Synthesis of Novel Non-Isocyanate Polyurethane/Functionalized Boron Nitride Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.2.1. Preparation of Bi-Cyclic Carbonate
2.2.2. Functionalization of Hexagonal Boron Nitride
2.2.3. Preparation of PHU Films and Nanocomposites
2.3. Methods
3. Results and Discussion
3.1. Synthesis of Bi-Cyclic Five-Membered Carbonate
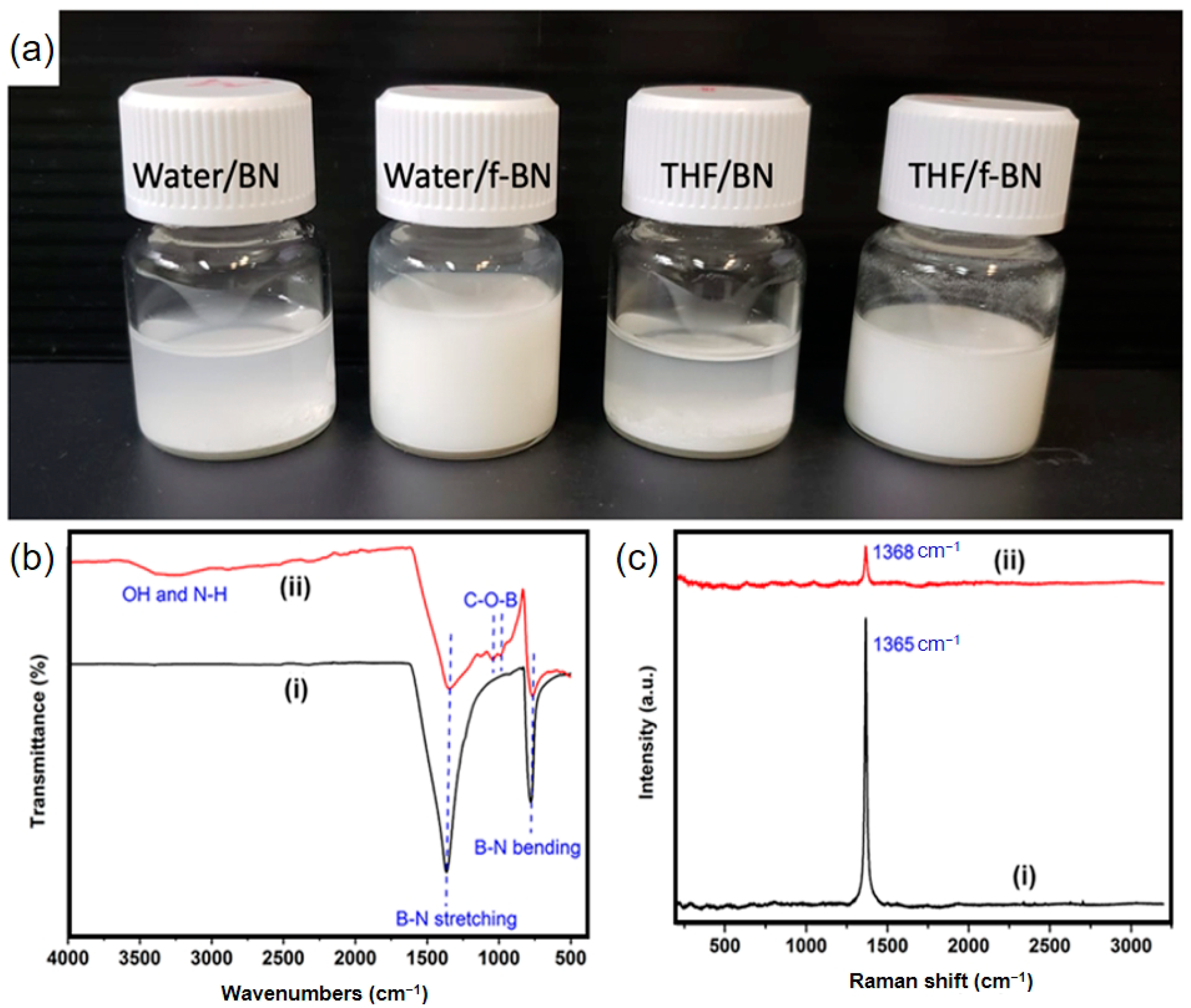
3.2. Functionalization of Boron Nitride
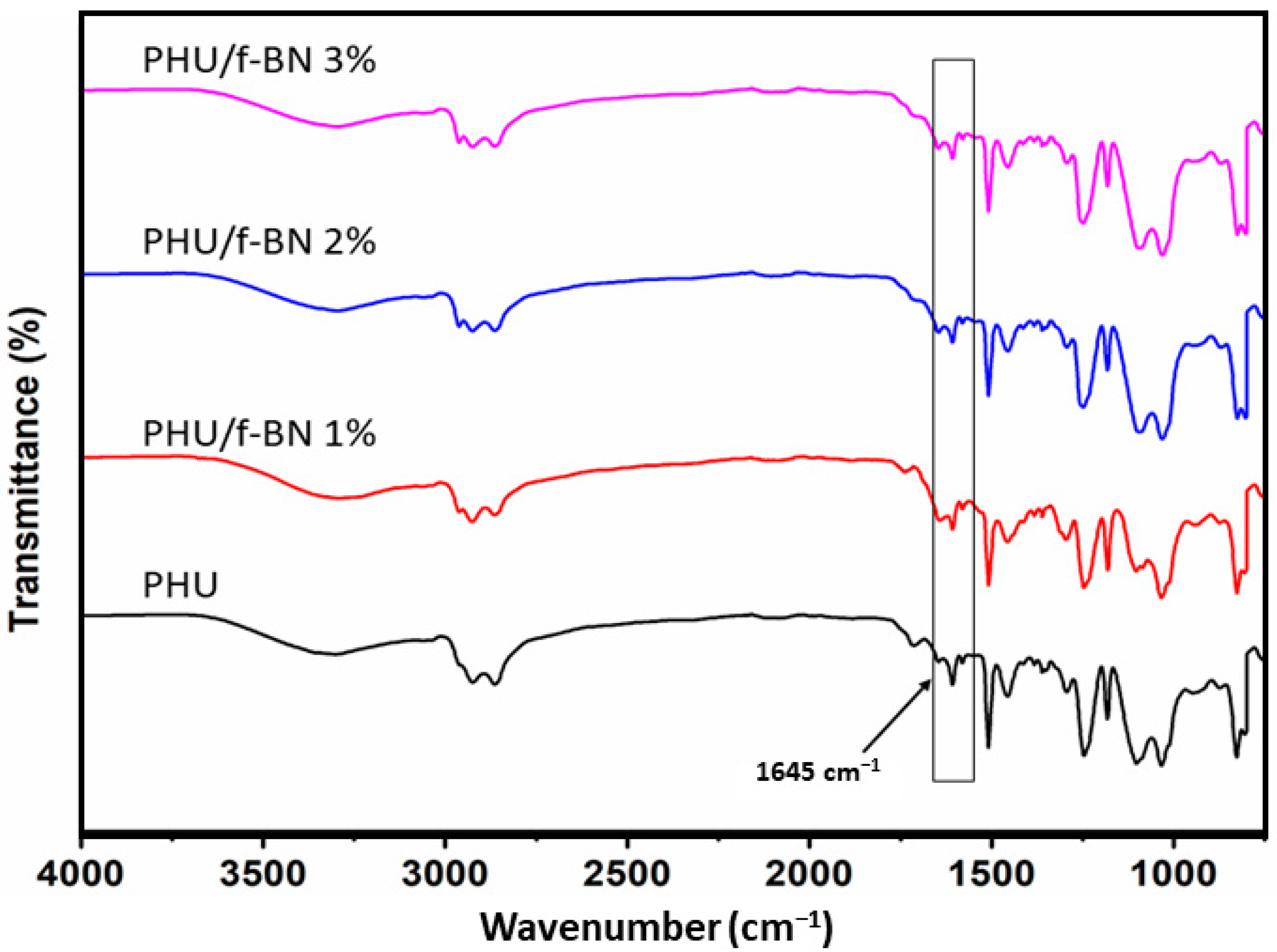
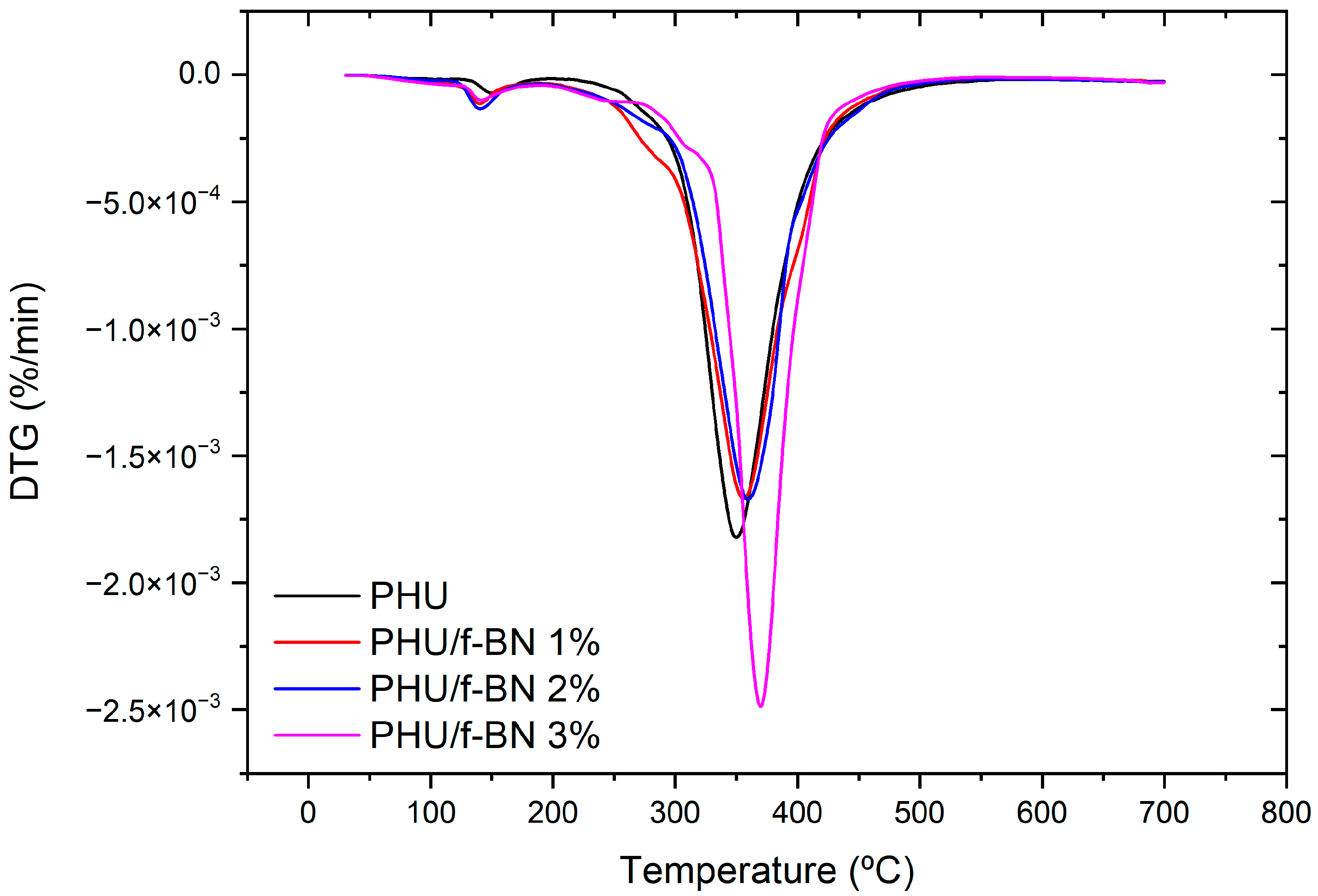
3.3. PHU Nanocomposite Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane Types, Synthesis and Ap-plications—A Review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Blattmann, H.; Fleischer, M.; Bähr, M.; Mülhaupt, R. Isocyanate- and Phosgene-Free Routes to Polyfunctional Cyclic Carbonates and Green Polyurethanes by Fixation of Carbon Dioxide. Macromol. Rapid Commun. 2014, 35, 1238–1254. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, A.; Elizalde, F.; Calvo, I.; Sardon, H. Trends in Non-Isocyanate Polyurethane (NIPU) Development. Chem. Commun. 2021, 57, 12254–12265. [Google Scholar] [CrossRef]
- Maisonneuve, L.; Lamarzelle, O.; Rix, E.; Grau, E.; Cramail, H. Isocyanate-Free Routes to Polyurethanes and Poly(Hydroxy Urethane)S. Chem. Rev. 2015, 115, 12407–12439. [Google Scholar] [CrossRef] [PubMed]
- Kathalewar, M.S.; Joshi, P.B.; Sabnis, A.S.; Malshe, V.C. Non-Isocyanate Polyurethanes: From Chemistry to Applications. RSC Adv. 2013, 3, 4110–4129. [Google Scholar] [CrossRef]
