Domain Structure, Thermal and Mechanical Properties of Polycaprolactone-Based Multiblock Polyurethane-Ureas under Control of Hard and Soft Segment Lengths
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Segmented PUU
2.3. Methods for Characterizing Segmented PUU
2.3.1. IR Spectroscopy
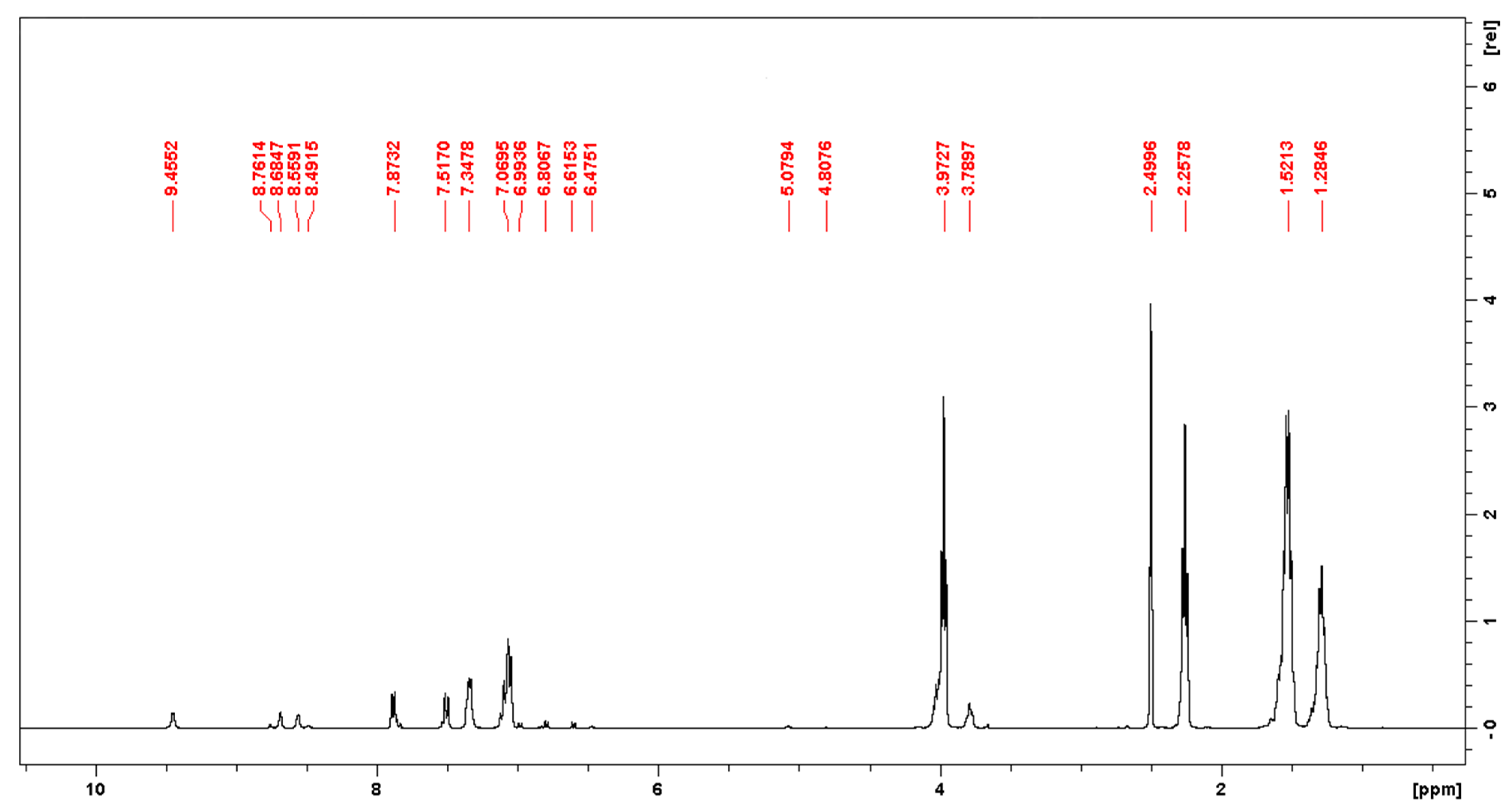
2.3.2. NMR Spectroscopy
2.3.3. X-ray Diffaction Analysis
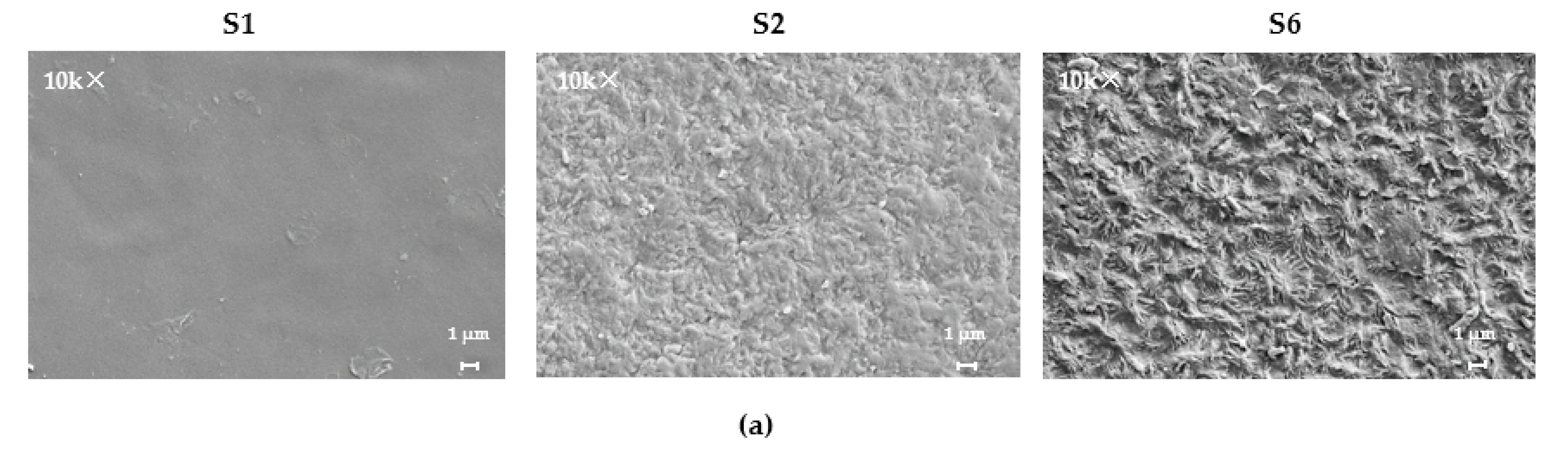
2.3.4. Scanning Electron Microscopy
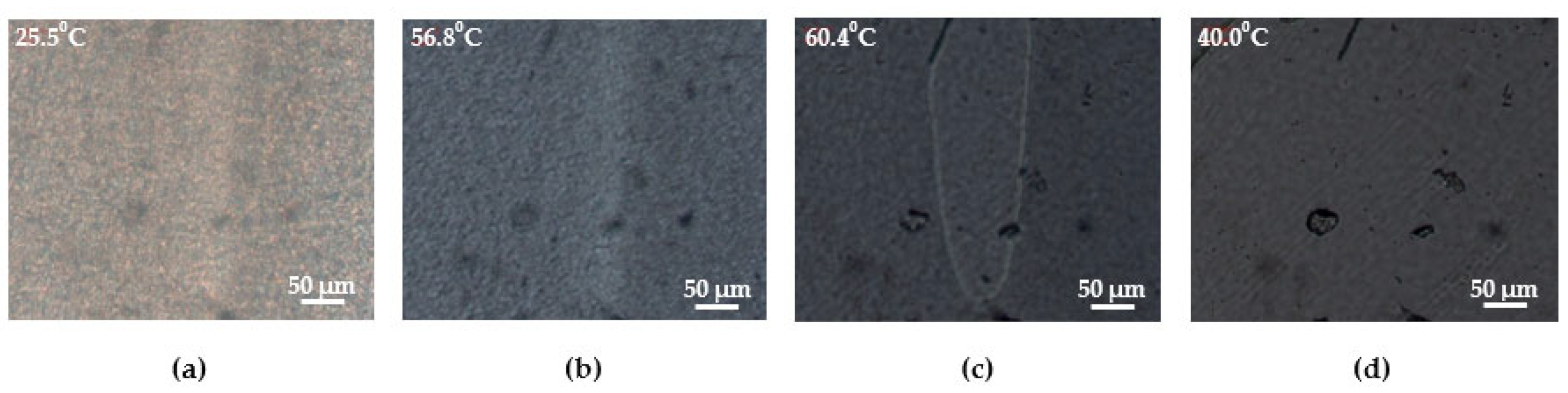
2.3.5. Polarizing Optical Microscopy
2.3.6. Differential Scanning Calorimetry
2.3.7. Dynamic Mechanical Analysis
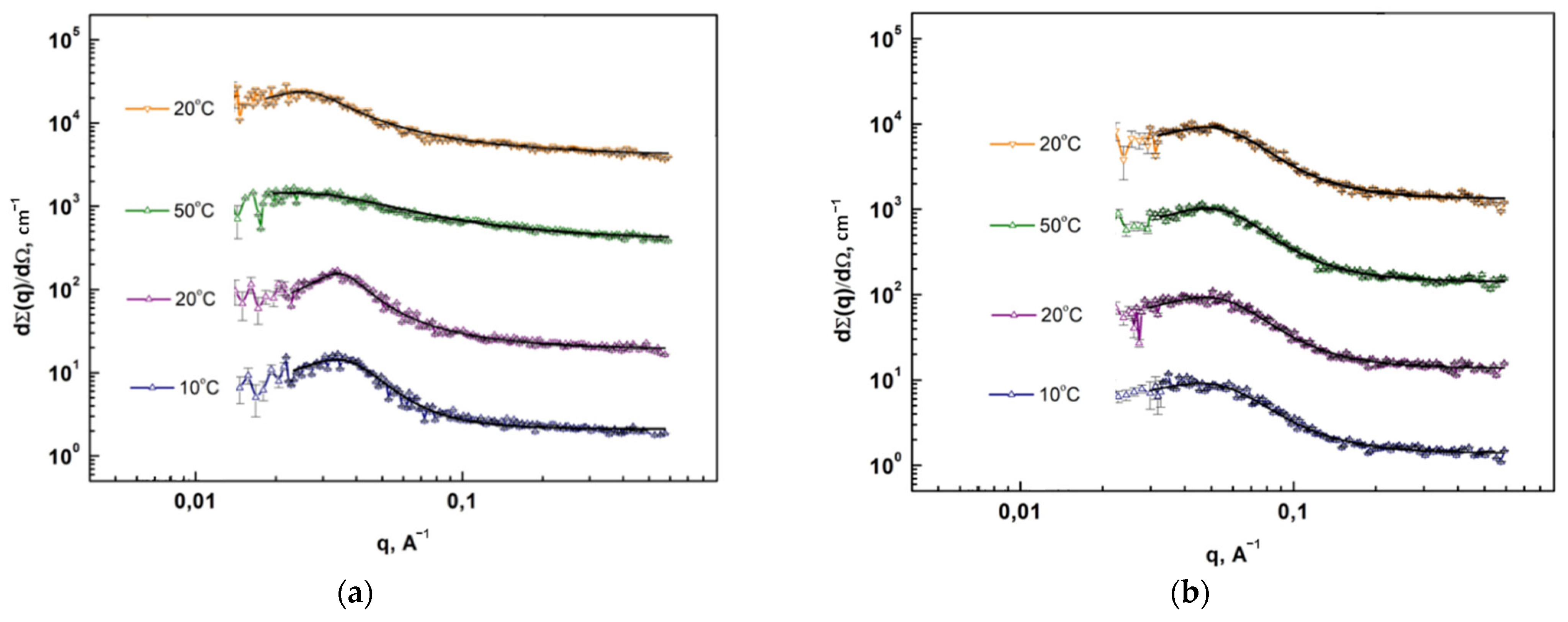
2.3.8. Small-Angle Neutron and X-ray Scattering Methods
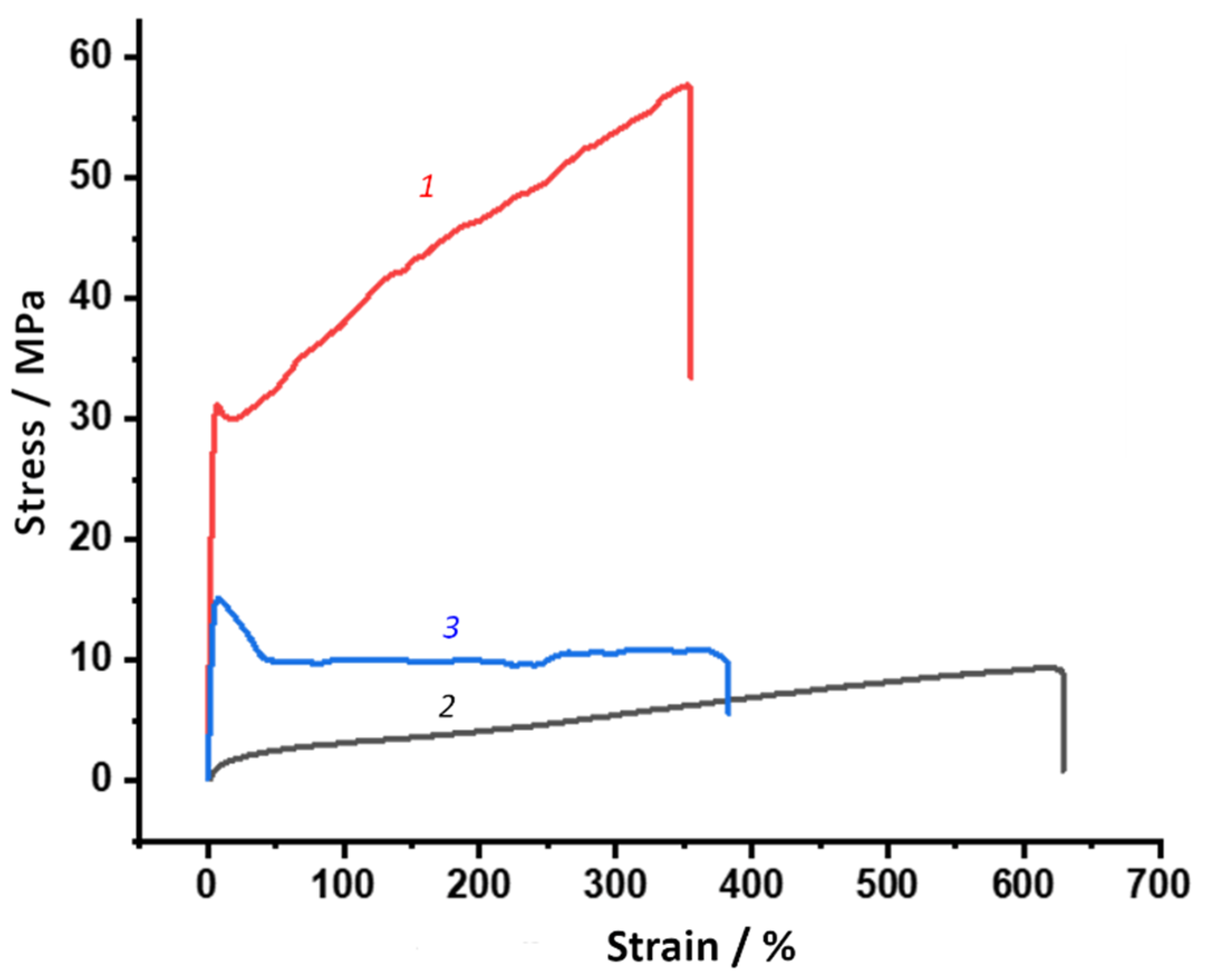
2.3.9. Mechanical Testing
3. Results and Discussion
3.1. Chemical Structure and Hydrogen Bonding Interactions in PUU
3.2. Relaxation and Phase Transitions in Multiblock PUU
3.3. Structural and Morphological Features of Multiblock PUU
3.4. Deformation-Strength and Thermomechanical Properties of PUU
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schollenberger, C.S.; Scott, H.; Moore, G.R. Polyurethane VC, a virtually cross-linked elastomer. Rubber World Abbreviated J. Name 1988, 137, 549–555. [Google Scholar]
- Aneja, A.; Wilkes, G.L. A systematic series of ‘model’ PTMO based segmented polyurethanes reinvestigated using atomic force microscopy. Polymer 2003, 44, 7221–7228. [Google Scholar] [CrossRef]
- Hepburn, C. Polyurethane Elastomers, 2nd ed.; Elsevier: London, UK, 1991; pp. 1–256. [Google Scholar]
- Nitta, K.-H.; Kuriyagawa, M. Thermoplastic Polyurethanes. In Handbook of Thermoplastics, 2nd ed.; Olabisi, O., Adewale, K., Eds.; CRC Press: NW, Boca Raton, FL, USA, 2015; Chapter 12; pp. 387–397. [Google Scholar]
- Yildirim, E.; Yurtsever, M.; Yilgor, E.; Yilgor, I.; Wilkes, G.L. Temperature-dependent changes in the hydrogen bonded hard segment network and microphase morphology in a model polyurethane: Experimental and simulation studies. J. Polym. Sci. Part B Polym. Phys. 2017, 56, 182–192. [Google Scholar] [CrossRef]
- Xiao, Y.; Jiang, L.; Liu, Z.; Yuan, Y.; Yan, P.; Zhou, C.; Lei, J. Effect of phase separation on the crystallization of soft segments of green waterborne polyurethanes. Polym. Test. 2017, 60, 160–165. [Google Scholar] [CrossRef]
- Paik Sung, C.S.; Hu, C.B.; Wu, C.S. Properties of segmented poly(urethaneureas) based on 2,4-toluene diisocyanate. 1. Thermal transitions, x-ray studies, and comparison with segmented poly(urethanes). Macromolecules 1980, 13, 111–116. [Google Scholar] [CrossRef]
- Velankar, S.; Cooper, S.L. Microphase separation and rheological properties of polyurethane melts. 2. Effect of block incompatibility on the microstructure. Macromolecules 2000, 33, 382–394. [Google Scholar] [CrossRef]
