefin with polar monomers. Coord. Chem. Rev. 2021, 435, 213802. [Google Scholar] [CrossRef]
- Tan, C.; Chen, C. Emerging Palladium and Nickel Catalysts for Copolymerization of Olefins with Polar Monomers. Angew. Chem. Int. Ed. 2019, 58, 7192–7200. [Google Scholar] [CrossRef] [PubMed]
- Gladysz, J.A.; Ball, Z.T.; Bertrand, G.; Blum, S.A.; Dong, V.M.; Dorta, R.; Hahn, F.E.; Humphrey, M.G.; Jones, W.D.; Klosin, J.; et al. Organometallics Roundtable 2011. Organometallics 2012, 31, 1–18. [Google Scholar] [CrossRef]
- Liu, G.; Huang, Z. Recent Advances in Coordination-Insertion Copolymerization of Ethylene with Polar Functionalized Comonomers. Chin. J. Chem. 2020, 38, 1445–1448. [Google Scholar] [CrossRef]
- Ito, S.; Nozaki, K. Coordination–Insertion Copolymerization of Polar Vinyl Monomers by Palladium Catalysts. Chem. Rec. 2010, 10, 315–325. [Google Scholar] [CrossRef]
- Nakamura, A.; Anselment, T.M.J.; Claverie, J.; Goodall, B.; Jordan, R.F.; Mecking, S.; Rieger, B.; Sen, A.; Leeuwen, P.W.N.M.V.; Nozaki, K. Ortho-Phosphinobenzenesulfonate: A Superb Ligand for Palladium-Catalyzed Coordination-Insertion Copolymerization of Polar Vinyl Monomers. Acc. Chem. Res. 2013, 46, 1438–1449. [Google Scholar] [CrossRef]
- Qasim, M.; Bashir, M.S.; Iqbal, S.; Mahmood, Q. Recent advancements in α-diimine-nickel and -palladium catalysts for ethylene polymerization. Eur. Polym. J. 2021, 160, 110783. [Google Scholar] [CrossRef]
- Birajdar, R.S.; Chikkali, S.H. Insertion copolymerization of functional olefins: Quo Vadis? Eur. Polym. J. 2021, 143, 110183. [Google Scholar] [CrossRef]
- Ittel, S.D.; Johnson, L.K. Late-Metal Catalysts for Ethylene Homo- and Copolymerization. Chem. Rev. 2000, 100, 1169–1203. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, H.; Wang, X.; Pan, L.; Li, Y. Elaborate Tuning in Ligand Makes a Big Difference in Catalytic Performance: Bulky Nickel Catalysts for (Co)polymerization of Ethylene with Promising Vinyl Polar Monomers. ChemCatChem 2019, 11, 2329–2340. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Breuil, P.A.R.; Magna, L.; Michel, T.; Espada Pastor, M.F.; Delcroix, D. Nickel Catalyzed Olefin Oligomerization and Dimerization. Chem. Rev. 2020, 120, 7919–7983. [Google Scholar] [CrossRef]
- Chen, Z.; Brookhart, M. Exploring Ethylene/Polar Vinyl Monomer Copolymerizations Using Ni and Pd alpha-Diimine Catalysts. Acc. Chem Res. 2018, 51, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Goudari, S.B.; Chen, C. A simple and versatile nickel platform for the generation of branched high molecular weight polyolefins. Nat. Commun. 2020, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Younkin, T.R.; Connor, E.F.; Henderson, J.I.; Friedrich, S.K.; Grubbs, R.H.; Bansleben, D.A. Neutral, Single-Component Nickel (11) Polyolefin Catalysts That Tolerate Heteroatoms. Science 2000, 287, 460–462. [Google Scholar] [CrossRef]
- Xin, B.S.; Sato, N.; Tanna, A.; Oishi, Y.; Konishi, Y.; Shimizu, F. Nickel Catalyzed Copolymerization of Ethylene and Alkyl Acrylates. J. Am. Chem. Soc. 2017, 139, 3611–3614. [Google Scholar] [CrossRef]
- Ito, S.; Ota, Y.; Nozaki, K. Ethylene/allyl monomer cooligomerization by nickel/phosphine-sulfonate catalysts. Dalton. Trans. 2012, 41, 13807–13809. [Google Scholar] [CrossRef]
- Jung, J.; Yasuda, H.; Nozaki, K. Copolymerization of Nonpolar Olefins and Allyl Acetate Using Nickel Catalysts Bearing a Methylene-Bridged Bisphosphine Monoxide Ligand. Macromolecules 2020, 53, 2547–2556. [Google Scholar] [CrossRef]
- Tao, W.; Nakano, R.; Ito, S.; Nozaki, K. Copolymerization of Ethylene and Polar Monomers by Using Ni/IzQO Catalysts. Angew. Chem. Int. Ed. 2016, 55, 2835–2839. [Google Scholar] [CrossRef]
- Wang, F.; Chen, C. A continuing legend: The Brookhart-type α-diimine nickel and palladium catalysts. Polym. Chem. 2019, 10, 2354–2369. [Google Scholar] [CrossRef]
- Chen, M.; Chen, C. The Synthesis of Functionalized Polyolefin Materials and Research on Material Properties. Polym. Bull. 2021, 6, 26–34. [Google Scholar] [CrossRef]
- Chen, M.; Chen, C. Polar and Functionalized Polyolefins: New Catalysts, New Modulation Strategies and New Materials. Acta. Polym. Sin. 2018, 11, 1372–1384. [Google Scholar] [CrossRef]
- Jian, Z. Synthesis of Functionalized Polyolefins: Design from Catalysts to Polar Monomers. Acta. Polym. Sin. 2018, 11, 1359–1371. [Google Scholar] [CrossRef]
- Zhou, G.; Cui, L.; Mu, H.; Jian, Z. Custom-made polar monomers utilized in nickel and palladium promoted olefin copolymerization. Polym. Chem. 2021, 12, 3878–3892. [Google Scholar] [CrossRef]
- Nozaki, K. Copolymerization of ethylene with non-vinyl polar monomers. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2022, 98, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Pan, L.; Song, D.; Li, Y. Neutral Nickel Catalysts for Olefin Homo- and Copolymerization: Relationships between Catalyst Structures and Catalytic Properties. Chem. Rev. 2015, 115, 12091–12137. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, F.; Li, Y. Late Transition Metal Complexes for Olefin Copolymerization with Polar Monomers. Chin. J. Org. Chem. 2021, 41, 1396–1433. [Google Scholar] [CrossRef]