- Martínez de Sarasa Buchaca, M.; de la Cruz-Martínez, F.; Francés-Poveda, E.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Garcés, A.; Castro-Osma, J.A.; Lara-Sánchez, A. Synthesis of Nonisocyanate Poly(hydroxy)urethanes from Bis(cyclic carbonates) and Polyamines. Polymers 2022, 14, 2719. [Google Scholar] [CrossRef]
- Alves, M.; Grignard, B.; Mereau, R.; Jerome, C.; Tassaing, T.; Detrembleur, C. Organocatalyzed Coupling of Carbon Dioxide with Epoxides for the Synthesis of Cyclic Carbonates: Catalyst Design and Mechanistic Studies. Catal. Sci. Technol. 2017, 7, 2651–2684. [Google Scholar] [CrossRef]
- Claver, C.; Yeamin, M.B.; Reguero, M.; Masdeu-Bultó, A.M. Recent Advances in the Use of Catalysts Based on Natural Products for the Conversion of CO2 into Cyclic Carbonates. Green Chem. 2020, 22, 7665–7706. [Google Scholar] [CrossRef]
- Song, B.; Qin, A.; Tang, B.Z. Syntheses, Properties, and Applications of CO2-Based Functional Polymers. Cell Rep. Phys. Sci. 2022, 3, 100719. [Google Scholar] [CrossRef]
- Stachak, P.; Łukaszewska, I.; Hebda, E.; Pielichowski, K. Recent Advances in Fabrication of non-isocyanate Polyure-thane-based composite materials. Materials 2021, 14, 3497. [Google Scholar] [CrossRef]
- Ecochard, Y.; Caillol, S. Hybrid polyhydroxyurethanes: How to overcome limitations and reach cutting edge properties? Eur. Polym. J. 2020, 137, 109915. [Google Scholar] [CrossRef]
- Panchireddy, S.; Grignard, B.; Thomassin, J.M.; Jerome, C.; Detrembleur, C. Bio-Based Poly(Hydroxyurethane) Glues for Metal Substrates. Polym. Chem. 2018, 9, 2650–2659. [Google Scholar] [CrossRef]
