Freeze Moisture Treatment and Ozonation of Adlay Starch (Coix lacryma-jobi): Effect on Functional, Pasting, and Physicochemical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Adlay Starch
2.3. Modification of Adlay Starch by Freeze Moisture Treatment (FMT) and Ozonation
2.4. Analysis of the Morphology of Starch Granule Particles
2.5. Analysis of Functional Properties
2.6. Analysis of Pasting Properties
2.7. Analysis of Color
2.8. Analysis of Functional Groups
2.9. Statistical Analysis
3. Results and Discussion
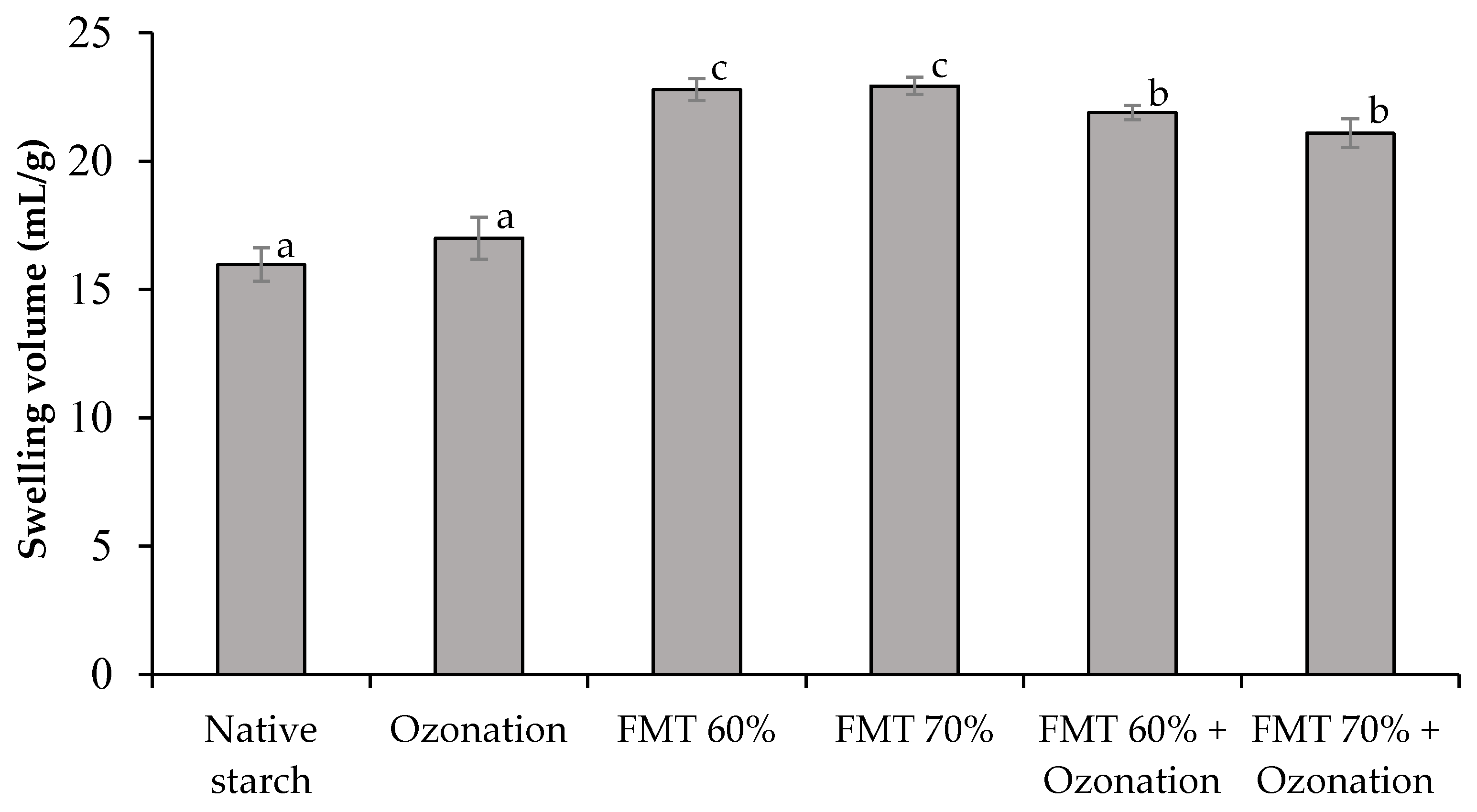
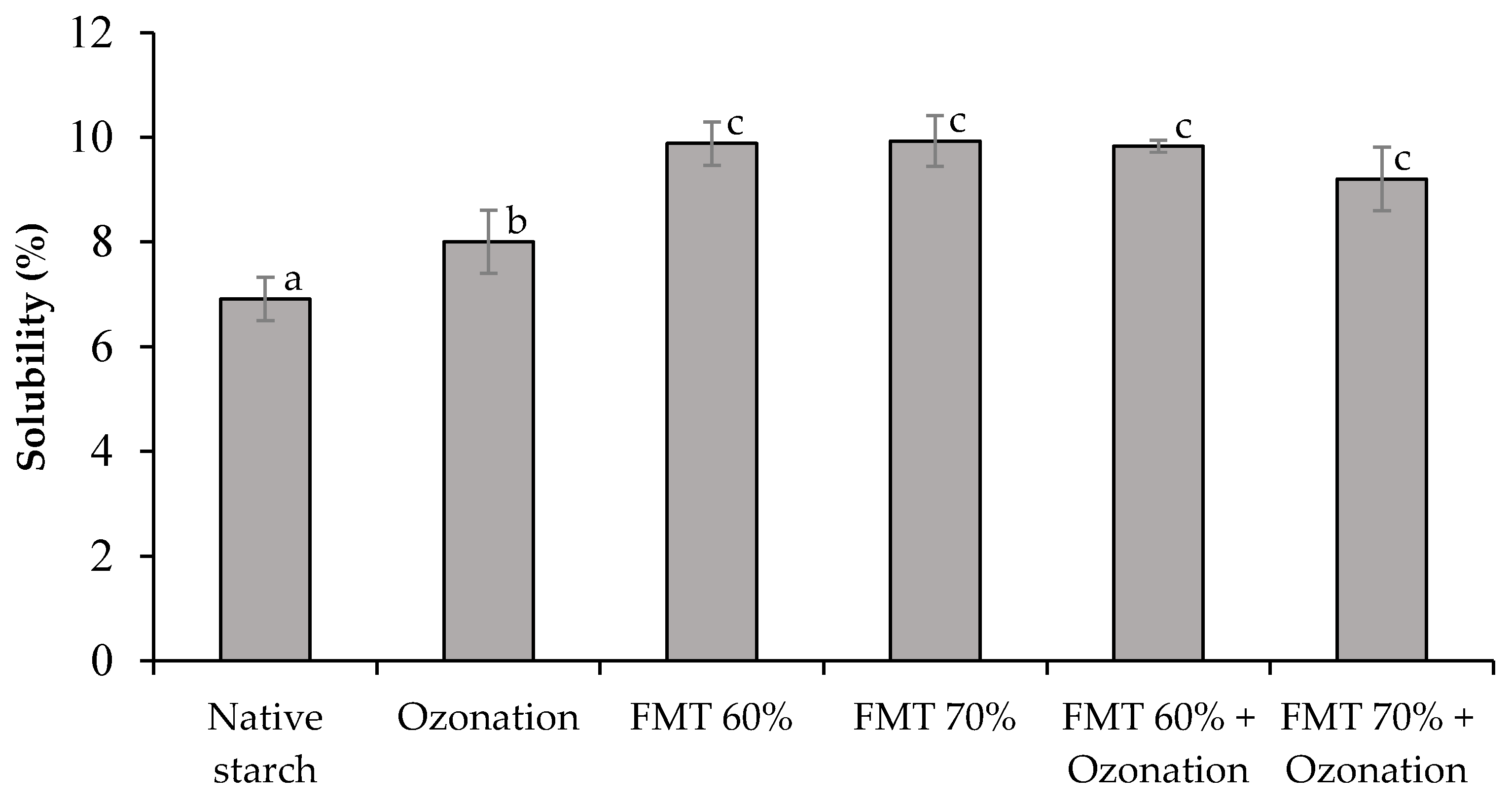
3.1. Swelling Volume (SV) and Solubility
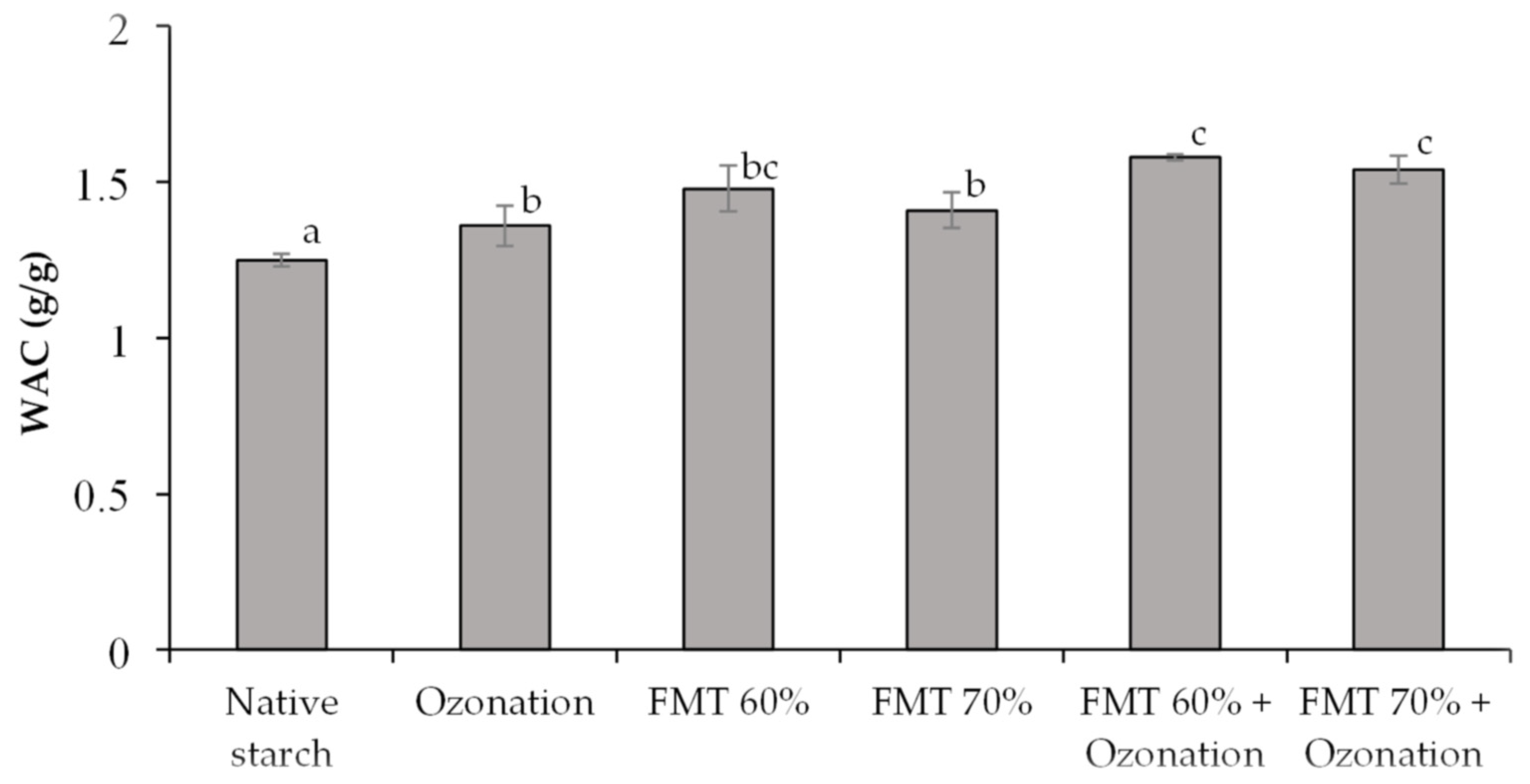
3.2. Water Absorption Capacity (WAC)
3.3. Color
3.4. Pasting Properties
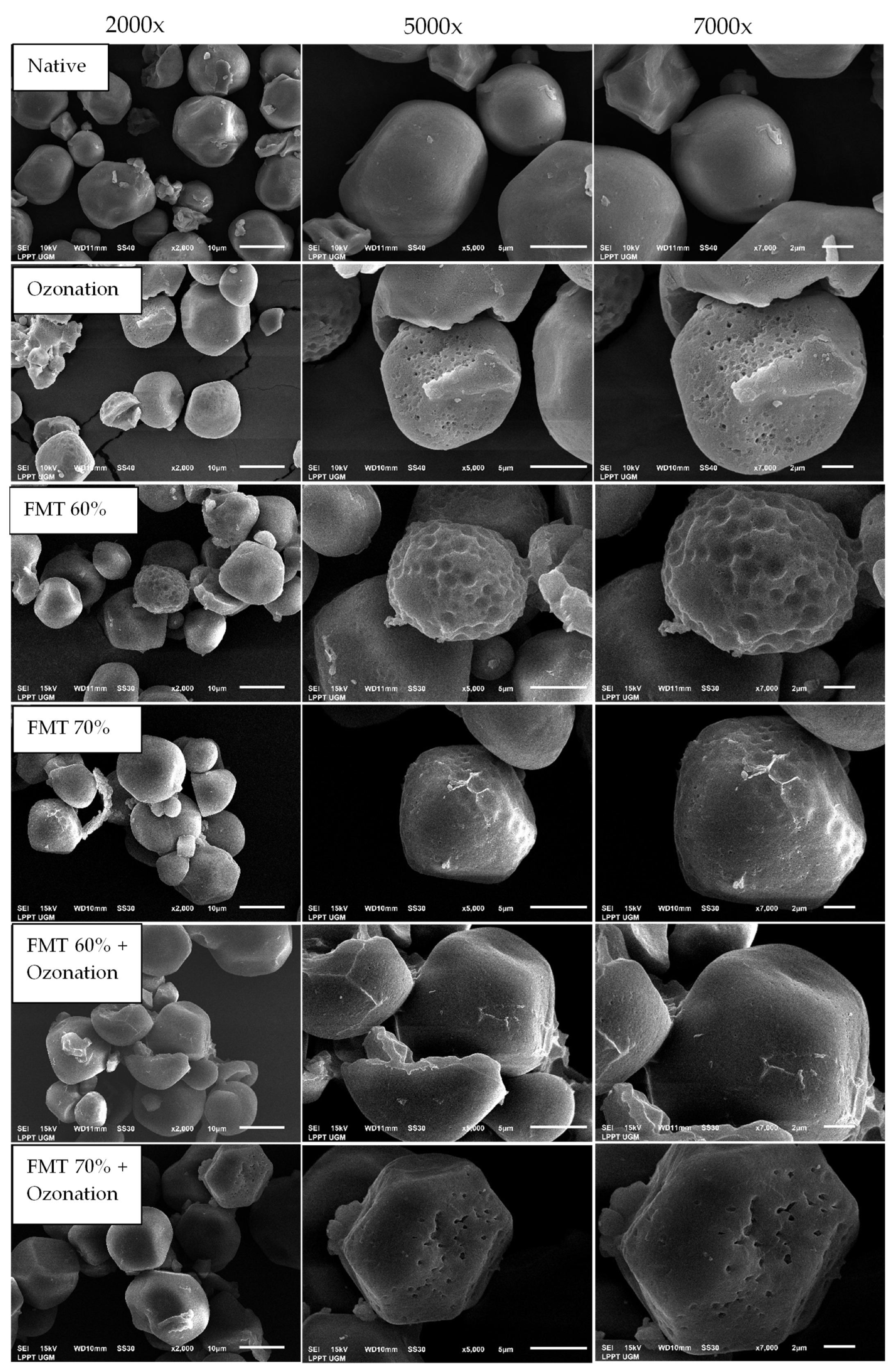
3.5. Starch Granule Morphology
3.6. Functional Groups of Starch
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masahid, A.D.; Belgis, M.; Agesti, H.V. Functional Properties of Adlay Flour (Coix Lacryma-Jobi L. var. Ma-Yuen) Resulting from Modified Durations of Fermentation Using Rhizopus Oligosporus. Int. J. Food Agric. Nat. Resour. 2021, 2, 1–6. [Google Scholar] [CrossRef]
- Tensiska; Cahyana, Y.; Nurhadi, B.; Desani, W.D.; Subroto, E. In Vitro Digestibility and the Characteristics of Puffed Products from Different Degrees of Milling and Moisture Content of Adlay Grain (Coix Lacryma-Jobi L.). Food Res. 2021, 5, 371–378. [Google Scholar] [CrossRef]
- Emmambux, M.N.; Taylor, J.R.N. Morphology, Physical, Chemical, and Functional Properties of Starches from Cereals, Legumes, and Tubers Cultivated in Africa: A Review. Starch-Stärke 2013, 65, 715–729. [Google Scholar] [CrossRef]
