Potential Applications of an Exopolysaccharide Produced by Bacillus xiamenensis RT6 Isolated from an Acidic Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Bacterial Strain and PCR Amplification
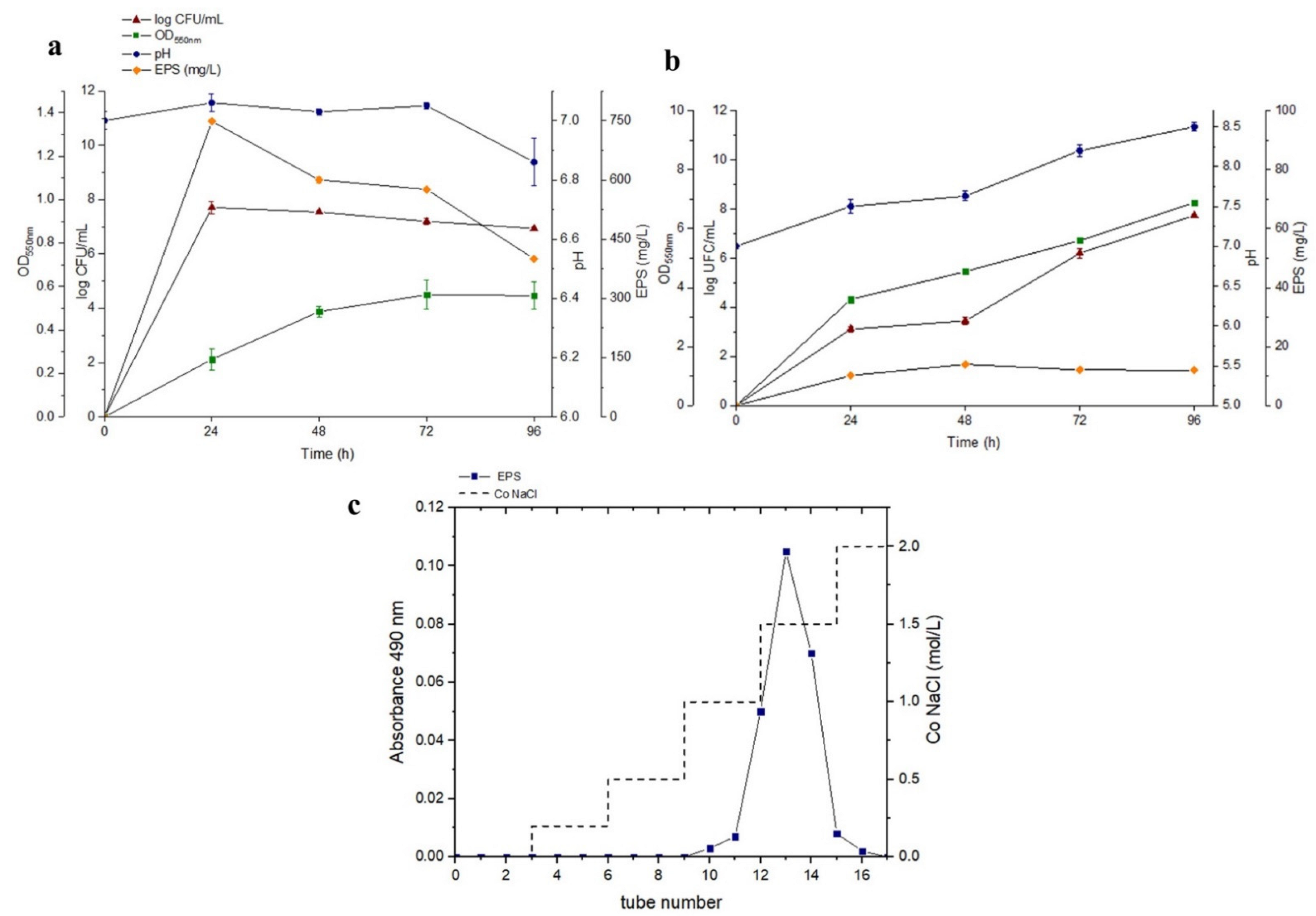
2.3. Analysis of Bacterial Growth, Colony Forming Units (CFU)/mL, pH and EPS Production
2.4. Isolation, Purification and Molecular Weight of EPS
2.5. Compositional Analysis and Characterisation of the EPS
2.5.1. Compositional Analysis
2.5.2. Attenuated Total Reflectance/FT-Infrared Spectroscopy (ATR-FTIR)
2.5.3. Thermogravimetric (TGA) and Differential Scanning Calorimetry (DSC) Analysis
2.6. Antioxidant Activity Tests
2.6.1. DPPH (1,1–Diphenyl−2–picryl–hydrazyl) Free Radical Scavenging Activity
2.6.2. Hydroxyl Radical Scavenging Activity
2.6.3. Superoxide Anion Scavenging Activity
2.6.4. Metal Ion Chelating Activity
2.7. Biocompatibility Studies
2.7.1. Culture of Cells
2.7.2. Cytotoxicity Assay
2.8. Determination of the Antioxidant Ability on Cellular Level
2.8.1. Establishment of Injury Model
2.8.2. Determination of the Protection of EPSt on HeLa Cells against Oxidative Stress
2.9. Emulsifying Properties
2.10. Statistical Analysis
3. Results and Discussion
3.1. Strain Identification and EPS Optimisation
3.2. Compositional Analysis and Characterisation of EPSt Exopolymer
3.2.1. Molecular Weight Determination for EPSt
3.2.2. Gas Chromatography GC-MS Analysis for EPSt
3.2.3. ATR–FTIR Analysis for EPSt
3.2.4. Characterisation of the Thermal Properties of EPSt
3.3. Biotechnological Applications
3.3.1. Antioxidant Activity Tests
3.3.2. Biocompatibility Studies and Antioxidant Ability on Cellular Level
3.3.3. Emulsifying Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Barcelos, M.C.; Vespermann, K.A.; Pelissari, F.M.; Molina, G. Current status of biotechnological production and applications of microbial exopolysaccharides. Crit. Rev. Food Sci. Nutr. 2020, 60, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, Y.A.; El-Naggar, M.E.; Abdel-Megeed, A.; El-Newehy, M. Recent advancements in microbial polysaccharides: Synthesis and applications. Polymers 2021, 13, 4136. [Google Scholar] [CrossRef] [PubMed]
- Kopperi, H.; Amulya, K.; Mohan, S.V. Simultaneous biosynthesis of bacterial polyhydroxybutyrate (PHB) and extracellular polymeric substances (EPS): Process optimization and Scale-up. Bioresour. Technol. 2021, 341, 125735. [Google Scholar] [CrossRef] [PubMed]
- Chouchane, H.; Boutiti, S.; Ouertani, A.; Hassen, W.; Guesmi, S.; Neifar, M.; Jelassi, H.; Sghaier, H.; Masmoudi, A.S.E.; Cherif, A. Effect of gamma irradiation on enhanced biological activities of exopolysaccharide from Halomonas desertis G11: Biochemical and genomic Insights. Polymers 2021, 13, 3798. [Google Scholar] [CrossRef]
- Boujida, N.; Palau, M.; Charfi, S.; El Moussaoui, N.; Manresa, A.; Miñana-Galbis, D.; Senhaji, N.S.; Abrini, J. Isolation and characterization of halophilic bacteria producing exopolymers with emulsifying and antioxidant activities. Biocatal. Agric. Biotechnol. 2018, 16, 631–637. [Google Scholar] [CrossRef]