- Panchireddy, S.; Thomassin, J.M.; Grignard, B.; Damblon, C.; Tatton, A.; Jerome, C.; Detrembleur, C. Reinforced Poly(Hydroxyurethane) Thermosets as High Performance Adhesives for Aluminum Substrates. Polym. Chem. 2017, 8, 5897–5909. [Google Scholar] [CrossRef]
- Türünç, O.; Kayaman-Apohan, N.; Kahraman, M.V.; Menceloǧlu, Y.; Güngör, A. Nonisocyanate Based Polyurethane/Silica Nanocomposites and Their Coating Performance. J. Sol-Gel Sci. Technol. 2008, 47, 290–299. [Google Scholar] [CrossRef]
- Blattmann, H.; Mülhaupt, R. Multifunctional POSS Cyclic Carbonates and Non-Isocyanate Polyhydroxyurethane Hybrid Materials. Macromol. 2016, 49, 742–751. [Google Scholar] [CrossRef]
- Liu, W.; Hang, G.; Mei, H.; Li, L.; Zheng, S. Nanocomposites of Polyhydroxyurethane with POSS Microdomains: Synthesis via Non-Isocyanate Approach, Morphologies and Reprocessing Properties. Polymers 2022, 14, 1331. [Google Scholar] [CrossRef]
- Kathalewar, M.; Sabnis, A.; Waghoo, G. Effect of incorporation of surface treated zinc oxide on non-isocyanate polyurethane based nano-composite coatings. Prog. Org. Coat. 2013, 76, 1215–1229. [Google Scholar] [CrossRef]
- Li, L.; Zhao, B.; Wang, H.; Gao, Y.; Hu, J.; Zheng, S. Nanocomposites of Polyhydroxyurethane with Fe3O4 Nanoparticles: Synthesis, Shape Memory and Reprocessing Properties. Compos. Sci. Technol. 2021, 215, 109009. [Google Scholar] [CrossRef]