- Capule, A.B.; Trinidad, T.P. Isolation and Characterization of Native and Modified Starch from Adlay (Coix Lacryma Jobi-L.). Int. Food Res. J. 2016, 23, 1199–1206. [Google Scholar]
- Tarahi, M.; Shahidi, F.; Hedayati, S. A Novel Starch from Bitter Vetch (Vicia ervilia) Seeds: A Comparison of Its Physicochemical, Structural, Thermal, Rheological, and Pasting Properties with Conventional Starches. Int. J. Food Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Zhang, G.; Ding, Y.; Ni, C.; Ban, Q.; Xu, L.; Guo, L.; Cheng, J. Physicochemical and Morphological Properties of Extruded Adlay (Coix Lachryma-Jobi L.) Flour. J. Chem. 2019, 2019, 6239870. [Google Scholar] [CrossRef]
- Tarahi, M.; Hedayati, S.; Shahidi, F. Effects of Mung Bean (Vigna radiata) Protein Isolate on Rheological, Textural, and Structural Properties of Native Corn Starch. Polymers 2022, 14, 3012. [Google Scholar] [CrossRef]
- Subroto, E.; Indiarto, R.; Djali, M.; Rosyida, H.D. Production and Application of Crosslinking- Modified Starch as Fat Replacer: A Review. Int. J. Eng. Trends Technol. 2020, 68, 26–30. [Google Scholar] [CrossRef]
- Mohamed, I.O. Effects of Processing and Additives on Starch Physicochemical and Digestibility Properties. Carbohydr. Polym. Technol. Appl. 2021, 2, 100039. [Google Scholar] [CrossRef]
- Marta, H.; Cahyana, Y.; Djali, M.; Pramafisi, G. The Properties, Modification, and Application of Banana Starch. Polymers 2022, 14, 3092. [Google Scholar] [CrossRef]
- Hj. Latip, D.N.; Samsudin, H.; Utra, U.; Alias, A.K. Modification Methods toward the Production of Porous Starch: A Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2841–2862. [Google Scholar] [CrossRef] [PubMed]
