Curcumin and Diclofenac Therapeutic Efficacy Enhancement Applying Transdermal Hydrogel Polymer Films, Based on Carrageenan, Alginate and Poloxamer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. Film Characterization
2.3.1. Infrared Spectroscopy
2.3.2. Texture Analysis
2.3.3. Scanning Electron Microscopy
2.3.4. XRD Analysis
2.3.5. Thermogravimetric Analysis
2.3.6. Differential Scanning Calorimetry
2.3.7. Encapsulation Efficiency of Drugs
2.4. In Vitro Drug Release
2.5. Drug Release Kinetics
2.6. Computational Details
2.7. Antibacterial Activity of Films
2.8. Cell Viability Study
2.9. In Vivo Study
3. Results and Discussion
3.1. Film Characterization
3.1.1. Basic Characteristics of Films
3.1.2. FTIR Spectroscopy
3.1.3. Texture Analysis
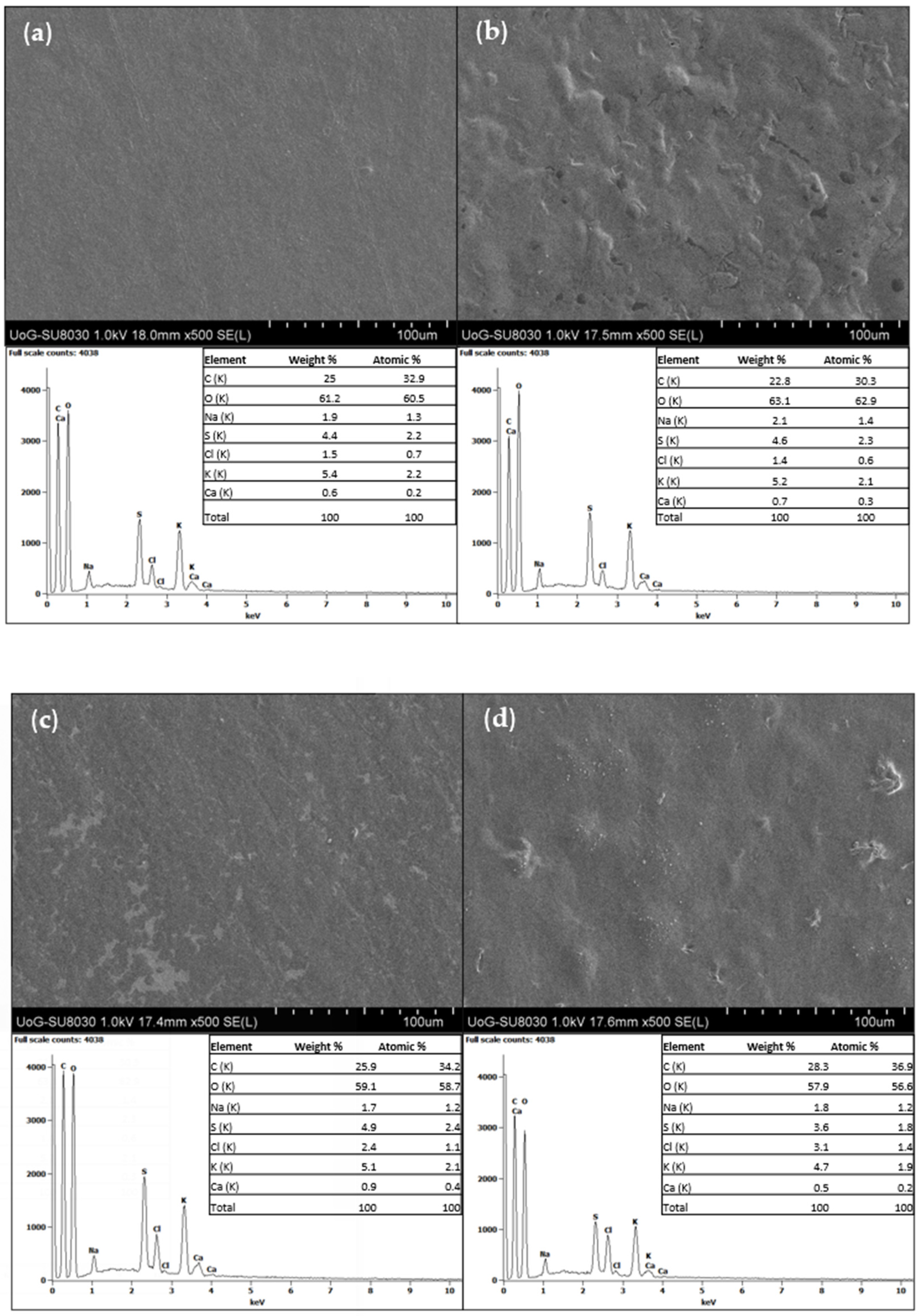
3.1.4. SEM Analysis
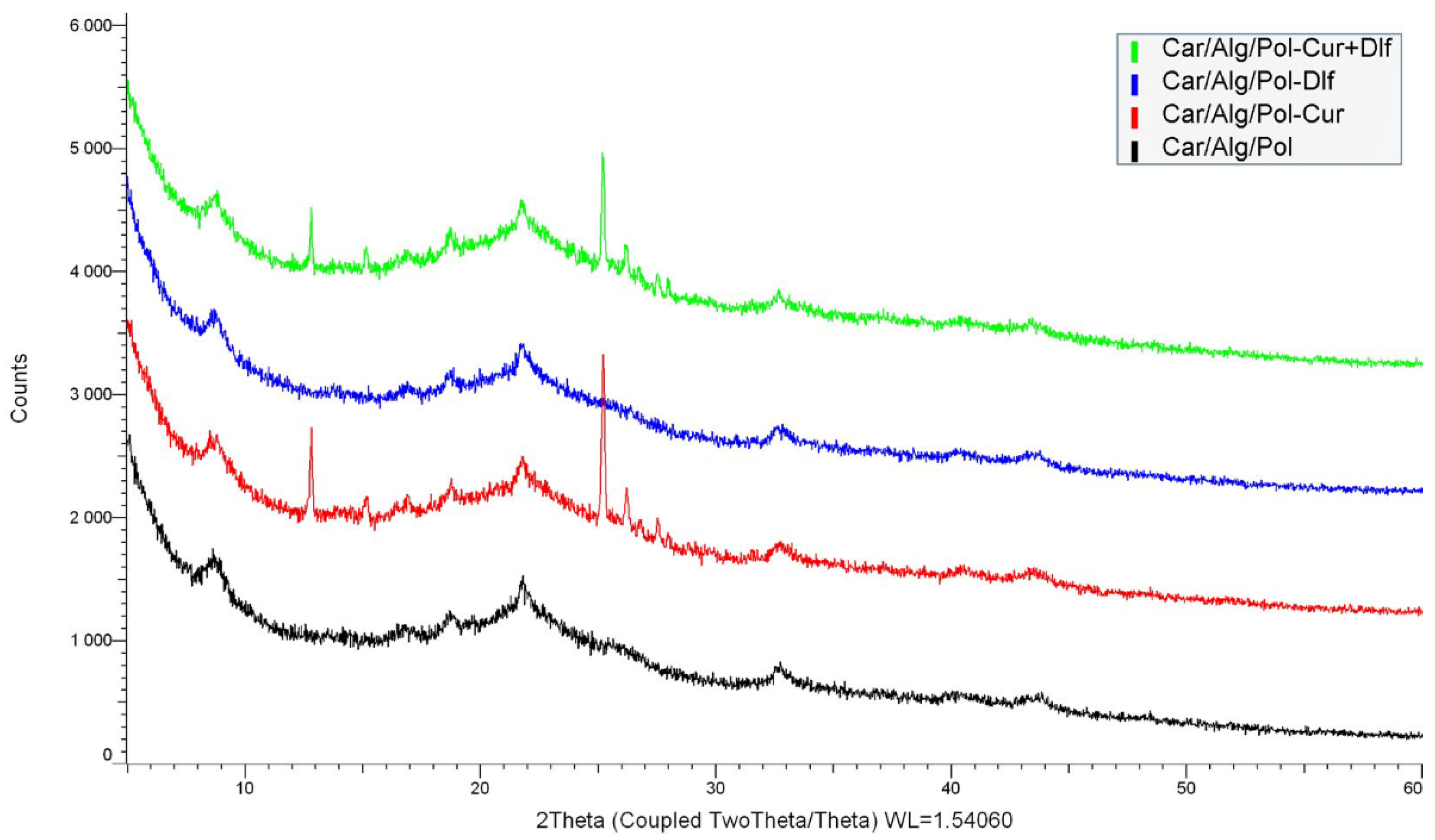
3.1.5. XRD Analysis
3.1.6. Thermogravimetric Analysis
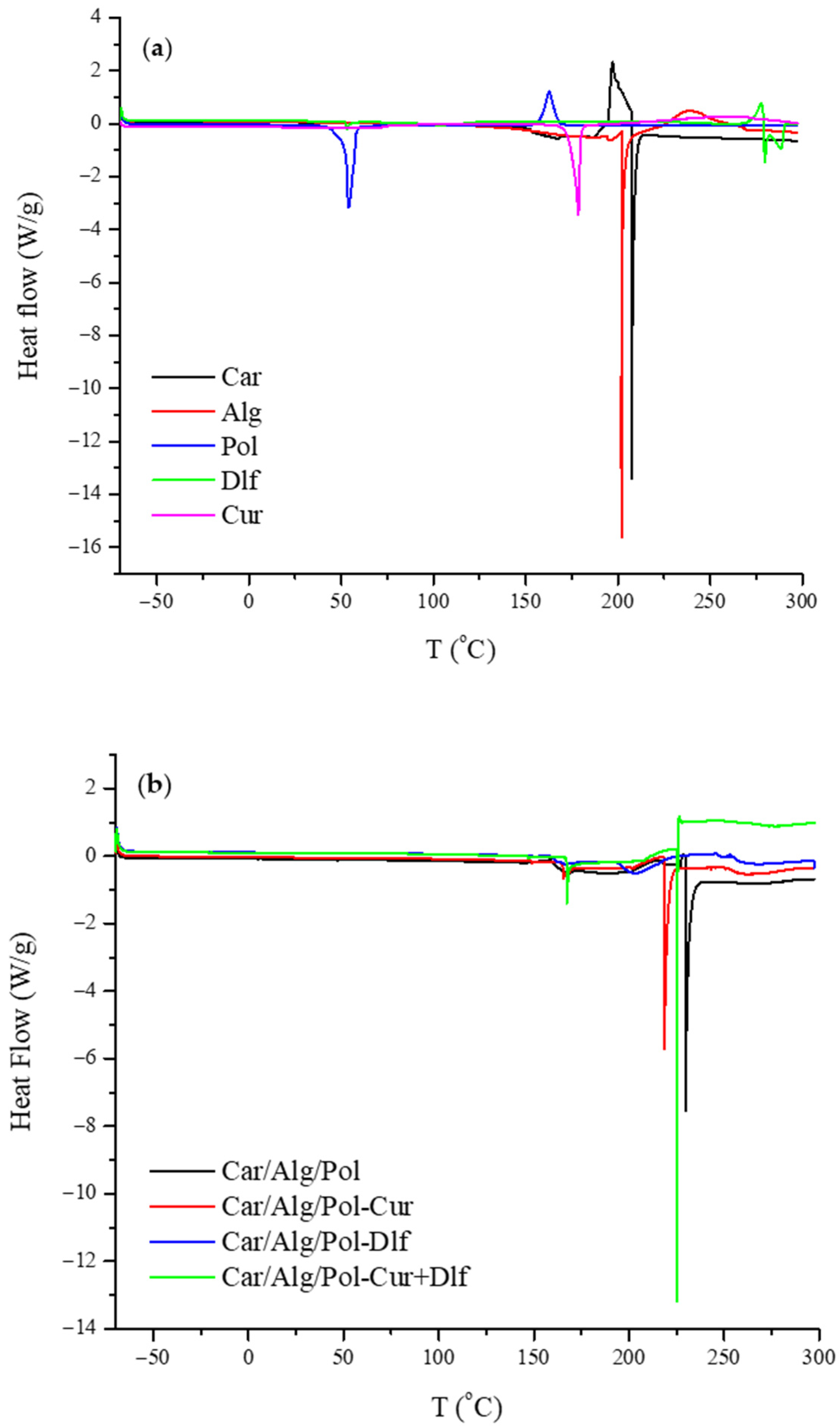
3.1.7. Differential Scanning Calorimetry
3.1.8. Drug Encapsulation Efficiency
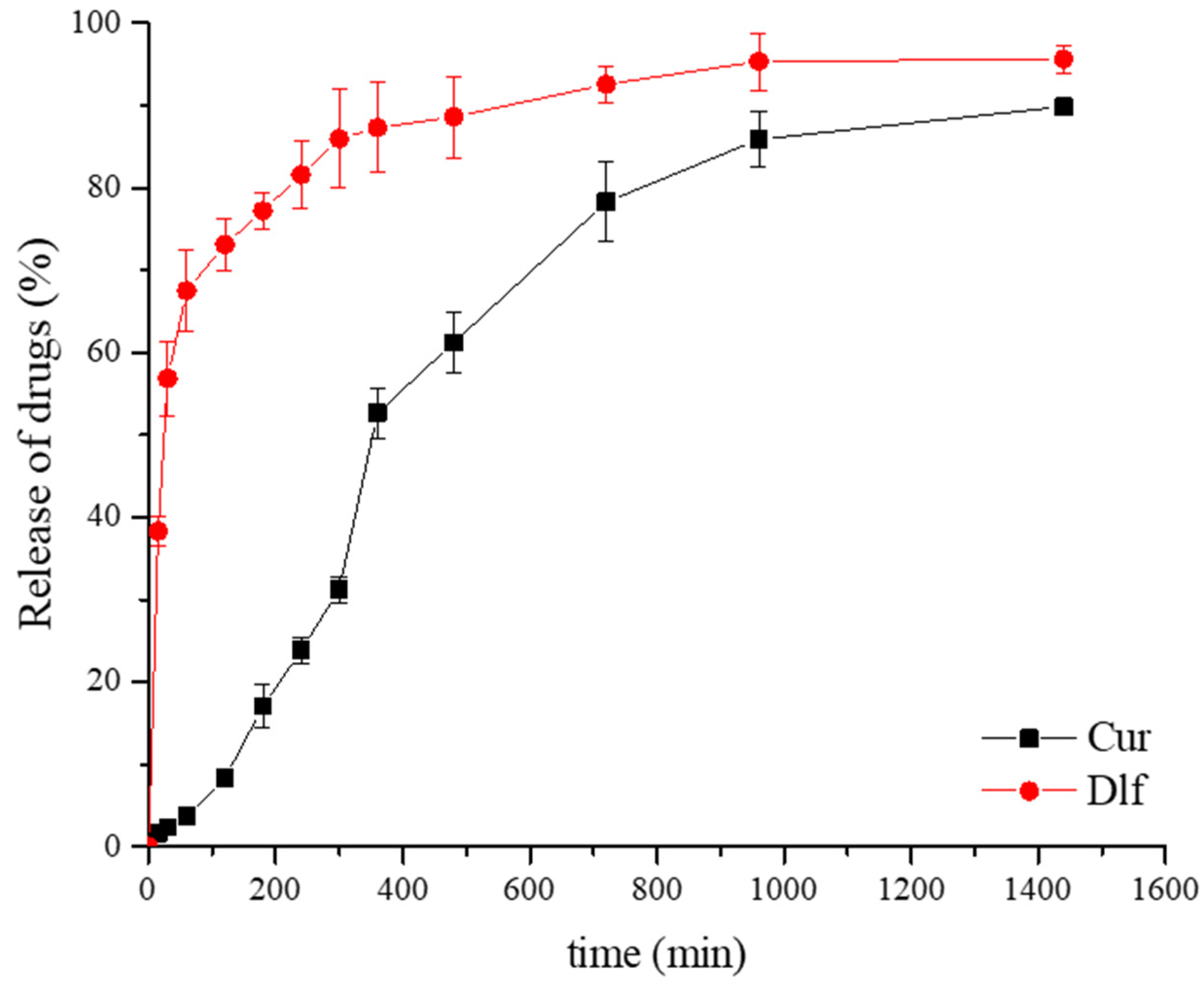
3.2. In Vitro Release Study
3.3. Drug Release Kinetics
3.4. Theoretical Study of Component Interaction in Developed Films
3.5. Antibacterial Activity of Films
3.6. Cell Viability Assay and In Vivo Wound Healing Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Schultz, G.S. Molecular regulation of wound healing. In Acute and Chronic Wounds: Nursing Management, 2nd ed.; Mosby: St. Loius, MO, USA, 1999; pp. 413–429. [Google Scholar]
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin acute wound healing: A comprehensive review. Int. J. Inflamm. 2019, 2019, 3706315. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, I.R.; Miraftab, M.; Collyer, G. A critical review of modern and emerging absorbent dressings used to treat exuding wounds. Int. Wound J. 2012, 9, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Eaglstein, W.H.; Davis, S.C.; Mehle, A.L.; Mertz, P.M. Optimal use of an occlusive dressing to enhance healing: Effect of delayed application and early removal on wound healing. Arch. Dermatol. 1988, 124, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef]
- Farahani, M.; Shafiee, A. Wound healing: From passive to smart dressings. Adv. Healthc. Mater. 2021, 10, 2100477. [Google Scholar] [CrossRef]
- Boateng, J.; Catanzano, O. Advanced therapeutic dressings for effective wound healing—A review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef]
- Ávila-Salas, F.; Marican, A.; Pinochet, S.; Carreño, G.; Valdés, O.; Venegas, B.; Donoso, W.; Cabrera-Barjas, G.; Vijayakumar, S.; Durán-Lara, E.F. Film dressings based on hydrogels: Simultaneous and sustained-release of bioactive compounds with wound healing properties. Pharmaceutics 2019, 11, 447. [Google Scholar] [CrossRef]
