Chemical Modification, Characterization, and Activity Changes of Land Plant Polysaccharides: A Review
Abstract
:1. Introduction
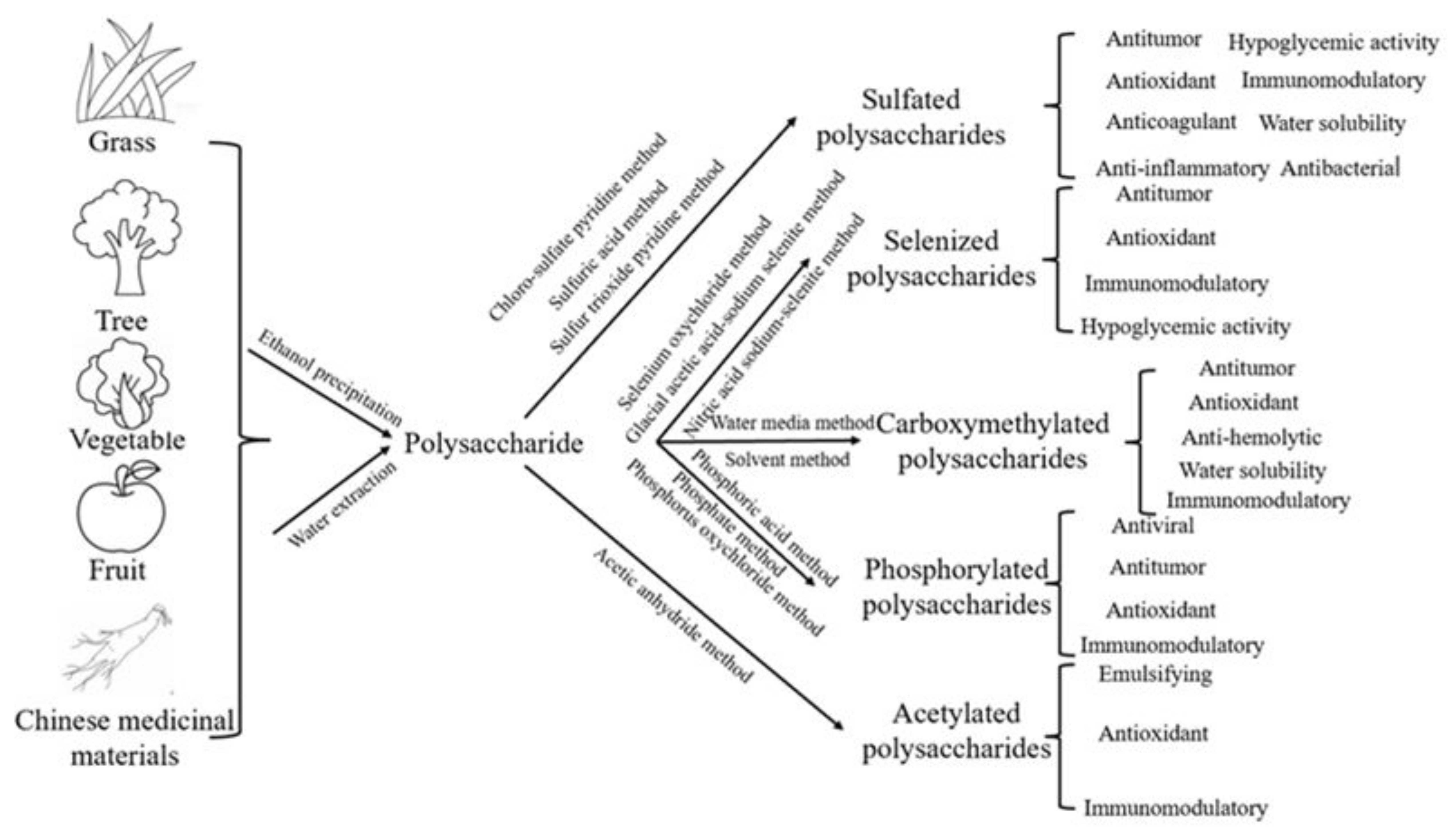
2. Polysaccharide Modification
2.1. Sulfation of Polysaccharides
2.1.1. Chloro-Sulfate Pyridine Method
2.1.2. Sulfur Trioxide Pyridine Method
2.1.3. Sulfuric Acid Method
| Source | Extraction Methods | B-Mw (kda) | A-Mw (kda) | Modification Method | Main Modifying Conditions | DS | B-CHO (%) | A-CHO (%) | Biological Activity | Refer |
|---|---|---|---|---|---|---|---|---|---|---|
| Alfalfa (AP) | Hot-water extraction | 22 | 25 | Chloro-sulfonic acid pyridine method | AP (200 mg) N, N-dimethylformamide (20 mL) Ratio of chlorosulfonic acid to pyridine (1.5:1) Reaction at 55 °C for 2.25 h | 0.724 | 90.2 | 70.3 | Antioxidant, Antibacterial Antiobesity | [21] |
| Opuntia ficus indica cladodes (PC) | Ultrasonic-assisted extraction | 7.89 | 2.1–3.87 | Sulfur trioxide pyridine method | PC (400 mg) Formamide (16 mL) Ratio of chlorosulfonic acid to N, N-dimethylformamide (1:6) Reaction at 50 °C for 3 h | 0.12–0.46 | 54.2 | 21.44–52.72 | Anticoagulant | [22] |
| Amana edulis (AEPS) | Acidic extraction Hot-water extraction | N | N | Sulfuric acid method | AEPS (500 mg) Ratio of concentrated sulfuric acid to n-butanol (3:1) (NH4)2SO4 (125 mg) Reaction at 0 °C for 30 min | 1.256–2.134 | 56.53–65.61 | 56.48–63.41 | Antioxidant | [23] |
| Persimmon fruits (PFP) | Hot-water extraction | 130 | 48–53 | Chlorosulfonic acid pyridine method | PAS (500 mg) Formamide (20 mL) Ratio of chlorosulfonic acid to pyridine (1:8, 1:4, 1:2) Reaction at 50 °C for 2 h | 0.8–2.5 | N | N | Immunomodulatory | [25] |
| Longan (LP) | Hot-water extraction | 118 | 105 | Sulfuric acid method | LP (500 mg) Ratio of sulfuric acid to butanol complex (3:1) Ammonium sulfate (125 mg) Reaction at 10 °C for 3 h | 2.011 | N | N | Immunomodulatory Antitumor | [26] |
| Dendrobium huoshanense (DHPD) | Hot-water extraction | 8.09 × 103 | 1.01–1.10 × 104 | Chlorosulfonic acid pyridine method | DHPD (500 mg) Formamide (5 mL) Ratio of chlorosulfonic acid to pyridine (1:2) Reaction at 60 °C for 30 min, 60 min | 0.475–0.94 | 92.89 | 37.7–56.35 | Antiglycation | [27] |
| Astragalus (APS) | N | N | N | Chlorosulfonic acid pyridine method | APS (400 mg) Ratio of chlorosulfonic acid to pyridine (1:6) Reaction at 95 °C for 1 h | 1.4 | 97 | N | Immunomodulatory | [28] |
| Cyclina sinensis (CSPS-1) | Hot-water extraction | N | N | Chlorosulfonic acid pyridine method | ASP (100 mg) N, N-dimethylformamide (20 mL) Ratio of chlorosulfonic acid to pyridine (1:4) Reaction at 65 °C for 2 h | 0.3–1.02 | N | N | Antitumor | [29] |
| Lyciumbarbarum (LBPS) | N | 11.87–13.12 | 18.58–29.06 | Chlorosulfonic acid pyridine method | LBPS (400 mg) Ratio of chlorosulfonic acid to pyridine (1:8) Reaction at 80 °C for 2 h | N | N | 35.37–63.21 | Immunomodulatory | [30] |
| Artemisia sphaerocephala (ASP) | Microwave-assisted extraction | 73.48 | 18.06–32.72 | Chlorosulfonic acid pyridine method | ASP (500 mg) Formamide (20 mL) Ratio of chlorosulfonic acid to pyridine (2:1) Reaction at 60 °C for 3 h | 0.44–0.63 | 90.2 | N | Antitumor | [31,32] |
| Cyclocarya paliurus (CP) | Hot-water extraction | 1.16 × 103 | 0.97–1.07 × 103 | Chlorosulfonic acid pyridine method | CP (600 mg) Formamide (60 mL) Ratio of chlorosulfonic acid to pyridine (1:4, 1:8) Reaction at 60 °C for 4 h | 0.12–0.42 | N | 42.41–63.77 | Antioxidant Immunomodulatory | [33] |
| Sphallerocarpus gracilis (SGP) | Hot-water extraction | 218 | 59 | Chlorosulfonic acid pyridine method | SGP (200 mg) Formamide (15 mL) Ratio of chlorosulfonic acid to pyridine (1.3:1) Reaction at 65 °C for 3.4 h | 0.99 | N | N | Antioxidant | [34] |
| Borojoa sorbilis cuter (BP) | Ultrahigh pressure extraction | 35.8 | N | Sulfur trioxide pyridine method | BP (100 mg) DMSO (5 mL) SO3⋅Pyr (400 mg) Reaction at 60 °C for 7 h | 1.18 | N | N | Antitumor | [35] |
| Cyclocarya paliurus (CP) | Hot-water extraction | 1.16 × 103 | 0.97–1.12 × 103 | Chlorosulfonic acid pyridine method | CP (600 mg) CSA to Pyr at Ratios of 1:1, 1:4, 1:6, and 1:8 N, N-dimethylformamide (60 mL) Reaction at 60 °C for 4 h | 0.12–0.55 | 60.62 | 35.87–49.71 | Antioxidant | [36] |
| Cyclocarya paliurus (CP) | Hot-water extraction (pretreatment degreasing) | 139 | 212 | Chlorosulfonic acid pyridine method | CP (600 mg) Formamide (20 mL) Ratio of chlorosulfonic acid to pyridine (1:4) Reaction at 60 °C for 4 h | 0.42 | 63.77 | 42.41 | Anti-inflammatory Antioxidant | [37] |
| Blackcurrant (BCP) | Ultrasonic-assisted extraction | 17.6 × 103 | 17.6–18.5 × 103 | Aminosul-fonic acid method | BCP (100 mg) N, N-dimethylformamide (40 mL) 4-dimethylamino-pyridine (162.2 mg) Ratio of amino sulfonic acid to BCP (8:1 to 30:1) reaction at different temperatures (60–100 °C) for various periods (1–5 h) | 0.53–1.28 | 84.89 | N | Antioxidant Hypoglycemic activity | [38] |
| Artemisia sphaerocephala (PAS) | Microwave-assisted extraction | 139.8 | 103–760 | Chloro-sulfonic acid pyridine method | PAS (500 mg) Formamide (30 mL) Ratio of chlorosulfonic acid to pyridine (2:1) Reaction at 60 °C for 15 to 300 min | 0.63–0.86 | N | N | Antitumor | [39] |
| Pumpkin (N) | Hot-water extraction | 10.18 | 3.84–7.7 | Chlorosulfonic acid pyridine method | Polysaccharide (250 mg) Formamide (10 mL) Reaction at 60 °C for 3 h | 0.26–0.45 | 95.17 | 7.53–12.29 | Anticoagulant | [40] |
| Abelmoschus manihot (Linn.) Medicus (LAMP) | Hot-water extraction | N | 264.2–1044.2 | Aminosul-fonic acid method | Polysaccharide (40 mg) N, N-dimethylformamide (15 mL) Aminosul-fonic acid (40, 80, 120 mg) Reaction at 80 °C for 3 h | 0.25 | 99.76 | 52.1 | Immunomodulatory | [41] |
| Bupleurum chinense (BCP) | Hot-water extraction | 29 | 37.6–51.7 | Chlorosulfonic acid pyridine method | BP (200 mg) Ratio of chlorosulfonic acid to pyridine (1:4, 1:8) Reaction at 80 °C for 2 h | 0.38–0.61 | 97.5 | N | Antioxidant Antisenescence | [42] |
| Polygonatum sibiricum (N) | Hot-water extraction | 132.6 | 82.1–117.0 | Sulfur trioxide pyridine method | Reaction at 80 °C for 1 h, 3 h, 6 h | 0.5–1.9 | 94 | 78.5–88 | Immunomodulatory | [43] |
| Lycium barbarum L. (LBP) | Enzyme extraction | 80.00 | 131.78 | Chlorosulfonic acid pyridine method | LBP (100 mg) Formamide (5 mL) Ratio of chlorosulfonic acid to pyridine (3:1) Reaction at 60 °C for 3 h | 1.43 | N | N | Antiangiogenic | [44] |
| Cucumber (N) | Hot-water extraction | N | N | Chloro-sulfonic acid pyridine method | Polysaccharide (500 mg) N, N-dimethyl formamide (10 mL) Reaction at 80 °C for 3 h | 0.65 | 20.6 | 31.2 | Antioxidant | [45] |
| Pumpkin (N) | Hot-water extraction | N | N | Chloro-sulfonic acid pyridine method | polysaccharide (500 mg) N, N-dimethylformamide (30 mL) Ratio of chlorosulfonic acid to pyridine (2:5) Reaction at 100 °C for 1 h | 0.35 | 81 | 61.8 | Antioxidant | [46] |
| Chinese yam (CYP) | Enzyme-assisted hot-water extraction | 19.5 | 29.6 | Chloro-sulfonic acid pyridine method | Ratio of chlorosulfonic acid to pyridine (1:5) Reaction at 70 °C | 0.44 | 35.77 | 33.27 | Immunomodulatory | [47] |