- He, X.; Xu, X.; Bo, G.; Yan, Y. Studies on the Effects of Different Multiwalled Carbon Nanotube Functionalization Techniques on the Properties of Bio-Based Hybrid Non-Isocyanate Polyurethane. RSC Adv. 2020, 10, 2180–2190. [Google Scholar] [CrossRef]
- Adeel, M.; Zhao, B.; Li, L.; Zheng, S. Nanocomposites of Poly(Hydroxyurethane)s with Multiwalled Carbon Nanotubes: Synthesis, Shape Memory, and Reprocessing Properties. ACS Appl. Polym. Mater. 2020, 2, 1711–1721. [Google Scholar] [CrossRef]
- Ge, W.; Zhao, B.; Li, L.; Nie, K.; Zheng, S. Nanocomposites of Polyhydroxyurethane with Nanocrystalline Cellulose: Syn-thesis, Thermomechanical and Reprocessing Properties. Eur. Polym. J. 2021, 149, 110287. [Google Scholar] [CrossRef]
- Białkowsk, A.; Bakar, M.; Przybyłek, M. Effect of Nonisocyanate Polyurethane and Nanoclay on the Mechanical Properties of an Epoxy Resin. Mech. Compos. Mater. 2018, 54, 665–674. [Google Scholar] [CrossRef]
- Gennen, S.; Grignard, B.; Thomassin, J.M.; Gilbert, B.; Vertruyen, B.; Jerome, C.; Detrembleur, C. Polyhydroxyurethane Hydrogels: Synthesis and Characterizations. Eur. Polym. J. 2016, 84, 849–862. [Google Scholar] [CrossRef]
- Yang, Y.; Pössel, B.; Mülhaupt, R. Graphenated Ceramic Particles as Functional Fillers for Nonisocyanate Polyhydroxyure-thane Composites. Macromol. Mater. Eng. 2020, 305, 2000203. [Google Scholar] [CrossRef]
- Doley, S.; Sarmah, A.; Sarkar, C.; Dolui, S.K. In Situ Development of Bio-Based Polyurethane-Blend-Epoxy Hybrid Materials and Their Nanocomposites with Modified Graphene Oxide via Non-Isocyanate Route. Polym. Int. 2018, 67, 1062–1069. [Google Scholar] [CrossRef]
