Effect of Nitrogen Gas Post-Curing and Printer Type on the Mechanical Properties of 3D-Printed Hard Occlusal Splint Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flexural Strength and Modulus Testing
2.2. Surface Microhardness (Vickers Hardness)
2.3. Surface Polymerization (Degree of Double Bond Conversion; DC)
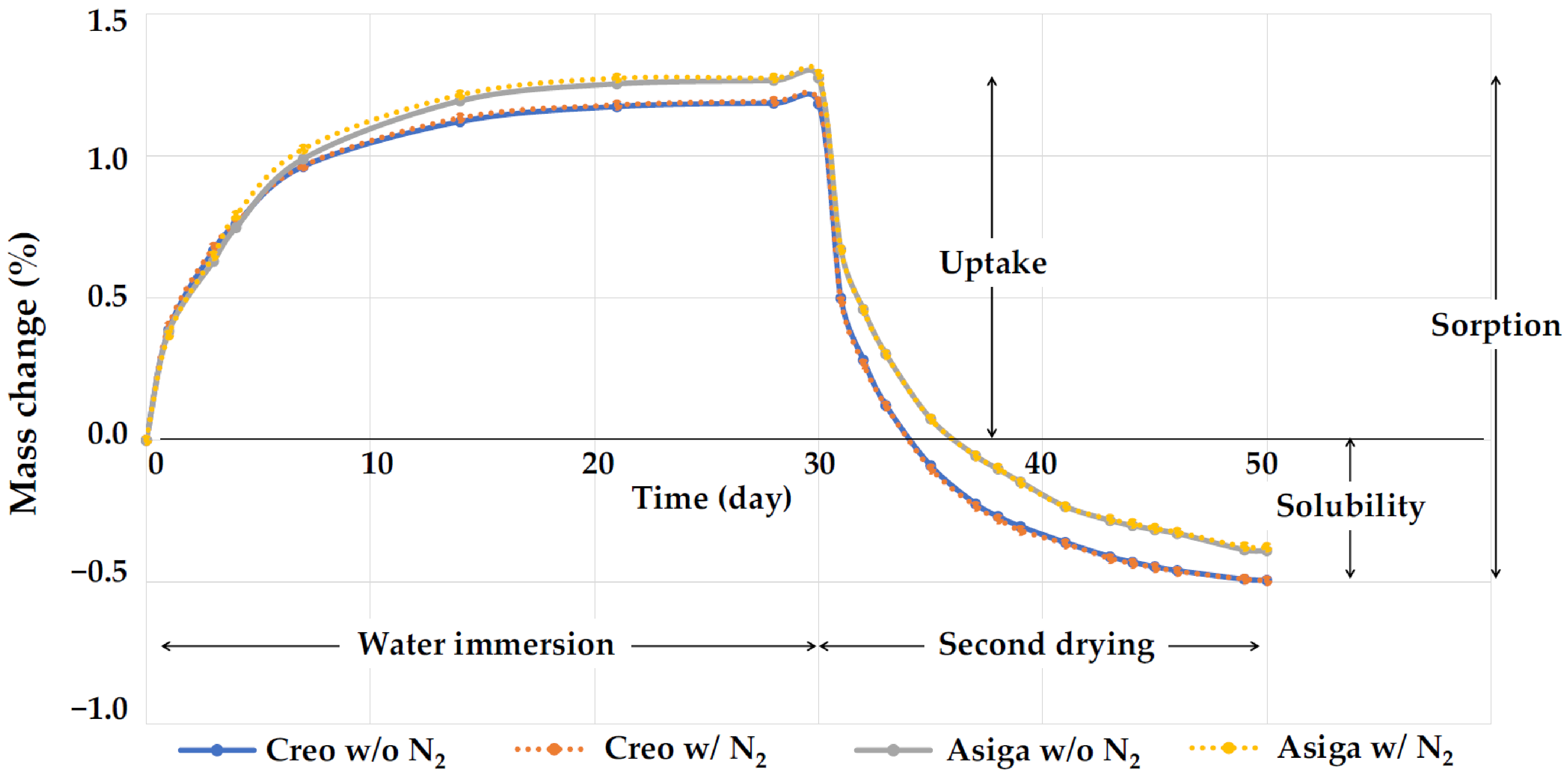
2.4. Water Sorption and Solubility
2.5. Three-Dimensional Microlayer Structure
2.6. Fracture Toughness (KIC)
f(x) = 3x1/2[1.99 − x(1 − x)(2.15 − 3.93x + 2.7x2)]/[2(1 + 2x)(1 − x)3/2]
and 0 < x < 1 with x = a/W
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dao, T.T.; Lavigne, G.J. Oral splints: The crutches for temporomandibular disorders and bruxism? Crit. Rev. Oral. Biol. Med. 1998, 9, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Klasser, G.D.; Greene, C.S. Oral appliances in the management of temporomandibular disorders. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Fricton, J.; Look, J.O.; Wright, E.; Alencar, F.G.P., Jr.; Chen, H.; Lang, M.; Ouyang, W.; Velly, A.M. Systematic review and meta-analysis of randomized controlled trials evaluating intraoral orthopedic appliances for temporomandibular disorders. J. Orofac. Pain. 2010, 24, 237–254. [Google Scholar]
- Uchida, H.; Wada, J.; Watanabe, C.; Nagayama, T.; Mizutani, K.; Mikami, R.; Inukai, S.; Wakabayashi, N. Effect of night dentures on tooth mobility in denture wearers with sleep bruxism: A pilot randomized controlled trial. J. Prosthodont Res. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Kuzmanovic, P.J.; Dodic, S.; Lazic, V.; Trajkovic, G.; Milic, N.; Milicic, B. Occlusal stabilization splint for patients with temporomandibular disorders: Meta-analysis of short and long term effects. PLoS ONE 2017, 12, e0171296. [Google Scholar] [CrossRef] [PubMed]
- Leib, A.M. Patient preference for light-cured composite bite splint compared to heat-cured acrylic bite splint. J. Periodontol. 2001, 72, 1108–1112. [Google Scholar] [CrossRef]
- Dedem, P.; Türp, J.C. Digital Michigan splint—From intraoral scanning to plasterless manufacturing. Int. J. Comput. Dent. 2016, 19, 63–76. [Google Scholar]