- Kazak, H.; Oner, E.T.; Dekker, R.F. Extremophiles as sources of exopolysaccharides. In Handbook on Carbohydrate Polymers: Development, Properties and Applications; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010; pp. 605–619. [Google Scholar]
- Li, W.; Ji, J.; Chen, X.; Jiang, M.; Rui, X.; Dong, M. Structural elucidation and antioxidant activities of exopolysaccharides from Lactobacillus helveticus MB2–1. Carbohydr. Polym. 2014, 102, 351–359. [Google Scholar] [CrossRef]
- Andrew, M.; Jayaraman, G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr. Res. 2020, 487, 107881. [Google Scholar] [CrossRef]
- Xu, X.; Qiao, Y.; Peng, Q.; Shi, B.; Dia, V.P. Antioxidant and immunomodulatory properties of partially purified exopolysaccharide from Lactobacillus casei isolated from Chinese Northeast Sauerkraut. Immunol. Investig. 2022, 51, 748–765. [Google Scholar] [CrossRef]
- Nazli, F.; Mustafa, A.; Ahmad, M.; Hussain, A.; Jamil, M.; Wang, X.; Shakeel, Q.; Imtiaz, M.; El-Esawi, M.A. A review on practical application and potentials of phytohormone-producing plant growth-promoting rhizobacteria for inducing heavy metal tolerance in crops. Sustainability 2020, 12, 9056. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef] [Green Version]
- Petrova, P.; Arsov, A.; Ivanov, I.; Tsigoriyna, L.; Petrov, K. New Exopolysaccharides Produced by Bacillus licheniformis 24 Display Substrate-Dependent Content and Antioxidant Activity. Microorganisms 2021, 9, 2127. [Google Scholar] [CrossRef] [PubMed]
- Razack, S.A.; Velayutham, V.; Thangavelu, V. Medium optimization and in vitro antioxidant activity of exopolysaccharide produced by Bacillus subtilis. Korean J. Chem. Eng. 2014, 31, 296–303. [Google Scholar] [CrossRef]
- Yang, H.; Deng, J.; Yuan, Y.; Fan, D.; Zhang, Y.; Zhang, R.; Han, B. Two novel exopolysaccharides from Bacillus amyloliquefaciens C−1: Antioxidation and effect on oxidative stress. Curr. Microbiol. 2015, 70, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Kumar, R.; Yadav, P.; Singh, A.; Yadav, A.; Chowdhary, P.; Raj, A. Biofilm formation and extracellular polymeric substance (EPS) production by Bacillus haynesii and influence of hexavalent chromium. Bioresour. Technol. 2022, 352, 127109. [Google Scholar] [CrossRef]
- Karamać, M. Chelation of Cu (II), Zn (II), and Fe (II) by tannin constituents of selected edible nuts. Int. J. Mol. Sci. 2009, 10, 5485–5497. [Google Scholar] [CrossRef] [PubMed]
- Salehizadeh, H.; Shojaosadati, S.A. Removal of metal ions from aqueous solution by polysaccharide produced from Bacillus firmus. Water Res. 2003, 37, 4231–4235. [Google Scholar] [CrossRef]
- Mathivanan, K.; Chandirika, J.U.; Mathimani, T.; Rajaram, R.; Annadurai, G.; Yin, H. Production and functionality of exopolysaccharides in bacteria exposed to a toxic metal environment. Ecotoxicol. Environ. Saf. 2021, 208, 111567. [Google Scholar] [CrossRef]
- Gangalla, R.; Gattu, S.; Palaniappan, S.; Ahamed, M.; Macha, B.; Thampu, R.K.; Fais, A.; Cincotti, A.; Gatto, G.; Dama, M. Structural characterisation and assessment of the novel Bacillus amyloliquefaciens RK3 exopolysaccharide on the improvement of cognitive function in Alzheimer’s disease mice. Polymers 2021, 13, 2842. [Google Scholar] [CrossRef]