- Chen, C. Redox-Controlled Polymerization and Copolymerization. ACS Catal. 2018, 8, 5506–5514. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Luo, Y.; Chen, C. A Second-Coordination-Sphere Strategy to Modulate Nickel- and Palladium-Catalyzed Olefin Polymerization and Copolymerization. Angew. Chem. Int. Ed. 2017, 56, 11604–11609. [Google Scholar] [CrossRef]
- Tran, T.V.; Karas, L.J.; Wu, J.I.; Do, L.H. Elucidating Secondary Metal Cation Effects on Nickel Olefin Polymerization Catalysts. ACS Catal. 2020, 10, 10760–10772. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, C. Influence of Polyethylene Glycol Unit on Palladium- and Nickel-Catalyzed Ethylene Polymerization and Copolymerization. Angew. Chem. Int. Ed. 2017, 56, 14672–14676. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Y.; Jian, Z. Tunable branching and living character in ethylene polymerization using “polyethylene glycol sandwich” α-diimine nickel catalysts. Polym. Chem. 2021, 12, 1236–1243. [Google Scholar] [CrossRef]
- Berkefeld, A.; Drexler, M.; Moller, H.M.; Mecking, S. Mechanistic Insights on the Copolymerization of Polar Vinyl Monomers with Neutral Ni(II) Catalysts. J. Am. Chem. Soc. 2009, 131, 12613–12622. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, Z.; Zhao, Y.; Luo, Y.; He, S. Multivariate Linear Regression Models to Predict Monomer Poisoning Effect in Ethylene/Polar Monomer Copolymerization Catalyzed by Late Transition Metals. Inorganics 2022, 10, 26. [Google Scholar] [CrossRef]
- Zhu, L.; Dai, Y.; Schrage, B.R.; Ziegler, C.J.; Jia, L. Ligand and solvent effects on the catalytic activity and lifetime of zwitterionic Nickel(II) catalysts for alternating CO-Ethylene copolymerization. J. Organomet. Chem. 2021, 952, 122045. [Google Scholar] [CrossRef]
- Baur, M.; Lin, F.; Morgen, T.O.; Odenwald, L.; Mecking, S. Polyethylene materials with in-chain ketones from nonalternating catalytic copolymerization. Science 2021, 374, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Pan, R.C.; Chen, M.; Liu, Y.; Chen, C.; Lu, X.B. Synthesis of Nonalternating Polyketones Using Cationic Diphosphazane Monoxide-Palladium Complexes. J. Am. Chem. Soc. 2021, 143, 10743–10750. [Google Scholar] [CrossRef]
- Chen, S.Y.; Ren, B.H.; Li, S.H.; Song, Y.H.; Jiao, S.; Zou, C.; Chen, C.; Lu, X.B.; Liu, Y. Cationic P,O-Coordinated Nickel(II) Catalysts for Carbonylative Polymerization of Ethylene: Unexpected Productivity via Subtle Electronic Variation. Angew. Chem. Int. Ed. 2022, 61, e202204126. [Google Scholar] [CrossRef]
- Tempel, D.J.; Johnson, L.K.; Huff, R.L.; White, P.S.; Brookhart, M. Mechanistic Studies of Pd(II)-α-Diimine-Catalyzed Olefin Polymerizations. J. Am. Chem. Soc. 2000, 122, 6686–6700. [Google Scholar] [CrossRef]
- Li, S.; Dai, S. Highly efficient incorporation of polar comonomers in copolymerizations with ethylene using iminopyridyl palladium system. J. Catal. 2021, 393, 51–59. [Google Scholar] [CrossRef]
- Guo, L.; Hu, X.; Lu, W.; Xu, G.; Liu, Q.; Dai, S. Investigations of ligand backbone effects on bulky diarylmethyl-based nickel(II) and palladium(II) catalyzed ethylene polymerization and copolymerization. J. Organomet. Chem. 2021, 952, 122046. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Pan, R.-C.; Liu, Y.; Lu, X.-B. Bulky o-Phenylene-Bridged Bimetallic α-Diimine Ni(II) and Pd(II) Catalysts in Ethylene (Co)polymerization. Organometallics 2021, 40, 3703–3711. [Google Scholar] [CrossRef]
- Ge, Y.; Li, S.; Wang, H.; Dai, S. Synthesis of Branched Polyethylene and Ethylene-MA Copolymers Using Unsymmetrical Iminopyridyl Nickel and Palladium Complexes. Organometallics 2021, 40, 3033–3041. [Google Scholar] [CrossRef]
- Liu, Y.; Harth, E. Distorted Sandwich α-Diimine Pd(II) Catalyst: Linear Polyethylene and Synthesis of Ethylene/Acrylate Elastomers. Angew. Chem. Int. Ed. 2021, 60, 24107–24115. [Google Scholar] [CrossRef]
- Zheng, H.; Zhong, L.; Du, C.; Du, W.; Cheung, C.S.; Ruan, J.; Gao, H. Combining hydrogen bonding interactions with steric and electronic modifications for thermally robust α-diimine palladium catalysts toward ethylene (co)polymerization. Catal. Sci. Technol. 2021, 11, 124–135. [Google Scholar] [CrossRef]
- Ge, Y.; Li, S.; Fan, W.; Dai, S. Flexible “Sandwich” (8-Alkylnaphthyl α-Diimine) Catalysts in Insertion Polymerization. Inorg. Chem. 2021, 60, 5673–5681. [Google Scholar] [CrossRef]
- Alberoni, C.; D’Alterio, M.C.; Balducci, G.; Immirzi, B.; Polentarutti, M.; Pellecchia, C.; Milani, B. Tunable “In-Chain” and “At the End of the Branches” Methyl Acrylate Incorporation in the Polyolefin Skeleton through Pd(II) Catalysis. ACS Catal. 2022, 12, 3430–3443. [Google Scholar] [CrossRef]
- Dai, S.; Li, G.; Lu, W.; Liao, Y.; Fan, W. Suppression of chain transfer via a restricted rotation effect of dibenzosuberyl substituents in polymerization catalysis. Polym. Chem. 2021, 12, 3240–3249. [Google Scholar] [CrossRef]