- Maniglia, B.C.; Castanha, N.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Starch Modification through Environmentally Friendly Alternatives: A Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2482–2505. [Google Scholar] [CrossRef]
- Neelam, K.; Vijay, S.; Lalit, S. Various Techniques for the Modification of Starch and the Applications of Its Derivatives. Int. Res. J. Pharm. 2012, 3, 25–31. [Google Scholar]
- Subroto, E.; Indiarto, R.; Marta, H.; Shalihah, S. Effect of Heat—Moisture Treatment on Functional and Pasting Properties of Potato (Solanum tuberosum L. var. Granola) Starch. Food Res. 2019, 3, 469–476. [Google Scholar] [CrossRef]
- Adiyanti, T.; Subroto, E. Modifications of Banana Starch and Its Characteristics: A Review. Int. J. Sci. Technol. Res. 2020, 9, 3–6. [Google Scholar]
- Cahyana, Y.; Wijaya, E.; Halimah, T.S.; Marta, H.; Suryadi, E.; Kurniati, D. The Effect of Different Thermal Modifications on Slowly Digestible Starch and Physicochemical Properties of Green Banana Flour (Musa acuminata colla). Food Chem. 2019, 274, 274–280. [Google Scholar] [CrossRef]
- Hottot, A.; Vessot, S.; Andrieu, J. A Direct Characterization Method of the Ice Morphology. Relationship Between Mean Crystals Size and Primary Drying Times of Freeze-Drying Processes. Dry. Technol. 2004, 22, 2009–2021. [Google Scholar] [CrossRef]
- Nowak, D.; Jakubczyk, E. The Freeze-Drying of Foods—The Characteristic of the Process Course and the Effect of Its Parameters on the Physical Properties of Food Materials. Foods 2020, 9, 1488. [Google Scholar] [CrossRef]
- Zhang, B.; Cui, D.; Liu, M.; Gong, H.; Huang, Y.; Han, F. Corn Porous Starch: Preparation, Characterization and Adsorption Property. Int. J. Biol. Macromol. 2012, 50, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Ankita; Prasad, K. Studies on Spinach Powder as Affected by Dehydration Temperature and Process of Blanching. Int. J. Agric. Food Sci. Technol. 2013, 4, 309–316. [Google Scholar]