- Meziani, M.J.; Sheriff, K.; Parajuli, P.; Priego, P.; Bhattacharya, S.; Rao, A.M.; Quimby, J.L.; Qiao, R.; Wang, P.; Hwu, S.-J.; et al. Advances in Studies of Boron Nitride Nanosheets and Nanocomposites for Thermal Transport and Related Applications. ChemPhysChem 2022, 23, e202100645. [Google Scholar] [CrossRef]
- Rasul, M.G.; Kiziltas, A.; Arfaei, B.; Shahbazian-Yassar, R. 2D Boron Nitride Nanosheets for Polymer Composite Materials. npj 2D Mater. Appl. 2021, 5, 56. [Google Scholar] [CrossRef]
- Hayat, A.; Sohail, M.; Hamdy, M.S.; Taha, T.A.; AlSalem, H.S.; Alenad, A.M.; Amin, M.A.; Shah, R.; Palamanit, A.; Khan, J.; et al. Fabrication, Characteristics, and Applications of Boron Nitride and Their Composite Nanomaterials. Surf. Interfaces 2022, 29, 101725. [Google Scholar] [CrossRef]
- Chen, S.; Xu, R.; Liu, J.; Zou, X.; Qiu, L.; Kang, F.; Liu, B.; Cheng, H.M. Simultaneous Production and Functionalization of Boron Nitride Nanosheets by Sugar-Assisted Mechanochemical Exfoliation. Adv. Mater. 2019, 31, 1804810. [Google Scholar] [CrossRef]
- Caló, V.; Nacci, A.; Monopoli, A.; Fanizzi, A. Cyclic Carbonate Formation from Carbon Dioxide and Oxiranes in Tetrabu-tylammonium Halides as Solvents and Catalysts. Org. Lett. 2002, 4, 2561–2563. [Google Scholar] [CrossRef]
- North, M.; Pasquale, R. Mechanism of Cyclic Carbonate Synthesis from Epoxides and CO2. Angew. Chemie-Int. Ed. 2009, 48, 2946–2948. [Google Scholar] [CrossRef] [PubMed]