- Lauren, M.; McIntyre, F. A new computer-assisted method for design and fabrication of occlusal splints. Am. J. Orthod. Dentofac. Orthop. 2008, 133, S130–S135. [Google Scholar] [CrossRef]
- Salmi, M.; Paloheimo, K.S.; Tuomi, J.; Ingman, T.; Mäkitie, A. A digital process for additive manufacturing of occlusal splints: A clinical pilot study. J. R Soc. Interface. 2013, 10, 20130203. [Google Scholar] [CrossRef]
- Berntsen, C.; Kleven, M.; Heian, M.; Hjortsjö, C. Clinical comparison of conventional and additive manufactured stabilization splints. Acta Biomater. Odontol. Scand. 2018, 4, 81–89. [Google Scholar] [CrossRef]
- Gonçalves, F.A.M.M.; Fonseca, A.C.; Domingos, M.; Gloria, A.; Serra, A.C.; Coelho, J.F.J. The potential of unsaturated polyesters in biomedicine and tissue engineering: Synthesis, structure-properties relationships and additive manufacturing. Prog. Polym. Sci. 2017, 68, 1–34. [Google Scholar] [CrossRef]
- Väyrynen, V.O.; Tanner, J.; Vallittu, P.K. The anisotropicity of the flexural properties of an occlusal device material processed by stereolithography. J. Prosthet Dent. 2016, 116, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Zinser, M.J.; Mischkowski, R.A.; Sailer, H.F.; Zöller, J.E. Computer-assisted orthognathic surgery: Feasibility study using multiple CAD/CAM surgical splints. Oral Surg. Oral Med. Oral Patho.l Oral Radiol. 2012, 113, 673–687. [Google Scholar] [CrossRef] [PubMed]
- van Noort, R. The future of dental devices is digital. Dent. Mater. 2012, 28, 3–12. [Google Scholar] [CrossRef]
- Wickström, H.; Hilgert, E.; Nyman, J.O.; Desai, D.; Karaman, D.Ş.; de Beer, T.; Sandler, N.; Rosenholm, J.M. Inkjet Printing of Drug-Loaded Mesoporous Silica Nanoparticles-A Platform for Drug Development. Molecules 2017, 22, 2020. [Google Scholar] [CrossRef]
- Panwar, A.; Tan, L.P. Current Status of Bioinks for Micro-Extrusion-Based 3D Bioprinting. Molecules 2016, 21, 685. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef]