- Hai, Z.; Lu, Z.; Li, S.; Cao, Z.; Dai, S. The synergistic effect of rigid and flexible substituents on insertion polymerization with α-diimine nickel and palladium catalysts. Polym. Chem. 2021, 12, 4643–4653. [Google Scholar] [CrossRef]
- Zhong, S.; Tan, Y.; Zhong, L.; Gao, J.; Liao, H.; Jiang, L.; Gao, H.; Wu, Q. Precision Synthesis of Ethylene and Polar Monomer Copolymers by Palladium-Catalyzed Living Coordination Copolymerization. Macromolecules 2017, 50, 5661–5669. [Google Scholar] [CrossRef]
- Dai, S.; Li, S. Effect of aryl orientation on olefin polymerization in iminopyridyl catalytic system. Polymer 2020, 200, 122607. [Google Scholar] [CrossRef]
- Guo, L.; Dai, S.; Sui, X.; Chen, C. Palladium and Nickel Catalyzed Chain Walking Olefin Polymerization and Copolymerization. ACS Catal. 2015, 6, 428–441. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Zhong, L.; Du, C.; Du, W.; Zheng, H.; Cheung, C.S.; Wang, L.; Gao, H. Unprecedented Steric and Positioning Effects of Comonomer Substituents on α-Diimine Palladium-Catalyzed Vinyl Arene/CO Copolymerization. Macromolecules 2021, 54, 687–695. [Google Scholar] [CrossRef]
- Xiao, Z.; Zheng, H.; Du, C.; Zhong, L.; Liao, H.; Gao, J.; Gao, H.; Wu, Q. Enhancement on Alternating Copolymerization of Carbon Monoxide and Styrene by Dibenzobarrelene-Based α-Diimine Palladium Catalysts. Macromolecules 2018, 51, 9110–9121. [Google Scholar] [CrossRef]
- Wang, G.; Li, M.; Pang, W.; Chen, M.; Tan, C. Lewis acids in situ modulate pyridazine-imine Ni catalysed ethylene (co)polymerisation. New J. Chem. 2019, 43, 13630–13634. [Google Scholar] [CrossRef]
- Tan, C.; Zou, C.; Chen, C. An Ionic Cluster Strategy for Performance Improvements and Product Morphology Control in Metal-Catalyzed Olefin–Polar Monomer Copolymerization. J. Am. Chem. Soc. 2022, 144, 2245–2254. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Gao, H.; Du, C.; Wang, L.; Zhong, L.; Gao, H.; Wu, Q. Bulky Backbone Strategy in α-Diimine Nickel and Palladium Catalyzed-Olefin Polymerization. Polym. Bull. 2021, 6, 81–93. [Google Scholar] [CrossRef]
- Hu, X.; Wang, C.; Jian, Z. Comprehensive studies of the ligand electronic effect on unsymmetrical α-diimine nickel(ii) promoted ethylene (co)polymerizations. Polym. Chem. 2020, 11, 4005–4012. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Zhang, Y.; Jian, Z. Unsymmetrical Strategy Makes Significant Differences in α-Diimine Nickel and Palladium Catalyzed Ethylene (Co)Polymerizations. ChemCatChem 2020, 12, 2497–2505. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Wang, C.-Q.; Hu, X.-Q.; Xia, Y.; Chi, Y.; Zhang, Y.-X.; Jian, Z.-B. Benzosuberyl Substituents as a “Sandwich-like” Function in Olefin Polymerization Catalysis. Chin. J. Polym. Sci. 2021, 39, 984–993. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Li, B.; Jian, Z. Horizontally and Vertically Concerted Steric Strategy in α-Diimine Nickel Promoted Ethylene (Co)Polymerization. Chin. J. Chem. 2021, 39, 2829–2836. [Google Scholar] [CrossRef]
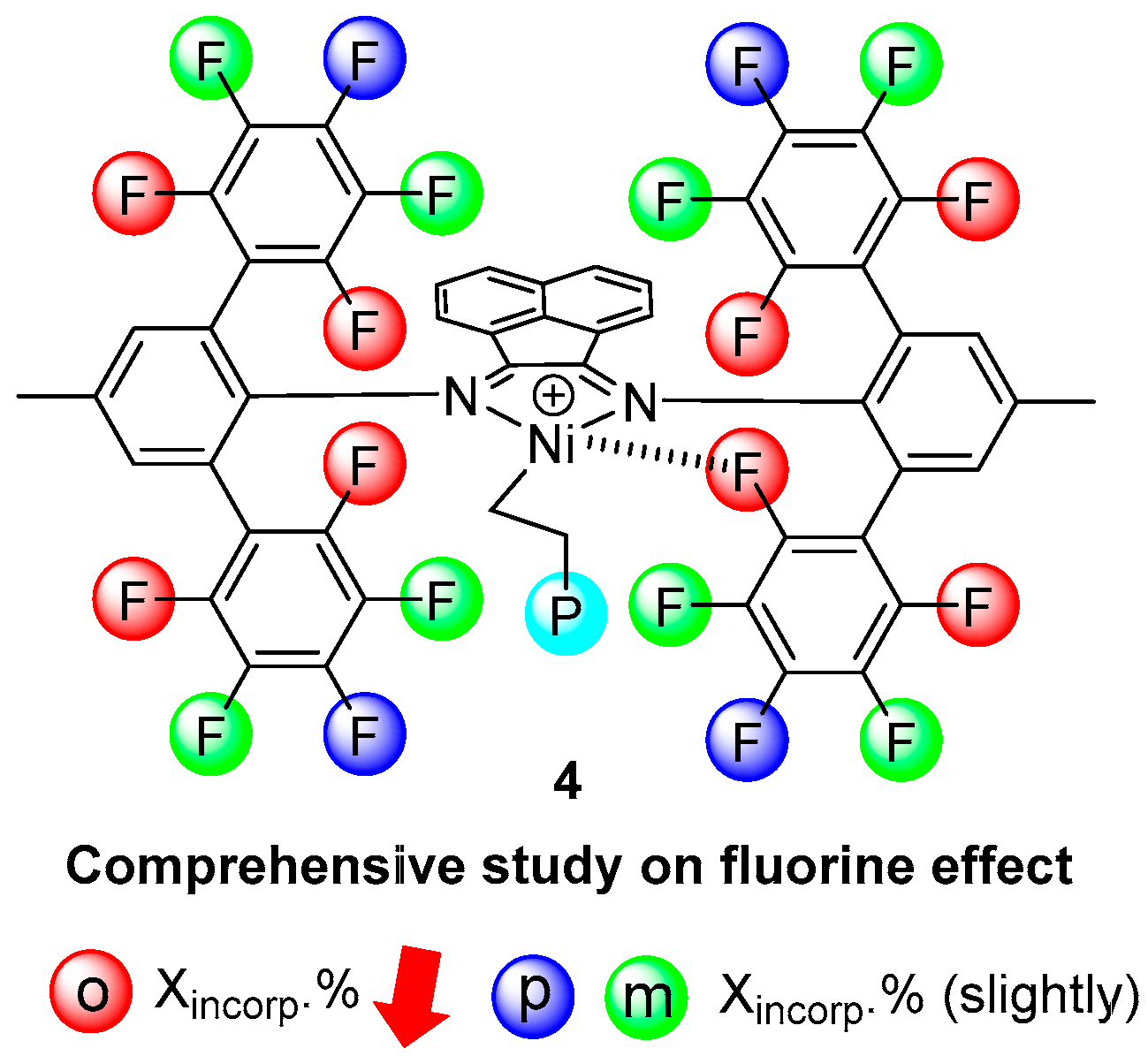
- Hu, X.; Zhang, Y.; Li, B.; Jian, Z. Fluorinated α-Diimine Nickel Mediated Ethylene (Co)Polymerization. Chem. Eur. J. 2021, 27, 11935–11942. [Google Scholar] [CrossRef]
- Zhong, L.; Zheng, H.; Du, C.; Du, W.; Liao, G.; Cheung, C.S.; Gao, H. Thermally robust α-diimine nickel and palladium catalysts with constrained space for ethylene (co)polymerizations. J. Catal. 2020, 384, 208–217. [Google Scholar] [CrossRef]
- Kanai, Y.; Foro, S.; Plenio, H. Bispentiptycenyl–Diimine–Nickel Complexes for Ethene Polymerization and Copolymerization with Polar Monomers. Organometallics 2019, 38, 544–551. [Google Scholar] [CrossRef]
- Peng, D.; Chen, C. Photoresponsive Palladium and Nickel Catalysts for Ethylene Polymerization and Copolymerization. Angew. Chem. Int. Ed. 2021, 60, 22195–22200. [Google Scholar] [CrossRef]
- Huo, P.; Liu, W.; He, X.; Chen, Y. Norbornene/n-Butyl methacrylate copolymerization over α-Diimine nickel and palladium catalysts supported on multiwalled carbon nanotubes. J. Polyme. Sci. A Polym. Chem. 2014, 52, 3213–3220. [Google Scholar] [CrossRef]
- Liang, C.; Yang, J.; Luo, G.; Luo, Y. Benchmark study of density functionals for the insertions of olefin and polar monomers catalyzed by α–diimine palladium complexes. Comput. Theor. Chem. 2020, 1187, 112942. [Google Scholar] [CrossRef]
- Olscher, F.; Gottker-Schnetmann, I.; Monteil, V.; Mecking, S. Role of Radical Species in Salicylaldiminato Ni(II) Mediated Polymer Chain Growth: A Case Study for the Migratory Insertion Polymerization of Ethylene in the Presence of Methyl Methacrylate. J. Am. Chem. Soc. 2015, 137, 14819–14828. [Google Scholar] [CrossRef]
- Schiebel, E.; Santacroce, S.; Falivene, L.; Göttker-Schnetmann, I.; Caporaso, L.; Mecking, S. Tailored Strength Neighboring Group Interactions Switch Polymerization to Dimerization Catalysis. ACS Catal. 2019, 9, 3888–3894. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Li, Y.; Chen, Y.; Hu, N. Copolymerization of Ethylene with Methyl Methacrylate with Neutral Nickel(II) Complexes Bearing β-Ketoiminato Chelate Ligands. Organometallics 2005, 24, 2502–2510. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, L.; Tanaka, R.; Shiono, T.; Cai, Z. Highly Robust Nickel Catalysts Containing Anilinonaphthoquinone Ligand for Copolymerization of Ethylene and Polar Monomers. Macromolecules 2017, 50, 9216–9221. [Google Scholar] [CrossRef]