- Mahendran, S.; Saravanan, S.; Vijayabaskar, P.; Anandapandian, K.; Shankar, T. Antibacterial potential of microbial exopolysaccharide from Ganoderma lucidum and Lysinibacillus fusiformis. Int. J. Recent Sci. Res. 2013, 4, 501–505. [Google Scholar]
- Sánchez-León, E.; Bello-Morales, R.; López-Guerrero, J.A.; Poveda, A.; Jiménez-Barbero, J.; Gironès, N.; Abrusci, C. Isolation and characterization of an exopolymer produced by Bacillus licheniformis: In vitro antiviral activity against enveloped viruses. Carbohydr. Polym. 2020, 248, 116737. [Google Scholar] [CrossRef]
- Aullybux, A.A.; Puchooa, D.; Bahorun, T.; Jeewon, R.; Wen, X.; Matin, P. Antioxidant and cytotoxic activities of exopolysaccharides from Alcaligenes faecalis species isolated from the marine environment of Mauritius. J. Polym. Environ. 2022, 30, 1462–1477. [Google Scholar] [CrossRef]
- Castellane, T.C.L.; Persona, M.R.; Campanharo, J.C.; de Macedo Lemos, E.G. Production of exopolysaccharide from rhizobia with potential biotechnological and bioremediation applications. Int. J. Biol. Macromol. 2015, 74, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Gurav, R.; Choi, Y.; Choi, T.; Kim, H.; Song, H.; Lee, S.M.; Park, S.L.; Lee, H.S.; Kim, Y. Bioprospecting of exopolysaccharide from marine Sphingobium yanoikuyae BBL01: Production, characterization, and metal chelation activity. Bioresour. Technol. 2021, 324, 124674. [Google Scholar] [CrossRef] [PubMed]
- Haddar, A.; Hamed, M.; Bouallegue, A.; Bastos, R.; Coelho, E.; Coimbra, M.A. Structural elucidation and interfacial properties of a levan isolated from Bacillus mojavensis. Food Chem. 2021, 343, 128456. [Google Scholar] [CrossRef] [PubMed]
- Vinothkanna, A.; Sathiyanarayanan, G.; Rai, A.K.; Mathivanan, K.; Saravanan, K.; Sudharsan, K.; Kalimuthu, P.; Ma, Y.; Sekar, S. Exopolysaccharide produced by probiotic Bacillus albus DM−15 isolated from ayurvedic fermented dasamoolarishta: Characterization, antioxidant, and anticancer activities. Front. Microbiol. 2022, 13, 832109. [Google Scholar] [CrossRef]
- Aguilera, A.; Amils, R. Tolerance to cadmium in Chlamydomonas sp. (Chlorophyta) strains isolated from an extreme acidic environment, the Tinto River (SW, Spain). Aquat. Toxicol. 2005, 75, 316–329. [Google Scholar] [CrossRef]
- Nazli, F.; Wang, X.; Ahmad, M.; Hussain, A.; Dar, A.; Nasim, M.; Jamil, M.; Panpluem, N.; Mustafa, A. Efficacy of indole acetic acid and exopolysaccharides-producing Bacillus safensis strain FN13 for inducing Cd-stress tolerance and plant growth promotion in Brassica juncea (L.). Appl. Sci. 2021, 11, 4160. [Google Scholar] [CrossRef]
- Xia, Y.; Farooq, M.A.; Javed, M.T.; Kamran, M.A.; Mukhtar, T.; Ali, J.; Tabassum, T.; ur Rehman, S.; Munis, M.F.H.; Sultan, T. Multi-stress tolerant PGPR Bacillus xiamenensis PM14 activating sugarcane (Saccharum officinarum L.) red rot disease resistance. Plant Physiol. Biochem. 2020, 151, 640–649. [Google Scholar]
- Beveridge, T.J. Use of the Gram stain in microbiology. Biotech. Histochem. 2001, 76, 111–118. [Google Scholar] [CrossRef]
- Blanc, D.S.; Lugeon, C.; Wenger, A.; Siegrist, H.H.; Francioli, P. Quantitative antibiogram typing using inhibition zone diameters compared with ribotyping for epidemiological typing of methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 1994, 32, 2505–2509. [Google Scholar] [CrossRef]