- Zhang, J.; Deubler, R.; Hartlieb, M.; Martin, L.; Tanaka, J.; Patyukova, E.; Topham, P.D.; Schacher, F.H.; Perrier, S. Evolution of microphase separation with variations of segments of sequence-controlled multiblock copolymers. Macromolecules 2017, 50, 7380–7387. [Google Scholar] [CrossRef] [Green Version]
- Sokolova, M.P.; Bugrov, A.N.; Smirnov, M.A.; Smirnov, A.V.; Lahderanta, E.; Svetlichnyi, V.M.; Toikka, A.M. Effect of domain structure of segmented poly(urethane-imide) membranes with polycaprolactone soft blocks on dehyration of n-propanol via pervaporation. Polymers 2018, 10, 1222. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhu, M.; Hao, C.; Dai, R.; Huang, M.; Liu, H.; He, S.; Liu, W. Development of semicrystalline polyurethane with self-healing and body temperature-responsive shape memory properties. Eur. Polym. J. 2022, 167, 111060. [Google Scholar] [CrossRef]
- Gorbunova, M.A.; Anokhin, D.V.; Badamshina, E.R. Recent advances in the synthesis and application of thermoplastic semicrystalline shape memory polyurethanes. Polym. Sci. Ser. B 2020, 62, 427–450. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, J.; Nedolosa, C.; Saiani, A.; Assender, H.E. Phase separation and crystallization in high hard block content polyurethane thin films. Macromolecules 2015, 48, 5358–5366. [Google Scholar] [CrossRef]
- Kalajahi, A.E.; Rezaei, M.; Abbasi, F. Preparation, characterization, and thermo-mechanical properties of poly (e-caprolactone)-piperazine-based polyurethane-urea shape memory polymers. J. Mater. Sci. 2016, 51, 4379–4389. [Google Scholar] [CrossRef]
- Kong, Z.; Tian, Q.; Zhang, R.; Yin, J.; Shi, L.; Ying, W.B.; Hu, H.; Yao, C.; Wang, K.; Zhu, J. Reexamination of the microphase separation in MDI and PTMG based polyurethane: Fast and continuous association/dissociation processes of hydrogen bonding. Polymer 2019, 185, 121943. [Google Scholar] [CrossRef]
- Chu, B.; Gao, T.; Li, Y.; Wang, J.; Desper, C.R.; Byrne, C.A. Microphase separation kinetics in segment polyurethanes: Effect of soft segment length and structure. Macromolecules 1992, 25, 5724–5729. [Google Scholar] [CrossRef]
- Terban, M.W.; Seidel, K.; Poöselt, E.; Malfois, M.; Baumann, R.-P.; Sander, R.; Paulus, D.; Hinrichsen, B.; Dinnebier, R.E. Cross-examining polyurethane nanodomain formation and internal structure. Macromolecules 2020, 53, 9065–9073. [Google Scholar] [CrossRef] [PubMed]
- Kojio, K.; Kugumiya, S.; Uchiba, Y.; Nishino, Y.; Furukawa, M. The microphase-separated structure of polyurethane bulk and thin films. Polym. J. 2009, 41, 118–124. [Google Scholar] [CrossRef]
- Baeza, G.P. Recent advances on the structure-properties relationship of multiblock copolymers. J. Polym. Sci. 2021, 59, 2405–2433. [Google Scholar] [CrossRef]
- Yanagihara, Y.; Osaka, N.; Murayama, S.; Saito, H. Thermal annealing behavior and structure development of crystalline hard segment domain in a melt-quenched thermoplastic polyurethane. Polymer 2013, 54, 2183–2189. [Google Scholar] [CrossRef]