- Song, S.; Liu, Z.; Abubaker, M.A.; Ding, L.; Zhang, J.; Yang, S.; Fan, Z. Antibacterial polyvinyl alcohol/bacterial cellulose/nano-silver hydrogels that effectively promote wound healing. Mater. Sci. Eng. C 2021, 126, 112171. [Google Scholar] [CrossRef]
- Sharaf, S.M.; Al-Mofty, S.E.-D.; El-Sayed, E.-S.M.; Omar, A.; Dena, A.S.A.; El-Sherbiny, I.M. Deacetylated cellulose acetate nanofibrous dressing loaded with chitosan/propolis nanoparticles for the effective treatment of burn wounds. Int. J. Biol. Macromol. 2021, 193, 2029–2037. [Google Scholar] [CrossRef]
- Langer, R. Polymeric delivery systems for controlled drug release. Chem. Eng. Commun. 1980, 6, 1–48. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Mogosanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef]
- Pereira, R.; Carvalho, A.; Vaz, D.C.; Gil, M.H.; Mendes, A.; Bártolo, P. Development of novel alginate based hydrogel films for wound healing applications. Int. J. Biol. Macromol. 2013, 52, 221–230. [Google Scholar] [CrossRef]
- Jaiswal, L.; Shankar, S.; Rhim, J.W. Carrageenan-based functional hydrogel film reinforced with sulfur nanoparticles and grapefruit seed extract for wound heal-ing application. Carbohydr. Polym. 2019, 224, 115191. [Google Scholar] [CrossRef]
- Postolović, K.; Ljujić, B.; Kovačević, M.M.; Đorđević, S.; Nikolić, S.; Živanović, S.; Stanić, Z. Optimization, characterization, and evaluation of carrageenan/alginate/poloxamer/curcumin hydrogel film as a functional wound dressing material. Mater. Today Commun. 2022, 31, 103528. [Google Scholar] [CrossRef]
- Todd, P.A.; Sorkin, E.M. Diclofenac sodium—A reappraisal of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs 1988, 35, 244–285. [Google Scholar] [CrossRef]
- Goh, C.F.; Lane, M.E. Formulation of diclofenac for dermal delivery. Int. J. Pharm. 2014, 473, 607–616. [Google Scholar] [CrossRef]
- Salem-Milani, A.; Balaei-Gajan, E.; Rahimi, S.; Moosavi, Z.; Abdollahi, A.; Zakeri-Milani, P.; Bolourian, M. Antibacterial effect of diclofenac sodium on Enterococcus faecalis. J. Dent. 2013, 10, 16. [Google Scholar]
- Dutta, N.K.; Dastidar, S.G.; Kumar, A.; Mazumdar, K.; Ray, R.; Chakrabarty, A.N. Antimycobacterial activity of the antiinflammatory agent diclofenac sodium, and its synergism with streptomycin. Braz. J. Microbiol. 2004, 35, 316–323. [Google Scholar] [CrossRef][Green Version]
