Assessment of Oxaliplatin-Loaded Iodine Nanoparticles for Chemoradiotherapy of Human Colorectal Cancer (HT-29) Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Iodine Nanoparticles
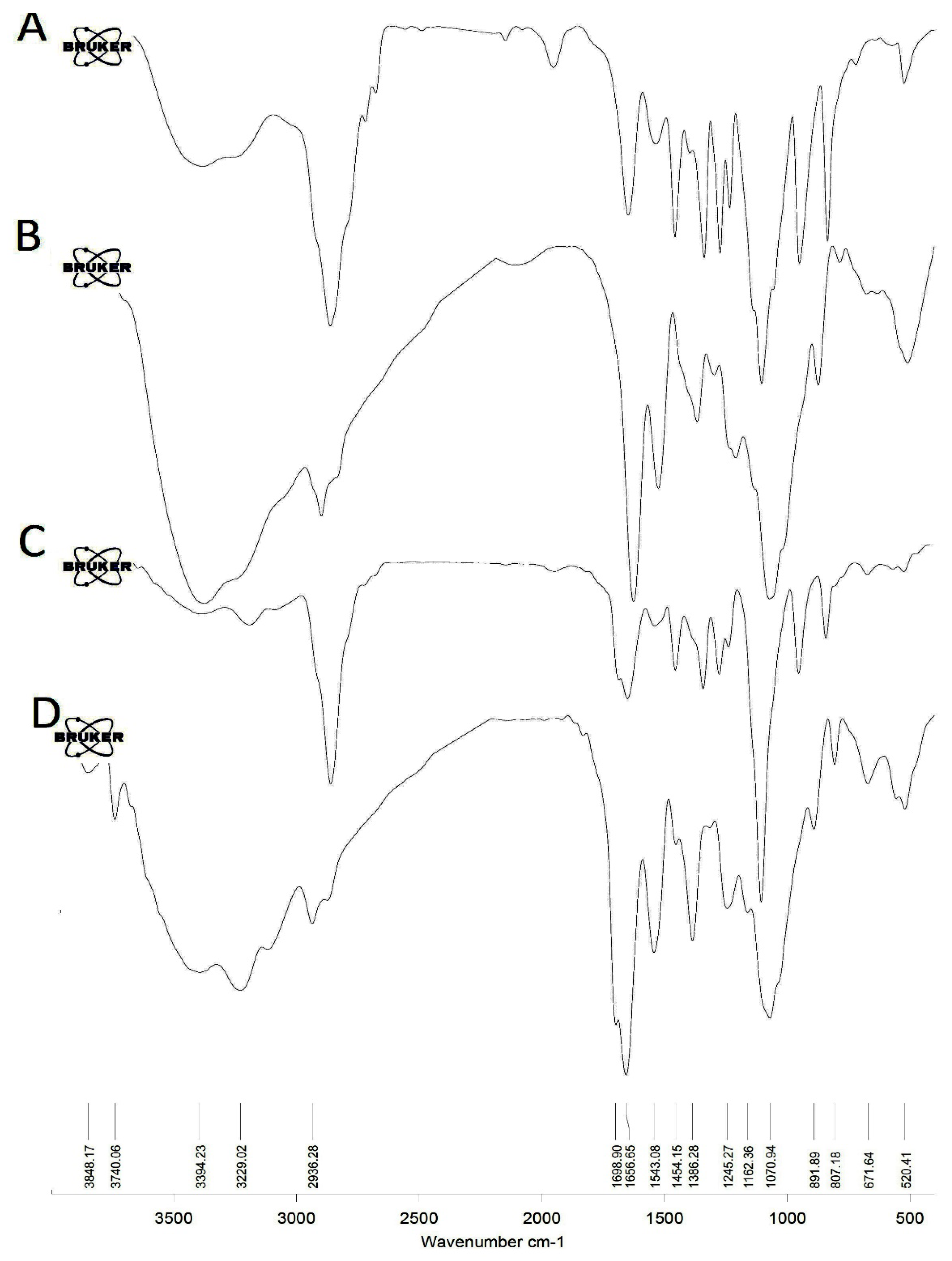
2.3. FT-IR Analysis
2.4. Characterization of Iodine Nanoparticles
2.5. Entrapment Efficiency Spectrophotometric Assay
2.6. In Vitro MTT Assays and Cytotoxicity (IC50)
2.7. Cell Apoptosis Study
2.8. Cell Cycle Determination
2.9. Statistical Analysis
3. Results
3.1. Synthesis of Iodine Nanoparticles
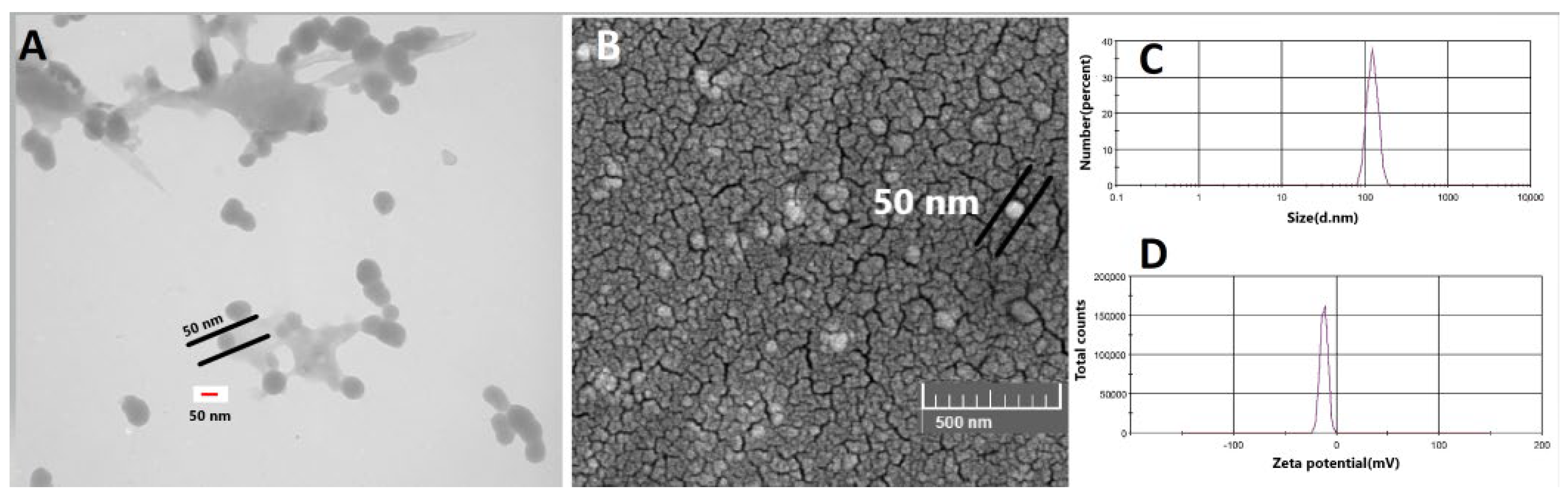
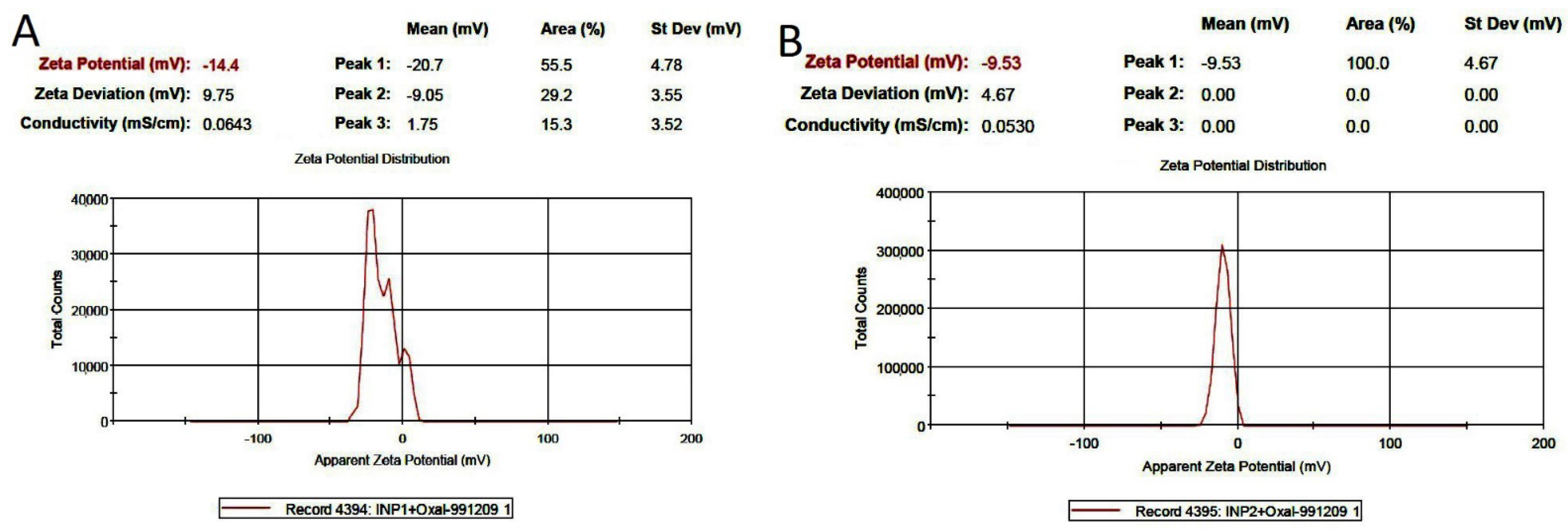
3.2. Morphology and Size of Iodine Nanoparticles
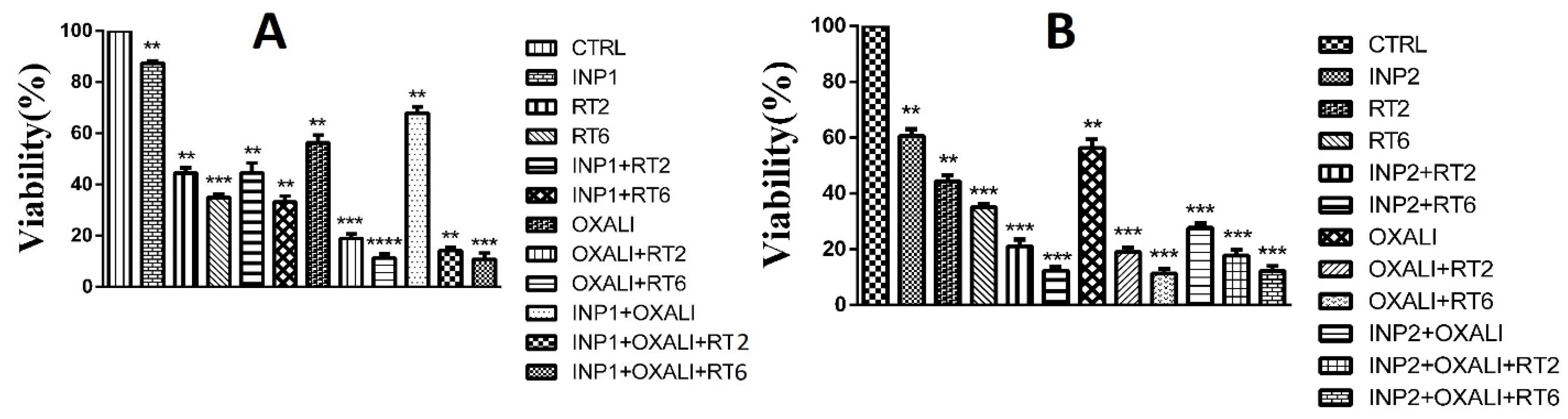
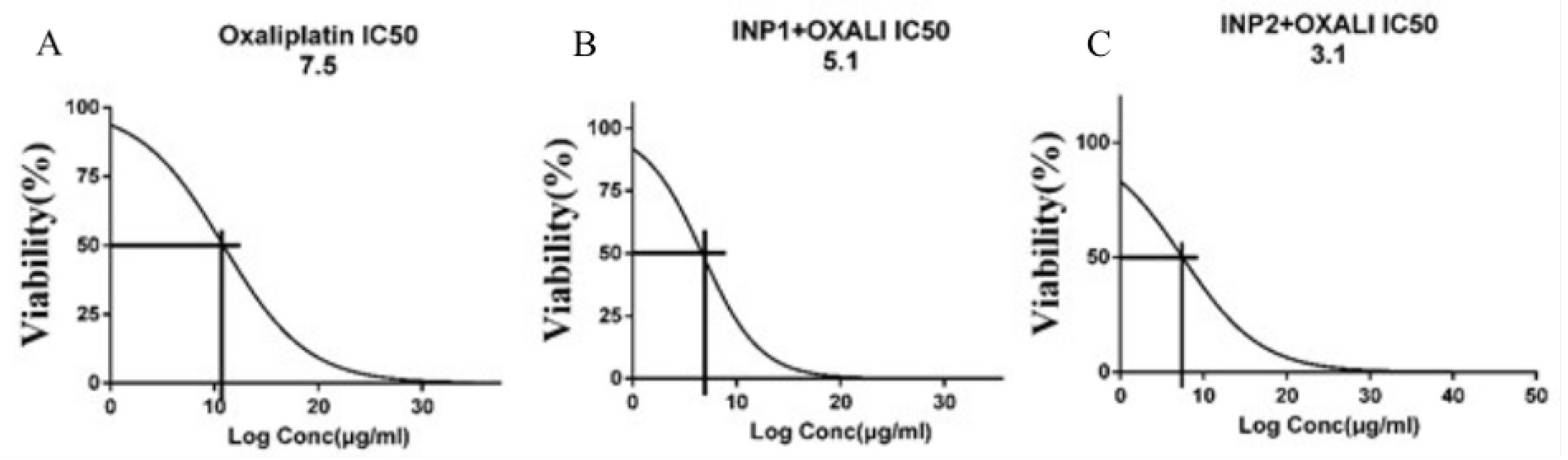
3.3. Cytotoxicity Assay
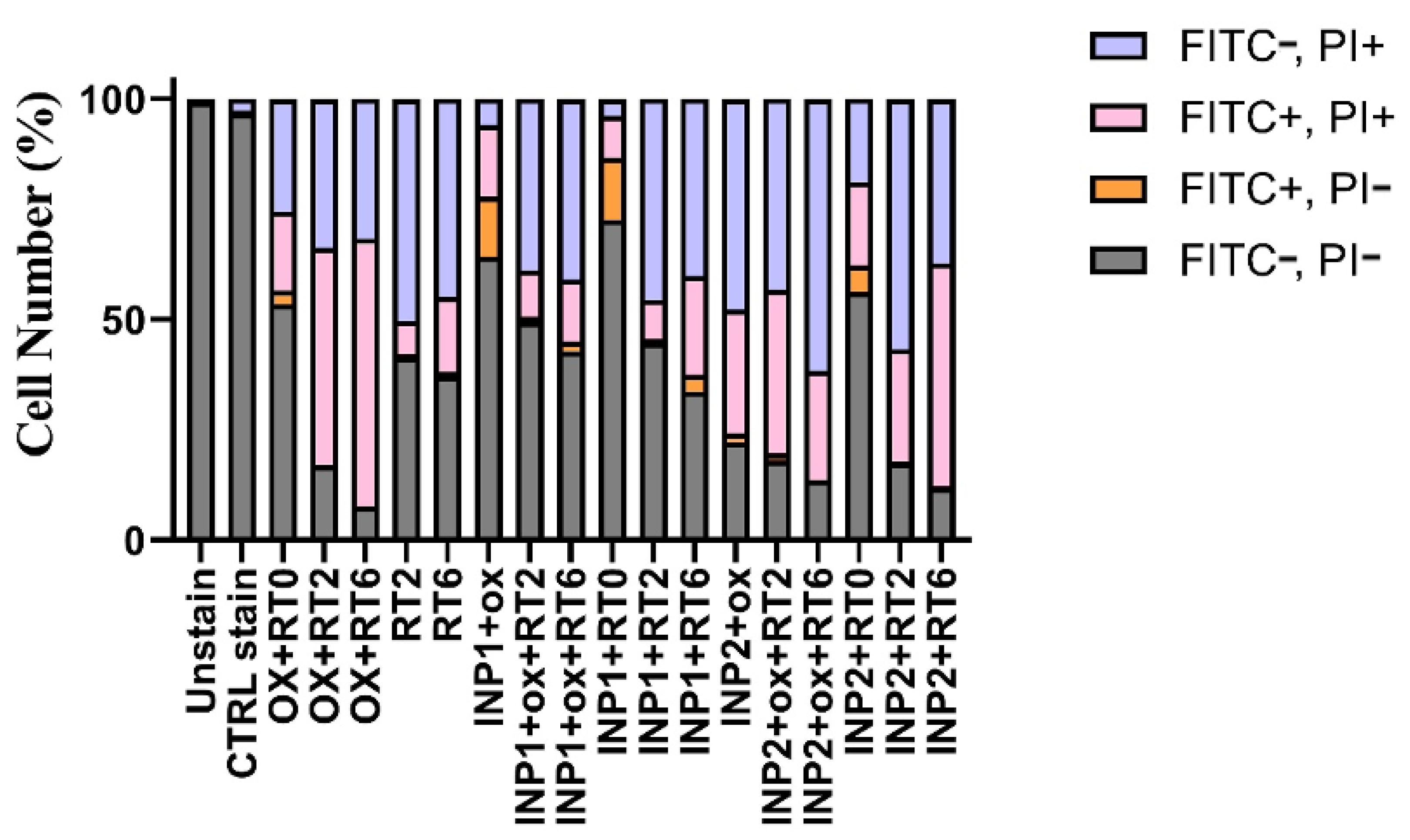
3.4. In Vitro Apoptosis Assay on HT-29 Cells
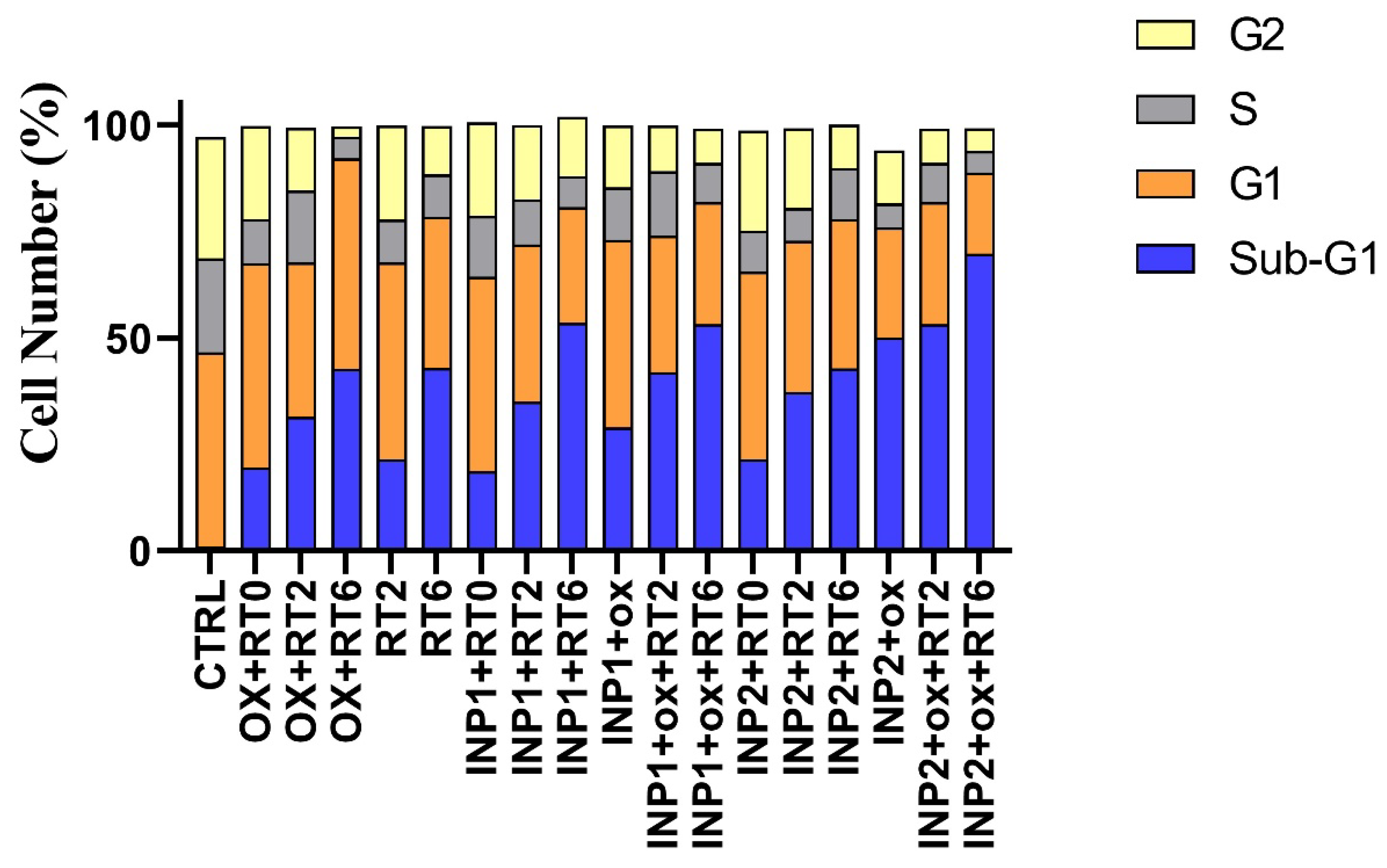
3.5. Cell Cycle Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, J.Y.; Jang, G.B.; Choi, J.H.; Kim, J.H.; Lee, C.J.; Lee, S.; Baek, J.H.; Park, K.K.; Kim, J.M.; et al. Aberrant activation of the CD45-Wnt signaling axis promotes stemness and therapy resistance in colorectal cancer cells. Theranostics 2021, 11, 8755. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.; Connolly, C.; Schettino, G.; Butterworth, K.T.; Prise, K.M. Biological mechanisms of gold nanoparticle radiosensitization. Cancer Nanotechnol. 2017, 8, 2. [Google Scholar] [CrossRef]