- Jung, Y.; Lee, B.-H.; Yoo, S.-H. Physical Structure and Absorption Properties of Tailor-Made Porous Starch Granules Produced by Selected Amylolytic Enzymes. PLoS ONE 2017, 12, e0181372. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Liu, J.; Xu, X. Preparation, Characterization, Physicochemical Property and Potential Application of Porous Starch: A Review. Int. J. Biol. Macromol. 2020, 148, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- WeiRong, Y.; HuiYuan, Y. Adsorbent Characteristics of Porous Starch. Starch-Stärke 2002, 54, 260–263. [Google Scholar] [CrossRef]
- Guzel-Seydim, Z.B.; Greene, A.K.; Seydim, A.C. Use of Ozone in the Food Industry. LWT-Food Sci. Technol. 2004, 37, 453–460. [Google Scholar] [CrossRef]
- Botondi, R.; Barone, M.; Grasso, C. A Review into the Effectiveness of Ozone Technology for Improving the Safety and Preserving the Quality of Fresh-Cut Fruits and Vegetables. Foods 2021, 10, 748. [Google Scholar] [CrossRef]
- Brodowska, A.J.; Nowak, A.; Śmigielski, K. Ozone in the Food Industry: Principles of Ozone Treatment, Mechanisms of Action, and Applications: An Overview. Crit. Rev. Food Sci. Nutr. 2018, 58, 2176–2201. [Google Scholar] [CrossRef] [PubMed]
- Satmalawati, E.M.; Pranoto, Y.; Marseno, D.W.; Marsono, Y. Oxidation of Cassava Starch at Different Dissolved Ozone Concentration: Effect on Functional and Structural Properties. Food Res. 2020, 4, 1896–1904. [Google Scholar] [CrossRef]
- Castanha, N.; da Matta Junior, M.D.; Augusto, P.E.D. Potato Starch Modification Using the Ozone Technology. Food Hydrocoll. 2017, 66, 343–356. [Google Scholar] [CrossRef]