| Jerusalem artichoke (JAP) | N | 2.6 | N | Sulfur trioxide pyridine method | JAP (200 mg) Pyridine (5 mL) SO3⋅Pyr (600 mg) Reaction at 95 °C for 4 h | 0.56 | N | N | Antitumor | [48] |
| Chinese yam (CYP) | Enzymatic-assisted extraction | 33.33 | 37.04 | Chloro-sulfonic acid pyridine method | CYP (400 mg) Formamide (100 mL) Ratio of chlorosulfonic acid to pyridine (1:3) Reaction at 70 °C for 3 h | 0.51 | 47.45 | 36.77 | Immunomodulatory | [49] |
| Cyclocarya paliurus (CPP, CPP0.05) | Hot-water extraction (pretreatment degreasing) | 35.8 30.1 | 12.64–52.62 | Chloro-sulfonic acid pyridine method | CPP, CPP0.05 (20 mg) Formamide (20 mL) Ratio of chlorosulfonic acid to pyridine (1:6) Reaction at 60 °C for 2 h | 0.18–0.32 | 62.75 | 50.14–54.42 | Immunomodulatory | [50] |
| Tamarind seed (TSP) | Hot-water extraction | 1370 | 1340 | Sulfur trioxide pyridine method | TSP (1 g) Reaction at 50 °C for 4 h | 0.31 | N | N | Osteogenic activities | [51] |
| Jujube (JP) | Hot-water extraction | 275 | 317 | Chloro-sulfonic acid pyridine method | JP (500 mg) Formamide (100 mL) Ratio of chlorosulfonic acid to pyridine (1:1) Reaction at 75 °C for 1 h | 0.664 | 75.4 | 63.40 | Antioxidant Antibacterial | [52] |
| Orchis chusua D. Don (SP) | Hot-water extraction | 369 | 318 | Sulfur trioxide pyridine method | SP (200 mg) N, N-dimethylformamide (20 mL) SO3⋅Pyr (100 mg) Reaction at 80 °C for 3 h | 0.12 | 47.93 | 71.55 | Antioxidant Probiotic ability | [53] |
| Cyclocarya paliurus (CP) | Hot-water extraction | 139.6 | 161.5 | Chloro-sulfonic acid pyridine method | CP sample (400 mg) formamide (40 mL) Ratio of chlorosulfonic acid to pyridine (1:7) Reaction at 60 °C for 4 h | 0.17 | N | N | Antioxidant Antitumor | [54] |
| Cardamine hupingshanensis (CHP) | Hot-water extraction | N | 22.2 | Sulfur trioxide pyridine method | CHP (600 mg) DMSO (180 mL) SO3⋅Pyr (15 g) Reaction at 55 °C for 2 h | N | N | N | Antioxidant | [55] |
2.2. Selenization of Polysaccharides
2.2.1. Glacial Acetic Acid/Sodium Selenite Method
2.2.2. Nitric Acid Sodium/Selenite Method
2.2.3. Selenium Oxychloride Method
2.3. Phosphorylation of Polysaccharides
2.3.1. Phosphate Method
2.3.2. Phosphoric Acid Method
2.3.3. Phosphorus Oxychloride Method
2.4. Carboxymethylation of Polysaccharides
2.4.1. Water Media Method
2.4.2. Solvent Method
2.5. Acetylation of Polysaccharides
Acetic Anhydride Method
3. Characterization of Polysaccharides
3.1. DS
- A: Consumption of sodium hydroxide per gram of sample
- V0: Consumption of sodium hydroxide
- C0: Concentration of sodium hydroxide
- V1: Consumption of hydrochloric acid
- C1: Concentration of hydrochloric acid
- W: Weight of sample
- V1: Volume of hydrochloric acid required for the polysaccharide before modification
- V2: Volume of hydrochloric acid required for the polysaccharide sample after modification
- M: Molar concentration of hydrochloric acid
- W: Weight of sample
3.2. Mw
3.3. FT-IR Spectra
3.4. Monosaccharide Composition
3.5. NMR
4. Biological Activity
4.1. Bioactivity of Sulfated Polysaccharides
4.2. Bioactivity of Selenized Polysaccharides
4.3. Bioactivity of Phosphorylated Polysaccharides
4.4. Bioactivity of Carboxymethylated Polysaccharides
4.5. Bioactivity of Acetylated Polysaccharides
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chai, Y.-Y.; Kan, L.-B.; Zhao, M. Enzymatic extraction optimization, anti-HBV and antioxidant activities of polysaccharides from Viscum coloratum (Kom.) Nakai. Int. J. Biol. Macromol. 2019, 134, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Rostami, H.; Gharibzahedi, S.M.T. Cellulase-assisted extraction of polysaccharides from Malva sylvestris: Process optimization and potential functionalities. Int. J. Biol. Macromol. 2017, 101, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-C.; Zhao, Y.-N.; Yang, Y.; Zhang, H.-F.; Ding, C.-B.; Hu, C.; Zhou, L.-J.; Zhang, Z.-W.; Yuan, S.; Chen, Y.-E.; et al. Microwave-assisted extraction, physicochemical characterization and bioactivity of polysaccharides from Camptotheca acuminata fruits. Int. J. Biol. Macromol. 2019, 133, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-G.; Liu, J.-C.; Liu, X.-F.; Zhang, X.; Xu, Y.; Leng, F.-F.; Avwenagbiku, M.O. Kinetic modeling of the ultrasonic-assisted extraction of polysaccharide from Nostoc commune and physicochemical properties analysis. Int. J. Biol. Macromol. 2019, 128, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-J.; Wang, M.-J.; Xie, M.H.; Wan, P.; Chen, D.; Hu, B.; Ye, H.; Zeng, X.-X.; Liu, Z.-H. Evaluation of chemical property, cytotoxicity and antioxidant activity in vitro and in vivo of polysaccharides from Fuzhuan brick teas. Int. J. Biol. Macromol. 2018, 116, 120–127. [Google Scholar] [CrossRef]
- Yu, X.-H.; Liu, Y.; Wu, X.-L.; Liu, L.-Z.; Fu, W.; Song, D.D. Isolation, purification, characterization and immunostimulatory activity of polysaccharides derived from American ginseng. Carbohydr. Polym. 2017, 156, 9–18. [Google Scholar] [CrossRef]
- Mi, Y.-Q.; Tan, W.-Q.; Zhang, J.-J.; Guo, Z. Modification of hydroxypropyltrimethyl ammonium chitosan with organic acid: Synthesis, characterization, and antioxidant activity. Polymers 2020, 12, 2460. [Google Scholar] [CrossRef]
- Jiao, Y.-K.; Zhang, M.-L.; Wang, S.-M.; Yan, C.-Y. Consumption of guava may have beneficial effects in type 2 diabetes: A bioactive perspective. Int. J. Biol. Macromol. 2017, 101, 543–552. [Google Scholar] [CrossRef]
- Kravchenko, A.O.; Anastyuk, S.D.; Sokolova, E.V.; Isakov, V.V.; Glazunov, V.P.; Helbert, W.; Yermak, I.M. Analysis and cytokine-induced activity of gelling sulfated polysaccharide from the cystocarpic plants of Ahnfeltiopsis flabelliformis. Carbohydr. Polym. 2016, 151, 523–534. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Presa, F.B.; Marques, M.L.M.; Viana, R.L.S.; Nobre, L.T.D.B.; Costa, L.S.; Rocha, H.A.O. The protective role of sulfated polysaccharides from green seaweed udotea flabellum in cells exposed to oxidative damage. Mar. Drugs 2018, 16, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.-T.; Wang, W.-X.; Liao, D.-D.; Chen, D.-M.; Zhu, P.-P.; Cai, G.-X.; Kiyoshi, A. Polysaccharides from enteromorpha prolifera improve glucose metabolism in diabetic rats. J. Diabetes Res. 2015, 2015, 675201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaich, H.; Ben Amira, A.; Abbes, F.; Bouaziz, M.; Besbes, S.; Richel, A.; Blecker, C.; Attia, H.; Garna, H. Effect of extraction procedures on structural, thermal and antioxidant properties of ulvan from Ulva lactuca collected in Monastir coast. Int. J. Biol. Macromol. 2017, 105, 1430–1439. [Google Scholar] [CrossRef]
- Guo, M.; Ma, Y.-F.; Wang, C.-G.; Liu, H.Z.; Li, Q.; Fei, M. Synthesis, antioxidant activity, and biodegradability of a novel recombinant polysaccharide derived from chitosan and lactose. Carbohydr. Polym. 2015, 118, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-J.; Xiong, Q.-P.; Lai, X.-P.; Li, X.; Wan, M.; Zhang, J.-N.; Yan, Y.-J.; Cao, M.; Lu, L.; Guan, J.-M.; et al. Molecular modification of polysaccharides and resulting bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Muneeb, A.M. Recent trends in chemical modification and antioxidant activities of plants-based polysaccharides: A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100045. [Google Scholar]