- Boateng, J.S.; Pawar, H.V.; Tetteh, J. Polyox and carrageenan based composite film dressing containing anti-microbial and anti-inflammatory drugs for effective wound healing. Int. J. Pharm. 2013, 441, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Pawar, H.V.; Tetteh, J.; Boateng, J.S. Preparation, optimisation and characterisation of novel wound healing film dressings loaded with streptomycin and diclofenac. Colloids Surf. B 2013, 102, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, F.Y.; Aleanizy, F.S.; El Tahir, E.; Alquadeib, B.T.; Alsarra, I.A.; Alanazi, J.S.; Abdelhady, H.G. Preparation, characterization, and antibacterial activity of diclofenac-loaded chitosan nanoparticles. Saudi Pharm. J. 2019, 27, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.N.; Ullah, A.; Haider, M.K.; Hussain, N.; Ullah, S.; Hashmi, M.; Khan, M.Q.; Kim, I.S. Evaluating antibacterial efficacy and biocompatibility of PAN nanofibers loaded with diclofenac sodium salt. Polymers 2021, 13, 510. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef] [PubMed]
- Stanić, Z. Curcumin, a compound from natural sources, a true scientific challenge—A review. Plant Foods Hum. Nutr. 2017, 72, 1–12. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Strimpakos, A.S.; Sharma, R.A. Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid. Redox Signal. 2008, 10, 511–546. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Preparation of carbohydrate-based functional composite films incorporated with curcumin. Food Hydrocoll. 2020, 98, 105302. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Antioxidant and antimicrobial poly (vinyl alcohol)-based films incorporated with grapefruit seed extract and curcumin. J. Environ. Chem. Eng. 2021, 9, 104694. [Google Scholar] [CrossRef]
- Imlay, J. Pathways of oxidative damage. Annu. Rev. Microbiol. 2003, 57, 395–418. [Google Scholar] [CrossRef]
- Mohanty, C.; Das, M.; Sahoo, S.K. Sustained wound healing activity of curcumin loaded oleic acid based polymeric bandage in a rat model. Mol. Pharm. 2012, 9, 2801–2811. [Google Scholar] [CrossRef]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol. Cell. Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Sharad, S.; Maheshwari, R.K. Skin regenerative potentials of curcumin. Biofactors 2013, 39, 141–149. [Google Scholar] [CrossRef]
- Mohanty, C.; Sahoo, S.K. Curcumin and its topical formulations for wound healing applications. Drug Discov. Today 2017, 22, 1582–1592. [Google Scholar] [CrossRef]
- Joe, B.; Vijaykumar, M.; Lokesh, B.R. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit. Rev. Food Sci. Nutr. 2004, 44, 97–111. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef]
- Duan, Y.; Li, K.; Wang, H.; Wu, T.; Zhao, Y.; Li, H.; Tang, H.; Yang, W. Preparation and evaluation of curcumin grafted hyaluronic acid modified pullulan polymers as a functional wound dressing material. Carbohydr. Polym. 2020, 238, 116195. [Google Scholar] [CrossRef]
- Wathoni, N.; Motoyama, K.; Higashi, T.; Okajima, M.; Kaneko, T.; Arima, H. Enhancement of curcumin wound healing ability by complexation with 2-hydroxypropyl-γ-cyclodextrin in sacran hydrogel film. Int. J. Biol. Macromol. 2017, 98, 268–276. [Google Scholar] [CrossRef]