- Lutz, A.M.; Hampe, R.; Roos, M.; Lümkemann, N.; Eichberger, M.; Stawarczyk, B. Fracture resistance and 2-body wear of 3-dimensional-printed occlusal devices. J. Prosthet Dent. 2019, 121, 166–172. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, D.H.; Huang, S.C.; Lin, Y.M. Comparison of flexural properties and cytotoxicity of interim materials printed from mono-LCD and DLP 3D printers. J. Prosthet Dent. 2021, 26, 703–708. [Google Scholar] [CrossRef]
- Revilla-León, M.; Özcan, M. Additive Manufacturing Technologies Used for Processing Polymers: Current Status and Potential Application in Prosthetic Dentistry. J. Prosthodont. 2019, 28, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Tulcan, A.; Vasilescu, M.D.; Tulcan, L. Study of the Influence of Technological Parameters on Generating Flat Part with Cylindrical Features in 3D Printing with Resin Cured by Optical Processing. Polymers 2020, 2, 1941. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Kalberer, N.; Kamnoedboon, P.; Mekki, M.; Durual, S.; Özcan, M.; Müller, F. CAD-CAM complete denture resins: An evaluation of biocompatibility, mechanical properties, and surface characteristics. J. Dent. 2021, 114, 103785. [Google Scholar] [CrossRef] [PubMed]
- Zinelis, S.; Panayi, N.; Polychronis, G.; Papageorgiou, S.N.; Eliades, T. Comparative analysis of mechanical properties of orthodontic aligners produced by different contemporary 3D printers. Orthod Craniofac Res. 2022, 25, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Kunjan, C.; Jawahar, N.; Chandrasekhar, U. Influence of layer thickness on mechanical properties in stereolithography. Rapid Prototyp. J. 2006, 12, 106–113. [Google Scholar]
- Al Mortadi, N.; Eggbeer, D.; Lewis, J.; Williams, R.J. CAD/CAM/AM applications in the manufacture of dental appliances. Am. J. Orthod. Dentofacial Orthop. 2012, 142, 727–733. [Google Scholar] [CrossRef]
- Reymus, M.; Lümkemann, N.; Stawarczyk, B. 3D-printed material for temporary restorations: Impact of print layer thickness and post-curing method on degree of conversion. Int. J. Comput. Dent. 2019, 22, 231–237. [Google Scholar]