- Chowdhury, S.I.; Tanaka, R.; Nakayama, Y.; Shiono, T. Synthesis of norbornene/divinylbenzene copolymers catalyzed by anilinonaphthoquinone-ligated nickel complexes and their applications for the synthesis of graft polymers. J. Polym. Sci. 2020, 58, 1564–1570. [Google Scholar] [CrossRef]
- Chowdhury, S.I.; Tanaka, R.; Nakayama, Y.; Shiono, T. Coordination-Insertion Copolymerization of Norbornene and p-Substituted Styrenes Using Anilinonaphthoquinone-Ligated Nickel Complexes. Macromol. Chem. Phys. 2020, 221, 1900494. [Google Scholar] [CrossRef]
- Cheng, H.; Cai, Z. (Anilino)anthraquinone Nickel-Catalyzed Random Copolymerization of Norbornene and Ethylene. ChemCatChem 2018, 10, 497–500. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, H.; Li, Y.; Hu, Y.; Zhang, X.; Cai, Z. Structurally simple dinuclear nickel catalyzed olefin copolymerization with polar monomers. J. Catal. 2018, 368, 291–297. [Google Scholar] [CrossRef]
- Cheng, H.; Su, Y.; Hu, Y.; Zhang, X.; Cai, Z. Ethylene Polymerization and Copolymerization with Polar Monomers Using Nickel Complexes Bearing Anilinobenzoic Acid Methyl Ester Ligand. Polymers 2018, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Dai, S.; Chen, C. Ethylene Polymerization and Copolymerization Using Nickel 2-Iminopyridine-N-oxide Catalysts: Modulation of Polymer Molecular Weights and Molecular-Weight Distributions. Macromolecules 2017, 51, 49–56. [Google Scholar] [CrossRef]
- Li, M.; Cai, Z.; Eisen, M.S. Neutral Nickel(II) Complexes Bearing Aryloxide Imidazolin-2-imine Ligands for Efficient Copolymerization of Norbornene and Polar Monomers. Organometallics 2018, 37, 4753–4762. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Xiao, R.; Cai, Z. Bis(N-acylated imidazolin-2-imine) nickel catalyzed norbornene copolymerization with methyl acrylate. Polym. Chem. 2020, 11, 5542–5547. [Google Scholar] [CrossRef]
- Gao, J.; Yang, B.; Chen, C. Sterics versus electronics: Imine/phosphine-oxide-based nickel catalysts for ethylene polymerization and copolymerization. J. Catal. 2019, 369, 233–238. [Google Scholar] [CrossRef]
- Zou, C.; Tan, C.; Pang, W.; Chen, C. Amidine/Phosphine-Oxide-Based Nickel Catalysts for Ethylene Polymerization and Copolymerization. ChemCatChem 2019, 11, 5339–5344. [Google Scholar] [CrossRef]
- Cai, Z.; Do, L.H. Customizing Polyolefin Morphology by Selective Pairing of Alkali Ions with Nickel Phenoxyimine-Polyethylene Glycol Catalysts. Organometallics 2017, 36, 4691–4698. [Google Scholar] [CrossRef]
- Bie, Z.; Li, B.G.; Hu, J.; Yao, Z. Studies on Alternating Copolymerization of Ethylene and Carbon Monoxide Using Nickel-Based Catalyst: Cocatalyst and the Polarity of Solvent. Macromol. React. Eng. 2021, 16, 2100047. [Google Scholar] [CrossRef]
- Saki, Z.; D’Auria, I.; Dall’Anese, A.; Milani, B.; Pellecchia, C. Copolymerization of Ethylene and Methyl Acrylate by Pyridylimino Ni(II) Catalysts Affording Hyperbranched Poly(ethylene-co-methyl acrylate)s with Tunable Structures of the Ester Groups. Macromolecules 2020, 53, 9294–9305. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, H.; Xiao, R.; Cai, Z. Rational design of nickel catalysts containing N-acylated imidazoline-2-imine ligand for ethylene copolymerization with polar monomer. J. Catal. 2020, 383, 117–123. [Google Scholar] [CrossRef]
- Li, K.; Mu, H.; Kang, X.; Jian, Z. Suppression of Chain Transfer and Promotion of Chain Propagation in Neutral Anilinotropone Nickel Polymerization Catalysis. Macromolecules 2022, 55, 2533–2541. [Google Scholar] [CrossRef]
- Cui, L.; Hu, X.; Zhang, Y.; Jian, Z. Systematic Study of Fluorine Effect on α-Ketiminato Nickel Catalyzed Ethylene (Co)Polymerization. Acta. Polym. Sin. 2021, 52, 531–540. [Google Scholar] [CrossRef]
- Chua, Y.; Hu, X.; Zhang, Y.; Liu, D.; Zhang, Y.; Jian, Z. Influence of Backbone and Axial Substituent of Catalyst on α-Imino-ketone Nickel Mediated Ethylene (Co)Polymerization. Chin. J. Polym. Sci. 2022, 40, 469–477. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, C.; Zhao, H.; Cai, Z.; Chen, C. Hydrogen-Bonding-Induced Heterogenization of Nickel and Palladium Catalysts for Copolymerization of Ethylene with Polar Monomers. Angew. Chem. Int. Ed. 2021, 60, 17446–17451. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, X.; Gong, X.; Xu, D.; Ma, Y. Macrocyclic Trinuclear Nickel Phenoxyimine Catalysts for High-Temperature Polymerization of Ethylene and Isospecific Polymerization of Propylene. Macromolecules 2017, 50, 6561–6568. [Google Scholar] [CrossRef]
- Ji, G.; Chen, Z.; Wang, X.; Ning, X.; Xu, C.; Zhang, X.; Tao, W.; Li, J.; Gao, Y.; Shen, Q.; et al. Direct copolymerization of ethylene with protic comonomers enabled by multinuclear Ni catalysts. Nat. Commun. 2021, 12, 6283. [Google Scholar] [CrossRef]