- Castro-Osma, J.A.; North, M.; Wu, X. Synthesis of Cyclic Carbonates Catalysed by Chromium and Aluminium Salphen Complexes. Chem.-Eur. J. 2016, 22, 2100–2107. [Google Scholar] [CrossRef] [PubMed]
- Ohkawara, T.; Suzuki, K.; Nakano, K.; Mori, S.; Nozaki, K. Facile Estimation of Catalytic Activity and Selectivities in Co-polymerization of Propylene Oxide with Carbon Dioxide Mediated by Metal Complexes with Planar Tetradentate Ligand. J. Am. Chem. Soc. 2014, 136, 10728–10735. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Ebitani, K.; Yoshida, T.; Yoshida, H.; Kaneda, K. Mg-Al Mixed Oxides as Highly Active Acid-Base Catalysts for Cycloaddition of Carbon Dioxide to Epoxides. J. Am. Chem. Soc. 1999, 121, 4526–4527. [Google Scholar] [CrossRef]
- Dai, W.L.; Luo, S.L.; Yin, S.F.; Au, C.T. The Direct Transformation of Carbon Dioxide to Organic Carbonates over Heteroge-neous Catalysts. Appl. Catal. A Gen. 2009, 366, 2–12. [Google Scholar] [CrossRef]
- Qu, J.; Cao, C.Y.; Dou, Z.F.; Liu, H.; Yu, Y.; Li, P.; Song, W.G. Synthesis of Cyclic Carbonates: Catalysis by an Iron-Based Composite and the Role of Hydrogen Bonding at the Solid/Liquid Interface. ChemSusChem 2012, 5, 652–655. [Google Scholar] [CrossRef]
- Carré, C.; Bonnet, L.; Avérous, L. Original Biobased Nonisocyanate Polyurethanes: Solvent- and Catalyst-Free Synthesis, Thermal Properties and Rheological Behaviour. RSC Adv. 2014, 4, 54018–54025. [Google Scholar] [CrossRef]
- Van Velthoven, J.L.J.; Gootjes, L.; Van Es, D.S.; Noordover, B.A.J.; Meuldijk, J. Poly(Hydroxy Urethane)s Based on Renewable Diglycerol Dicarbonate. Eur. Polym. J. 2015, 70, 125–135. [Google Scholar] [CrossRef]