- Bedini, E.; Laezza, A.; Parrilli, M.; Iadonisi, A. A review of chemical methods for the selective sulfation and desulfation of polysaccharides. Carbohydr. Polym. 2017, 174, 1224–1239. [Google Scholar] [CrossRef]
- Zhao, B.-T.; Tao, F.-Q.; Wang, J.-L.; Zhang, J. The sulfated modification and antioxidative activity of polysaccharides from Potentilla anserine L. New J. Chem. 2020, 44, 4726–4735. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Xiong, Q.; Yu, Y.; Peng, L.C. Sulfated modification, characterization, and potential bioactivities of polysaccharide from the fruiting bodies of Russula virescens. Int. J. Biol. Macromol. 2020, 154, 1438–1447. [Google Scholar] [CrossRef]
- Zhao, X.-N.; Hu, Y.-L.; Wang, D.-Y.; Guo, L.-W.; Yang, S.-J.; Fan, Y.-P.; Zhao, B.-K.; Wang, Y.-L.; Abula, S. Optimization of sulfated modification conditions of tremella polysaccharide and effects of modifiers on cellular infectivity of NDV. Int. J. Biol. Macromol. 2011, 49, 44–49. [Google Scholar] [CrossRef]
- Li, Z.-W.; Wei, Y.-H.; Wang, Y.-W.; Zhang, R.; Zhang, C.-J.; Wang, C.-X.; Yan, X.-B. Preparation of highly substituted sulfated alfalfa polysaccharides and evaluation of their biological activity. Foods 2022, 11, 737. [Google Scholar] [CrossRef]
- Chaouch, M.A.; Hammi, K.M.; Dhahri, M.; Ben Mansour, M.; Maaroufi, M.R.; Le Cerf, D.; Majdoub, H. Access to new anticoagulant by sulfation of pectin-like polysaccharides isolated from Opuntia ficus indica cladodes. Int. J. Biol. Macromol. 2018, 120, 1794–1800. [Google Scholar] [CrossRef]
- Cao, Y.-Y.; Ji, Y.-H.; Liao, A.-M.; Huang, J.-H.; Thakur, K.; Li, X.-L.; Hu, F.; Zhang, J.-G.; Wei, Z.-J. Effects of sulfated, phosphorylated and carboxymethylated modifications on the antioxidant activities in-vitro of polysaccharides sequentially extracted from Amana edulis. Int. J. Biol. Macromol. 2020, 146, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, J.; Li, Y.-Y.; Shen, J.-M.; Zhao, T.; Zhang, H.-X. Sulfated modification and cytotoxicity of Portulaca oleracea L. polysaccharides. Glycoconj. J. 2010, 27, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhang, Y.; Guo, H. Effect of polysaccharides from fresh persimmon (Diospyros kaki) fruits and its sulfated derivates on the immunomodulatory activity of mouse peritoneal macrophage cells. Adv. Mater. Res. 2013, 2215, 108–115. [Google Scholar] [CrossRef]
- Jiang, J.; Meng, F.-Y.; He, Z.; Ning, Y.-L.; Li, X.-H.; Song, H.; Wang, J.; Zhou, R. Sulfated modification of longan polysaccharide and its immunomodulatory and antitumor activity in vitro. Int. J. Biol. Macromol. 2014, 67, 323–329. [Google Scholar] [CrossRef]
- Li, X.-L.; Xiao, J.-J.; Zha, X.-Q.; Pan, L.-H.; Asghar, M.N.; Luo, J.-P. Structural identification and sulfated modification of an antiglycation Dendrobium huoshanense polysaccharide. Carbohydr. Polym. 2014, 106, 247–254. [Google Scholar] [CrossRef]
- Wang, X.-F.; Li, Y.-L.; Shen, J.; Wang, S.-Y.; Yao, J.-H.; Yang, X.-J. Effect of astragalus polysaccharide and its sulfated derivative on growth performance and immune condition of lipopolysaccharide-treated broilers. Int. J. Biol. Macromol. 2015, 76, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.-X.; Xiong, Q.-P.; Li, S.-L.; Zhao, X.-R.; Zeng, X.-X. Structural characterization, sulfation and antitumor activity of a polysaccharide fraction from Cyclina sinensis. Carbohydr. Polym. 2015, 115, 200–206. [Google Scholar] [CrossRef]
- Wang, J.-M.; Ge, B.-L.; Du, C.-Y.; Xue, J.-L.; Zhuang, Y.-W.; Xue, K. Sulfated modification promotes the immunomodulatory bioactivities of lyciumbarbarum polysaccharides in vitro. Int. J. Clin. Exp. Med. 2015, 8, 20380–20390. [Google Scholar]
- Wang, J.-L.; Yang, W.; Wang, J.-C.; Wang, X.; Wu, F.; Yao, J.; Zhang, J.; Lei, Z.-Q. Regioselective sulfation of artemisia sphaerocephala polysaccharide: Characterization of chemical structure. Carbohydr. Polym. 2015, 133, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Bao, A.-J.; Wang, Q.; Guo, H.-Y.; Zhang, Y.-D.; Liang, J.-Y.; Kong, W.-B.; Yao, J.; Zhang, J. Sulfation can enhance antitumor activities of Artemisia sphaerocephala polysaccharide in vitro and vivo. Int. J. Biol. Macromol. 2018, 107, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, M.-Y.; Wang, Z.-J.; Wang, Y.-X.; Xie, M.-Y.; Xie, J.-H. Sulfated polysaccharide from Cyclocarya paliurus enhances the immunomodulatory activity of macrophages. Carbohydr. Polym. 2017, 174, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-F.; Song, S.; Wei, Y.-X.; Wang, F.-X.; Zhao, M.; Guo, J.; Zhang, J. Sulfated modification of the polysaccharide from Sphallerocarpus gracilis and its antioxidant activities. Int. J. Biol. Macromol. 2016, 87, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.-F.; Liao, K.-S.; Wu, Y.-S.; Pan, Q.; Wu, L.-L.; Jiao, H.; Guo, D.-A.; Li, B.; Liu, B. Optimization, characterization, sulfation and antitumor activity of neutral polysaccharides from the fruit of Borojoa sorbilis cute. Carbohydr. Polym. 2016, 151, 364–372. [Google Scholar] [CrossRef]
- Xie, J.H.; Wang, Z.J.; Shen, M.Y.; Nie, S.P.; Gong, B.; Li, H.S.; Zhao, Q.; Li, W.J.; Xie, M.Y. Sulfated modification, characterization and antioxidant activities of polysaccharide from Cyclocarya paliurus. Food Hydrocolloid. 2016, 53, 7–15. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, J.; Yang, Y.; Zhang, F.; Wang, S.; Wu, T.; Shen, M.; Xie, M. Sulfated Cyclocarya paliurus polysaccharides markedly attenuates inflammation and oxidative damage in lipopolysaccharide-treated macrophage cells and mice. Sci. Rep. 2017, 7, 40402. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.-Q.; Gao, Y.-K.; Liu, F.; Niu, X.-J.; Wang, L.-B.; Li, X.-G.; Chen, H.-C.; Yang, Y. Sulfated modification of the polysaccharides from blackcurrant and their antioxidant and alpha-amylase inhibitory activities. Int. J. Biol. Macromol. 2018, 109, 1344–1354. [Google Scholar] [CrossRef]
- Wang, J.-L.; Bao, A.-J.; Meng, X.-H.; Guo, H.-Y.; Zhang, Y.-D.; Zhao, Y.-L.; Kong, W.-B.; Liang, J.-Y.; Yao, J.; Zhang, J. An efficient approach to prepare sulfated polysaccharide and evaluation of anti-tumor activities in vitro. Carbohydr. Polym. 2018, 184, 366–375. [Google Scholar] [CrossRef]
- Liang, L.; Ao, L.; Ma, T.; Ni, Y.-Y.; Liao, X.-J.; Hu, X.-S.; Song, Y. Sulfated modification and anticoagulant activity of pumpkin (Cucurbita pepo, Lady Godiva) polysaccharide. Int. J. Biol. Macromol. 2018, 106, 447. [Google Scholar] [CrossRef]
- Pan, X.-X.; Tao, J.-H.; Jiang, S.; Zhu, Y.; Qian, D.-W.; Duan, J.-A. Characterization and immunomodulatory activity of polysaccharides from the stems and leaves of Abelmoschus manihot and a sulfated derivative. Int. J. Biol. Macromol. 2018, 107, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.-B.; Zheng, X.-L.; Song, J.-X.; Liu, J.; Ren, T.; Zhang, X.; Huang, L.-Q.; Wu, M.J. Radical scavenging activity of sulfated Bupleurum chinense polysaccharides and their effects against oxidative stress-induced senescence. Carbohydr. Polym. 2018, 192, 143–149. [Google Scholar] [CrossRef]