- Ghahremani, F.; Shahbazi-Gahrouei, D.; Kefayat, A.; Motaghi, H.; Mehrgardi, M.A.; Javanmard, S.H. AS1411 aptamer conjugated gold nanoclusters as a targeted radiosensitizer for megavoltage radiation therapy of 4T1 breast cancer cells. RSC Adv. 2018, 8, 4249–4258. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Smilowitz, H.M.; O’Connor, M.J.; Dilmanian, F.A.; Slatkin, D.N. Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomedicine 2013, 8, 1601–1609. [Google Scholar] [CrossRef]
- Kuncic, Z.; Lacombe, S. Nanoparticle radio-enhancement: Principles, progress, and application to cancer treatment. Phys. Med. Biol. 2018, 63, 02TR1. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Zhang, Z.; Wang, B.; Xu, Q.; Mao, W.; Tian, J.; Yang, K.; Wang, F. Radionuclide imaging-guided chemo-radioisotope synergistic therapy using a 131I-labeled polydopamine multifunctional nanocarrier. Mol. Ther. 2018, 26, 1385–1393. [Google Scholar] [CrossRef]
- Butzmann, C.M.; Technau-Hafsi, K.; Bross, F. “Silver man” argyria of the skin after ingestion of a colloidal silver solution. JDDG J. Der Dtsch. Dermatol. Ges. 2015, 13, 1030–1032. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Ridwan, S.M.; Stanishevskiy, Y.; Smilowitz, N.R.; Davis, J.; Smilowitz, H.M. Small long blood half-life iodine nanoparticle for vascular and tumor imaging. Sci. Rep. 2018, 8, 13803. [Google Scholar] [CrossRef]
- Mansouri, M.; Shahbazi-Gahrouei, D. A review on theranostic applications of iodine nanoparticles: Recent findings and perspectives. Nanomed. J. 2021, 8, 234–240. [Google Scholar]
- Adam, J.; Balosso, J.; Renier, M.; Elleaume, H.; Estève, F.; Berkvens, P.; Nemoz, C.; Brochard, T.; Tessier, A.; Verry, C.; et al. Synchrotron stereotactic radiation therapy: A report on phase 1/2 clinical trial achievements, ongoing developments, and long-term prospects. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, E624–E625. [Google Scholar] [CrossRef][Green Version]
- Hainfeld, J.F.; Ridwan, S.M.; Stanishevskiy, Y.; Panchal, R.; Slatkin, D.N.; Smilowitz, H.M. Iodine nanoparticles enhance radiotherapy of intracerebral human glioma in mice and increase the efficacy of chemotherapy. Sci. Rep. 2019, 9, 4505. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.; Jia, Z.; Wu, H.; Gu, K. Oxaliplatin-based regimen is superior to cisplatin-based regimen in tumor remission as first-line chemotherapy for advanced gastric cancer: A meta-analysis. J. Cancer 2019, 10, 1923. [Google Scholar] [CrossRef]