- Keim, W. Oligomerization of ethylene to alpha-olefins: Discovery and development of the shell higher olefin process (SHOP). Angew. Chem. Int. Ed. 2013, 52, 12492–12496. [Google Scholar] [CrossRef]
- Gibson, V.C.; Tomov, A. Functionalized polyolefin synthesis using [P., O] Ni catalysts. Chem. Commun. 2001, 19, 1964–1965. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, A.; Grau, E.; Broyer, J.-P.; Boisson, C.; Spitz, R.; Monteil, V. Homo- and Copolymerizations of (Meth)Acrylates with Olefins (Styrene, Ethylene) Using Neutral Nickel Complexes: A Dual Radical/Catalytic Pathway. Macromolecules 2011, 44, 3293–3301. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, H.; Pan, L.; Wang, X.; Li, Y. Robust Bulky [P, O] Neutral Nickel Catalysts for Copolymerization of Ethylene with Polar Vinyl Monomers. ACS Catal. 2018, 8, 5963–5976. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Pan, L.; Wang, B.; Li, Y. Facile Synthesis of High-Molecular-Weight Vinyl Sulfone (Sulfoxide) Modified Polyethylenes via Coordination–Insertion Copolymerization. Macromolecules 2020, 53, 5177–5187. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Li, W.W.; Li, B.X.; Mu, H.L.; Li, Y.S. Well-defined phosphino-phenolate neutral nickel(II) catalysts for efficient (co)polymerization of norbornene and ethylene. Dalton Trans. 2015, 44, 7382–7394. [Google Scholar] [CrossRef]
- Mu, H.; Li, Y.; Li, Y. Ethylene Homo- and Copolymerization by Single Component Phosphinophenolate Neutral Nickel Catalysts. Chin. J. Appl. Chem. 2012, 12, 1381–1388. [Google Scholar] [CrossRef]
- Konishi, Y.; Tao, W.-j.; Yasuda, H.; Ito, S.; Oishi, Y.; Ohtaki, H.; Tanna, A.; Tayano, T.; Nozaki, K. Nickel-Catalyzed Propylene/Polar Monomer Copolymerization. ACS Macro Lett. 2018, 7, 213–217. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Chi, Y.; Jian, Z. Influence of initiating groups on phosphino-phenolate nickel catalyzed ethylene (co)polymerization. Dalton Trans. 2020, 49, 2636–2644. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Wang, F.; Pan, L.; Wang, B.; Li, Y. Robust and Reactive Neutral Nickel Catalysts for Ethylene Polymerization and Copolymerization with a Challenging 1,1-Disubstituted Difunctional Polar Monomer. ACS Catal. 2021, 11, 2902–2911. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Pan, L.; Wang, F.; Luo, S.; Li, Y. Reactivity of Phosphino-naphtholate Nickel Complexes and Their Catalysis of Copolymerization with Polar Monomers. ChemCatChem 2022, 14, e202101736. [Google Scholar] [CrossRef]
- Xiong, S.; Shoshani, M.M.; Zhang, X.; Spinney, H.A.; Nett, A.J.; Henderson, B.S.; Miller III, T.F.; Agapie, T. Efficient Copolymerization of Acrylate and Ethylene with Neutral P, O-Chelated Nickel Catalysts: Mechanistic Investigations of Monomer Insertion and Chelate Formation. J. Am. Chem. Soc. 2021, 143, 6516–6527. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.V.; Nguyen, Y.H.; Do, L.H. Development of highly productive nickel–sodium phenoxyphosphine ethylene polymerization catalysts and their reaction temperature profiles. Polym. Chem. 2019, 10, 3718–3721. [Google Scholar] [CrossRef]
- Zhang, Y.; Jian, Z. 2-Phosphine-pyridine-N-oxide palladium and nickel catalysts for ethylene polymerization and copolymerization with polar monomers. Polymer 2020, 194, 122410. [Google Scholar] [CrossRef]
- Cui, L.; Jian, Z. A N-bridged strategy enables hemilabile phosphine–carbonyl palladium and nickel catalysts to mediate ethylene polymerization and copolymerization with polar vinyl monomers. Polym. Chem. 2020, 11, 6187–6193. [Google Scholar] [CrossRef]
- Cui, L.; Chu, Y.; Liu, D.; Han, Y.; Mu, H.; Jian, Z. Enhancement on Hemilabile Phosphine-Amide Palladium and Nickel Catalysts for Ethylene (Co)Polymerization with Polar Monomers Using a Cyclizing Strategy. Chin. J. Polym. Sci. 2022, 40, 241–247. [Google Scholar] [CrossRef]
- Zhu, N.; Liang, T.; Huang, Y.; Pang, W.; Chen, M.; Tan, C. Influences of Ligand Backbone Substituents on Phosphinecarbonylpalladium and -nickel Catalysts for Ethylene Polymerization and Copolymerization with Polar Monomers. Inorg. Chem. 2021, 60, 13080–13090. [Google Scholar] [CrossRef]
- Hu, X.; Ma, X.; Jian, Z. Coordination–insertion polymerization of polar allylbenzene monomers. Polym. Chem. 2019, 10, 1912–1919. [Google Scholar] [CrossRef]
- Wang, X.; Nozaki, K. Selective Chain-End Functionalization of Polar Polyethylenes: Orthogonal Reactivity of Carbene and Polar Vinyl Monomers in Their Copolymerization with Ethylene. J. Am. Chem. Soc. 2018, 140, 15635–15640. [Google Scholar] [CrossRef]
- Carrow, B.P.; Nozaki, K. Transition-Metal-Catalyzed Functional Polyolefin Synthesis: Effecting Control through Chelating Ancillary Ligand Design and Mechanistic Insights. Macromolecules 2014, 47, 2541–2555. [Google Scholar] [CrossRef]
- Chen, M.; Chen, C. Rational Design of High-Performance Phosphine Sulfonate Nickel Catalysts for Ethylene Polymerization and Copolymerization with Polar Monomers. ACS Catal. 2017, 7, 1308–1312. [Google Scholar] [CrossRef] [Green Version]
- Song, G.; Pang, W.; Li, W.; Chen, M.; Chen, C. Phosphine-sulfonate-based nickel catalysts: Ethylene polymerization and copolymerization with polar-functionalized norbornenes. Polym. Chem. 2017, 8, 7400–7405. [Google Scholar] [CrossRef]