- Piegat, A.; Fray, M. Thermoplastic elastomers: Materials for heart assist devices. Polim. Med. 2016, 46, 79–87. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, P.; Tanwar, S.; Varshney, G.; Yadav, S. Assessment of bio-based polyurethanes: Perspective on applications and bio-degradation. Macromol 2022, 2, 19. [Google Scholar] [CrossRef]
- Ramezani, M.; Moroe, M.B.B. Biostable segmented thermoplastic polyurethane shape memory polymers for smart biomedical applications. ACS Appl. Polym. Mater. 2022, 4, 1956–1965. [Google Scholar] [CrossRef]
- Frohn-Sorensen, P.; Schreiber, F.; Manns, M.; Knoche, J.; Engel, B. Additive manufacturing of TPU Pneu-nets as soft robotic actuators. In Towards Sustainable Customization: Bridging Smart Products and Manufacturing Systems; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Tobushi, H.; Hara, H.; Yamada, E.; Hayashi, S. Thermomechanical properties of shape memory polymer of polyurethane series and their applications. J. Phys. IV France 1996, 6, 377–384. [Google Scholar]
- Rahmawati, R.; Nozaki, S.; Kojio, K.; Takahara, A.; Shinohara, N.; Yamasaki, S. Microphase-separated structure and mechanical properties of cycloaliphatic diisocyanate-based thiourethane elastomers. Polym. J. 2019, 51, 265–273. [Google Scholar] [CrossRef]
- Ma, Z.; Hong, Y.; Nelson, D.M.; Pichamuthu, J.E.; Leeson, C.E.; Wagner, W.R. Biodegradable polyurethane ureas with variable polyester or polycarbonate soft segments: Effects of crystallinity, molecular weight, and composition on mechanical properties. Biomacromolecules 2011, 12, 3265–3274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skarja, G.A.; Woodhouse, K.A. Structure-property relationships of degradable polyurethane elastomers containing an amino acid-based chain extender. J. Appl. Polym. Sci. 2000, 75, 1522–1534. [Google Scholar] [CrossRef]
- Gisselfalt, K.; Helgee, B. Effect of soft segment length and chain extender structure on phase separation and morphology in poly(urethane urea)s. Macromol. Mater. Eng. 2003, 288, 265–271. [Google Scholar] [CrossRef]
- Asensio, M.; Costa, V.; Nohales, A.; Bianchi, O.; Gómez, C.M. Tunable structure and properties of segmented thermoplastic polyurethanes as a function of flexible segment. Polymers 2019, 11, 1910. [Google Scholar] [CrossRef] [Green Version]
- Korley, L.T.J.; Pate, B.D.; Thomas, E.L.; Hammond, P.T. Effect of the degree of soft and hard segment ordering on the morphology and mechanical behavior of semicrystalline segmented polyurethanes. Polymer 2006, 47, 3073–3082. [Google Scholar] [CrossRef]
- Jin, X.; Guo, N.; You, Z.; Tan, Y. Design and performance of polyurethane elastomers composed with different soft segments. Materials 2020, 13, 991. [Google Scholar] [CrossRef]
- Touchet, T.J.; Cosgriff-Hernandez, E.M. Hierarchal structure–property relationships of segmented polyurethanes. In Advances in Polyurethane Biomaterials; Cooper, S.L., Guan, J., Eds.; Woodhead Publishing: Duxford, UK, 2016; Chapter 1; pp. 3–22. [Google Scholar]