- Montagnani, F.; Turrisi, G.; Marinozzi, C.; Aliberti, C.; Fiorentini, G. Effectiveness and safety of oxaliplatin compared to cisplatin for advanced, unresectable gastric cancer: A systematic review and meta-analysis. Gastric Cancer 2011, 14, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Kachnic, L.A.; Li, L.; Fournier, L.; Willers, H. Fanconi anemia pathway heterogeneity revealed by cisplatin and oxaliplatin treatments. Cancer Lett. 2010, 292, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Hayat, M.M.; Sohail, M.; Ashraf, M. Spectrophotometric determination of cisplatin, carboplatin, and oxaliplatin in pure and injectable dosage forms. Biomed. Res. 2019, 3, 557–562. [Google Scholar]
- Bahuguna, A.; Khan, I.; Bajpai, V.K.; Kang, S.C. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J. Pharmacol. 2017, 12, 115–118. [Google Scholar] [CrossRef]
- Anitha, A.; Maya, S.; Deepa, N.; Chennazhi, K.; Nair, S.; Tamura, H.; Jayakumar, R. Efficient water soluble O-carboxymethyl chitosan nanocarrier for the delivery of curcumin to cancer cells. Carbohydr. Polym. 2011, 83, 452–461. [Google Scholar] [CrossRef]