- Liang, T.; Chen, C. Position Makes the Difference: Electronic Effects in Nickel-Catalyzed Ethylene Polymerizations and Copolymerizations. Inorg. Chem. 2018, 57, 14913–14919. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, Y.; Zhang, J.; Jian, Z. High-Performance Neutral Phosphine-Sulfonate Nickel(II) Catalysts for Efficient Ethylene Polymerization and Copolymerization with Polar Monomers. Organometallics 2019, 38, 1118–1126. [Google Scholar] [CrossRef]
- Behzadi, S.; Zou, C.; Yang, B.-P.; Tan, C.; Chen, C.-L. Styrene-containing Phosphine-sulfonate Ligands for Nickel- and Palladium-catalyzed Ethylene Polymerization. Chin. J. Polym. Sci. 2020, 39, 447–454. [Google Scholar] [CrossRef]
- Noda, S.; Nakamura, A.; Kochi, T.; Chung, L.; Morokuma, K.; Nozaki, K. Mechanistic Studies on the Formation of Linear Polyethylene Chain Catalyzed by Palladium Phosphine-Sulfonate Complexes: Experiment and Theoretical Studies. J. Am. Chem. Soc. 2009, 131, 14088–14100. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, A.; Xu, X.; Raza, W.; Kukkar, D.; Kim, K.; Luo, Y. Computational study of the copolymerization mechanism of ethylene with methyl 2-acetamidoacrylate catalyzed by phosphine-sulfonate palladium complexes. New J. Chem. 2021, 45, 16670–16678. [Google Scholar] [CrossRef]
- Tan, C.; Qasim, M.; Pang, W.; Chen, C. Ligand–metal secondary interactions in phosphine–sulfonate palladium and nickel catalyzed ethylene (co)polymerization. Polym. Chem. 2020, 11, 411–416. [Google Scholar] [CrossRef]
- Xu, M.; Chen, C. A disubstituted-norbornene-based comonomer strategy to address polar monomer problem. Sci. Bull. 2021, 66, 1429–1436. [Google Scholar] [CrossRef]
- Xia, J.; Han, Y.-F.; Kou, S.; Zhang, Y.; Jian, Z. Exploring steric effect of electron-donating group in palladium and nickel mediated ethylene polymerization and copolymerization with polar monomers. Eur. Polym. J. 2021, 160, 110781. [Google Scholar] [CrossRef]
- Zou, C.; Zhang, H.; Tan, C.; Cai, Z. Polyolefins with Intrinsic Antimicrobial Properties. Macromolecules 2021, 54, 64–70. [Google Scholar] [CrossRef]
- Chen, M.; Chen, C. Direct and Tandem Routes for the Copolymerization of Ethylene with Polar Functionalized Internal Olefins. Angew. Chem. Int. Ed. 2020, 59, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.R.; Deshmukh, S.S.; Koshti, V.S.; Poddar, S.; Gonnade, R.G.; Rajamohanan, P.R.; Chikkali, S.H. Reactivity of Difunctional Polar Monomers and Ethylene Copolymerization: A Comprehensive Account. Macromolecules 2017, 50, 5748–5758. [Google Scholar] [CrossRef]
- Gaikwad, S.R.; Deshmukh, S.S.; Gonnade, R.G.; Rajamohanan, P.R.; Chikkali, S.H. Insertion Copolymerization of Difunctional Polar Vinyl Monomers with Ethylene. ACS Macro Lett. 2015, 4, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.; Chen, C. Catechol Functionalized Polyolefins. Angew. Chem. Int. Ed. 2020, 59, 7953–7959. [Google Scholar] [CrossRef] [PubMed]
- Carrow, B.P.; Nozaki, K. Synthesis of Functional Polyolefins Using Cationic Bisphosphine Monoxide–Palladium Complexes. J. Am. Chem. Soc. 2012, 134, 8802–8805. [Google Scholar] [CrossRef] [PubMed]
- Mitsushige, Y.; Carrow, B.P.; Ito, S.; Nozaki, K. Ligand-controlled insertion regioselectivity accelerates copolymerisation of ethylene with methyl acrylate by cationic bisphosphine monoxide–palladium catalysts. Chem. Sci. 2016, 7, 737–744. [Google Scholar] [CrossRef]
- Wilders, A.M.; Contrella, N.D.; Sampson, J.R.; Zheng, M.; Jordan, R.F. Allosteric Effects in Ethylene Polymerization Catalysis. Enhancement of Performance of Phosphine-Phosphinate and Phosphine-Phosphonate Palladium Alkyl Catalysts by Remote Binding of B(C6F5)3. Organometallics 2017, 36, 4990–5002. [Google Scholar] [CrossRef]
- Zhang, W.; Waddell, P.M.; Tiedemann, M.A.; Padilla, C.E.; Mei, J.; Chen, L.; Carrow, B.P. Electron-Rich Metal Cations Enable Synthesis of High Molecular Weight, Linear Functional Polyethylenes. J. Am. Chem. Soc. 2018, 140, 8841–8850. [Google Scholar] [CrossRef]
- Sui, X.; Dai, S.; Chen, C. Ethylene Polymerization and Copolymerization with Polar Monomers by Cationic Phosphine Phosphonic Amide Palladium Complexes. ACS Catal. 2015, 5, 5932–5937. [Google Scholar] [CrossRef]
- Mu, H.-L.; Ye, J.-H.; Zhou, G.-L.; Li, K.-K.; Jian, Z.-B. Ethylene Polymerization and Copolymerization with Polar Monomers by Benzothiophene-bridged BPMO-Pd Catalysts. Chin. J. Polym. Sci. 2019, 38, 579–586. [Google Scholar] [CrossRef]
- Li, K.; Ye, J.; Wang, Z.; Mu, H.; Jian, Z. Indole-bridged bisphosphine-monoxide palladium catalysts for ethylene polymerization and copolymerization with polar monomers. Polym. Chem. 2020, 11, 2740–2748. [Google Scholar] [CrossRef]
- Ye, J.; Mu, H.; Wang, Z.; Jian, Z. Heteroaryl Backbone Strategy in Bisphosphine Monoxide Palladium-Catalyzed Ethylene Polymerization and Copolymerization with Polar Monomers. Organometallics 2019, 38, 2990–2997. [Google Scholar] [CrossRef]