- Oprea, S. Effect of composition and hard-segment content on thermo-mechanical properties of cross-linked polyurethane copolymers. High Perform. Polym. 2009, 21, 353–370. [Google Scholar] [CrossRef]
- Klinedinst, D.B.; Yilgor, I.; Yilgor, E.; Zhang, M.; Wilkes, G.L. The effect of varying soft and hard segment length on the structure-property relationships of segmented polyurethanes based on a linear symmetric diisocyanate, 1,4-butanediol and PTMO soft segments. Polymer 2012, 53, 5358–5366. [Google Scholar] [CrossRef]
- Sheth, J.P.; Klinedinst, D.B.; Wilkes, G.L.; Yilgor, I.; Yilgor, E. Role of chain symmetry and hydrogen bonding in segmented copolymers with monodisperse hard segments. Polymer 2005, 46, 7317–7322. [Google Scholar] [CrossRef]
- Yilgor, I.; Yilgor, E.; Guler, I.G.; Ward, T.C.; Wilkes, G.L. FTIR investigation of the influence of diisocyanate symmetry on the morphology development in model segmented polyurethanes. Polymer 2006, 47, 4105–4114. [Google Scholar] [CrossRef]
- Saenz-Perez, M.; Lizundia, E.; Laza, J.M.; Garcia-Barrasa, J.; Vilas, J.L.; Leon, L.M. Methylene diphenyl diisocyanate (MDI) and toluene diisocyanate (TDI) based polyurethanes: Thermal, shape-memory and mechanical behavior. RSC Adv. 2016, 6, 69094–69102. [Google Scholar] [CrossRef]
- Yudin, V.E.; Bugrov, A.N.; Didenko, A.L.; Smirnova, V.E.; Gofman, I.V.; Kononova, S.V.; Kremnev, R.V.; Popova, E.N.; Svetlichnyi, V.M.; Kudryavtseva, V.V. Composites of multiblock (segmented) aliphatic poly(ester imide) with zirconia nanoparticles: Synthesis, mechanical properties, and pervaporation behavior. Polym. Sci. Ser. B. 2014, 56, 859–866. [Google Scholar] [CrossRef]
- Runov, V.V.; Bugrov, A.N.; Smyslov, R.Y.; Kopitsa, G.P.; Runova, M.K.; Vasil’ev, B.V.; Popova, E.N.; Kirillova, S.A.; Feoktystov, F. Mesostructure of composite materials based on segmented poly(urethane imide) containing ferrite nanoparticles. Russ. J. Inorg. Chem. 2021, 66, 225–236. [Google Scholar] [CrossRef]
- Heydarnezhad, H.R.; Mohammadi, N.; Arbe, A.; Alegria, A. How does microstructural design affect the dynamics and rheology of segmented polyurethanes? Macromolecules 2020, 53, 5381–5398. [Google Scholar] [CrossRef]
- Morozov, I.A. Nanoindentation of polyurethane with phase-separated fibrillar structure. Polym. Test. 2021, 94, 107038. [Google Scholar] [CrossRef]
- Cheng, B.-X.; Gao, W.-C.; Ren, X.-M.; Ouyang, X.-Y.; Zhao, Y.; Zhao, H.; Wu, W.; Huang, C.-X.; Liu, Y.; Liu, X.-Y.; et al. A review of microphase separation of polyurethane: Characterization and applications. Polym. Test. 2022, 107, 107489. [Google Scholar] [CrossRef]
- The software OriginPro 2022b (build 9.9.5.167) from OriginLab Corporation. Available online: https://www.originlab.com/index.aspx?go=Support/DocumentationAndHelpCenter/Installation/Multi-userDeployment/MSIInstall (accessed on 23 September 2022).