- Bernal, M.M.; Molenberg, I.; Estravis, S.; Rodriguez-Perez, M.A.; Huynen, I.; Lopez-Manchado, M.A.; Verdejo, R. Comparing the Effect of Carbon-Based Nanofillers on the Physical Properties of Flexible Polyurethane Foams. J. Mater. Sci. 2012, 47, 5673–5679. [Google Scholar] [CrossRef]
- Yin, S.; Ren, X.; Lian, P.; Zhu, Y.; Mei, Y. Synergistic Effects of Black Phosphorus/Boron Nitride Nanosheets on Enhancing the Flame-Retardant Properties of Waterborne Polyurethane and Its Flame-Retardant Mechanism. Polymers 2020, 12, 1487. [Google Scholar] [CrossRef]
- Kim, K.; Kim, M.; Kim, J. Enhancement of the Thermal and Mechanical Properties of a Surface-Modi Fi Ed Boron Nitride–Polyurethane Composite. Polym. Adv. Technol. 2014, 25, 791–798. [Google Scholar] [CrossRef]
- Kim, K.; Kim, M.; Kim, J. Fabrication of UV-Curable Polyurethane Acrylate Composites Containing Surface-Modified Boron Nitride for Underwater Sonar Encapsulant Application. Ceram. Int. 2014, 40, 10933–10943. [Google Scholar] [CrossRef]
- Bashir, M.A. Use of Dynamic Mechanical Analysis (DMA) for Characterizing Interfacial Interactions in Filled Polymers. Solids 2021, 2, 108–120. [Google Scholar] [CrossRef]
- Landel, R.F.; Nielsen, L.E. Mechanical Properties of Polymers and Composites, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 1994. [Google Scholar]
- Gholami, M.; Shakeri, A.; Zolghadr, M.; Yamini, G. Non-Isocyanate Polyurethane from the Extracted Tannin of Sumac Leaves: Synthesis, Characterization, and Optimization of the Reaction Parameters. Ind. Crops Prod. 2021, 161, 113195. [Google Scholar] [CrossRef]