- Li, P.; Lambart, A.L.; Stawarczyk, B.; Reymus, M.; Spintzyk, S. Postpolymerization of a 3D-printed denture base polymer: Impact of post-curing methods on surface characteristics, flexural strength, and cytotoxicity. J. Dent. 2021, 115, 103856. [Google Scholar] [CrossRef]
- Aati, S.; Akram, Z.; Shrestha, B.; Patel, J.; Shih, B.; Shearston, K.; Ngo, H.; Fawzy, A. Effect of post-curing light exposure time on the physico-mechanical properties and cytotoxicity of 3D-printed denture base material. Dent. Mater. 2022, 38, 57–67. [Google Scholar] [CrossRef]
- Soto-Montero, J.; de Castro, E.F.; Romano, B.C.; Nima, G.; Shimokawa, C.A.K.; Giannini, M. Color alterations, flexural strength, and microhardness of 3D printed resins for fixed provisional restoration using different post-curing times. Dent. Mater. 2022, 38, 1271–1282. [Google Scholar] [CrossRef]
- Song, G.; Son, J.W.; Jang, J.H.; Choi, S.H.; Jang, W.H.; Lee, B.N.; Park, C. Comparing volumetric and biological aspects of 3D-printed interim restorations under various post-curing modes. J. Adv. Prosthodont. 2021, 13, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Huettig, F.; Kustermann, A.; Kuscu, E.; Geis-Gerstorfer, J.; Spintzyk, S. Polishability and wear resistance of splint material for oral appliances produced with conventional, subtractive, and additive manufacturing. J. Mech. Behav. Biomed. Mater. 2017, 75, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Perea-Lowery, L.; Gibreel, M.; Vallittu, P.K.; Lassila, L. Evaluation of the mechanical properties and degree of conversion of 3D printed splint material. J. Mech. Behav. Biomed. Mater. 2021, 115, 104254. [Google Scholar] [CrossRef] [PubMed]
- Gibreel, M.; Perea-Lowery, L.; Vallittu, P.K.; Lassila, L. Characterization of occlusal splint materials: CAD-CAM versus conventional resins. J. Mech. Behav. Biomed. Mater. 2021, 124, 104813. [Google Scholar] [CrossRef]
- Moon, W.; Kim, S.; Lim, B,S.; Park, Y,S.; Kim, R.J.; Chung, S.H. Dimensional Accuracy Evaluation of Temporary Dental Restorations with Different 3D Printing Systems. Materials 2021, 14, 1487. [Google Scholar] [CrossRef]
- Ozcan, M.; Barbosa, S.H.; Melo, R.M.; Galhano, G.A.; Bottino, M.A. Effect of surface conditioning methods on the microtensile bond strength of resin composite to composite after aging conditions. Dent. Mater. 2007, 23, 1276–1282. [Google Scholar] [CrossRef]