- Castanha, N.; e Santos, D.N.; Cunha, R.L.; Augusto, P.E.D. Properties and Possible Applications of Ozone-Modified Potato Starch. Food Res. Int. 2019, 116, 1192–1201. [Google Scholar] [CrossRef]
- Martinez-Bustos, F.; Amaya-Liano, S.L.; Carbajal-Arteaga, J.A.; Chang, Y.K.; de J Zazueta-Morales, J. Physicochemical Properties of Cassava, Potato and Jicama Starches Oxidised with Organic Acids. J. Sci. Food Agric. 2007, 1243, 1237–1243. [Google Scholar] [CrossRef]
- Obadi, M.; Zhu, K.X.; Peng, W.; Sulieman, A.A.; Mohammed, K.; Zhou, H.M. Effects of Ozone Treatment on the Physicochemical and Functional Properties of Whole Grain Flour. J. Cereal Sci. 2018, 81, 127–132. [Google Scholar] [CrossRef]
- Collado, L.S.; Corke, H. Heat-Moisture Treatment Effects on Sweetpotato Starches Differing in Amylose Content. Food Chem. 1999, 65, 339–346. [Google Scholar] [CrossRef]
- Kadan, R.S.; Bryant, R.J.; Pepperman, A.B. Functional Properties of Extruded Rice Flours. J. Food Sci. 2003, 68, 1669–1672. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chang, S.M. Characterization Of Red Bean Starch And Its Noodle Quality. Cereal Chem. 2005, 73, 302–308. [Google Scholar]
- Fonseca, L.M.; Gonçalves, J.R.; El Halal, S.L.M.; Pinto, V.Z.; Dias, A.R.G.; Jacques, A.C.; da Rosa Zavareze, E. Oxidation of Potato Starch with Different Sodium Hypochlorite Concentrations and Its Effect on Biodegradable Films. LWT-Food Sci. Technol. 2015, 60, 714–720. [Google Scholar] [CrossRef] [Green Version]
- Lawal, O.S. Composition, Physicochemical Properties and Retrogradation Characteristics of Native, Oxidised, Acetylated and Acid-Thinned New Cocoyam (Xanthosoma sagittifolium) Starch. Food Chem. 2004, 87, 205–218. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Lawal, O.S. Effect of Annealing and Heat Moisture Conditioning on the Physicochemical Characteristics of Bambarra Groundnut (Voandzeia subterranea) Starch. Nahrung-Food 2002, 46, 311–316. [Google Scholar] [CrossRef]