- Niemczyk, A.; Piegat, A.; Olalla, Á.S.; Fray, M. New approach to evaluate microphase separation in segmented polyurethanes containing carbonate macrodiol. Eur. Polym. J. 2017, 93, 182–191. [Google Scholar] [CrossRef]
- Cognet-Georjon, E.; Mйchin, F.; Pascault, J.-P. New polyurethanes based on 4,4′-diphenylmethane diisocyanate and 1,4:3,6 dianhydrosorbitol, 2. Synthesis and properties of segmented polyurethane elastomers. Macromol. Chem. Phys. 1996, 197, 3593–3612. [Google Scholar] [CrossRef]
- Kopal, I.; Harničárová, M.; Valíček, J.; Kušnerová, M. Modeling the Temperature Dependence of Dynamic Mechanical Properties and Visco-Elastic Behavior of Thermoplastic Polyurethane Using Artificial Neural Network. Polymers 2017, 9, 519. [Google Scholar] [CrossRef] [PubMed]
- Stefanović, I.S.; Džunuzović, J.V.; Džunuzović, E.S.; Dapčević, A.; Šešlija, S.I.; Balanč, B.D.; Dobrzyńska-Mizera, M. Composition-property relationship of polyurethane networks based on polycaprolactone diol. Polym. Bull. 2021, 78, 7103–7128. [Google Scholar] [CrossRef]
- Kandi, R.; Pandey, P.M.; Majood, M.; Mohanty, S. Fabrication and characterization of customized tubular scaffolds for tracheal tissue engineering by using solvent based 3D printing on predefined template. Rapid Prototyp. J. 2021, 27, 421–428. [Google Scholar] [CrossRef]
- Doucet, M.; Cho, J.H.; Alina, G.; Bakker, J.; Bouwman, W.; Butler, P.; Campbell, K.; Gonzales, M.; Heenan, G.; Jackson, A.; et al. SasView Version 4.2.1. Zenodo. 10 Febuary 2019. Available online: https://zenodo.org/record/2561236#.YzqZInZByUk (accessed on 23 September 2022).