- Brendeke, J.; Ozcan, M. Effect of physicochemical aging conditions on the composite-composite repair bond strength. J. Adhes Dent. 2007, 9, 399–406. [Google Scholar]
- Egilmez, F.; Ergun, G.; Cekic-Nagas, I.; Vallittu, P.K.; Lassila, L.V.J. Does artificial aging affect mechanical properties of CAD/CAM composite materials. J. Prosthodont Res. 2018, 62, 65–74. [Google Scholar] [CrossRef]
- Lo Giudice, R.; Famà, F. Health Care and Health Service Digital Revolution. Int. J. Environ. Res. Public Health. 2020, 17, 4913. [Google Scholar] [CrossRef]



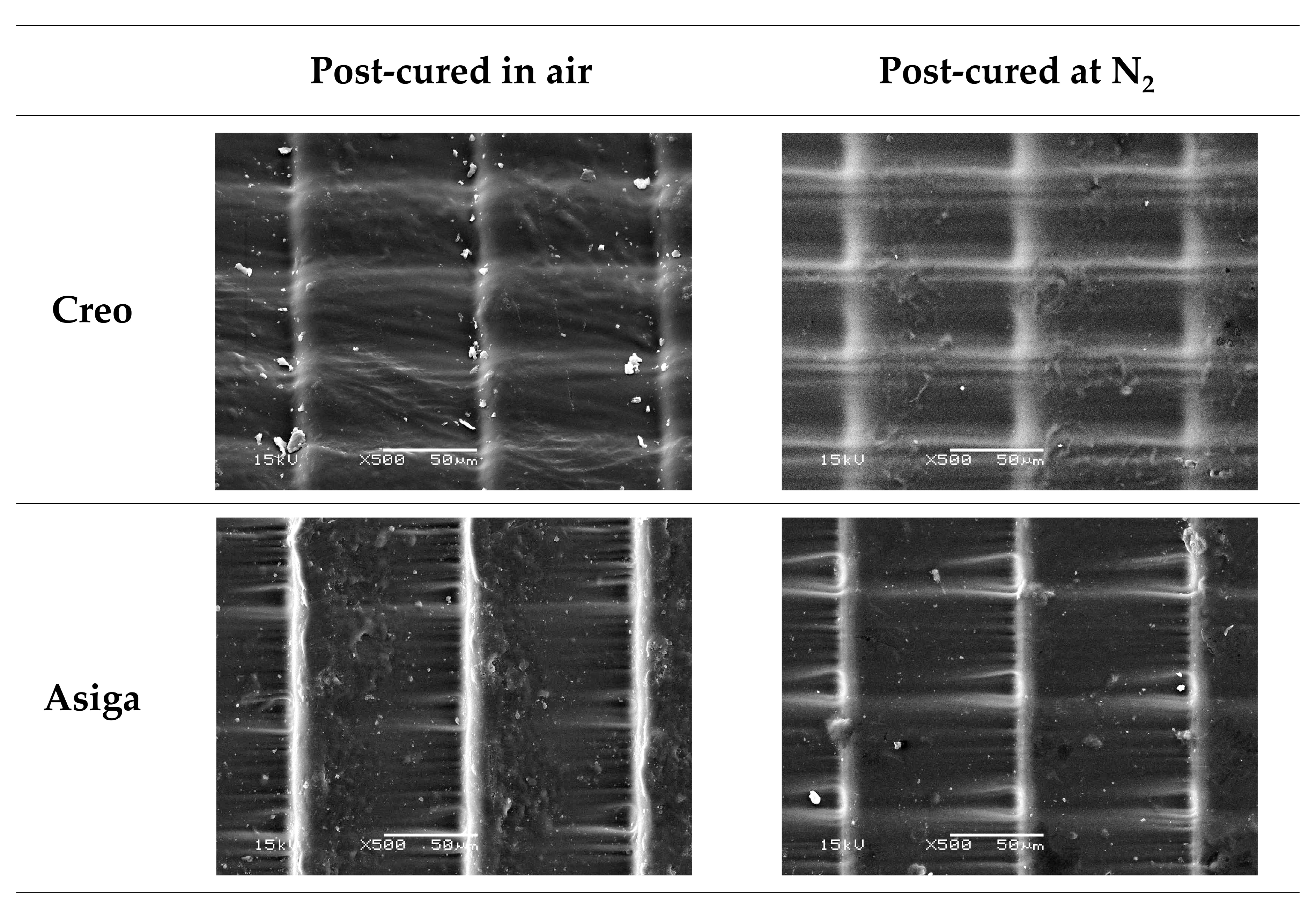
| Variable | Flexural Strength | Flexural Modulus | Vickers Hardness Number (VHN) | Fracture Toughness | Degree of Double Bond Conversion (DC) | Three-Dimensional (3D) Microlayer Structure | Water Sorption | Water Solubility | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Width | Length | Height | ||||||||
| Post-curing method | <0.001 | <0.001 | <0.001 | 0.069 | <0.001 | 0.335 | 0.127 | 0.907 | <0.001 | 0.968 |
| Printer type | 0.323 | 0.220 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Aging in boiling water | <0.001 | <0.001 | <0.001 | <0.001 | - | - | - | - | - | - |
| Printer Type | Post-Curing | Aging in BW | Flexural Strength (MPa) | Flexural Modulus (GPa) | Broken Specimens (%) | VHN | Fracture Toughness (MPa m1/2) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | # | ## | # | # | ||||||||
| Creo | SS | - | 92.5 ± 3.1 | ac | 2.21 ± 0.11 | acd | 30 | a | 12.5 ± 0.4 | abf | 2.22 ± 0.13 | ac |
| + | 66.3 ± 3.2 | b | 1.70 ± 0.14 | be | 40 | ab | 12.1 ± 0.4 | abd | 0.70 ± 0.06 | bg | ||
| SS + N2 | - | 90.6 ± 4.0 | c | 2.13 ± 0.13 | ac | 60 | ab | 15.7 ± 0.5 | c | 2.28 ± 0.12 | acf | |
| + | 80.5 ± 1.5 | d | 2.11 ± 0.07 | ac | 40 | ab | 15.3 ± 0.5 | c | 0.69 ± 0.05 | bg | ||
| Asiga | SS | - | 92.1 ± 2.8 | ac | 2.30 ± 0.09 | ad | 0 | a | 11.8 ± 0.6 | bd | 2.59 ± 0.11 | d |
| + | 73.6 ± 1.8 | e | 1.79 ± 0.05 | bef | 100 | b | 11.6 ± 0.4 | d | 0.85 ± 0.05 | eg | ||