- Sumardiono, S.; Cahyono, H.; Jos, B.; Pudjihastuti, I.; Yafiz, A.M.; Rachmasari, M. Physicochemical Properties of Sago Ozone Oxidation: The Effect of Reaction Time, Acidity, and Concentration of Starch. Foods 2021, 10, 1309. [Google Scholar] [CrossRef]
- Klein, B.; Vanier, N.L.; Moomand, K.; Pinto, V.Z.; Colussi, R.; da Rosa Zavareze, E.; Dias, A.R.G. Ozone Oxidation of Cassava Starch in Aqueous Solution at Different PH. Food Chem. 2014, 155, 167–173. [Google Scholar] [CrossRef]
- Sánchez-Rivera, M.M.; García-Suárez, F.J.L.; Velázquez Del Valle, M.; Gutierrez-Meraz, F.; Bello-Pérez, L.A. Partial Characterization of Banana Starches Oxidized by Different Levels of Sodium Hypochlorite. Carbohydr. Polym. 2005, 62, 50–56. [Google Scholar] [CrossRef]
- Lewandowicz, J.; Le Thanh-Blicharz, J.; Szwengiel, A. The Effect of Chemical Modification on the Rheological Properties and Structure of Food Grade Modified Starches. Processes 2022, 10, 938. [Google Scholar] [CrossRef]
- Sangseethong, K.; Lertphanich, S.; Sriroth, K. Physicochemical Properties of Oxidized Cassava Starch Prepared under Various Alkalinity Levels. Starch-Stärke 2009, 61, 92–100. [Google Scholar] [CrossRef]
- Pukkahuta, C.; Suwannawat, B.; Shobsngob, S.; Varavinit, S. Comparative Study of Pasting and Thermal Transition Characteristics of Osmotic Pressure and Heat-Moisture Treated Corn Starch. Carbohydr. Polym. 2008, 72, 527–536. [Google Scholar] [CrossRef]
- Sukhija, S.; Singh, S.; Riar, C.S. Molecular Characteristics of Oxidized and Cross-Linked Lotus (Nelumbo nucifera) Rhizome Starch. Int. J. Food Prop. 2017, 20, S1065–S1081. [Google Scholar] [CrossRef]