- Li, X.; Nan, K.; Li, L.; Zhang, Z.; Chen, H. In vivo evaluation of curcumin nanoformulation loaded methoxy poly (ethylene glycol)-graft-chitosan composite film for wound healing application. Carbohydr. Polym. 2012, 88, 84–90. [Google Scholar] [CrossRef]
- Sajjad, W.; He, F.; Ullah, M.W.; Ikram, M.; Shah, S.M.; Khan, R.; Khan, T.; Khan, A.; Khalid, A.; Yang, G.; et al. Fabrication of bacterial cellulose-curcumin nanocomposite as a novel dressing for partial thickness skin burn. Front. Bioeng. Biotechnol. 2020, 8, 553037. [Google Scholar] [CrossRef]
- Alven, S.; Nqoro, X.; Aderibigbe, B.A. Polymer-based materials loaded with curcumin for wound healing applications. Polymers 2020, 12, 2286. [Google Scholar] [CrossRef]
- Sharifi, S.; Fathi, N.; Memar, M.Y.; Khatibi, S.M.H.; Khalilov, R.; Negahdari, R.; Vahed, S.Z.; Dizaj, S.M. Anti-microbial activity of curcumin nanoformulations: New trends and future perspectives. Phytother. Res. 2020, 34, 1926–1946. [Google Scholar] [CrossRef]
- Hadizadeh, M.; Naeimi, M.; Rafienia, M.; Karkhaneh, A. A bifunctional electrospun nanocomposite wound dressing containing surfactin and curcumin: In vitro and in vivo studies. Mater. Sci. Eng. C 2021, 129, 112362. [Google Scholar] [CrossRef]
- De Paz-Campos, M.A.; Ortiz, M.I.; Piña, A.E.C.; Zazueta-Beltrán, L.; Castañeda-Hernández, G. Synergistic effect of the interaction between curcumin and diclofenac on the formalin test in rats. Phytomedicine 2014, 21, 1543–1548. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Costa, M.P.; Prates, L.M.; Baptista, L.; Cruz, M.T.; Ferreira, I.L. Interaction of polyelectrolyte complex between sodium alginate and chitosan dimers with a single glyphosate molecule: A DFT and NBO study. Carbohydr. Polym. 2018, 198, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Emamian, S.; Lu, T.; Kruse, H.; Emamian, H. Exploring nature and predicting strength of hydrogen bonds: A correlation analysis between atoms-in-molecules descriptors, binding energies, and energy components of symmetry-adapted perturbation theory. J. Comput. Chem. 2019, 40, 2868. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Andrews, J.M. BSAC standardized disc susceptibility testing method (version 4). J. Antimicrob. Chemother. 2005, 56, 60–76. [Google Scholar] [CrossRef]
- EUCAST—The European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 9.0. 2019. Available online: http://www.eucast.org (accessed on 14 June 2022).