- Arab-Bafrani, Z.; Shahbazi-Gahrouei, D.; Abbasian, M.; Fesharaki, M. Multiple MTS assay as the alternative method to determine survival fraction of the irradiated HT-29 colon cancer cells. J. Med. Sign. Sens. 2016, 6, 112–116. [Google Scholar]
- Mesía, R.; Henke, M.; Fortin, A.; Minn, H.; Ancona, A.C.Y.; Cmelak, A.; Markowitz, A.V.; Hotte, S.J.; Singh, S.; Chan, A.T.C.; et al. Chemoradiotherapy with or without panitumumab in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-1): A randomized, controlled, open-label phase 2 trial. Lancet Oncol. 2015, 16, 208–220. [Google Scholar] [CrossRef]
- Arienti, C.; Zoli, W.; Pignatta, S.; Carloni, S.; Paganelli, G.; Ulivi, P.; Romeo, A.; Menghi, E.; Sarnelli, A.; Medri, L.; et al. Efficacy of Different Sequences of Radio-and Chemotherapy in Experimental Models of Human Melanoma. J. Cellular Physiol. 2014, 229, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.S.; Nam, B.H.; Kim, K.P.; Kim, J.E.; Park, S.J.; Park, Y.S.; Park, J.O.; Kim, S.Y.; Kim, T.U.; Kim, P.H.; et al. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): An open-label, multicentre, phase 2, randomized controlled trial. Lancet Oncol. 2014, 15, 1245–1253. [Google Scholar] [CrossRef]