- Mitsushige, Y.; Yasuda, H.; Carrow, B.P.; Ito, S.; Kobayashi, M.; Tayano, T.; Watanabe, Y.; Okuno, Y.; Hayashi, S.; Kuroda, J.; et al. Methylene-Bridged Bisphosphine Monoxide Ligands for Palladium-Catalyzed Copolymerization of Ethylene and Polar Monomers. ACS Macro Lett. 2018, 7, 305–311. [Google Scholar] [CrossRef]
- Hong, C.; Sui, X.; Li, Z.; Pang, W.; Chen, M. Phosphine phosphonic amide nickel catalyzed ethylene polymerization and copolymerization with polar monomers. Dalton. Trans. 2018, 47, 8264–8267. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, C. A Versatile Ligand Platform for Palladium- and Nickel-Catalyzed Ethylene Copolymerization with Polar Monomers. Angew. Chem. Int. Ed. 2018, 57, 3094–3098. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, M.; Luo, G.; Chen, C.; Luo, Y. Diphosphazane-monoxide and Phosphine-sulfonate Palladium Catalyzed Ethylene Copolymerization with Polar Monomers: A Computational Study. Organometallics 2019, 38, 638–646. [Google Scholar] [CrossRef]
- Xu, M.; Yu, F.; Li, P.; Xu, G.; Zhang, S.; Wang, F. Enhancing Chain Initiation Efficiency in the Cationic Allyl-Nickel Catalyzed (Co)Polymerization of Ethylene and Methyl Acrylate. Inorg. Chem. 2020, 59, 4475–4482. [Google Scholar] [CrossRef]
- Zou, C.; Liao, D.; Pang, W.; Chen, M.; Tan, C. Versatile PNPO ligands for palladium and nickel catalyzed ethylene polymerization and copolymerization with polar monomers. J. Catal. 2021, 393, 281–289. [Google Scholar] [CrossRef]
- Xiao, D.; Cai, Z.; Do, L.H. Accelerating ethylene polymerization using secondary metal ions in tetrahydrofuran. Dalton. Trans. 2019, 48, 17887–17897. [Google Scholar] [CrossRef]
- Tahmouresilerd, B.; Xiao, D.; Do, L.H. Rigidifying Cation-Tunable Nickel Catalysts Increases Activity and Polar Monomer Incorporation in Ethylene and Methyl Acrylate Copolymerization. Inorg. Chem. 2021, 60, 19035–19043. [Google Scholar] [CrossRef]
- Nakano, R.; Nozaki, K. Copolymerization of Propylene and Polar Monomers Using Pd/IzQO Catalysts. J. Am. Chem. Soc. 2015, 137, 10934–10937. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Wang, X.; Ito, S.; Nozaki, K. Palladium complexes bearing an N-heterocyclic carbene–sulfonamide ligand for cooligomerization of ethylene and polar monomers. J. Polym. Sci. A Polym. Chem. 2018, 57, 474–477. [Google Scholar] [CrossRef]
- Akita, S.; Nakano, R.; Ito, S.; Nozaki, K. Synthesis and Reactivity of Methylpalladium Complexes Bearing a Partially Saturated IzQO Ligand. Organometallics 2018, 37, 2286–2296. [Google Scholar] [CrossRef]
- Tao, W.; Akita, S.; Nakano, R.; Ito, S.; Hoshimoto, Y.; Ogoshi, S.; Nozaki, K. Copolymerisation of ethylene with polar monomers by using palladium catalysts bearing an N-heterocyclic carbene-phosphine oxide bidentate ligand. Chem. Commun. 2017, 53, 2630–2633. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Nakano, R.; Ito, S.; Nozaki, K. Palladium/IzQO-Catalyzed Coordination-Insertion Copolymerization of Ethylene and 1,1-Disubstituted Ethylenes Bearing a Polar Functional Group. J. Am. Chem. Soc. 2018, 140, 1876–1883. [Google Scholar] [CrossRef]
- Park, D.-A.; Byun, S.; Ryu, J.Y.; Lee, J.; Lee, J.; Hong, S. Abnormal N-Heterocyclic Carbene–Palladium Complexes for the Copolymerization of Ethylene and Polar Monomers. ACS Catal. 2020, 10, 5443–5453. [Google Scholar] [CrossRef]
- Seidel, F.W.; Nozaki, K. A Ni(0) sigma-Borane Complex Bearing a Rigid Bidentate Borane/Phosphine Ligand: Boryl Complex Formation by Oxidative Dehydrochloroborylation and Catalytic Activity for Ethylene Polymerization. Angew. Chem. Int. Ed. 2022, 61, e202111691. [Google Scholar] [CrossRef]
- Ulbrich, A.H.D.P.S.; Milani, J.L.S.; Roisnel, T.; Carpentier, J.-F.; Casagrande, O.L. Zwitterionic Ni(ii) complexes bearing pyrazolyl-ether-imidazolium ligands: Synthesis, structural characterization and use in ethylene oligomerization. New J. Chem. 2015, 39, 7234–7242. [Google Scholar] [CrossRef]
- Hameury, S.; de Frémont, P.; Breuil, P.-A.R.; Olivier-Bourbigou, H.; Braunstein, P. Bis(ether-functionalized NHC) Nickel(II) Complexes, Trans to Cis Isomerization Triggered by Water Coordination, and Catalytic Ethylene Oligomerization. Organometallics 2014, 34, 2183–2201. [Google Scholar] [CrossRef]
- Li, M.; Shu, X.; Cai, Z.; Eisen, M.S. Synthesis, Structures, and Norbornene Polymerization Behavior of Neutral Nickel(II) and Palladium(II) Complexes Bearing Aryloxide Imidazolidin-2-imine Ligands. Organometallics 2018, 37, 1172–1180. [Google Scholar] [CrossRef]
- Yang, D.; Dong, J.; Wang, B. Homo- and copolymerization of norbornene with tridentate nickel complexes bearing o-aryloxide-N-heterocyclic carbene ligands. Dalton. Trans. 2018, 47, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Cho, K.; Iwai, A.; Ito, T.; Iwasawa, N. Development of N-Phosphinomethyl-Substituted NHC-Nickel(0) Complexes as Robust Catalysts for Acrylate Salt Synthesis from Ethylene and CO2. Chemistry 2019, 25, 13504–13508. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hahm, H.; Ryu, J.Y.; Byun, S.; Park, D.-A.; Lee, S.H.; Lim, H.; Lee, J.; Hong, S. Pyridine-Chelated Imidazo [1,5-a] Pyridine N-Heterocyclic Carbene Nickel(II) Complexes for Acrylate Synthesis from Ethylene and CO2. Catalysts 2020, 10, 758. [Google Scholar] [CrossRef]