- Jorgensen, J.H.; Turnidge, J.D. Susceptibility test methods: Dilution and disc diffusion methods. In Manual of Clinical Microbiology, 8th ed.; American Society of Microbiology: Washington, DC, USA, 2003; pp. 1119–1125. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. lmmunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Li, H.; Xue, Y.; Jia, B.; Bai, Y.; Zuo, Y.; Wang, S.; Zhao, Y.; Yang, W.; Tang, H. The preparation of hyaluronic acid grafted pullulan polymers and their use in the formation of novel biocompatible wound healing film. Carbohydr. Polym. 2018, 188, 92–100. [Google Scholar] [CrossRef]
- Mohan, P.K.; Sreelakshmi, G.; Muraleedharan, C.V.; Joseph, R. Water soluble complexes of curcumin with cyclodextrins: Characterization by FT-Raman spectros-copy. Vib. Spectrosc. 2012, 62, 77–84. [Google Scholar] [CrossRef]
- Aielo, P.B.; Borges, F.A.; Romeira, K.M.; Miranda, M.C.R.; Arruda, L.B.D.; Filho, P.N.L.; Drago, B.C.; Herculano, R.D. Evaluation of sodium diclofenac release using natural rubber latex as carrier. Mater. Res. 2014, 17, 146–152. [Google Scholar] [CrossRef]
- Rezvanian, M.; Ahmad, N.; Amin, M.C.I.M.; Ng, S.F. Optimization, charac-terization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 2017, 97, 131–140. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Preparation of bioactive functional poly (lactic acid)/curcumin composite film for food packaging application. Int. J. Biol. Macromol. 2020, 162, 1780–1789. [Google Scholar] [CrossRef]
- Luo, N.; Varaprasad, K.; Reddy, G.V.S.; Rajulu, A.V.; Zhang, J. Preparation and characterization of cellulose/curcumin composite films. RSC Adv. 2012, 2, 8483–8488. [Google Scholar] [CrossRef]
- Xie, Q.; Zheng, X.; Li, L.; Ma, L.; Zhao, Q.; Chang, S.; You, L. Effect of curcumin addition on the properties of biodegradable pectin/chitosan films. Molecules 2021, 26, 215. [Google Scholar] [CrossRef]
- Thorat, A.A.; Dalvi, S.V. Solid-state phase transformations and storage sta-bility of curcumin polymorphs. Cryst. Growth Des. 2015, 15, 1757–1770. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.W. pH-responsive pectin-based multifunctional films incorporated with curcumin and sulfur nanoparticles. Carbohydr. Polym. 2020, 23, 115638. [Google Scholar] [CrossRef]
- Xiao, C.; Liu, H.; Lu, Y.; Zhang, L. Blend films from sodium alginate and gela-tin solutions. J. Macromol. Sci. A 2001, 38, 317–328. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Y.; Bai, R.; Zhang, X.; Yuan, L.; Liu, J. Preparation of pH-sensitive and antioxidant packaging films based on κ-carrageenan and mulberry polyphenolic extract. Int. J. Biol. Macromol. 2019, 134, 993–1001. [Google Scholar] [CrossRef]
- Postolović, K.S.; Antonijević, M.D.; Ljujić, B.; Miletić Kovačević, M.; Gazdić Janković, M.; Stanić, Z.D. pH-Responsive hydrogel beads based on alginate, κ-carrageenan and poloxamer for enhanced curcumin, natural bioactive compound, encapsulation and controlled release efficiency. Molecules 2022, 27, 4045. [Google Scholar] [CrossRef]