- Yelithao, K.; Surayot, U.; Park, W.; Lee, S.; Lee, D.H.; You, S. Effect of sulfation and partial hydrolysis of polysaccharides from Polygonatum sibiricum on immune-enhancement. Int. J. Biol. Macromol. 2019, 122, 10–18. [Google Scholar] [CrossRef]
- Zhou, L.-S.; Huang, L.-L.; Yue, H.; Ding, K. Structure analysis of a heteropolysaccharide from fruits of Lycium barbarum L. and anti-angiogenic activity of its sulfated derivative. Int. J. Biol. Macromol. 2018, 108, 47–55. [Google Scholar] [CrossRef]
- Chen, S.; Huang, H.; Huang, G. Extraction, derivatization and antioxidant activity of cucumber polysaccharide. Int. J. Biol. Macromol. 2019, 140, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, G. Antioxidant activities of sulfated pumpkin polysaccharides. Int. J. Biol. Macromol. 2019, 126, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Xie, L.; Xie, J.; Shen, M. Sulfated chinese yam polysaccharide enhances the immunomodulatory activity of RAW 264.7 cells via the TLR4-MAPK/NF-κB signaling pathway. Food Funct. 2022, 13, 1316–1326. [Google Scholar] [CrossRef]
- Shao, T.-L.; Yuan, P.-C.; Zhang, W.-Z.; Dou, D.-Y.; Wang, F.-G.; Hao, C.-Y.; Liu, C.-Y.; Han, J.; Chen, K.-S.; Wang, G.-D. Preparation and characterization of sulfated inulin-type fructans from Jerusalem artichoke tubers and their antitumor activity. Carbohydr. Res. 2021, 509, 108422. [Google Scholar] [CrossRef]
- Huang, R.; Shen, M.-Y.; Yu, Y.; Liu, X.; Xie, J.-H. Physicochemical characterization and immunomodulatory activity of sulfated chinese yam polysaccharide. Int. J. Biol. Macromol. 2020, 165, 635–644. [Google Scholar] [CrossRef]
- Han, Y.; Ouyang, K.H.; Li, J.-G.; Liu, X.; An, Q.; Zhao, M.; Chen, S.; Li, X.; Ye, X.-M.; Zhao, Z.-T.; et al. Sulfated modification, characterization, immunomodulatory activities and mechanism of the polysaccharides from Cyclocarya paliurus on dendritic cells. Int. J. Biol. Macromol. 2020, 159, 108–116. [Google Scholar] [CrossRef]
- Nguyen, M.T.H.; Tran, C.V.; Nguyen, P.H.; Tran, Q.; Kim, M.S.; Jung, W.K.; Nguyen, P.T.M. In vitro osteogenic activities of sulfated derivative of polysaccharide extracted from Tamarindus indica L. Biol. Chem. 2021, 402, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.F.; Wang, N.; Kou, J.J.; An, X.W.; Meng, F.H.; Zheng, X.J.; Wang, W.W.; Wang, L.L.; Wang, Z.H.; Liu, M.J.; et al. Sulfated modification, characterization and potential bioactivities of polysaccharide from Ziziphus jujuba cv. Jinsixiaozao. Nat. Prod. Commun. 2021, 16, 10. [Google Scholar] [CrossRef]
- Nuerxiati, R.; Mutailipu, P.; Abuduwaili, A.; Dou, J.; Aisa, H.A.; Yili, A. Effects of different chemical modifications on the structure and biological activities of polysaccharides from Orchis chusua D. Don. J. Food Sci. 2021, 86, 2434–2444. [Google Scholar] [CrossRef]
- Xie, L.-M.; Huang, Z.-B.; Qin, L.; Yu, Q.; Chen, Y.; Zhu, H.-B.; Xie, J.-H. Effects of sulfation and carboxymethylation on Cyclocarya paliurus polysaccharides: Physicochemical properties, antitumor activities and protection against cellular oxidative stress. Int. J. Biol. Macromol. 2022, 204, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, X.; Li, S.; Luo, K. Determining the structure and in vitro antioxidant activity of sulfated polysaccharides from cardamine hupingshanensis. Starch-Starke 2021, 74, e2100203. [Google Scholar] [CrossRef]
- William, M.; Margot, L.; Gunnar, E.; Pascal, L.L.; Daren, K.H. High-dose intravenous selenium does not improve clinical outcomes in the critically ill: A systematic review and meta-analysis. Crit. Care 2016, 20, 356. [Google Scholar]
- Lee, J.H.; Lee, Y.K.; Chang, Y.-H. Effects of selenylation modification on structural and antioxidant properties of pectic polysaccharides extracted from Ulmus pumila L. Int. J. Biol. Macromol. 2017, 104, 1124–1132. [Google Scholar] [CrossRef]
- Yang, W.-J.; Huang, G.-L.; Huang, H.-L. Preparation and structure of polysaccharide selenide. Ind. Crops Prod. 2020, 154, 112630. [Google Scholar] [CrossRef]
- Li, R.; Qin, X.-J.; Liu, S.; Zhang, X.-Y.; Zeng, X.-R.; Guo, H.-Y.; Wang, T.; Zhang, Y.-D.; Zhang, J.-P.; Zhang, J.; et al. [HNMP]HSO4 catalyzed synthesis of selenized polysaccharide and its immunomodulatory effect on RAW264.7 cells via MAPKs pathway. Int. J. Biol. Macromol. 2020, 160, 1066–1077. [Google Scholar] [CrossRef]
- Feng, Y.-Q.; Qiu, Y.-J.; Duan, Y.-Q.; He, Y.-Q.; Xiang, H.; Sun, W.-X.; Zhang, H.-H.; Ma, H.-L. Characterization, antioxidant, antineoplastic and immune activities of selenium modified Sagittaria sagittifolia L. polysaccharides. Food Res. Int. 2021, 153, 110913. [Google Scholar] [CrossRef]
- Zhu, S.-Y.; Hu, J.-H.; Liu, S.; Guo, S.-J.; Jia, Y.; Li, M.; Kong, W.-B.; Liang, J.-Y.; Zhang, J.; Wang, J.-L. Synthesis of Se-polysaccharide mediated by selenium oxychloride: Structure features and antiproliferative activity. Carbohydr. Polym. 2020, 246, 116545. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Chen, J.; Wang, D.; Hu, Y.; Wang, M.; Zhang, J.; Nguyen, T.Y.; Liu, C.; Liu, X. Optimization of selenylation conditions for Chinese angelica polysaccharide based on immune-enhancing activity. Carbohydr. Polym. 2013, 92, 645–650. [Google Scholar] [CrossRef]
- Qiu, S.; Chen, J.; Chen, X.; Fan, Q.; Zhang, C.; Wang, D.; Li, X.; Chen, X.; Chen, X.; Liu, C.; et al. Optimization of selenylation conditions for Lycium barbarum polysaccharide based on antioxidant activity. Carbohydr. Polym. 2014, 103, 148–153. [Google Scholar] [CrossRef]
- Qiu, S.; Chen, J.; Qin, T.; Hu, Y.; Wang, D.; Fan, Q.; Zhang, C.; Chen, X.; Chen, X.; Liu, C.; et al. Effects of selenylation modification on immune-enhancing activity of garlic polysaccharide. PLoS ONE 2014, 9, e86377. [Google Scholar] [CrossRef]
- Chen, W.; Chen, J.; Wu, H.; Gou, Y.; Hu, F.; Liu, L.; Gao, X.; Zhang, P. Optimization of selenylation conditions for a pectic polysaccharide and its structural characteristic. Int. J. Biol. Macromol. 2014, 69, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, X.; Yue, C.; Hou, R.; Chen, J.; Lu, Y.; Li, X.; Li, R.; Liu, C.; Gao, Z.; et al. Effect of selenylation modification on immune-enhancing activity of Atractylodes macrocephala polysaccharide. Int. J. Biol. Macromol. 2015, 72, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.-F.; Chen, T.; Yan, M.-F.; Zhao, W.-H.; Li, F.; Cheng, W.-D.; Yuan, L.-X. Synthesis, characterization, antioxidant activity and neuroprotective effects of selenium polysaccharide from Radix Hedy sari. Carbohydr. Polym. 2015, 125, 161–168. [Google Scholar] [CrossRef]
- Wang, J.-L.; Yang, X.-P.; Bao, A.J.; Liu, X.-L.; Zeng, J.-Y.; Liu, X.-R.; Yao, J.; Zhang, J.; Lei, Z.-Q. Microwave-assisted synthesis, structure and anti-tumor activity of selenized Artemisia sphaerocephala polysaccharide. Int. J. Biol. Macromol. 2017, 95, 1108–1118. [Google Scholar] [CrossRef] [Green Version]
- Hou, R.; Chen, J.; Yue, C.; Li, X.; Liu, J.; Gao, Z.; Liu, C.; Lu, Y.; Wang, D.; Li, H.; et al. Modification of lily polysaccharide by selenylation and the immune-enhancing activity. Carbohydr. Polym. 2016, 142, 73–81. [Google Scholar] [CrossRef]