- Morro, A.; Catalina, F.; Corrales, T.; Pablos, J.L.; Marin, I.; Abrusci, C. New blends of ethylene-butyl acrylate copolymers with thermoplastic starch. Characterization and bacterial biodegradation. Carbohydr. Polym. 2016, 149, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrusci, C.; Pablos, J.L.; Corrales, T.; López-Marín, J.; Marín, I.; Catalina, F. Biodegradation of photo-degraded mulching films based on polyethylenes and stearates of calcium and iron as pro-oxidant additives. Int. Biodeterior. Biodegrad. 2011, 65, 451–459. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.t.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Niknezhad, S.V.; Najafpour-Darzi, G.; Morowvat, M.H.; Ghasemi, Y. Exopolysaccharide production of Pantoea sp. BCCS 001 GH: Physical characterizations, emulsification, and antioxidant activities. Int. J. Biol. Macromol. 2018, 118, 1103–1111. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, F.; Shi, M.; Zhang, X.; Zhou, B.; Zhang, Y.; Chen, X. Characterization and biotechnological potential analysis of a new exopolysaccharide from the Arctic marine bacterium Polaribacter sp. SM1127. Sci. Rep. 2015, 5, 18435. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Prasad, B.; Rai, A.K.; Velappan, S.P.; Subbanna, M.N.; Narayan, B. In vitro antioxidant and antibacterial properties of hydrolysed proteins of delimed tannery fleshings: Comparison of acid hydrolysis and fermentation methods. Biodegradation 2011, 22, 287–295. [Google Scholar] [CrossRef]
- Tada, H.; Shiho, O.; Kuroshima, K.; Koyama, M.; Tsukamoto, K. An improved colorimetric assay for interleukin 2. J. Immunol. Methods 1986, 93, 157–165. [Google Scholar] [CrossRef]
- Zhou, L.; Luo, S.; Li, J.; Zhou, Y.; Chen, T.; Feng, S.; Ding, C. Simultaneous optimization of extraction and antioxidant activity from Blumea laciniata and the protective effect on Hela cells against oxidative damage. Arab. J. Chem. 2020, 13, 9231–9242. [Google Scholar] [CrossRef]
- Meneghine, A.K.; Moretto, C.; Castellane, T.C.L.; Carareto Alves, L.M. Production, characterization and bioemulsifying activity of an exopolysaccharide produced by Sphingomonas sp. isolated from freshwater. J. Polym. Environ. 2017, 25, 1080–1086. [Google Scholar] [CrossRef]
- Din, B.U.; Rafique, M.; Javed, M.T.; Kamran, M.A.; Mehmood, S.; Khan, M.; Sultan, T.; Munis, M.F.H.; Chaudhary, H.J. Assisted phytoremediation of chromium spiked soils by Sesbania sesban in association with Bacillus xiamenensis PM14: A biochemical analysis. Plant Physiol. Biochem. 2020, 146, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; Zettler, E.; Gómez, F.; Amaral-Zettler, L.; Rodríguez, N.; Amils, R. Distribution and seasonal variability in the benthic eukaryotic community of Rio Tinto (SW, Spain), an acidic, high metal extreme environment. Syst. Appl. Microbiol. 2007, 30, 531–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amils, R.; González-Toril, E.; Fernández-Remolar, D.; Gómez, F.; Aguilera, Á.; Rodríguez, N.; Malki, M.; García-Moyano, A.; Fairén, A.G.; de la Fuente, V. Extreme environments as Mars terrestrial analogs: The Rio Tinto case. Planet. Space Sci. 2007, 55, 370–381. [Google Scholar] [CrossRef]