- Pasquali, I.; Bettini, R.; Giordano, F. Thermal behaviour of diclofenac, diclofenac sodium and sodium bicarbonate compositions. J. Therm. Anal. Calorim. 2007, 90, 903–907. [Google Scholar] [CrossRef]
- Tudja, P.; Khan, M.Z.I.; Meštrovic, E.; Horvat, M.; Golja, P. Thermal behaviour of diclofenac sodium: Decomposition and melting characteristics. Chem. Pharm. Bull. 2001, 49, 1245–1250. [Google Scholar] [CrossRef]
- Kianvash, N.; Bahador, A.; Pourhajibagher, M.; Ghafari, H.; Nikoui, V.; Rezayat, S.M.; Dehpour, A.R.; Partoazar, A. Evaluation of propylene glycol nanoliposomes containing curcumin on burn wound model in rat: Biocompatibility, wound healing, and anti-bacterial effects. Drug Deliv. Transl. Res. 2017, 7, 654–663. [Google Scholar] [CrossRef]
- Shende, P.; Gupta, H. Formulation and comparative characterization of nanoparticles of curcumin using natural, synthetic and semi-synthetic polymers for wound healing. Life Sci. 2020, 253, 117588. [Google Scholar] [CrossRef]
- Gull, N.; Khan, S.M.; Butt, O.M.; Islam, A.; Shah, A.; Jabeen, S.; Khan, S.U.; Khan, A.; Khan, R.U.; Butt, M.T.Z. Inflammation targeted chitosan-based hydrogel for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2020, 162, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.M.; Ahmed, M.S.; El-Subruiti, G.M.; Khalifa, R.E.; Eltaweil, A.S. pH-Sensitive alginate/carboxymethyl chitosan/aminated chitosan microcapsules for efficient encapsulation and delivery of diclofenac sodium. Pharmaceutics 2021, 13, 338. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.U.; Raza, S.M.; Hussain, M. Formulation and in-vitro evaluation of cream containing diclofenac sodium and curcuma longa for the management of rheumatoid arthritis. Int. J. Pharma Sci. 2014, 4, 654–660. [Google Scholar]
- Mendes, A.C.; Gorzelanny, C.; Halter, N.; Schneider, S.W.; Chronakis, I.S. Hybrid electrospun chitosan-phospholipids nanofibers for transdermal drug delivery. Int. J. Pharm. 2016, 510, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Momoh, F.U.; Boateng, J.S.; Richardson, S.C.; Chowdhry, B.Z.; Mitchell, J.C. Development and functional characterization of alginate dressing as potential protein delivery system for wound healing. Int. J. Biol. Macromol. 2015, 81, 137–150. [Google Scholar] [CrossRef]
- Hajiali, H.; Summa, M.; Russo, D.; Armirotti, A.; Brunetti, V.; Bertorelli, R.; Athanassiou, A.; Mele, E. Alginate–lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J. Mater. Chem. B 2016, 4, 1686–1695. [Google Scholar] [CrossRef]
- Ferreira, H.; Matamá, T.; Silva, R.; Silva, C.; Gomes, A.C.; Cavaco-Paulo, A. Functionalization of gauzes with liposomes entrapping an anti-inflammatory drug: A strategy to improve wound healing. React. Funct. Polym. 2013, 73, 1328–1334. [Google Scholar] [CrossRef]