- Barton, M.B.; Jacob, S.; Shafiq, J.; Wong, K.; Thompson, S.R.; Hanna, T.P.; Delaney, G.P. Estimating the demand for radiotherapy from the evidence: A review of changes from 2003 to 2012. Radiother. Oncol. 2014, 112, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.T. Intravenous contrast medium administration and scan timing at CT: Considerations and approaches. Radiology 2010, 256, 32–61. [Google Scholar] [CrossRef]
- Liu, C.; Guo, J.; Yang, W.; Hu, W.; Wang, C.; Fu, S. Magnetic mesoporous silica microspheres with thermo-sensitive polymer shell for controlled drug release. J. Mater. Chem. 2009, 19, 4764–4770. [Google Scholar] [CrossRef]
- Lin, J.; Chen, H.; Ji, Y.; Zhang, Y. Functionally modified monodisperse core-shell silica nanoparticles: Silane coupling agent as capping and size tuning agent. Colloids Surf. A Physicochem. Eng. Aspm. 2012, 411, 111–121. [Google Scholar] [CrossRef]
- Ghahremani, F.; Kefayat, A.; Shahbazi-Gahrouei, D.; Motaghi, H.; Mehrgardi, M.A.; Javanmard, S.H. AS1411 Aptamer targeted gold nanoclusters effect on the enhancement of radiation therapy efficacy in 4T1 breast tumor-bearing mice. Nanomedicine 2018, 13, 2563–2578. [Google Scholar] [CrossRef]
- Fathy, M.M.; Mohamed, F.S.; Elbialy, N.; Elshemey, W.M. Multifunctional Chitosan-Capped Gold Nanoparticles for enhanced cancer chemo-radiotherapy: An in vitro study. Phys. Med. 2018, 48, 76–83. [Google Scholar] [CrossRef]