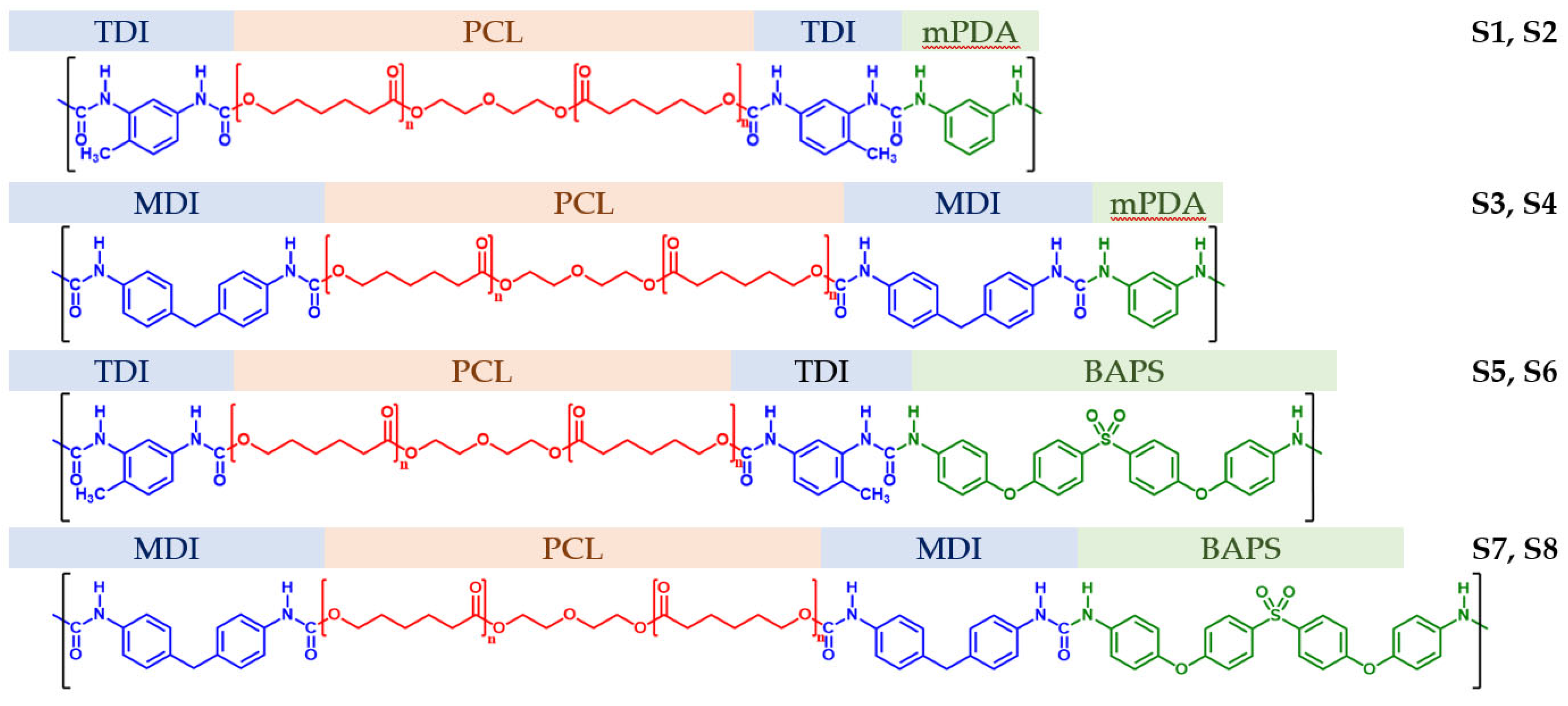
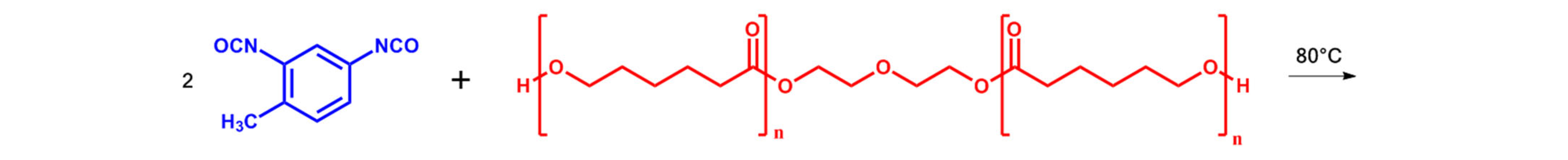
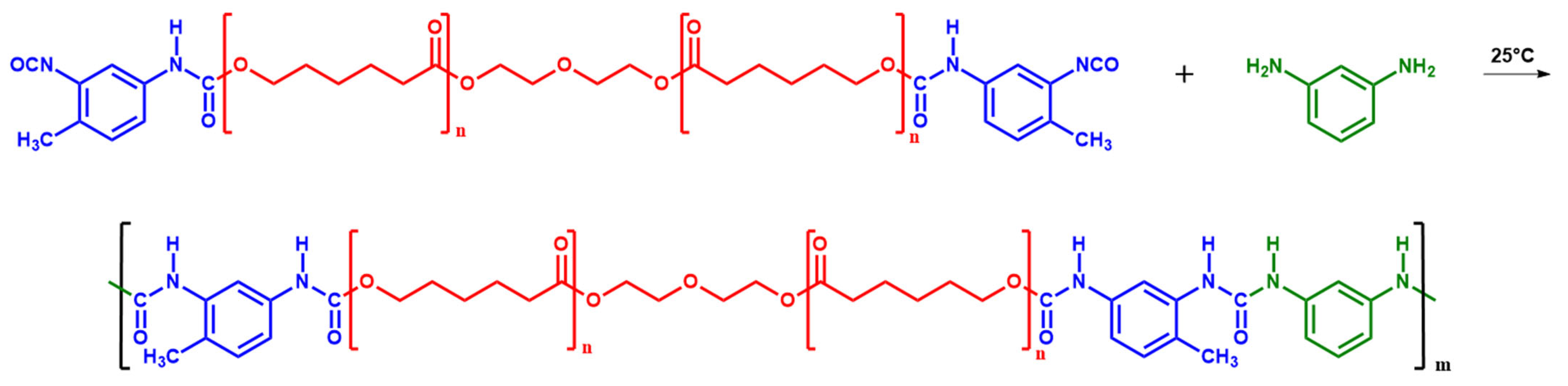
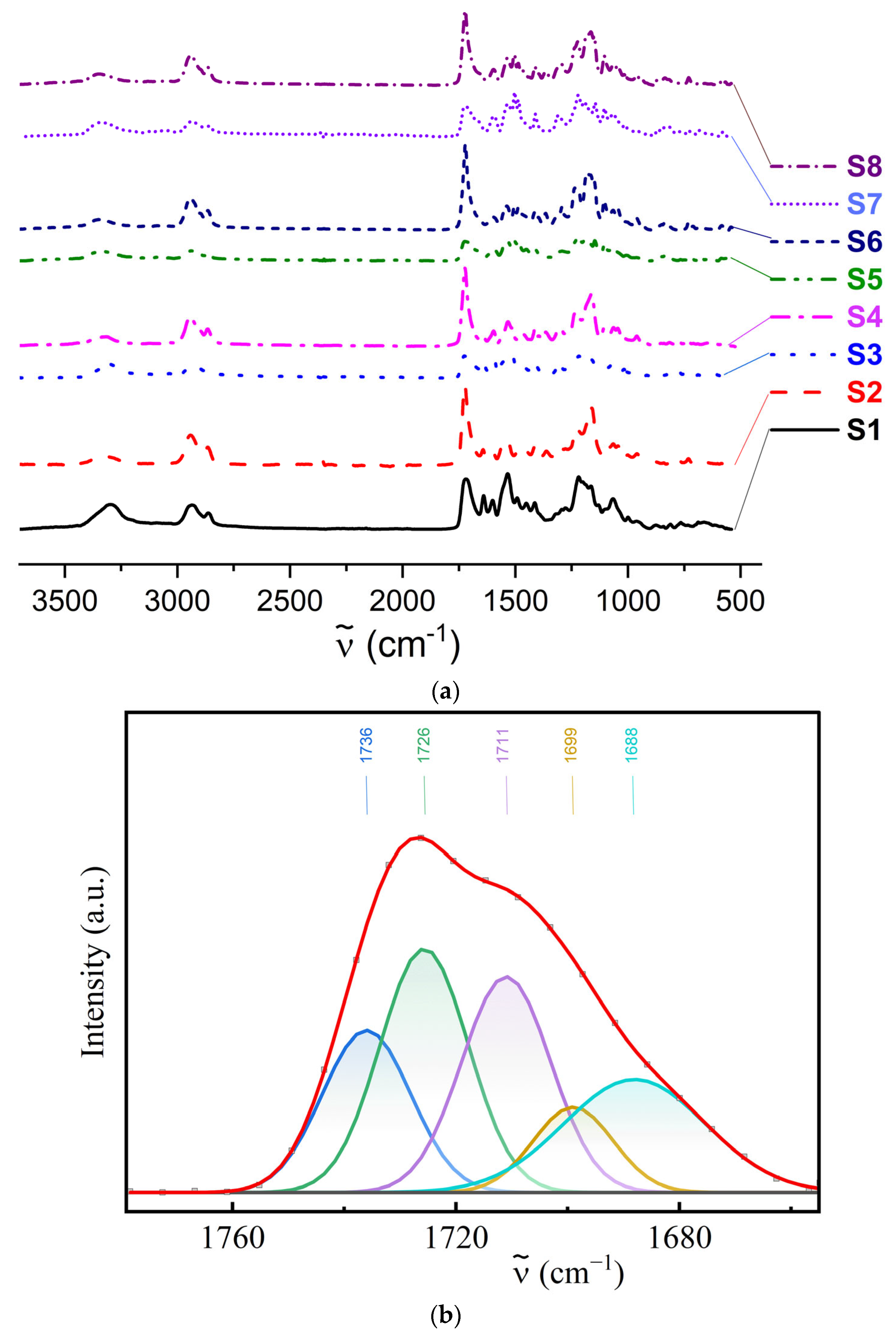


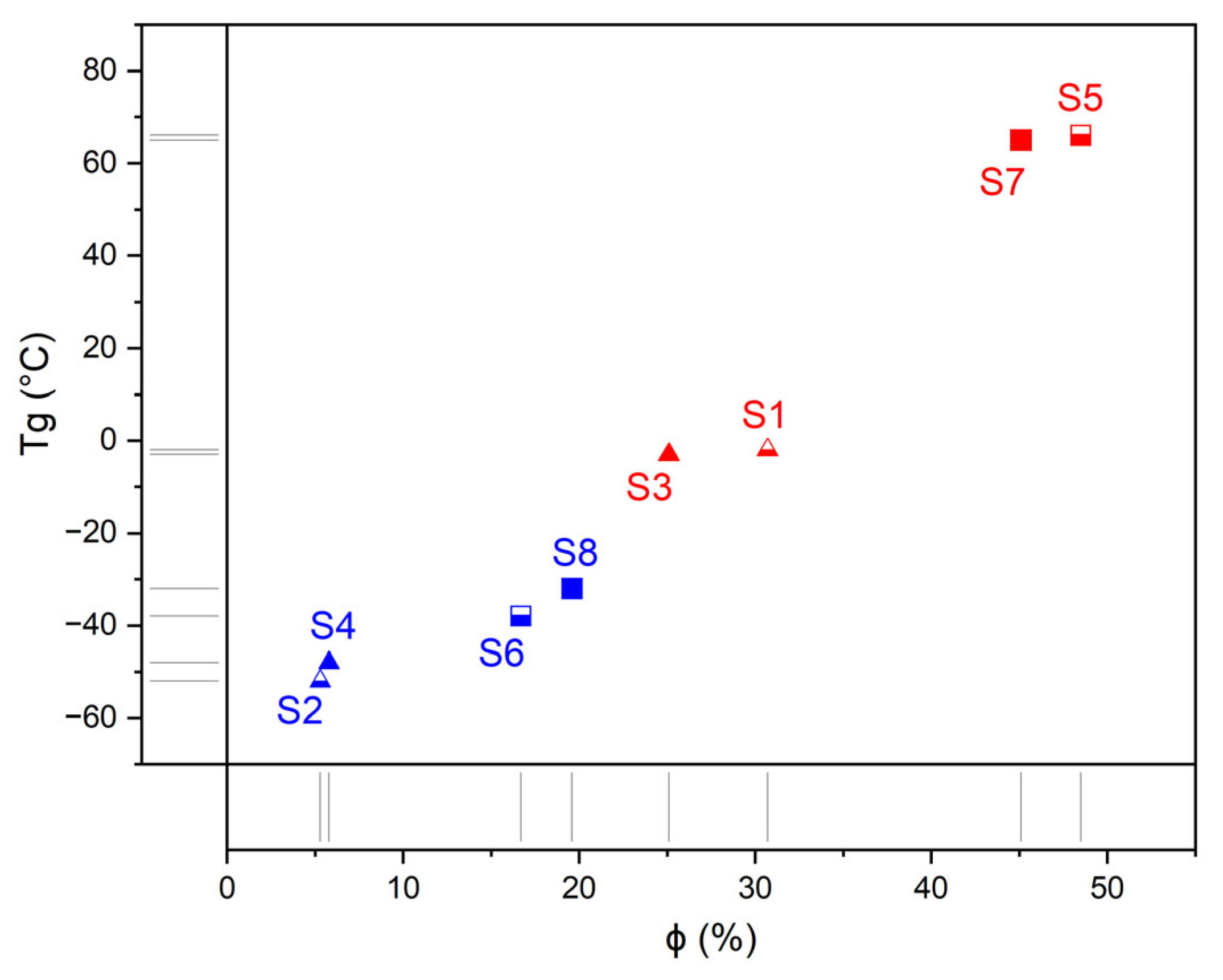


| Composition | Hard Segment | Mn PCL, g·mol−1 | Fraction of Hard Segments, CHS, % | Fraction of H-Bonded Urethane Groups, Cb, % | Fraction of Bonded Hard Segments, ϕ (1), % | |
|---|---|---|---|---|---|---|
| Group of I Stage | Group of II Stage | |||||
| S1 | TDI | mPDA | 530 | 46.3 | 66.3 | 30.7 |
| S2 | TDI | mPDA | 2000 | 18.6 | 28.3 | 5.3 |
| S3 | MDI | mPDA | 530 | 53.5 | 47.0 | 25.1 |
| S4 | MDI | mPDA | 2000 | 23.3 | 25.1 | 5.8 |
| S5 | TDI | BAPS | 530 | 59.6 | 81.4 | 48.5 |
| S6 | TDI | BAPS | 2000 | 28.1 | 59.4 | 16.7 |
| S7 | MDI | BAPS | 530 | 63.8 | 70.7 | 45.1 |
| S8 | MDI | BAPS | 2000 | 31.8 | 61.7 | 19.6 |
| Composition | Scan Number | Thermal Characteristics of the Films | ||||
|---|---|---|---|---|---|---|
| Tg, °C | Tcr, °C | ΔHcr, J/g | Tm, °C | ΔHm, J/g | ||
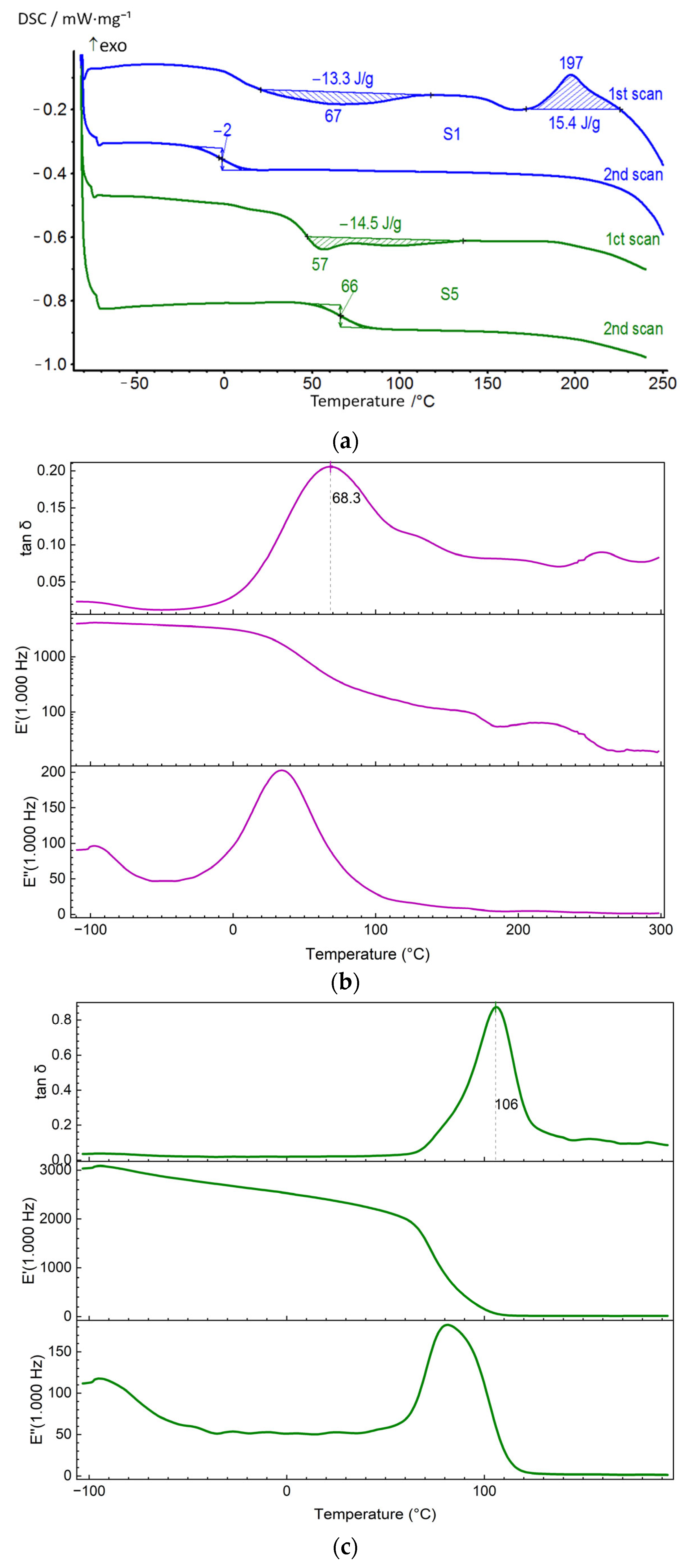
| S1 | 1 | 8 | 197 | 15.4 | 67 | −13.3 |
| 2 | −2 | - | - | - | - | |
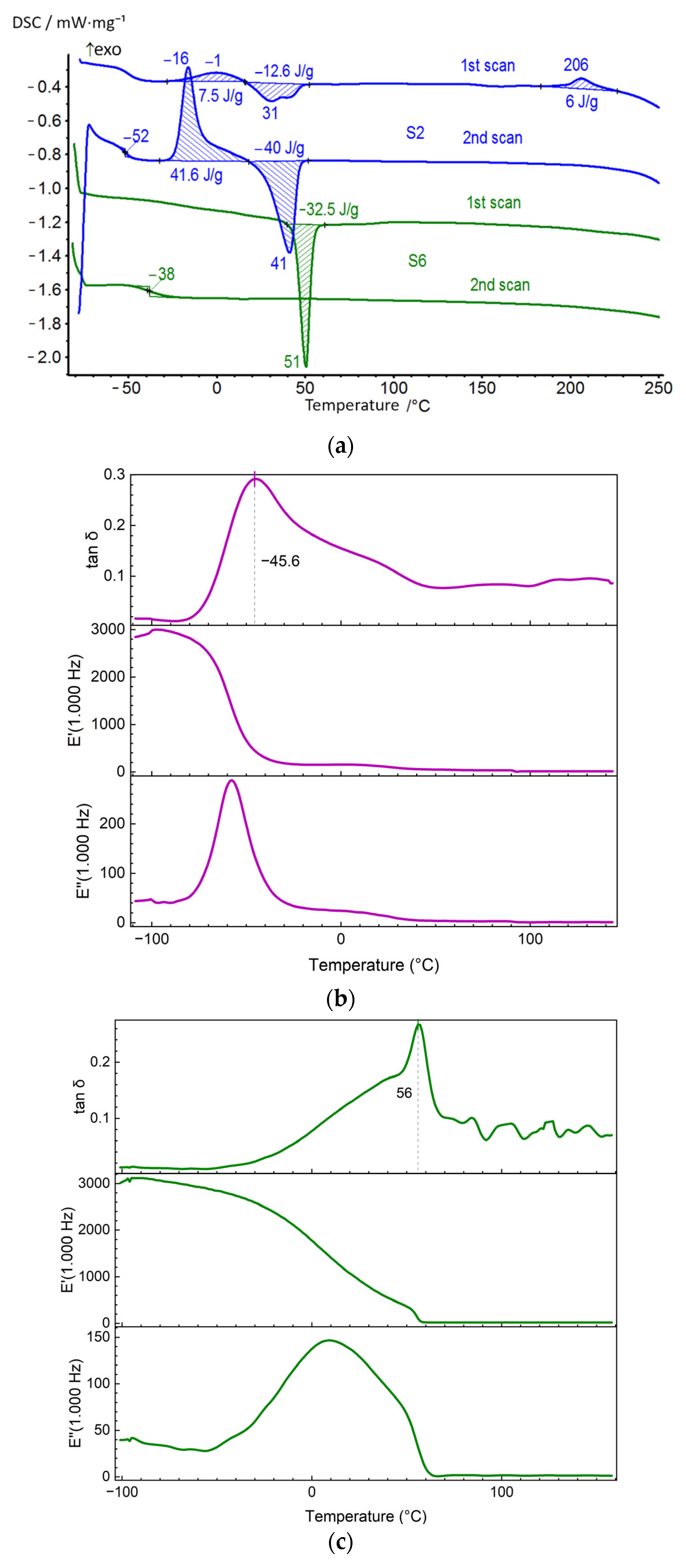
| S2 | 1 | −49 | −1; 206 | 7.5; 6 | 31 | −12.6 |
| 2 | −52 | −16 | 41.6 | 41 | −40 | |
| S3 | 1 | 5 | 203 | 2.5 | 58 | −5.4 |
| 2 | −3 | - | - | - | - | |
| S4 | 1 | −44 | - | - | 46 | −34.2 |
| 2 | −48 | −11 | 10.6 | 43 | −42.4 | |
| S5 | 1 | 11 | - | - | 57 | −14.5 |
| 2 | 66 | - | - | - | - | |
| S6 | 1 | - | - | - | 51 | −32.5 |