- Ghica, M.V.; Albu Kaya, M.G.; Dinu-Pîrvu, C.-E.; Lupuleasa, D.; Udeanu, D.I. Development, Optimization and in vitro/in vivo characterization of collagen-dextran spongious wound dressings loaded with flufenamic acid. Molecules 2017, 22, 1552. [Google Scholar] [CrossRef]
- Morgado, P.I.; Lisboa, P.F.; Ribeiro, M.P.; Miguel, S.P.; Simões, P.C.; Correia, I.J.; Aguiar-Ricardo, A. Poly(vinyl alcohol)/chitosan asymmetrical membranes: Highly controlled morphology toward the ideal wound dressing. J. Membr. Sci. Technol. 2017, 159, 262–271. [Google Scholar] [CrossRef]
- Tummalapalli, M.; Berthet, M.; Verrier, B.; Deopura, B.L.; Alam, M.S.; Gupta, B. Composite wound dressings of pectin and gelatin with aloe vera and curcumin as bioactive agents. Int. J. Biol. Macromol. 2016, 82, 104–113. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a natural antimicrobial agent with strain-specific activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef]
- Moghaddam, K.M.; Iranshahi, M.; Yazdi, M.C.; Shahverdi, A.R. The combination effect of curcumin with different antibiotics against Staphylococcus aureus. Int. J. Green Pharm. 2009, 3, 141–143. [Google Scholar]
- Amrouche, T.; Noll, K.S.; Wang, Y.; Huang, Q.; Chikindas, M.L. Antibacterial activity of subtilosin alone and combined with curcumin, poly-lysine and zinc lactate against listeriamonocytogenes strains. Probiotics Antimicrob. Proteins 2010, 2, 250–257. [Google Scholar] [CrossRef]
- Marathe, S.A.; Kumar, R.; Ajitkumar, P.; Nagaraja, V.; Chakravortty, D. Curcumin reduces the antimicrobial activity of ciprofloxacin against Salmonella Typhimurium and Salmonella Typhi. J. Antimicrob. Chemother. 2013, 68, 139–152. [Google Scholar] [CrossRef]
- Teow, S.Y.; Ali, S.A. Synergistic antibacterial activity of Curcumin with antibiotics against Staphylococcus aureus. Pak. J. Pharm. Sci. 2015, 28, 2109–2114. [Google Scholar]



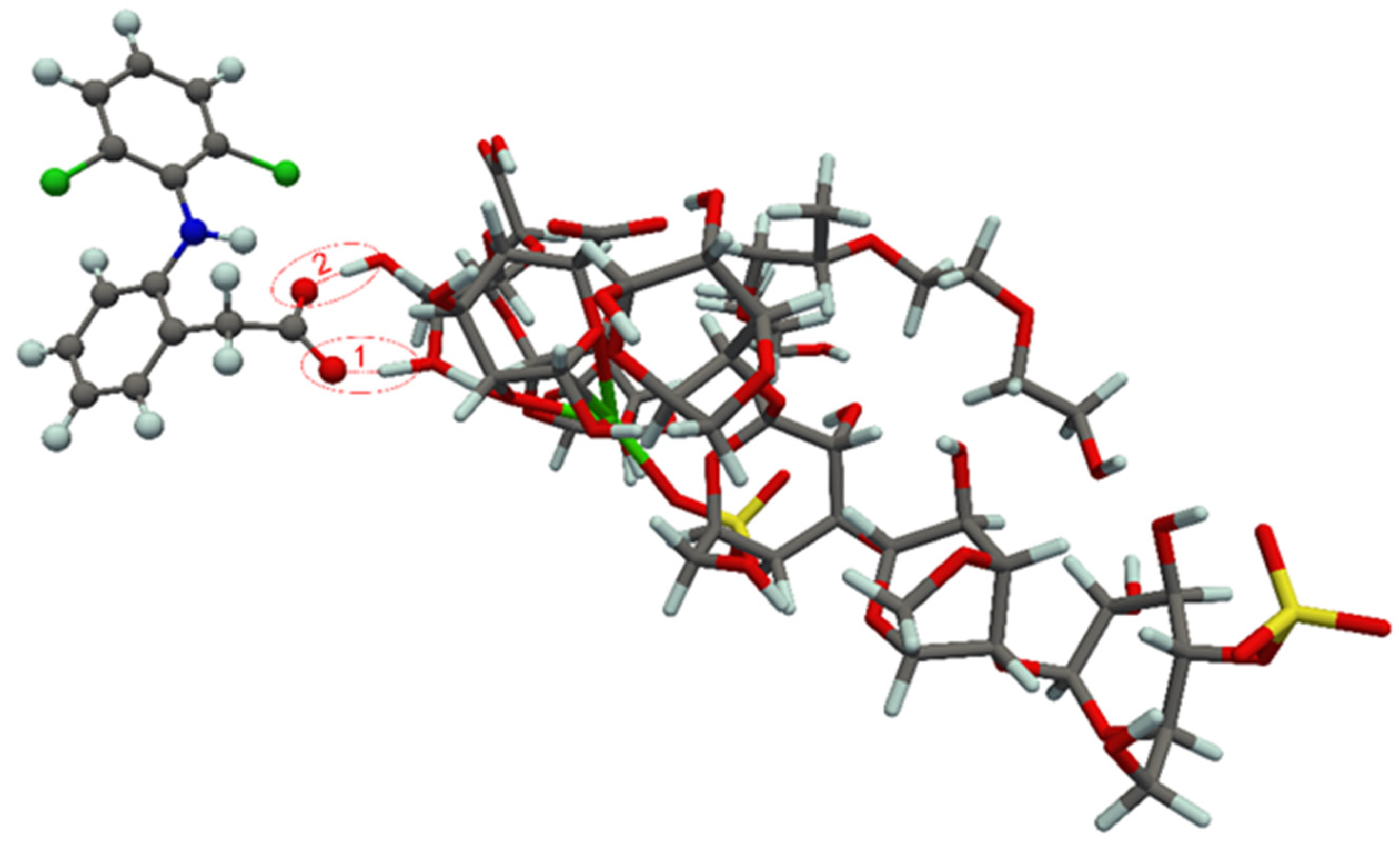
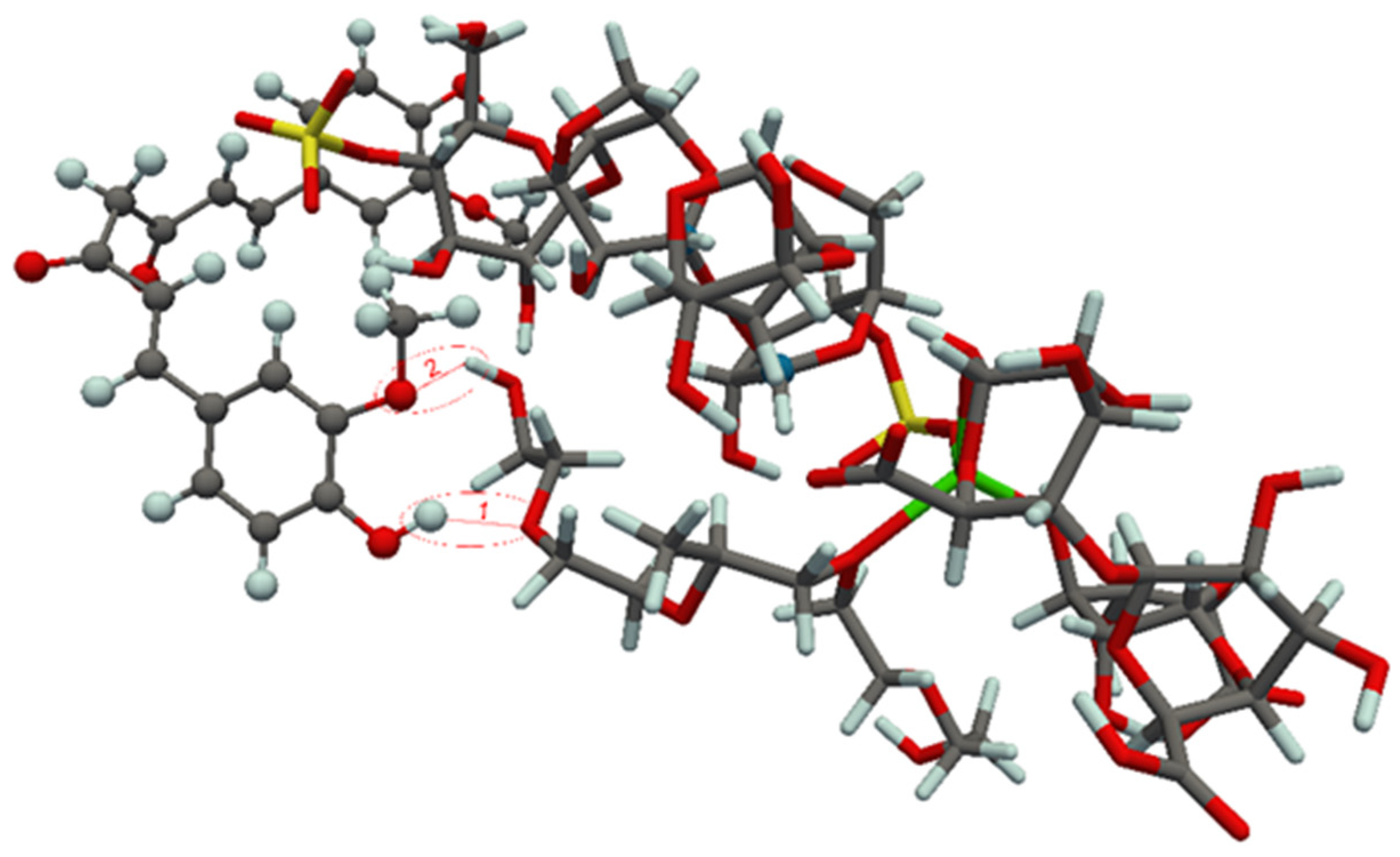


| Film | Mass of Film (mg/cm2) | Film Thickness (μm) | Mass of Drug (mg/cm2 of Carrier) |
|---|---|---|---|
| Car/Alg/Pol | 12.21 ± 0.65 [18] | 104.27 ± 3.35 [18] | / |
| Car/Alg/Pol-Dlf | 13.79 ± 0.65 | 121.12 ± 0.93 | 0.375 ± 0.012 |
| Car/Alg/Pol-Cur+Dlf | 14.29 ± 0.13 | 134.83 ± 2.17 | 0.718 ± 0.028 (Cur) |
| 0.400 ± 0.017 (Dlf) |
| Sample Name | Elongation at Break (% ± SD) | Tensile Strength (MPa ± SD) | Young’s Modulus (MPa ± SD) | Time to Break (s ± SD) |
|---|---|---|---|---|
| Car/Alg/Pol | 32.41 ± 1.02 | 34.60 ± 1.31 | 4.00 ± 0.04 | 3.09 ± 0.13 |
| Car/Alg/Pol-Cur | 27.36 ± 4.20 | 27.62 ± 2.63 | 3.86 ± 0.20 | 2.69 ± 0.40 |
| Car/Alg/Pol-Dlf | 25.19 ± 3.40 | 28.14 ± 1.63 | 4.41 ± 0.43 | 2.66 ± 0.39 |