- Çatal, H.; Ibanoǧlu, Ş. Effect of Aqueous Ozonation on the Pasting, Flow and Gelatinization Properties of Wheat Starch. LWT-Food Sci. Technol. 2014, 59, 577–582. [Google Scholar] [CrossRef]
- Kuakpetoon, D.; Wang, Y.-J. Locations of Hypochlorite Oxidation in Corn Starches Varying in Amylose Content. Carbohydr. Res. 2008, 343, 90–100. [Google Scholar] [CrossRef]
- Xie, Z.; Guan, J.; Chen, L.; Jin, Z.; Tian, Y. Effect of Drying Processes on the Fine Structure of A-, B-, and C-Type Starches. Starch-Stärke 2018, 70, 1700218. [Google Scholar] [CrossRef]
- Monroy, Y.; Rivero, S.; García, M.A. Microstructural and Techno-Functional Properties of Cassava Starch Modified by Ultrasound. Ultrason. Sonochem. 2018, 42, 795–804. [Google Scholar] [CrossRef]



| Treatment | L* | a* | b* | ΔE* |
|---|---|---|---|---|
| Native starch | 94.05 ± 0.30 a | 0.19 ± 0.02 c | 3.88 ± 0.08 a | 0.00 ± 0.00 a |
| Ozonation | 93.56 ± 0.65 ab | 0.20 ± 0.01 c | 3.65 ± 0.17 a | 0.54 ± 0.03 c |
| FMT 60% | 93.51 ± 0.03 b | 0.17 ± 0.01 b | 4.21 ± 0.08 b | 0.63 ± 0.05 c |
| FMT 70% | 93.50 ± 0.12 b | 0.15 ± 0.01 a | 3.95 ± 0.05 a | 0.56 ± 0.05 c |
| FMT 60% + ozonation | 94.20 ± 0.03 a | 0.20 ± 0.01 c | 3.86 ± 0.06 a | 0.15 ± 0.01 b |
| FMT 70% + ozonation | 94.02 ± 0.13 a | 0.17 ± 0.03 b | 3.71 ± 0.17 a | 0.17 ± 0.01 b |
| Treatment | Pasting Point (°C) | Peak Viscosity (cP) | Hold Viscosity (cP) | Final Viscosity (cP) | Breakdown Viscosity (cP) | Setback Viscosity (cP) |
|---|---|---|---|---|---|---|
| Native | 76.92 ± 0.25 ab | 5687.50 ± 163.34 a | 1322.00 ± 7.07 a | 3918.50 ± 70.00 a | 4365.50 ± 156.27 a | 2596.50 ± 62.93 a |