- Sutherland, I.W. Microbial polysaccharides from Gram-negative bacteria. Int. Dairy J. 2001, 11, 663–674. [Google Scholar] [CrossRef]
- Solmaz, K.B.; Ozcan, Y.; Mercan Dogan, N.; Bozkaya, O.; Ide, S. Characterization and production of extracellular polysaccharides (EPS) by Bacillus pseudomycoides U10. Environments 2018, 5, 63. [Google Scholar] [CrossRef]
- Vinothkanna, A.; Sathiyanarayanan, G.; Balaji, P.; Mathivanan, K.; Pugazhendhi, A.; Ma, Y.; Sekar, S.; Thirumurugan, R. Structural characterization, functional and biological activities of an exopolysaccharide produced by probiotic Bacillus licheniformis AG−06 from Indian polyherbal fermented traditional medicine. Int. J. Biol. Macromol. 2021, 174, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Vidhyalakshmi, R.; Valli, N.C.; Kumar, G.N.; Sunkar, S. Bacillus circulans exopolysaccharide: Production, characterization and bioactivities. Int. J. Biol. Macromol. 2016, 87, 405–414. [Google Scholar] [CrossRef]
- Krishna Leela, J.; Sharma, G. Studies on xanthan production from Xanthomonas campestris. Bioprocess Eng. 2000, 23, 687–689. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Gouveia, A.R.; Pinheiro, C.; Torres, C.A.; Grandfils, C.; Reis, M.A. Controlled production of exopolysaccharides from Enterobacter A47 as a function of carbon source with demonstration of their film and emulsifying abilities. Appl. Biochem. Biotechnol. 2014, 172, 641–657. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P.; Hugenholtz, J.; Zoon, P. An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int. Dairy J. 2002, 12, 163–171. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Manna, S.; Saha, P.; Basak, R.K.; Sen, R.; Roy, D.; Adhikari, B. Composition analysis and material characterization of an emulsifying extracellular polysaccharide (EPS) produced by Bacillus megaterium RB−05: A hydrodynamic sediment-attached isolate of freshwater origin. J. Appl. Microbiol. 2011, 111, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Kodali, V.P.; Perali, R.S.; Sen, R. Purification and partial elucidation of the structure of an antioxidant carbohydrate biopolymer from the probiotic bacterium Bacillus coagulans RK−02. J. Nat. Prod. 2011, 74, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Li, Y.; Wang, C.; Zhang, N.; Zhu, X.; Wu, R.; Wu, J. Purification, characterization and antitumor activity of an exopolysaccharide produced by Bacillus velezensis SN−1. Int. J. Biol. Macromol. 2020, 156, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Fan, Z.; Xie, P.; Guo, J. Bacillus cereus AR156 extracellular polysaccharides served as a novel micro-associated molecular pattern to induced systemic immunity to Pst DC3000 in Arabidopsis. Front. Microbiol. 2016, 7, 664. [Google Scholar] [CrossRef]
- Singh, R.P.; Shukla, M.K.; Mishra, A.; Kumari, P.; Reddy, C.; Jha, B. Isolation and characterization of exopolysaccharides from seaweed associated bacteria Bacillus licheniformis. Carbohydr. Polym. 2011, 84, 1019–1026. [Google Scholar] [CrossRef]
- Farag, M.M.; Moghannem, S.A.; Shehabeldine, A.M.; Azab, M.S. Antitumor effect of exopolysaccharide produced by Bacillus mycoides. Microb. Pathog. 2020, 140, 103947. [Google Scholar] [CrossRef]