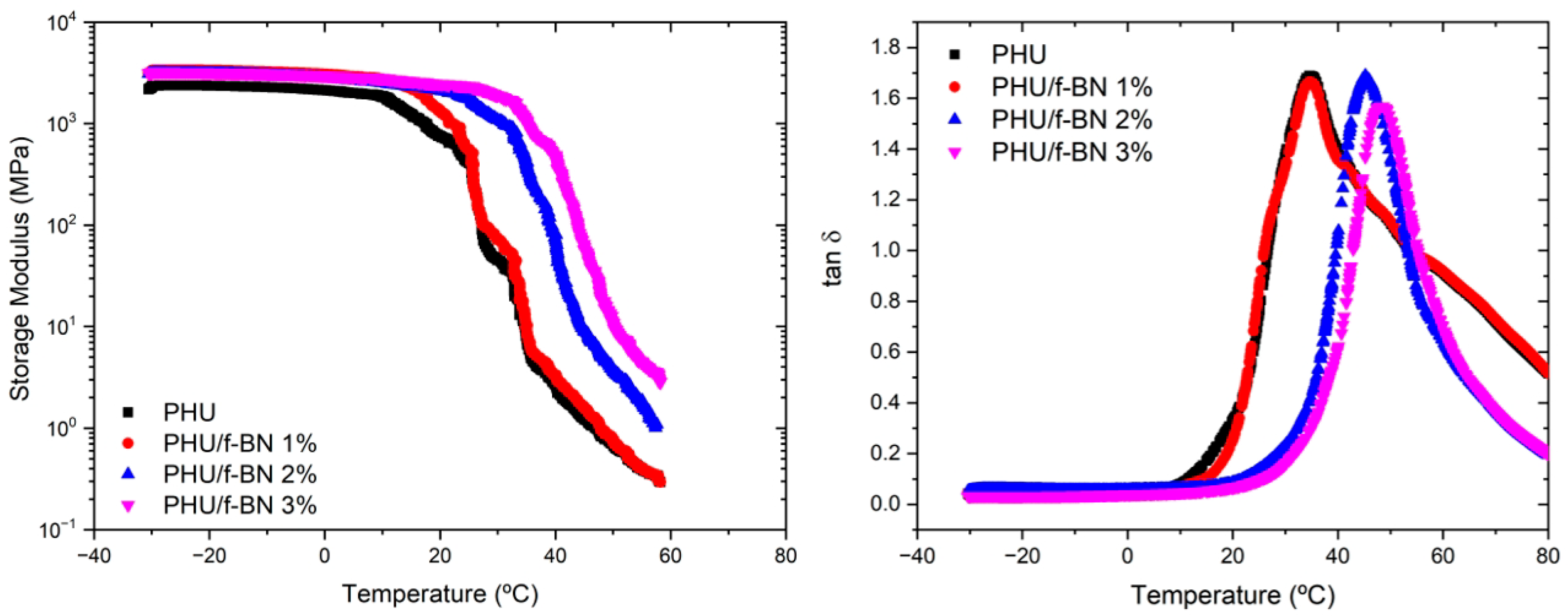
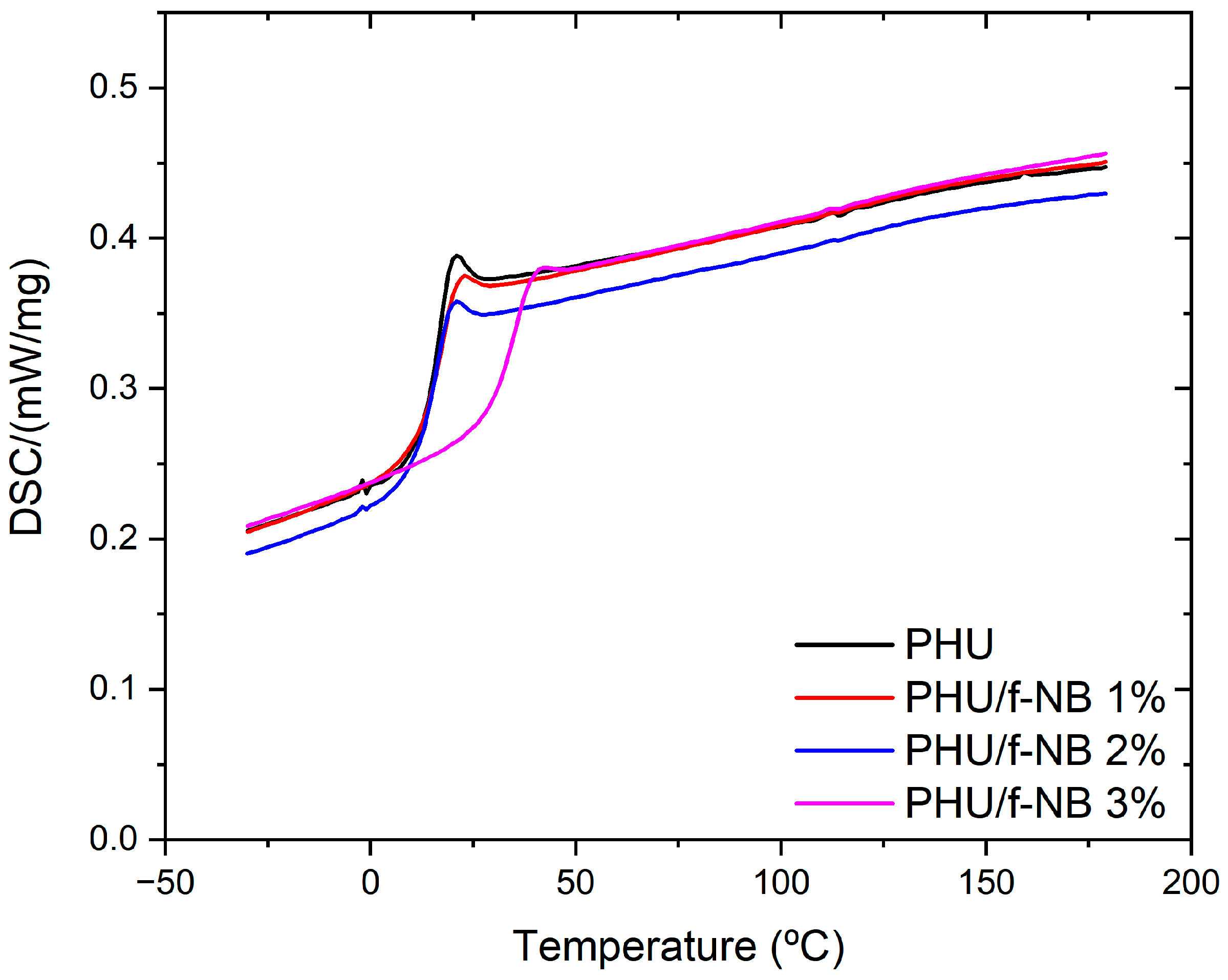
| Td (°C) * | Tg (°C) ** | Tα (°C) *** | E’ (MPa) *** | |
|---|---|---|---|---|
| PHU | 350 | 18.5 | 31.7 | 7.8 |
| PHU/f-BN 1% | 356 | 19.1 | 31.8 | 8.1 |
| PHU/f-BN 2% | 360 | 19.8 | 42.5 | 9.8 |
| PHU/f-BN 3% | 370 | 29.2 | 49.3 | 11.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Khezraji, S.; Chaib, M.; Thakur, S.; Raihane, M.; Lopez-Manchado, M.A.; Verdejo, R.; Lahcini, M. Synthesis of Novel Non-Isocyanate Polyurethane/Functionalized Boron Nitride Composites. Polymers 2022, 14, 3934. https://doi.org/10.3390/polym14193934
El Khezraji S, Chaib M, Thakur S, Raihane M, Lopez-Manchado MA, Verdejo R, Lahcini M. Synthesis of Novel Non-Isocyanate Polyurethane/Functionalized Boron Nitride Composites. Polymers. 2022; 14(19):3934. https://doi.org/10.3390/polym14193934
Chicago/Turabian StyleEl Khezraji, Said, Manal Chaib, Suman Thakur, Mustapha Raihane, Miguel A. Lopez-Manchado, Raquel Verdejo, and Mohammed Lahcini. 2022. "Synthesis of Novel Non-Isocyanate Polyurethane/Functionalized Boron Nitride Composites" Polymers 14, no. 19: 3934. https://doi.org/10.3390/polym14193934
APA StyleEl Khezraji, S., Chaib, M., Thakur, S., Raihane, M., Lopez-Manchado, M. A., Verdejo, R., & Lahcini, M. (2022). Synthesis of Novel Non-Isocyanate Polyurethane/Functionalized Boron Nitride Composites. Polymers, 14(19), 3934. https://doi.org/10.3390/polym14193934