- Wang, J.-L.; Li, Q.-Y.; Bao, A.-J.; Liu, X.-R.; Zeng, J.-Y.; Yang, X.-P.; Yao, J.; Zhang, J.; Lei, Z.-Q. Synthesis of selenium-containing Artemisia sphaerocephala polysaccharides: Solution conformation and anti-tumor activities in vitro. Carbohydr. Polym. 2016, 152, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Lian, K.-X.; Zhu, X.-Q.; Chen, J.; Liu, G.; Gu, X.-L. Selenylation modification: Enhancement of the antioxidant activity of a Glycyrrhiza uralensis polysaccharide. Glycoconj. J. 2018, 35, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-X.; Yu, C.; Han, Z.; Chen, Z.-Y.; Wei, X.-L.; Wang, Y.-F. Comparative analysis of existence form for selenium and structural characteristics in artificial selenium-enriched and synthetic selenized green tea polysaccharides. Int. J. Biol. Macromol. 2020, 154, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.-Y.; Bian, J.; Xu, S.-S.; Liu, C.-F.; Sun, Y.-Q.; Zhang, G.-L.; Li, D.-Q.; Liu, X.-G. Structural features, selenization modification, antioxidant and anti-tumor effects of polysaccharides from alfalfa roots. Int. J. Biol. Macromol. 2020, 149, 207–214. [Google Scholar] [CrossRef]
- Ru, Y.; Liu, K.-X.; Kong, X.-Y.; Li, X.-Y.; Shi, X.-R.; Chen, H.-M. Synthesis of selenylated polysaccharides from Momordica charantia L. and its hypoglycemic activity in streptozotocin-induced diabetic mice. Int. J. Biol. Macromol. 2020, 152, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.-Z.; Zhang, C.; Jing, L.-R.; Feng, M.; Li, R.; Yang, Y. The structural characterization and immune modulation activities comparison of Codonopsis pilosula polysaccharide (CPPS) and selenizing CPPS (sCPPS) on mouse in vitro and vivo. Int. J. Biol. Macromol. 2020, 160, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Sun, X.-Y.; Ouyang, J.-M. Preparation and characterization of selenized astragalus polysaccharide and its inhibitory effect on kidney stones. Mater. Sci. Eng. C 2020, 110, 110732. [Google Scholar]
- Zhao, M.; Bai, J.; Bu, X.; Yin, Y.; Wang, L.; Yang, Y.; Xu, Y. Characterization of selenized polysaccharides from Ribes nigrum L. and its inhibitory effects on α-amylase and α-glucosidase. Carbohydr. Polym. 2021, 259, 117729. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Gao, L.; Huang, J.; Yang, Y.; Xu, Y.; Liu, S.; Yu, W. Characterization, antioxidant and immunomodulatory effects of selenized polysaccharides from dandelion roots. Carbohydr. Polym. 2021, 260, 117796. [Google Scholar] [CrossRef]
- Guan, Q.-Y.; Lin, Y.-R.; Li, L.-Y.; Tang, Z.-M.; Zhao, X.-H.; Shi, J. In Vitro Immunomodulation of the polysaccharides from yam (Dioscorea opposita Thunb.) in response to a selenylation of lower extent. Foods 2021, 10, 2788. [Google Scholar] [CrossRef]
- Lin, Y.-R.; Guan, Q.-Y.; Li, L.-Y.; Tang, Z.-M.; Zhang, Q.; Zhao, X.-H. In vitro immuno-modulatory potentials of purslane (Portulaca oleracea L.) polysaccharides with a chemical selenylation. Foods 2021, 11, 14. [Google Scholar] [CrossRef]
- Bo, R.; Ji, X.; Yang, H.; Liu, M.; Li, J. The characterization of optimal selenized garlic polysaccharides and its immune and antioxidant activity in chickens. Int. J. Biol. Macromol. 2021, 182, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Q.; Chen, Y.; Guo, Y.; Wang, Q.; Wu, H.; Zhao, L. Effects of selenylation modification on the antioxidative and immunoregulatory activities of polysaccharides from the pulp of Rose laevigata michx fruit. Int. J. Biol. Macromol. 2022, 206, 242–254. [Google Scholar] [CrossRef]
- Xie, L.-M.; Shen, M.-Y.; Wen, P.-W.; Hong, Y.-Z.; Liu, X.; Xie, J.-H. Preparation, characterization, antioxidant activity and protective effect against cellular oxidative stress of phosphorylated polysaccharide from Cyclocarya paliurus. Food Chem. Toxicol. 2020, 145, 111754. [Google Scholar] [CrossRef]
- Deng, C.; Fu, H.-T.; Xu, J.-J.; Shang, J.-Y.; Cheng, Y.-M. Physiochemical and biological properties of phosphorylated polysaccharides from Dictyophora indusiate. Int. J. Biol. Macromol. 2015, 72, 894–899. [Google Scholar] [CrossRef]
- Song, Y.; Ni, Y.-Y.; Hu, X.-S.; Li, Q.-H. Effect of phosphorylation on antioxidant activities of pumpkin (Cucurbita pepo, Lady godiva) polysaccharide. Int. J. Biol. Macromol. 2015, 81, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Xu, L.; Wu, Q.; Wang, Q.; Kong, W.; Liang, J.; Yao, J.; Zhang, J. Synthesis and structural features of phosphorylated Artemisia sphaerocephala polysaccharide. Carbohydr. Polym. 2018, 181, 19–26. [Google Scholar] [CrossRef]
- Ming, K.; Chen, Y.; Shi, J.-T.; Yang, J.-J.; Yao, F.-K.; Du, H.-G.; Zhang, W.; Bai, J.-Y.; Liu, J.-G.; Wang, D.-Y.; et al. Effects of chrysanthemum indicum polysaccharide and its phosphate on anti-duck hepatitis a virus and alleviating hepatic injury. Int. J. Biol. Macromol. 2017, 102, 813–821. [Google Scholar] [CrossRef]
- Feng, H.-B.; Fan, J.; Yang, S.-P.; Zhao, X.-L.; Yi, X. Antiviral activity of phosphorylated radix Cyathulae officinalis polysaccharide against canine parvovirus in vitro. Int. J. Biol. Macromol. 2017, 99, 511–518. [Google Scholar] [CrossRef]
- Feng, H.; Mcdonough, S.; Fan, J.; Yang, S.; Zhao, X.; Lu, Y.; Gan, Y.; Yi, X.; Chang, Y.-F. Phosphorylated radix Cyathulae officinalis polysaccharides act as adjuvant via promoting dendritic cell maturation. Molecules 2017, 22, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, H.-B.; Fan, J.; Lin, L.; Liu, Y.-J.; Chai, D.-K.; Yang, J. Immunomodulatory effects of phosphorylated radix Cyathulae officinalis polysaccharides in immunosuppressed mice. Molecules 2019, 24, 4150. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Huang, G. Antioxidant activities of phosphorylated pumpkin polysaccharide. Int. J. Biol. Macromol. 2019, 125, 256–261. [Google Scholar] [CrossRef]
- Chakka, V.P.; Zhou, T. Carboxymethylation of polysaccharides: Synthesis and bioactivities. Int. J. Biol. Macromol. 2020, 165, 2425–2431. [Google Scholar] [CrossRef]
- Duan, S.-Y.; Zhao, M.-M.; Wu, B.-Y.; Wang, S.-J.; Yang, Y.; Xu, Y.-Q.; Wang, L.-B. Preparation, characteristics, and antioxidant activities of carboxymethylated polysaccharides from blackcurrant fruits. Int. J. Biol. Macromol. 2019, 155, 1114–1122. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Xie, J.-H.; Shen, M.-Y.; Tang, W.; Wang, H.; Nie, S.-P.; Xie, M.-Y. Carboxymethylation of polysaccharide from Cyclocarya paliurus and their characterization and antioxidant properties evaluation. Carbohydr. Polym. 2016, 136, 988–994. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G.-L. Extraction, derivatization and antioxidant activity of bitter gourd polysaccharide. Int. J. Biol. Macromol. 2019, 141, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, G. The antioxidant activities of carboxymethylated garlic polysaccharide and its derivatives. Int. J. Biol. Macromol. 2019, 140, 1054–1063. [Google Scholar] [CrossRef]