- Krishnamurthy, M.; Jayaraman Uthaya, C.; Thangavel, M.; Annadurai, V.; Rajendran, R.; Gurusamy, A. Optimization, compositional analysis, and characterization of exopolysaccharides produced by multi-metal resistant Bacillus cereus KMS3–1. Carbohydr. Polym. 2020, 227, 115369. [Google Scholar] [CrossRef]
- Min, W.; Fang, X.; Wu, T.; Fang, L.; Liu, C.; Wang, J. Characterization and antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103. J. Biosci. Bioeng. 2019, 127, 758–766. [Google Scholar] [CrossRef]
- Wang, Y.; Ahmed, Z.; Feng, W.; Li, C.; Song, S. Physicochemical properties of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir. Int. J. Biol. Macromol. 2008, 43, 283–288. [Google Scholar] [CrossRef]
- Goo, B.G.; Baek, G.; Choi, D.J.; Park, Y.I.; Synytsya, A.; Bleha, R.; Seong, D.H.; Lee, C.; Park, J.K. Characterization of a renewable extracellular polysaccharide from defatted microalgae Dunaliella tertiolecta. Bioresour. Technol. 2013, 129, 343–350. [Google Scholar]
- Liu, T.; Zhou, K.; Yin, S.; Liu, S.; Zhu, Y.; Yang, Y.; Wang, C. Purification and characterization of an exopolysaccharide produced by Lactobacillus plantarum HY isolated from home-made Sichuan Pickle. Int. J. Biol. Macromol. 2019, 134, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Bhat, B.; Bajaj, B.K. Hypocholesterolemic potential and bioactivity spectrum of an exopolysaccharide from a probiotic isolate Lactobacillus paracasei M7. Bioact. Carbohydr. Diet. Fibre 2019, 19, 100191. [Google Scholar] [CrossRef]
- Banerjee, A.; Rudra, S.G.; Mazumder, K.; Nigam, V.; Bandopadhyay, R. Structural and functional properties of exopolysaccharide excreted by a novel Bacillus anthracis (Strain PFAB2) of hot spring origin. Indian J. Microbiol. 2018, 58, 39–50. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Jiang, Y.; Zhao, X.; Hao, X.; Li, L.; Yang, Z. Characterization and antioxidant activity of the exopolysaccharide produced by Bacillus amyloliquefaciens GSBa−1. J. Microbiol. Biotechnol. 2018, 28, 1282–1292. [Google Scholar] [CrossRef]
- Bouallegue, A.; Casillo, A.; Chaari, F.; La Gatta, A.; Lanzetta, R.; Corsaro, M.M.; Bachoual, R.; Ellouz-Chaabouni, S. Levan from a new isolated Bacillus subtilis AF17: Purification, structural analysis and antioxidant activities. Int. J. Biol. Macromol. 2020, 144, 316–324. [Google Scholar] [CrossRef]
- Rahnama Vosough, P.; Habibi Najafi, M.B.; Edalatian Dovom, M.R.; Javadmanesh, A.; Mayo, B. Evaluation of antioxidant, antibacterial and cytotoxicity activities of exopolysaccharide from Enterococcus strains isolated from traditional Iranian Kishk. J. Food Meas. Charact. 2021, 15, 5221–5230. [Google Scholar] [CrossRef]
- Yang, F.; Chen, J.; Ye, S.; Liu, Z.; Ding, Y. Characterization of antioxidant activity of exopolysaccharides from endophytic Lysinibacillus sphaericus Ya6 under osmotic stress conditions. Process Biochem. 2022, 113, 87–96. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.; Fashogbon, R. In vitro antioxidant, antibacterial, in vivo immunomodulatory, antitumor and hematological potential of exopolysaccharide produced by wild type and mutant Lactobacillus delbureckii subsp. bulgaricus. Heliyon 2020, 6, e03268. [Google Scholar] [CrossRef]