| SS + N2 | - | 92.8 ± 1.8 | ac | 2.32 ± 0.10 | ad | 0 | a | 13.3 ± 0.5 | e | 2.39 ± 0.13 | cf | |
| + | 73.8 ± 1.5 | e | 1.86 ± 0.06 | ef | 100 | b | 12.8 ± 0.5 | af | 0.83 ± 0.05 | beg | ||
| Printer Type | Post-Curing Method | DC (%) | 3D Microlayer Structure (μm) | Water Sorption (%) | Water Solubility (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Width | Length | Height | |||||||||||
| Creo | SS | 64.7 ± 6.4 | a | 51.2 ± 0.4 | a | 97.7 ± 0.3 | a | 7.5 ± 1.2 | a | 1.685 ± 0.004 | a | 0.495 ± 0.020 | a |
| SS + N2 | 92.3 ± 4.5 | b | 51.3 ± 0.3 | a | 97.6 ± 0.4 | a | 7.3 ± 1.5 | a | 1.696 ± 0.006 | b | 0.496 ± 0.036 | a | |
| Asiga | SS | 56.7 ± 6.2 | a | 62.0 ± 0.5 | b | 100.6 ± 1.3 | b | 13.4 ± 0.8 | b | 1.664 ± 0.003 | c | 0.383 ± 0.006 | b |
| SS + N2 | 75.4 ± 4.5 | c | 62.3 ± 0.7 | b | 99.7 ± 1.5 | b | 13.7 ± 1.0 | b | 1.675 ± 0.009 | d | 0.379 ± 0.024 | b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, J.; Wada, K.; Gibreel, M.; Wakabayashi, N.; Iwamoto, T.; Vallittu, P.K.; Lassila, L. Effect of Nitrogen Gas Post-Curing and Printer Type on the Mechanical Properties of 3D-Printed Hard Occlusal Splint Material. Polymers 2022, 14, 3971. https://doi.org/10.3390/polym14193971
Wada J, Wada K, Gibreel M, Wakabayashi N, Iwamoto T, Vallittu PK, Lassila L. Effect of Nitrogen Gas Post-Curing and Printer Type on the Mechanical Properties of 3D-Printed Hard Occlusal Splint Material. Polymers. 2022; 14(19):3971. https://doi.org/10.3390/polym14193971
Chicago/Turabian StyleWada, Junichiro, Kanae Wada, Mona Gibreel, Noriyuki Wakabayashi, Tsutomu Iwamoto, Pekka K. Vallittu, and Lippo Lassila. 2022. "Effect of Nitrogen Gas Post-Curing and Printer Type on the Mechanical Properties of 3D-Printed Hard Occlusal Splint Material" Polymers 14, no. 19: 3971. https://doi.org/10.3390/polym14193971
APA StyleWada, J., Wada, K., Gibreel, M., Wakabayashi, N., Iwamoto, T., Vallittu, P. K., & Lassila, L. (2022). Effect of Nitrogen Gas Post-Curing and Printer Type on the Mechanical Properties of 3D-Printed Hard Occlusal Splint Material. Polymers, 14(19), 3971. https://doi.org/10.3390/polym14193971