- Zhao, T.; Guo, Y.-C.; Yan, S.-Y.; Li, N.; Ji, H.-C.; Hu, Q.-H.; Zhang, M.; Li, Q.; Gao, H.; Yang, L.-Q.; et al. Preparation, structure characterization of carboxymethylated schisandra polysaccharides and their intervention in immunotoxicity to polychlorinated biphenyls. Process Biochem. 2022, 115, 30–41. [Google Scholar] [CrossRef]
- Li, J.-J.; Hu, X.-Z.; Li, X.-P.; Ma, Z. Effects of acetylation on the emulsifying properties of Artemisia sphaerocephala Krasch. Polysaccharide. Carbohydr. Polym. 2016, 144, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-H.; Zhang, F.; Wang, Z.-J.; Shen, M.-Y.; Nie, S.-P.; Xie, M.-Y. Preparation, characterization and antioxidant activities of acetylated polysaccharides from Cyclocarya paliurus leaves. Carbohydr. Polym. 2015, 133, 596–604. [Google Scholar] [CrossRef]
- Liu, X.; Xie, J.-H.; Jia, S.; Huang, L.-X.; Wang, Z.-J.; Li, C.; Xie, M.-Y. Immunomodulatory effects of an acetylated Cyclocarya paliurus polysaccharide on murine macrophages RAW264.7. Int. J. Biol. Macromol. 2017, 98, 576–581. [Google Scholar] [CrossRef]
- Meng, Z.; Yi, H.; Li, J.-E.; Qi, A.; Ye, X.-M.; Xiang, L.; Zhao, Z.-T.; Yang, Z.; Jing, H.; Deng, Q.-H.; et al. Structural characterization and antioxidant activity of an acetylated Cyclocarya paliurus polysaccharide (Ac-CPP0.1). Int. J. Biol. Macromol. 2021, 171, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, G.-L. Preparation, characterization and antioxidant activity of acetylated garlic polysaccharide, and garlic polysaccharide-Zn (II) complex. J. Appl. Polym. Sci. 2021, 138, e51303. [Google Scholar] [CrossRef]
- Huang, Z.; Zong, M.-H.; Lou, W.-Y. Effect of acetylation modification on the emulsifying and antioxidant properties of polysaccharide from Millettia speciosa Champ. Food Hydrocoll. 2021, 124, 107217. [Google Scholar] [CrossRef]
- Ai, C.; Meng, H.-C.; Lin, J.W.; Tang, X.-Y.; Guo, X.-M. Emulsification properties of alkaline soluble polysaccharide from sugar beet pulp: Effect of acetylation and methoxylation. Food Hydrocoll. 2021, 124, 107361. [Google Scholar] [CrossRef]
- Xia, S.-L.; Zhai, Y.-C.; Wang, X.; Fan, Q.-R.; Dong, X.-Y.; Chen, M.; Han, T. Phosphorylation of polysaccharides: A review on the synthesis and bioactivities. Int. J. Biol. Macromol. 2021, 184, 946–954. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Xie, J.-H.; Shen, M.-Y.; Nie, S.-P.; Xie, M.-Y. Sulfated modification of polysaccharides: Synthesis, characterization and bioactivities. Trends Food Sci. Tech. 2018, 74, 147–157. [Google Scholar] [CrossRef]
- Zou, P.; Lu, X.-L.; Jing, C.-L.; Yuan, Y.; Lu, Y.; Zhang, C.-S.; Meng, L.; Zhao, H.-T.; Li, Y.-Q. Low molecular weightt polysaccharides from pyropia yezoensis enhance tolerance of wheat seedlings (Triticum aestivum L.) to salt Stress. Front. Plant Sci. 2018, 9, 427. [Google Scholar] [CrossRef] [Green Version]
- King, J.T.; Desai, U.R. A capillary electrophoretic method for fingerprinting low molecular weight heparins. Anal. Biochem. 2008, 380, 229–234. [Google Scholar] [CrossRef]
- Xiang, Z.-Y.; Tang, A.-G.; Ren, Y.-P.; Zhou, Q.-X.; Luo, X. Simultaneous determination of serum tryptophan metabolites in patients with systemic lupus erythematosus by high performance liquid chromatography with fluorescence detection. Clin. Chem. Lab. Med. 2010, 48, 513–517. [Google Scholar] [CrossRef]
- Quinlan, E.P.; Hanson, A.D.; Gregory, J.F. The analysis of folate and its metabolic precursors in biological samples. Anal. Biochem. 2006, 348, 163–184. [Google Scholar] [CrossRef]
- Han, S.-Y.; Li, P.-P. Progress of research in antitumor mchanisms with chinese medicine. Chin. J. Integr. Med. 2009, 15, 316–320. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, X.-K.; Liao, W.-F.; Fang, J.-P.; Chen, X.; Dong, Q.; Ding, K. A heteropolysaccharide, l-fuco-d-manno-1,6-α-d-galactan extracted from Grifola frondosa and antiangiogenic activity of its sulfated derivative. Carbohydr. Polym. 2014, 101, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Maas, N.C.; Gracher, A.H.P.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M.; Cipriani, T.R. Sulfation pattern of citrus pectin and its carboxy-reduced derivatives: Influence on anticoagulant and antithrombotic effects. Carbohydr. Polym. 2012, 89, 1081–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, I.Y.; Joe, Y.N.; Rha, H.J.; Lee, S.; Yoo, S.H.; Lee, H.G. Effect of sulfation on the physicochemical and biological properties of citrus pectins. Food Hydrocoll. 2009, 23, 1980–1983. [Google Scholar] [CrossRef]
- Wu, Z.; Ming, J.; Gao, R.-P.; Wang, Y.-X.; Liang, Q.; Yu, H.-G.; Zhao, G.-H. Characterization and antioxidant activity of the complex of tea polyphenols and oat β-glucan. J. Agric. Food Chem. 2011, 59, 10737–10746. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-G.; Xu, S.-S.; Ding, X.-D.; Yue, D.-D.; Bian, J.; Zhang, X.; Zhang, G.-L.; Gao, P.-Y. Structural characteristics of Medicago sativa L. polysaccharides and se-modified polysaccharides as well as their antioxidant and neuroprotective activities. Int. J. Biol. Macromol. 2019, 147, 1099–1106. [Google Scholar] [CrossRef]
- Li, J.; Shen, B.-X.; Nie, S.-L.; Duan, Z.-H.; Chen, K.-S. A combination of selenium and polysaccharides: Promising therapeutic potential. Carbohydr. Polym. 2018, 206, 163–173. [Google Scholar] [CrossRef]
- Chaiwong, N.; Leelapornpisid, P.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Sakdatorn, V.; Leksawasdi, N.; Phimolsiripol, Y. Antioxidant and moisturizing properties of carboxymethyl chitosan with different molecular weights. Polymers 2020, 12, 1445. [Google Scholar] [CrossRef]
- Song, J.-Y.; Chen, H.; Wei, Y.-F.; Liu, J. Synthesis of carboxymethylated β-glucan from naked barley bran and its antibacterial activity and mechanism against Staphylococcus aureus. Carbohydr. Polym. 2020, 242, 116418. [Google Scholar] [CrossRef]
- Hassabo, A.G.; Mohamed, A.L. Enhancement the thermos-regulating property of cellulosic fabric using encapsulated paraffins in modified pectin. Carbohydr. Polym. 2017, 165, 421–428. [Google Scholar] [CrossRef]
- Porfyris, A.; Papaspyrides, C.D.; Behabtu, N.; Lenges, C.; Kopatsis, A. High-Solids, Solvent-Free Modification of Engineered Polysaccharides. Molecules 2021, 26, 4058. [Google Scholar] [CrossRef] [PubMed]





| Source | Extraction Method | B-Mw (kda) | A-Mw (kda) | Modification Method | Main Modifying Conditions | Content | B-CHO (%) | A-CHO (%) | Biological Activity | Refer |
|---|---|---|---|---|---|---|---|---|---|---|
| Ulmus pumila L (PPU) | Hot-water extraction | 2.697 × 109 | 3.977–6.528 × 109 | Nitric acid/sodium selenite method | PPU (1 g) HNO3 (50 mL, 5%) Na2SeO3 (200, 400, 600, 800, 1000 mg) Reaction at room temperature for 24 h | 3.24–13.19 mg/g | 88.87 | N | Antioxidant | [57] |
| Artemisia sphaerocephala (PAS) | Hot-water extraction | 69.6 | 2.5–58.6 | Glacial acetic acid-selenous acid method | PAS (300 mg) DMSO (30 mL) H2SeO3 (40 mL) Reaction at (50, 70, 90 °C) for (4, 6) h | 139–8744 μg/g | N | N | Immunomodulatory | [59] |
| Sagittaria sagittifolia L. (PSSP) | Subcritical extraction | 47.12 | 16.82 | Nitric acid/sodium selenite method | PSSP (500 mg) HNO3 (50 mL, 0.6%) BaCl2 (5 mL, 0.1 M) Na2SeO3 (0.5 g) Reaction at 75 °C for 8 h | 2.89 μg/g | 77.67 | 82.26 | Antioxidant Immunomodulatory | [60] |