- Gangalla, R.; Sampath, G.; Beduru, S.; Sarika, K.; Govindarajan, R.K.; Ameen, F.; Alwakeel, S.; Thampu, R.K. Optimization and characterization of exopolysaccharide produced by Bacillus aerophilus rk1 and its in vitro antioxidant activities. J. King Saud Univ.-Sci. 2021, 33, 101470. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Gnanakan, A.; Lakshmana, S.S.; Meivelu, M.; Jeganathan, A. Structural characterization and anticancer activity of extracellular polysaccharides from ascidian symbiotic bacterium Bacillus thuringiensis. Carbohydr. Polym. 2018, 190, 113–120. [Google Scholar] [CrossRef]
- Rani, R.P.; Anandharaj, M.; Sabhapathy, P.; Ravindran, A.D. Physiochemical and biological characterization of novel exopolysaccharide produced by Bacillus tequilensis FR9 isolated from chicken. Int. J. Biol. Macromol. 2017, 96, 1–10. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Li, D.; Tian, J.; Xiao, L.; Kwok, L.; Li, W.; Sun, Z. Structure characterization, antioxidant capacity, rheological characteristics and expression of biosynthetic genes of exopolysaccharides produced by Lactococcus lactis subsp. lactis IMAU11823. Food Chem. 2022, 384, 132566. [Google Scholar] [CrossRef]
- Li, W.; Ji, J.; Rui, X.; Yu, J.; Tang, W.; Chen, X.; Jiang, M.; Dong, M. Production of exopolysaccharides by Lactobacillus helveticus MB2–1 and its functional characteristics in vitro. LWT-Food Sci. Technolog. 2014, 59, 732–739. [Google Scholar] [CrossRef]
- Liu, J.; Luo, J.; Ye, H.; Sun, Y.; Lu, Z.; Zeng, X. In vitro and in vivo antioxidant activity of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS−3. Carbohydr. Polym. 2010, 82, 1278–1283. [Google Scholar] [CrossRef]
- Mohamed, S.S.; Ibrahim, A.Y.; Asker, M.S.; Mahmoud, M.G.; El-Newary, S.A. Production, structural and biochemical characterization relevant to antitumor property of acidic exopolysaccharide produced from Bacillus sp. NRC5. Arch. Microbiol. 2021, 203, 4337–4350. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Li, X.L.; Zhou, A.G. Evaluation of antioxidant activity of the polysaccharides extracted from Lycium barbarum fruits in vitro. Eur. Polym. J. 2007, 43, 488–497. [Google Scholar] [CrossRef]
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M. Rhizosphere bacteria in plant growth promotion, biocontrol, and bioremediation of contaminated sites: A comprehensive review of effects and mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, L.; Jia, K.; Zhan, H.; Zhang, Z.; Shah, N.P.; Tao, X.; Wei, H. Sulfonation of Lactobacillus plantarum WLPL04 exopolysaccharide amplifies its antioxidant activities in vitro and in a Caco−2 cell model. J. Dairy Sci. 2019, 102, 5922–5932. [Google Scholar] [CrossRef]
- Choudhuri, I.; Khanra, K.; Pariya, P.; Maity, G.N.; Mondal, S.; Pati, B.R.; Bhattacharyya, N. Structural characterization of an exopolysaccharide isolated from Enterococcus faecalis, and study on its antioxidant activity, and cytotoxicity against HeLa cells. Curr. Microbiol. 2020, 77, 3125–3135. [Google Scholar] [CrossRef]