| Car/Alg/Pol-Cur+Dlf | 29.66 ± 3.38 | 32.66 ± 0.18 | 4.87 ± 0.43 | 2.77 ± 0.34 |
| Film | Zero-Order Kinetics | First-Order Kinetics | Highuchi Model | |||
|---|---|---|---|---|---|---|
| k0 | R2 | kI | R2 | kH | R2 | |
| Car/Alg/Pol-Dlf | 0.0156 | 0.3896 | 0.0202 | 0.3357 | 0.1083 | 0.6372 |
| Car/Alg/Pol-Cur+Dlf (Cur) | 0.0483 | 0.8529 | 0.1567 | 0.6112 | 0.2716 | 0.9370 |
| Car/Alg/Pol-Cur+Dlf (Dlf) | 0.0182 | 0.5347 | 0.0241 | 0.4304 | 0.1159 | 0.7541 |
| Hixon−Crowell Model | Korsmeyer−Peppas Model | |||||
| kHC | R2 | kKP | n | R2 | ||
| Car/Alg/Pol-Dlf | 0.0328 | 0.7955 | 0.7412 | 0.1400 | 0.8466 | |
| Car/Alg/Pol-Cur+Dlf (Cur) | 0.0431 | 0.9930 | 0.1427 | 0.6807 | 0.9213 | |
| Car/Alg/Pol-Cur+Dlf (Dlf) | 0.0321 | 0.9283 | 0.6861 | 0.1529 | 0.9069 | |
| Method | Diclofenac | Curcumin |
|---|---|---|
| B3LYP/def2-SVP | −36.5 | −28.5 |
| B3LYP-D3/def2-SVP | −44.9 | −56.9 |
| Bacteria | Bacillus subtilis ATCC 6633 | Staphylococcus aureus ATCC 25923 |
|---|---|---|
| Tested films | Inhibition zone (mm) ∑ | |
| Car/Alg/Pol-Dlf | 9.33 | 6.33 |
| Car/Alg/Pol-Cur+Dlf | 16.67 | 13.67 |
| Antibiotic disks | Inhibition zone (mm) ∑ and sensitivity category (S 1) | |
| A | 16.67—S | 26.67—S |
| T | 29.67—S | 23.33—S |
| S | 18.33—S | 16.67—S |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Postolović, K.S.; Antonijević, M.D.; Ljujić, B.; Radenković, S.; Miletić Kovačević, M.; Hiezl, Z.; Pavlović, S.; Radojević, I.; Stanić, Z. Curcumin and Diclofenac Therapeutic Efficacy Enhancement Applying Transdermal Hydrogel Polymer Films, Based on Carrageenan, Alginate and Poloxamer. Polymers 2022, 14, 4091. https://doi.org/10.3390/polym14194091
Postolović KS, Antonijević MD, Ljujić B, Radenković S, Miletić Kovačević M, Hiezl Z, Pavlović S, Radojević I, Stanić Z. Curcumin and Diclofenac Therapeutic Efficacy Enhancement Applying Transdermal Hydrogel Polymer Films, Based on Carrageenan, Alginate and Poloxamer. Polymers. 2022; 14(19):4091. https://doi.org/10.3390/polym14194091
Chicago/Turabian StylePostolović, Katarina S., Milan D. Antonijević, Biljana Ljujić, Slavko Radenković, Marina Miletić Kovačević, Zoltan Hiezl, Svetlana Pavlović, Ivana Radojević, and Zorka Stanić. 2022. "Curcumin and Diclofenac Therapeutic Efficacy Enhancement Applying Transdermal Hydrogel Polymer Films, Based on Carrageenan, Alginate and Poloxamer" Polymers 14, no. 19: 4091. https://doi.org/10.3390/polym14194091
APA StylePostolović, K. S., Antonijević, M. D., Ljujić, B., Radenković, S., Miletić Kovačević, M., Hiezl, Z., Pavlović, S., Radojević, I., & Stanić, Z. (2022). Curcumin and Diclofenac Therapeutic Efficacy Enhancement Applying Transdermal Hydrogel Polymer Films, Based on Carrageenan, Alginate and Poloxamer. Polymers, 14(19), 4091. https://doi.org/10.3390/polym14194091