| Artemisia sphaerocephala (ASP) | Microwave-assisted method | 73.5 | 11.4–331.5 | Selenium oxychloride method | ASP (500 mg) Formamide (20 mL) NaHSeO3 (1 g) SOCl2 (10 mL) Reaction at 60 °C for 10–60 min | 264- 22400 μg/g | N | N | Antitumor | [61] |
| Chinese angelica (CAP) | Hot-water extraction | N | N | Nitric acid/sodium selenite method | CAP (500 mg) HNO3 (50 mL, 5%) Na2SeO3 (200, 300, 400 mg) Reaction temperature (50, 70, 90 °C) Reaction time (6, 8, 10 h) | 6.41–12.98 mg/g | 92.7 | 23.5–63.2 | Immunomodulatory | [62] |
| Lycium barbarum (LBP) | Hot-water extraction | N | N | Nitric acid/sodium selenite method | LBP (500 mg) Na2SeO3 (200, 300, 400 mg) Reaction temperature (50, 70, 90 °C) Reaction time (6, 8, 10 h) | 7.65–13.66 mg/g | 87.1 | 19.2–44.6 | Antioxidant | [63] |
| Garlic (GPS) | Hot-water extraction | N | N | Nitric acid/sodium selenite method | GPS (500 mg) Na2SeO3 (200,300, 400 mg) Reaction temperature (50, 70, 90 °C) Reaction time (6, 8, 10 h) | 6.21–12.49 mg/g | 94.5 | 21.2–56.9 | Immunomodulatory | [64] |
| Codonopsis pilosula pectic (CPP1b) | Hot-water extraction | 148 | 195 | Nitric acid/sodium selenite method | CPP1b (100 mg) HNO3 (20 mL) Ratio of CPP1b to Na2SeO3 (2:1, 2:1.6, 2:2) Reaction temperature (60, 70, 80 °C) Reaction time (5, 7, 9 h) | 94.06–478 μg/g | N | N | Antitumor | [65] |
| Atractylodes macrocephala (AMP) | Hot-water extraction | N | N | Nitric acid/sodium selenite method | AMP (500 mg) Na2SeO3 (200,300, 400 mg) Reaction temperature (50, 70, 90 °C) Reaction time (6, 8, 10 h) | 6.12–12.23 mg/g | 84 | 36.1–62.99 | Immunomodulatory | [66] |
| Radix hedysari (RHP) | N | N | 27.7–62.7 | Nitric acid/sodium selenite method | RHP (400 mg) HNO3 (40 mL, 0.6%) Reaction at 65°C for (3, 5, 10, 15 h) | 1.04–3.29 mg/g | N | N | Antioxidant | [67] |
| Artemisia sphaerocephala (ASP) | Microwave-assisted extraction | 73.48 | 17.36- 46.67 | Nitric acid/sodium selenite method | ASP (500 mg) HNO3 (50 mL, 0.8%) H2SeO3 (1.0 g) BaCl2 (1.65 g) Reaction at 60 °C for (15–480) min by 300W ultrasonic powers | 111–264 μg/g | 90.2 | 69.8–86.8 | Antitumor | [68] |
| Lily (LP) | Hot-water extraction | N | N | Nitric acid/sodium selenite method | LP (500 mg) HNO3 (50 mL, 0.5%) Na2SeO3 (200,300, 400 mg) Reaction temperature (50, 70, 90 °C) Reaction time (6, 8, 10 h) | 11.8–39.2 mg/g | N | 56.31–77.14 | Immunomodulatory | [69] |
| Artemisia sphaerocephala (ASP) | Microwave-assisted extraction | 73.48 | 11.41–54.07 | Nitric acid/sodium selenite method | ASP (500 mg) HNO3 (50 mL, 0.4, 0,7, 1.2%) BaCl2 (1.65 g) Na2SeO3 (1000 mg) Reaction temperature (60, 70, 80 °C) Reaction time (6 h) | 168–1703 μg/g | 90.2 | N | Antitumor | [70] |
| Glycyrrhiza uralensis (GUP) | Hot-water extraction | 38.032 | 5.8 | Nitric acid/sodium selenite method | GUP (500 mg) HNO3 (50 mL, 0.5%) Na2SeO3 (200 mg) Reaction at 70 °C for 8 h | 1.34 mg/g | N | N | Antioxidant | [71] |
| Tea (TPS) | Hot-water extraction (pretreatment degreasing) | N | N | Nitric acid/sodium selenite method | TPS (500 mg) HNO3 (50 mL) Na2SeO3 (0.5 g) BaCl2 (0.1 mol/L) Reaction at 75 °C for 8 h | 2.12 mg/kg | 62.23 | 60.26 | Hypoglycemic activity | [72] |
| Alfalfa (RAPS) | Hot-water extraction (pretreatment degreasing) | 15.8 | 11.0 | Nitric acid/sodium selenite method | RAPS 50 mg HNO3 (10 mL,0.6%) Na2SeO3 (50 mg) Reaction at 70 °C for 10 h | 320 μg/g | 97.1 | N | Antioxidant Antitumor | [73] |
| Momordica charantia L. (MCPIIa) | Hot-water extraction | 13 | 40.038 | Nitric acid/sodium selenite method | MCPIIa (10 mg) Na2SeO3 solutions (2.5–10 mL, 0.05 M) Ascorbic acid solution (8 mL, 0.1 M). Reaction at 28 °C for 12 h | 445.0 μg/g | 93.99 | 92.12 | Hypoglycemic activity | [74] |
| Codonopsis pilosula (CPPS) | Hot-water extraction | 345 | 230.6 | Nitric acid/sodium selenite method | The ratio of sodium selenite to CPPS was 0.6:1 Reaction at 70°C for 8 h | 11.86 mg/g | 98.86 | N | Immunomodulatory | [75] |
| Astragalus (APS) | N | 12.314 | 10.042 | Nitric acid/sodium selenite method | APS (500 mg) HNO3 (50 mL, 5%) BaCl2 (1 g) Na2SeO3 (400 mg) Reaction at 70 °C for 6 h | 1.75 mg/g | N | N | Antioxidant | [76] |
| Ribes nigrum L. (RCP) | Ultrasonic-assisted extraction | 20.4 | 9.09–12.9 | Nitric acid/sodium selenite method | RCP (500 mg) BaCl2 (2.5 g) HNO3 (250 mL, 0.5 %) Na2SeO3 (500 mg) Reaction at (50, 80) °C for (3, 5, 7) h by (500, 800) W ultrasonic powers | 70–480 μg /g | 85.5 | 82.12–83.58 | Hypoglycemic activity | [77] |
| Dandelion roots (DRP) | Ultrasonic-assisted extraction | 8.7 | 5.6–7.9 | Nitric acid/sodium selenite method | DRP (500 mg) HNO3 (50 mL, 0.5%) BaCl2 (700 mg) Na2SeO3 (500 mg) Reaction at 40 °C for 4 h Reaction at 60 °C for 8 h | 170–710 μg/g | 94.24 | 96.31–96.72 | Immunomodulatory Antioxidant | [78] |
| Yam (YPS) | Hot-water extraction | N | N | Nitric acid/sodium selenite method | YPS (500 mg) HNO3 (8 mL, 0.5%) Na2SeO3 (25–50 mg) Reaction at 75 °C for 8 h | 715–1545 mg /kg | N | N | Immunomodulatory | [79] |
| Purslane (PSPO) | Hot-water extraction | N | N | Nitric acid/sodium selenite method | PSPO (300 mg) HNO3 (20 mL, 5%) Na2SeO3 (45 mg) Reaction at 75 °C for 8 h | 753–1325 mg/kg | N | N | Immunomodulatory | [80] |
| Garlic (GPS) | Hot-water extraction | N | N | Nitric acid/sodium selenite method | GPS (500 mg) HNO3 (0.5%,50 mL) Na2SeO3 (400 mg) Reaction at 70 °C for 6 h | 10.5–38.3 mg/kg | 95.26 | 31–54.8 | Antioxidant Immunomodulatory | [81] |
| Rose laevigata Michx fruit (PPRLMF-2) | Hot-water extraction | 137.1 | 82.158 | Nitric acid/sodium selenite method | PPRLMF-2 (500 mg) HNO3 (0.5%) Na2SeO3 (400 mg) Reaction at 70 °C for 8 h | 862 μg/g | N | 94.2 | Immunomodulatory | [82] |
| Source | Extraction Method | B-Mw (kda) | A-Mw (kda) | Modification Method | Main Modifying Conditions | DS | B-CHO (%) | A-CHO (%) | Biological Activity | Refer |
|---|---|---|---|---|---|---|---|---|---|---|
| Amana edulis (AEPs) | Acidic extraction Hot-water extraction | N | N | Phosphate method | CP (500 mg) Sodium tripolyphosphate 8.57 g Sodium trimetaphosphate 1.43 g Double-distilled water 100 mL 5% of sodium sulfate Reaction at 90 °C for 4 h | 0.42–0.54 | 56.53–65.61 | 53.35–63.17 | Antioxidant | [23] |
| Orchis chusua D. Don (SP) | Hot-water extraction | 369 | 355 | Phosphate method | SP (200 mg) Sodium tripolyphosphate (1.74 g) Sodium trimetaphosphate (0.286 g) Double-distilled water (20 mL) Sodium sulfate (1 g) Reaction at 90 °C for 2 h | 0.32 | 47.93 | 63.86 | Antioxidant Probiotic ability | [53] |
| Cyclocarya paliurus (CP) | Hot-water extraction | 139 | 155 | Phosphate method | CP (500 mg) Sodium tripolyphosphate (5.58 g) Sodium trimetaphosphate (2.23 g) Double-distilled water (100 mL) 5% of sodium sulfate Reaction at 65 °C for 5 h | 0.14 | 55.13 | 43.58 | Antioxidant | [83] |
| Dictyophora indusiata (DIP) | Acidic extraction (pretreatment degreasing) | N | 65 | Phosphoric acid method | DIP (4 g) 200 mL of DMSO containing 8 M urea Phosphoric acid (40 mL) Reaction at 100 °C for 6 h | 0.206 | N | N | Antioxidant Antitumor | [84] |