| 2 | −38 | - | - | - | - | |
| S7 | 1 | 54 | - | - | 97 | −3.2 |
| 2 | 65 | - | - | - | - | |
| S8 | 1 | - | - | - | 45 | -7 |
| 2 | −32 | - | - | - | - | |
| Composition | DMA Characteristics | |||||
|---|---|---|---|---|---|---|
| Tγ, °C | Tβ, °C | TαS, °C | TαH, °C | Tδ, °C | Plateau (E′), °C | |
| S1 | −98 | 34 | 68 | 130 | 205 | – |
| S2 | −95 | −58 | −45 | 29 | 90 | 100–140 |
| S3 | −74 | 28 | 47 | 160 | 255 | – |
| S4 | −60 | – | −48 | 20 | 117 | 50–80 |
| S5 | −90 | 50 | 106 | 155 | – | 130–190 |
| S6 | −95 | 15 | 56 | 80 | – | 60–160 |
| S7 | −96 | 50 | 108 | 178 | 219 | 140–230 |
| S8 | −98 | −14 | 55 | 125 | – | 65–160 |
| Composition | Method | , Å | d-Spacing, Å |
|---|---|---|---|
| S1 | SANS | 14 ± 2 | 63 |
| SAXS | 13 ± 1 | 60 | |
| S2 | SANS | 26 ± 5 | 78 |
| SAXS | 17 ± 1 | 74 | |
| S3 | SANS | 25 ± 3 | 94 |
| SAXS | 26 ± 1 | 100 | |
| S4 | SANS | 28 ± 4 | 134 |
| SAXS | 28 ± 4 | 119 | |
| S6 | SANS | 35 ± 15 | 299 |
| Composition | Films Properties | |||
|---|---|---|---|---|
| E, MPa | σbr, MPa | σy, MPa | εbr, % | |
| S1 | 995 ± 74 | 43 ± 14 | 44 ± 7 | 339 ± 135 |
| S2 | 14 ± 1 | 9 ± 1 | - | 664 ± 79 |
| S3 | 488 ± 37 | 48 ± 7 | 48 ± 6 | 196 ± 85 |
| S4 | 4 ± 1 | 27 ± 3 | - | 861 ± 115 |
| S5 | 1732 ± 113 | 66 ± 11 | 74 ± 3 | 266 ± 83 |
| S6 | 535 ± 25 | 11 ± 1 | 16 ± 1 | 391 ± 141 |
| S7 | 1721 ± 162 | 85 ± 12 | 75 ± 4 | 301 ± 75 |