| Ozonation | 76.47 ± 0.63 abc | 5214.67 ± 187.09 b | 1394.00 ± 109.30 ab | 3565.00 ± 332.45 a | 3820.67 ± 268.19 b | 2171.00 ± 260.48 b |
| FMT 60% | 76.38 ± 0.28 bc | 5587.00 ± 102.27 a | 1194.33 ± 227.98 ab | 3885.00 ± 171.50 a | 4392.67 ± 222.78 a | 2690.67 ± 322.06 ab |
| FMT 70% | 76.58 ± 0.48 abc | 5512.67 ± 71.44 a | 1401.00 ± 56.63 b | 3892.33 ± 71.65 a | 4111.67 ± 127.01 ab | 2491.33 ± 126.18 ab |
| FMT 60% + Ozonation | 75.99 ± 0.39 c | 5675.33 ± 40.81 a | 1453.00 ± 35.79 b | 3943.00 ± 46.29 a | 4222.33 ± 36.23 a | 2490.00 ± 28.58 a |
| FMT 70% + Ozonation | 76.02 ± 0.29 c | 5561.33 ± 116.52 a | 1502.33 ± 111.59 b | 3819.67 ± 147.82 a | 4079.00 ± 46.18 b | 2317.33 ± 129.11 b |
| Band Assignment | Wavenumbers (cm−1) |
|---|---|
| O–H stretch | 3397–3398 |
| C–H stretch | 2929–2930 |
| C–C stretch | 1412–1413 |
| C=O stretch (Carbonyl) | 1640–1643 |
| C–O stretch (Carboxyl) | 1304–1305 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subroto, E.; Sitha, N.; Filianty, F.; Indiarto, R.; Sukri, N. Freeze Moisture Treatment and Ozonation of Adlay Starch (Coix lacryma-jobi): Effect on Functional, Pasting, and Physicochemical Properties. Polymers 2022, 14, 3854. https://doi.org/10.3390/polym14183854
Subroto E, Sitha N, Filianty F, Indiarto R, Sukri N. Freeze Moisture Treatment and Ozonation of Adlay Starch (Coix lacryma-jobi): Effect on Functional, Pasting, and Physicochemical Properties. Polymers. 2022; 14(18):3854. https://doi.org/10.3390/polym14183854
Chicago/Turabian StyleSubroto, Edy, Nisyrah Sitha, Fitry Filianty, Rossi Indiarto, and Nandi Sukri. 2022. "Freeze Moisture Treatment and Ozonation of Adlay Starch (Coix lacryma-jobi): Effect on Functional, Pasting, and Physicochemical Properties" Polymers 14, no. 18: 3854. https://doi.org/10.3390/polym14183854