- Park, S.; Saravanakumar, K.; Sathiyaseelan, A.; Park, S.; Hu, X.; Wang, M. Cellular antioxidant properties of nontoxic exopolysaccharide extracted from Lactobacillales (Weissella cibaria) isolated from Korean kimchi. LWT 2022, 154, 112727. [Google Scholar] [CrossRef]
- Magdalena, E.L.; Timothy, S.; Stefan, L.; Jean, D.U. Antioxidant and DNA damage protective activities of selected endophytic actinobacteria isolated from Harpagophytum procumbens: A Kalahari desert-adapted plant. J. Med. Plants Res. 2022, 16, 66–81. [Google Scholar] [CrossRef]
- Fang, S.; Lin, F.; Qu, D.; Liang, X.; Wang, L. Characterization of purified red cabbage anthocyanins: Improvement in HPLC separation and protective effect against H2O2-induced oxidative stress in HepG2 cells. Molecules 2018, 24, 124. [Google Scholar] [CrossRef]
- Han, Y.; Liu, E.; Liu, L.; Zhang, B.; Wang, Y.; Gui, M.; Wu, R.; Li, P. Rheological, emulsifying and thermostability properties of two exopolysaccharides produced by Bacillus amyloliquefaciens LPL061. Carbohydr. Polym. 2015, 115, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Willumsen, P.A.; Karlson, U. Screening of bacteria, isolated from PAH-contaminated soils, for production of biosurfactants and bioemulsifiers. Biodegradation 1996, 7, 415–423. [Google Scholar] [CrossRef]
- Kanmani, P.; Suganya, K.; Yuvaraj, N.; Pattukumar, V.; Paari, K.A.; Arul, V. Synthesis and functional characterization of antibiofilm exopolysaccharide produced by Enterococcus faecium MC13 isolated from the gut of fish. Appl. Biochem. Biotechnol. 2013, 169, 1001–1015. [Google Scholar] [CrossRef]
- Furuhashi, H.; Higashiyama, M.; Okada, Y.; Kurihara, C.; Wada, A.; Horiuchi, K.; Hanawa, Y.; Mizoguchi, A.; Nishii, S.; Inaba, K. Dietary emulsifier polysorbate−80-induced small-intestinal vulnerability to indomethacin-induced lesions via dysbiosis. J. Gastroenterol. Hepatol. 2020, 35, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential commercial applications of microbial surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.H.; Chen, B.Y.; Shen, F.T.; Young, C.C. Optimization of exopolysaccharide production and diesel oil emulsifying properties in root nodulating bacteria. World J. Microbiol. Biotechnol. 2012, 28, 1367–1373. [Google Scholar] [CrossRef]
- Satpute, S.K.; Banat, I.M.; Dhakephalkar, P.K.; Banpurkar, A.G.; Chopade, B.A. Biosurfactants, bioemulsifiers and exopolysaccharides from marine microorganisms. Biotechnol. Adv. 2010, 28, 436–450. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang-Lin, E.; Sánchez-León, E.; Amils, R.; Abrusci, C. Potential Applications of an Exopolysaccharide Produced by Bacillus xiamenensis RT6 Isolated from an Acidic Environment. Polymers 2022, 14, 3918. https://doi.org/10.3390/polym14183918
Huang-Lin E, Sánchez-León E, Amils R, Abrusci C. Potential Applications of an Exopolysaccharide Produced by Bacillus xiamenensis RT6 Isolated from an Acidic Environment. Polymers. 2022; 14(18):3918. https://doi.org/10.3390/polym14183918
Chicago/Turabian StyleHuang-Lin, Elisa, Enrique Sánchez-León, Ricardo Amils, and Concepcion Abrusci. 2022. "Potential Applications of an Exopolysaccharide Produced by Bacillus xiamenensis RT6 Isolated from an Acidic Environment" Polymers 14, no. 18: 3918. https://doi.org/10.3390/polym14183918