| S8 | 311 ± 26 | 27 ± 5 | 11 ± 1 | 1043 ± 166 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bugrov, A.N.; Gorshkova, Y.E.; Ivan’kova, E.M.; Kopitsa, G.P.; Pavlova, A.A.; Popova, E.N.; Smirnova, V.E.; Smyslov, R.Y.; Svetlichnyi, V.M.; Vaganov, G.V.; et al. Domain Structure, Thermal and Mechanical Properties of Polycaprolactone-Based Multiblock Polyurethane-Ureas under Control of Hard and Soft Segment Lengths. Polymers 2022, 14, 4145. https://doi.org/10.3390/polym14194145
Bugrov AN, Gorshkova YE, Ivan’kova EM, Kopitsa GP, Pavlova AA, Popova EN, Smirnova VE, Smyslov RY, Svetlichnyi VM, Vaganov GV, et al. Domain Structure, Thermal and Mechanical Properties of Polycaprolactone-Based Multiblock Polyurethane-Ureas under Control of Hard and Soft Segment Lengths. Polymers. 2022; 14(19):4145. https://doi.org/10.3390/polym14194145
Chicago/Turabian StyleBugrov, Alexander N., Yulia E. Gorshkova, Elena M. Ivan’kova, Gennady P. Kopitsa, Alina A. Pavlova, Elena N. Popova, Valentina E. Smirnova, Ruslan Y. Smyslov, Valentin M. Svetlichnyi, Gleb V. Vaganov, and et al. 2022. "Domain Structure, Thermal and Mechanical Properties of Polycaprolactone-Based Multiblock Polyurethane-Ureas under Control of Hard and Soft Segment Lengths" Polymers 14, no. 19: 4145. https://doi.org/10.3390/polym14194145
APA StyleBugrov, A. N., Gorshkova, Y. E., Ivan’kova, E. M., Kopitsa, G. P., Pavlova, A. A., Popova, E. N., Smirnova, V. E., Smyslov, R. Y., Svetlichnyi, V. M., Vaganov, G. V., & Vasil’ev, B. V. (2022). Domain Structure, Thermal and Mechanical Properties of Polycaprolactone-Based Multiblock Polyurethane-Ureas under Control of Hard and Soft Segment Lengths. Polymers, 14(19), 4145. https://doi.org/10.3390/polym14194145