| Pumpkin (N) | Hot-water extraction | 10.1 | 9–17 | Phosphorus oxychloride method | Polysaccharides (500 mg) Pyridine (30 mL) N, N-Dimethylformamide (20 mL) POCl3 (4 mL) Reaction at 60 °C for 3 h | 0.33–0.52 | 97.42 | 44.7–65.38 | Antioxidant | [85] |
| Artemisia sphaerocephala (ASP) | Microwave-assisted extraction | 73.48 | 65.85–137.7 | Phosphorus oxychloride method | ASP (500 mg) N, N-Dimethylformamide (20 mL) Pyridine (10 mL) Time (1–6 h) Temperature (0–60 °C) | 034–0.54 | 90.2 | N | n | [86] |
| Chrysanthemum indicum (CIPS) | Hot-water extraction | N | N | Phosphate method | Ratio of sodium trimetaphosphate to sodium tripolyphosphate (5:2) Time (8 h) Temperature (70 °C) | 0.317 | N | N | Antiviral | [87] |
| Radix Cyathulae officinalis Kuan (RCPS) | Hot-water extraction | N | N | Phosphate method | Ratio of sodium trimetaphosphate to sodium tripolyphosphate (1:2, 1:4, 1:8) Time (2, 4, 8 h) Temperature (65, 80, 95 °C) | 0.31–0.77 | 96.6 | 78.9–96.6 | Antiviral Immunomodulatory | [88,89,90] |
| Pumpkin (N) | Hot-water extraction | N | N | Phosphorus oxychloride method | Polysaccharides (500 mg) Pyridine (7.5 mL) Chlorosulfonic acid (1 mL) N, N-Dimethylformamide (7.5 mL) Reaction at 80 °C for 3 h | 0.01–0.02 | 81 | 69–75 | Antioxidant | [91] |
| Source | Extraction Method | B-Mw (kda) | A-Mw (kda) | Modification Method | Main Modifying Conditions | DS | B-CHO (%) | A-CHO (%) | Biological Activity | Refer |
|---|---|---|---|---|---|---|---|---|---|---|
| Amana edulis (AEPs) | Acidic extraction Hot water extraction | N | N | Solvent method | AEPs (500 mg) Isopropanol (12.5 mL) React at 25 °C for 3 h followed by reaction at 60 °C which lasted for 1.5 h | 0.605–0.783 | 56.53–65.61 | 52.22–60.45 | Antioxidant | [23] |
| Cucumber (N) | Hot-water extraction | N | N | Water media method | Polysaccharide (500 mg) NaOH (6.5 g, 20%) Chloroacetic acid (4 g, 30%) Reaction at 70 °C for 4 h | 0.18 | 20.6 | 44.07 | Antioxidant | [45] |
| Orchis chusua D. Don (SP) | Hot-water extraction | 369 | 68 | Water media method | SP (200 mg) NaOH (15.2 mL, 20%) Chloroacetic acid (6.2 mL, 30%) Reaction at 70 °C for 2 h | 0.13 | 47.93 | 54.71 | Antioxidant | [53] |
| Cyclocarya paliurus (CP) | Hot-water extraction | 139.6 | 175.4 | Solvent method | CP (300 mg) Isopropanol (20 mL) Carboxymethylation reagent (10 mL, 20% NaOH, 3 g chloroacetic acid, and 20 mL isopropanol) Reaction at 55 °C for 5 h | 0.29 | N | N | Antioxidant Antitumor | [54] |
| Blackcurrant fruits (RNP) | Ultrasonic-assisted enzymatic extraction | 8.093 | 11.036–12.548 | Solvent method | RNP (100 mg) Isopropanol (5 mL) NaOH (2.0 mL, 20%) The resulting solution was heated at different temperatures (60–100 °C) at various ratios of MCA to polysaccharide (8:1–30:1) for various periods (20–60 min) | 0.44–1.1 | 51.95 | 47.61–52.47 | Antioxidant Anti-lipid peroxidation Anti-hemolytic | [93] |
| Cyclocarya paliurus (CP) | Hot-water extraction | N | 1.03–1.08 × 103 | Water media method | CP (500 mg) NaOH (38 mL, 20%) Chloroacetic acid (1, 2, 3 g) Reaction at 55 °C for 5 h | 0.025– 0.193 | 60.62 | 55.13–58.16 | Antioxidant | [94] |
| Bitter gourd (P) | Hot-water extraction | N | N | Water media method | P (1000 mg) NaOH (85 mL, 20%) Chloroacetic acid (15 mL, 4 mol/L) react at 55 °C for 5 h | 0.89 | 74 | 43.5 | Antioxidant | [95] |
| Garlic (P) | Hot-water extraction (Pretreatment degreasing) | N | N | Water media method | P (800 mg) NaOH (12.5 g, 20%) Chloroacetic acid (7.5 g, 30%) Reaction at 70 °C for 4 h | 0.92 | 76.67 | 60.27 | Antioxidant | [96] |
| Schisandra (SP) | Hot-water extraction | 143 | N | Solvent method | SP (500 mg) Isopropanol (20 mL) Chloroacetic acid (0.83 g) Reaction at 62.67 °C for 4.27 h | 0.88 | 82.5 | 89.5 | Immunomodulatory | [97] |
| Source | Extraction Method | B-Mw (kda) | A-Mw (kda) | Modification Method | Main Modifying Conditions | DS | B-CHO (%) | A-CHO (%) | Biological Activity | Refer |
|---|---|---|---|---|---|---|---|---|---|---|
| Orchis chusua D. Don (Salep) (SP) | Hot-water extraction | 369 | 331 | Acetic anhydride method | SP (200 mg) pH 9.0 Acetic anhydride (3 mL) pH of the reaction was maintained at 8.0–8.5 for 30 min at 60 °C | 0.56 | 58 | 47.93 | Probiotic ability | [53] |
| Bitter gourd (P) | Hot-water extraction | N | N | Acetic anhydride method | P (500 mg) pH 9.5 Acetic anhydride (0.6 mL) reacted at room temperature for 1 h | 0.27 | 74 | 62.3 | Antioxidant | [95] |
| Cyclocarya paliurus (CP) | Hot-water extraction | 900 | 1.05 × 103 | Acetic anhydride method | CP (500 mg) Acetic anhydride (1 mL) pH 8.0–8.5 | 0.13 | 60.62 | 64.89 | Immunomodulatory | [98] |
| Cyclocarya paliurus (CP) | Hot-water extraction | N | 1.05 × 103 1.08 × 103 1.09 × 103 | Acetic anhydride method | CP (500 mg) pH 9.0 Acetic anhydride (1, 4, 6 mL) pH of the reaction was maintained at 8.0–8.5 for 4 h | 0.13–0.57 | 60.62 | 64.89–66.91 | Antioxidant | [99] |
| Artemisia sphaerocephala Krasch. (ASKP) | Hot-water extraction | 525.9 | 321.7 446.8 799.0 1329 | Acetic anhydride method | ASKP (1000 mg) pH 8.0–8.5 Acetic anhydride (2.5 mL) Reaction at 25 °C for 2 h | 0.04–0.42 | N | N | Emulsifying | [100] |
| Cyclocarya paliurus (CCP) | Hot-water extraction (pretreatment degreasing) | 38.4 | 30.7 | Acetic anhydride method | CCP (200 mg) pH 8.0–8.5 Acetic anhydride (0.8 mL) Reaction at 40 °C for 2 h | 0.18 | 94.94 | 90.82 | Antioxidant | [101] |
| Garlic (PS) | Hot-water extraction (pretreatment degreasing) | N | N | Acetic anhydride method | PS (1500 mg) pH 11.0 Acetic anhydride (5 mL) pH of the reaction was maintained at 7–11 for 2.5 h at 30 °C | 0.5 | N | N | Antioxidant | [102] |
| Millettia speciosa Champ (MSCP) | Hot-water extraction | 15.6 | 9–18.8 | Acetic anhydride method | MSCP (300 mg) pH 8.0 Acetic anhydride (1, 3, 5 mL) pH of the reaction was maintained at 8.0–8.5 for 2 h | 0.1–0.56 | 80.25 | 70.43–76.21 | Emulsifying Antioxidant | [103] |
| Sugar beet pulp (ASP2) | Acidic extraction | 238 | 336 | Acetic anhydride method | ASP2 solution (1.5% w/w) pH 8.0 Acetic anhydride (1–6% w/w) pH of the reaction was maintained at 7.0–7.5 for 30 min | 0.86 | N | N | Emulsifying | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.-W.; Du, Z.-M.; Wang, Y.-W.; Feng, Y.-X.; Zhang, R.; Yan, X.-B. Chemical Modification, Characterization, and Activity Changes of Land Plant Polysaccharides: A Review. Polymers 2022, 14, 4161. https://doi.org/10.3390/polym14194161
Li Z-W, Du Z-M, Wang Y-W, Feng Y-X, Zhang R, Yan X-B. Chemical Modification, Characterization, and Activity Changes of Land Plant Polysaccharides: A Review. Polymers. 2022; 14(19):4161. https://doi.org/10.3390/polym14194161
Chicago/Turabian StyleLi, Zhi-Wei, Zhu-Mei Du, Ya-Wen Wang, Yu-Xi Feng, Ran Zhang, and Xue-Bing Yan. 2022. "Chemical Modification, Characterization, and Activity Changes of Land Plant Polysaccharides: A Review" Polymers 14, no. 19: 4161. https://doi.org/10.3390/polym14194161
APA StyleLi, Z.-W., Du, Z.-M., Wang, Y.-W., Feng, Y.-X., Zhang, R., & Yan, X.-B. (2022). Chemical Modification, Characterization, and Activity Changes of Land Plant Polysaccharides: A Review. Polymers, 14(19), 4161. https://doi.org/10.3390/polym14194161