- Si, G.; Na, Y.; Chen, C. Ethylene (co)Oligomerization by Phosphine-Pyridine Based Palladium and Nickel Catalysts. ChemCatChem 2018, 10, 5135–5140. [Google Scholar] [CrossRef]
- Kocen, A.L.; Brookhart, M.; Daugulis, O. A highly active Ni(II)-triadamantylphosphine catalyst for ultrahigh-molecular-weight polyethylene synthesis. Nat. Commun. 2019, 10, 438. [Google Scholar] [CrossRef]
- Du, W.; Zheng, H.; Li, Y.; Cheung, C.S.; Li, D.; Gao, H.; Deng, H.; Gao, H. Neutral Tridentate α-Sulfonato-β-diimine Nickel Catalyst for (Co)polymerizations of Ethylene and Acrylates. Macromolecules 2022, 55, 3096–3105. [Google Scholar] [CrossRef]
- Dai, S.; Chen, C. A Self-Supporting Strategy for Gas-Phase and Slurry-Phase Ethylene Polymerization using Late-Transition-Metal Catalysts. Angew. Chem. Int. Ed. 2020, 59, 14884–14890. [Google Scholar] [CrossRef]
- Zou, C.; Si, G.; Chen, C. A general strategy for heterogenizing olefin polymerization catalysts and the synthesis of polyolefins and composites. Nat. Commun. 2022, 13, 1954. [Google Scholar] [CrossRef]
- Jones, G.R.; Basbug Alhan, H.E.; Karas, L.J.; Wu, J.I.; Harth, E. Switching the Reactivity of Palladium Diimines with “Ancillary” Ligand to Select between Olefin Polymerization, Branching Regulation, or Olefin Isomerization. Angew. Chem. Int. Ed. 2020, 60, 1635–1640. [Google Scholar] [CrossRef]
- Zhang, Y.; Jian, Z. Polar Additive Triggered Branching Switch and Block Polyolefin Topology in Living Ethylene Polymerization. Macromolecules 2021, 54, 3191–3196. [Google Scholar] [CrossRef]
- Rajput, B.S.; Pawal, S.B.; Bodkhe, D.V.; Rao, I.N.; Sainath, A.V.S.; Chikkali, S.H. Renewing polyethylene: Insertion copolymerization of sugar derived hydrophilic monomers with ethylene. Eur. Polym. J. 2020, 134, 109775. [Google Scholar] [CrossRef]
- Magenau, A.J.D.; Strandwitz, N.C.; Gennaro, A.; Matyjaszewski, K. Electrochemically Mediated Atom Transfer Radical Polymerization. Science. 2011, 332, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Keyes, A.; Dau, H.; Alhan, H.E.B.; Ha, U.; Ordonez, E.; Jones, G.R.; Liu, Y.-S.; Tsogtgerel, E.; Loftin, B.; Wen, Z.; et al. Metal–organic insertion light initiated radical (MILRad) polymerization: Photo-initiated radical polymerization of vinyl polar monomers with various palladium diimine catalysts. Polym. Chem. 2019, 10, 3040–3047. [Google Scholar] [CrossRef]
- Zou, C.; Chen, C. Polar-Functionalized, Crosslinkable, Self-Healing and Photoresponsive Polyolefins. Angew. Chem. Int. Ed. 2020, 59, 395–402. [Google Scholar] [CrossRef]
- Akita, S.; Nozaki, K. Copolymerization of ethylene and methyl acrylate by palladium catalysts bearing IzQO ligands containing methoxyethyl ether moieties and salt effects for polymerization. Polym. J. 2021, 53, 1057–1060. [Google Scholar] [CrossRef]
- Wang, G.; Peng, D.; Sun, Y.; Chen, C. Interplay of Supramolecular Chemistry and Photochemistry with Palladium-Catalyzed Ethylene Polymerization. CCS Chem. 2020, 2, 2025–2034. [Google Scholar] [CrossRef]





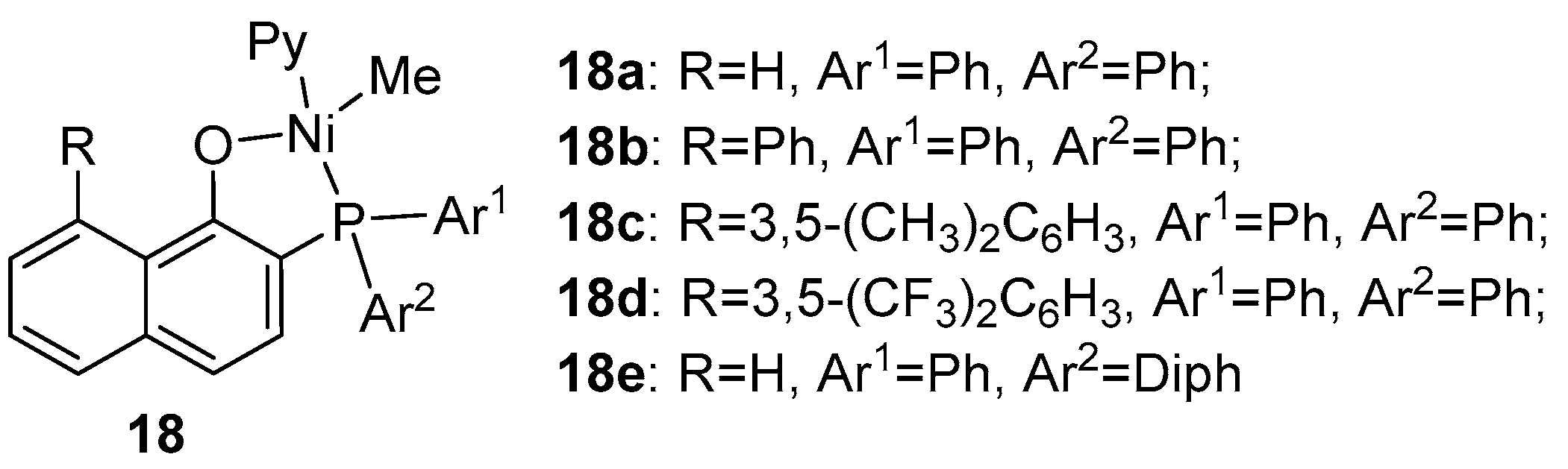

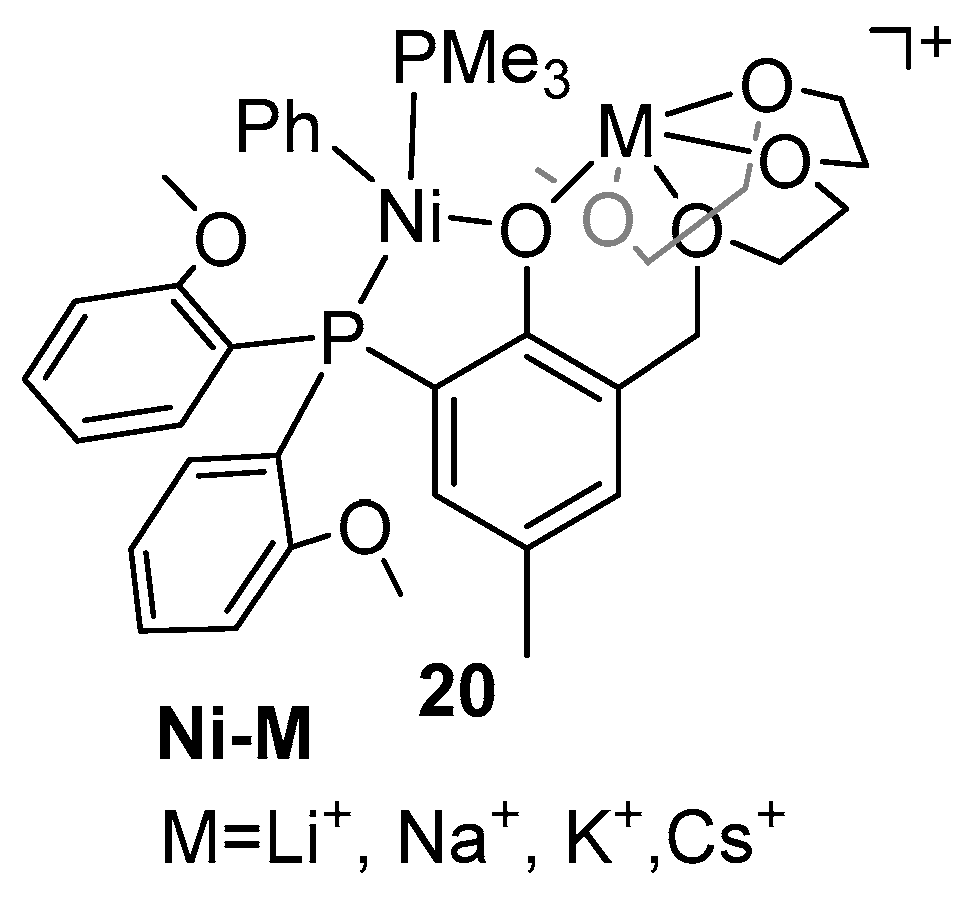
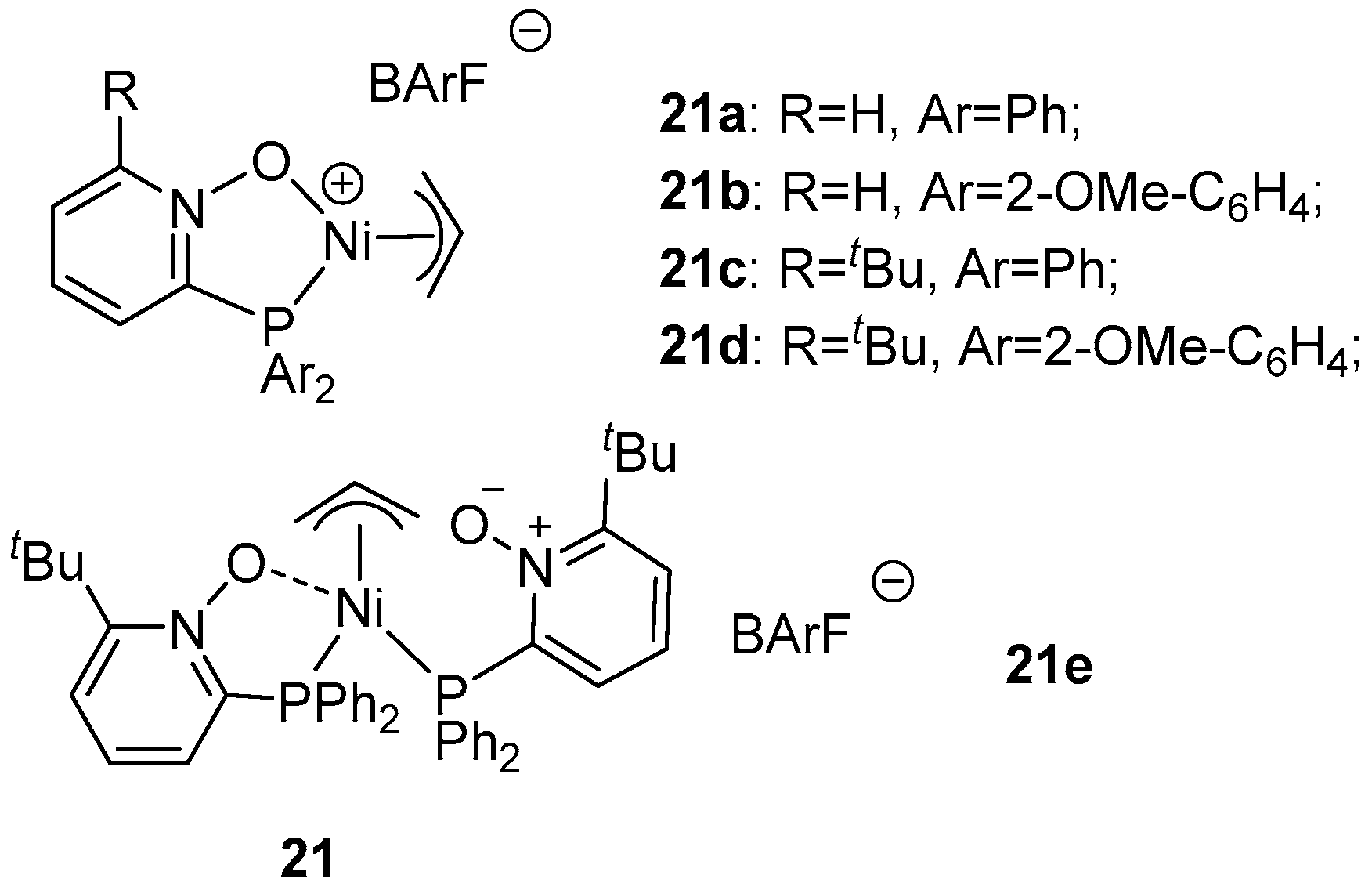


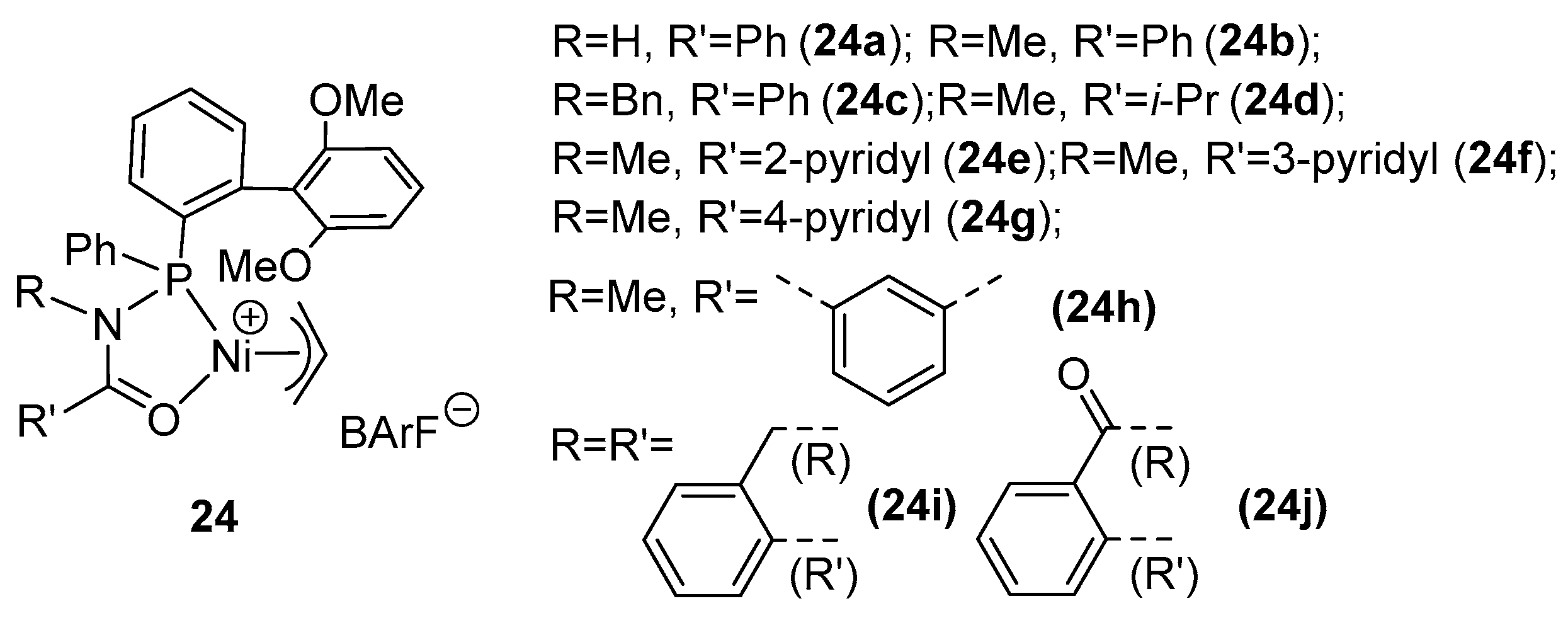
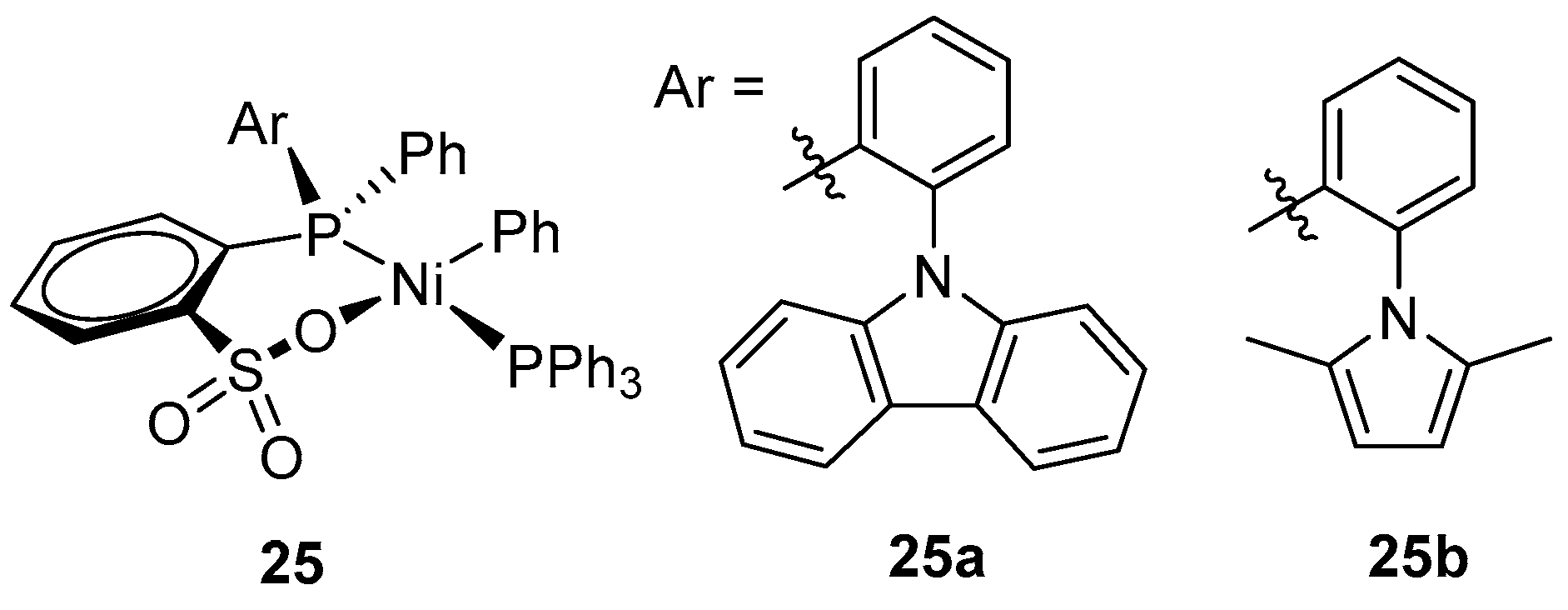

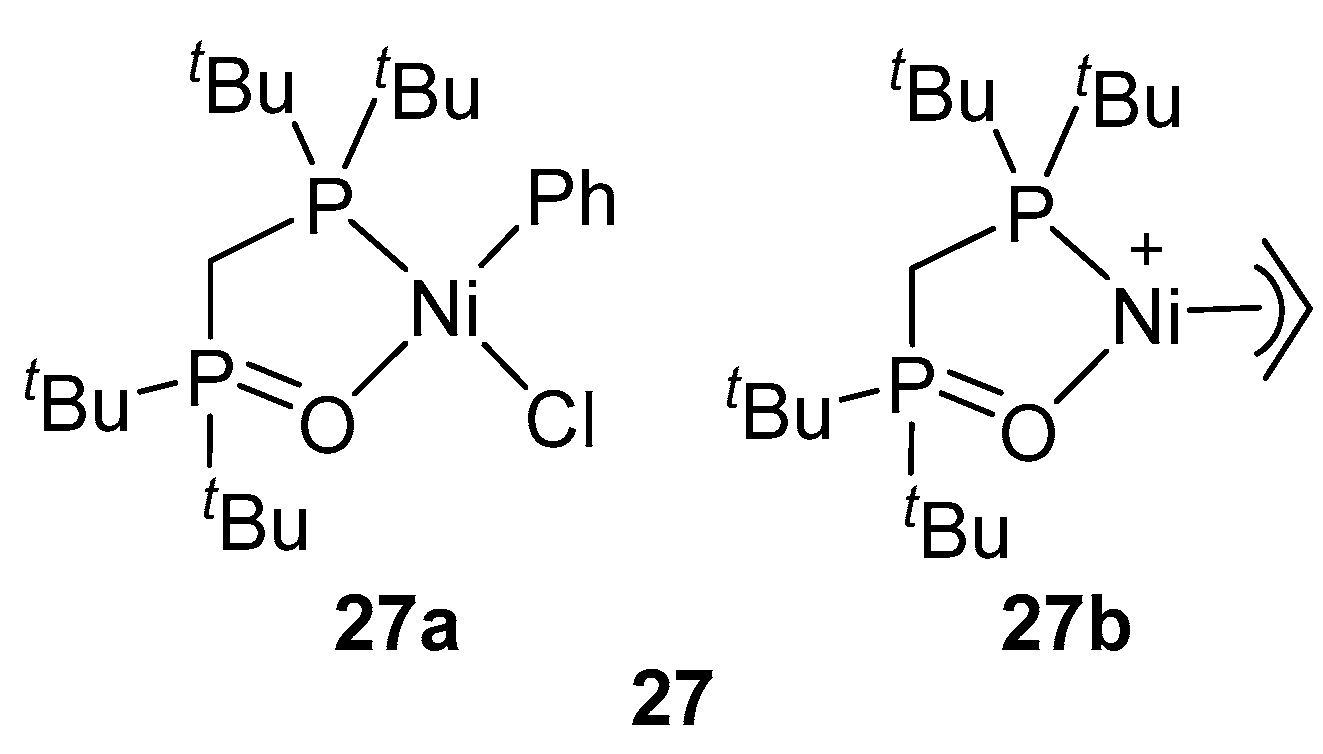
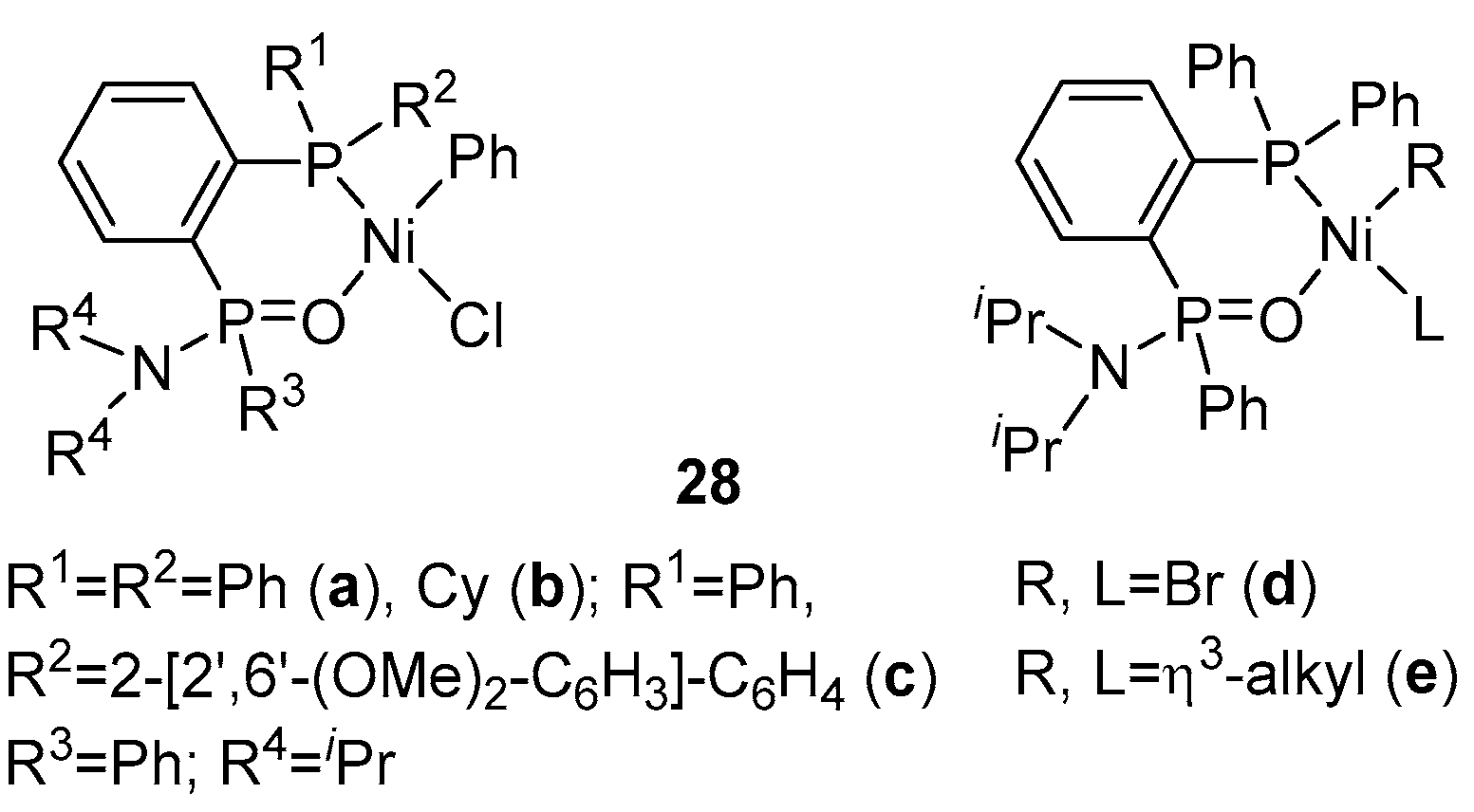
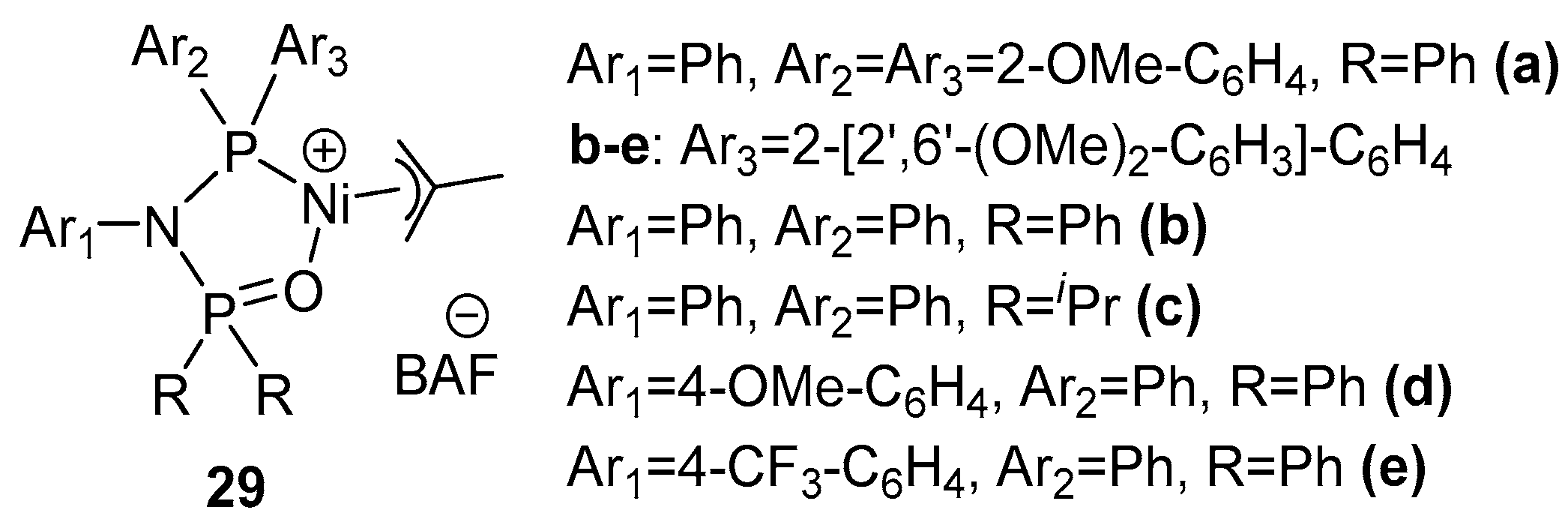





| Catalyst | Polar Monomers Used | Activity (g mol−1 h−1) | Mw Range (g mol−1) | Polymer Characteristics | Refs. |
|---|---|---|---|---|---|
 | UCOOMe, UCOOH, UOH, UCl, HO, VTMOS, HCl | ~105 | 104~105 | Branches | [66,67,68,69,70,71,72,73] |
 | HCl, UCOOMe, MA | 104~106 | 103~105 | Branches | [22,95,96] |
 | HAc, AAc, AAA, VA, AA | 104~106 | 103 ~ 105 | Branches | [79,93,94,99] |
 | MA, BA, tBA, DMAA, MMA, NVP, VTMOS, HCl, BuAE, | 103~105 | ~104 | Low branches | [108,109,111] |
 | UCOOMe, AA MA, AAc, ACl, BuVE | 103~105 | 103~104 | Low branches | [113,114,115,116] |
 | HCl, UCOOMe, UCOOH, UOH VTMOS, Norbornenes | 104~105 | 103~104 | Low branches to linear | [120,121,122,123,124,127,128,129] |
 | UCOOMe, HCl, PAc, PVE, BuAE | 103~105 | 103~104 | Linear | [144] |
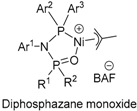 | MA, HCl, UCOOMe, NBCOOMe, VTMOS | 103~105 | ~103 | Linear | [145,147,148] |
 | AAc, AE | 102~103 | ~103 | Linear | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Gao, R.; Gou, Q.; Lai, J.; Li, X. Recent Advances in the Copolymerization of Ethylene with Polar Comonomers by Nickel Catalysts. Polymers 2022, 14, 3809. https://doi.org/10.3390/polym14183809
Zhang R, Gao R, Gou Q, Lai J, Li X. Recent Advances in the Copolymerization of Ethylene with Polar Comonomers by Nickel Catalysts. Polymers. 2022; 14(18):3809. https://doi.org/10.3390/polym14183809
Chicago/Turabian StyleZhang, Randi, Rong Gao, Qingqiang Gou, Jingjing Lai, and Xinyang Li. 2022. "Recent Advances in the Copolymerization of Ethylene with Polar Comonomers by Nickel Catalysts" Polymers 14, no. 18: 3809. https://doi.org/10.3390/polym14183809