- Arab-Bafrani, Z.; Zabihi, E.; Jafari, S.M.; Khoshbin-Khoshnazar, A.; Mousavi, E.; Khalili, M.; Babaei, A. Enhanced radiotherapy efficacy of breast cancer multicellular tumor spheroids through in-situ fabricated chitosan-zinc oxide bio-nanocomposites as radio-sensitizing agents. Int. J. Pharm. 2021, 605, 120828. [Google Scholar] [CrossRef]
- Rostami, A.; Lambie, M.; Caberry, W.Y.; Stambolic, V.; Waldron, J.N.; Bratman, S.V. Senescence, necrosis, and apoptosis govern circulating cell-free DNA release kinetics. Cell Rep. 2020, 31, 107830. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Azadmanesh, K.; Shokrgozar, M.A.; Journeay, W.S.; Laurent, S. Effect of nanoparticles on the cell life cycle. Chem. Rev. 2011, 111, 3407–3432. [Google Scholar] [CrossRef] [PubMed]
- Melancon, M.P.; Zhou, M.; Li, C. Cancer theranostics with near-infrared light-activatable multimodal nanoparticles. Acc. Chem. Res. 2011, 44, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Salehi, R.; Abyar, S.; Ramazani, F.; Khandar, A.A.; Hosseini-Yazdi, S.A.; White, J.M.; Edalati, M.; Kahroba, H.; Talebi, M. Enhanced anticancer potency with reduced nephrotoxicity of newly synthesized platin-based complexes compared with cisplatin. Sci. Rep. 2022, 12, 8316. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasouli, N.; Shahbazi-Gahrouei, D.; Hematti, S.; Baradaran, B.; Salehi, R.; Varshosaz, J.; Jafarizad, A. Assessment of Oxaliplatin-Loaded Iodine Nanoparticles for Chemoradiotherapy of Human Colorectal Cancer (HT-29) Cells. Polymers 2022, 14, 4131. https://doi.org/10.3390/polym14194131
Rasouli N, Shahbazi-Gahrouei D, Hematti S, Baradaran B, Salehi R, Varshosaz J, Jafarizad A. Assessment of Oxaliplatin-Loaded Iodine Nanoparticles for Chemoradiotherapy of Human Colorectal Cancer (HT-29) Cells. Polymers. 2022; 14(19):4131. https://doi.org/10.3390/polym14194131
Chicago/Turabian StyleRasouli, Naser, Daryoush Shahbazi-Gahrouei, Simin Hematti, Behzad Baradaran, Roya Salehi, Jaleh Varshosaz, and Abbas Jafarizad. 2022. "Assessment of Oxaliplatin-Loaded Iodine Nanoparticles for Chemoradiotherapy of Human Colorectal Cancer (HT-29) Cells" Polymers 14, no. 19: 4131. https://doi.org/10.3390/polym14194131
APA StyleRasouli, N., Shahbazi-Gahrouei, D., Hematti, S., Baradaran, B., Salehi, R., Varshosaz, J., & Jafarizad, A. (2022). Assessment of Oxaliplatin-Loaded Iodine Nanoparticles for Chemoradiotherapy of Human Colorectal Cancer (HT-29) Cells. Polymers, 14(19), 4131. https://doi.org/10.3390/polym14194131








