Comparative Thermo-Mechanical Properties of Sustainable Epoxy Polymer Networks Derived from Linseed Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Functionalization of the LO
2.3. Synthesis of the ELO-Based Polymeric Matrices
2.4. Characterization Techniques
3. Results and Discussion
3.1. ELO Synthesis and Structural Characterization
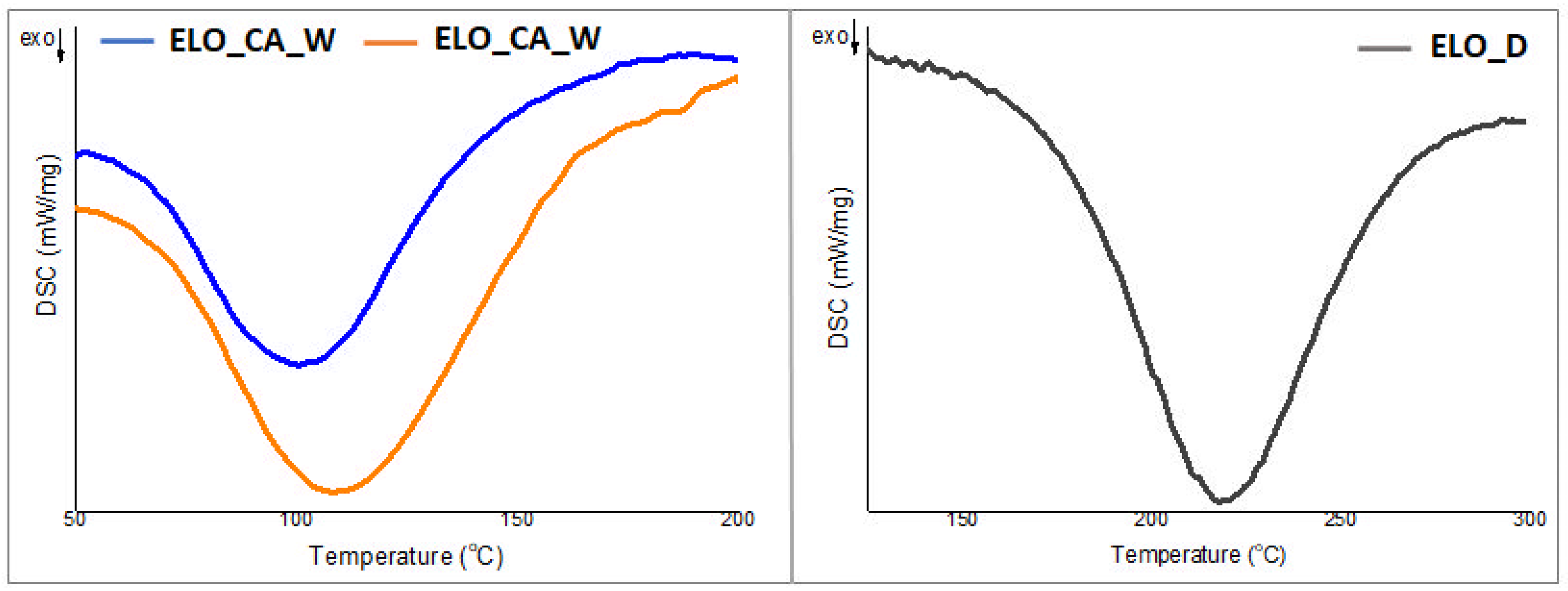
3.2. DSC Studies for the ELO-Based Initial Formulation
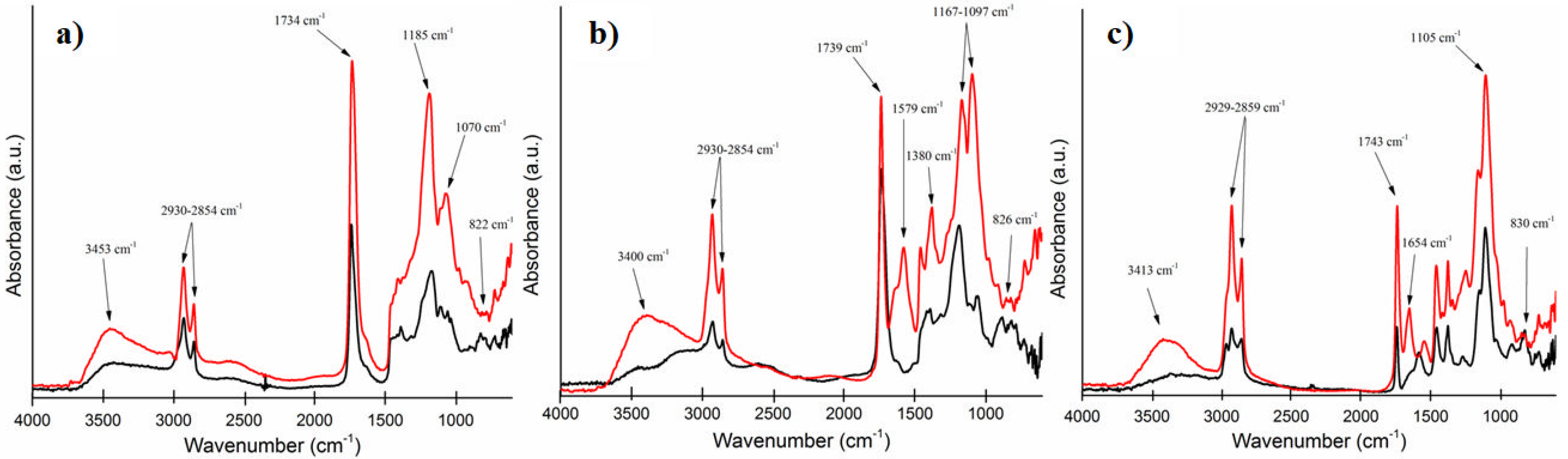
3.3. FTIR Spectrometry Studies
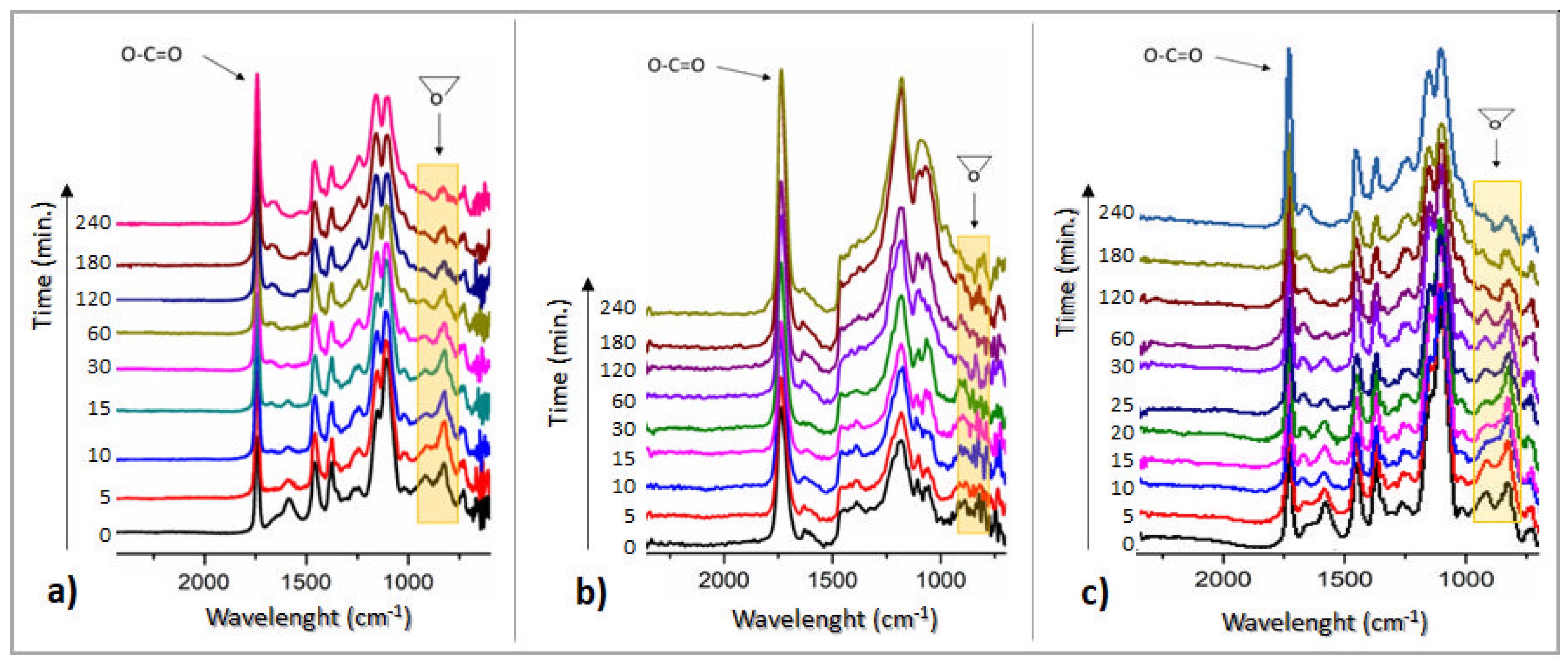
3.4. The Curing Kinetics for the Formulated ELO-Based Systems
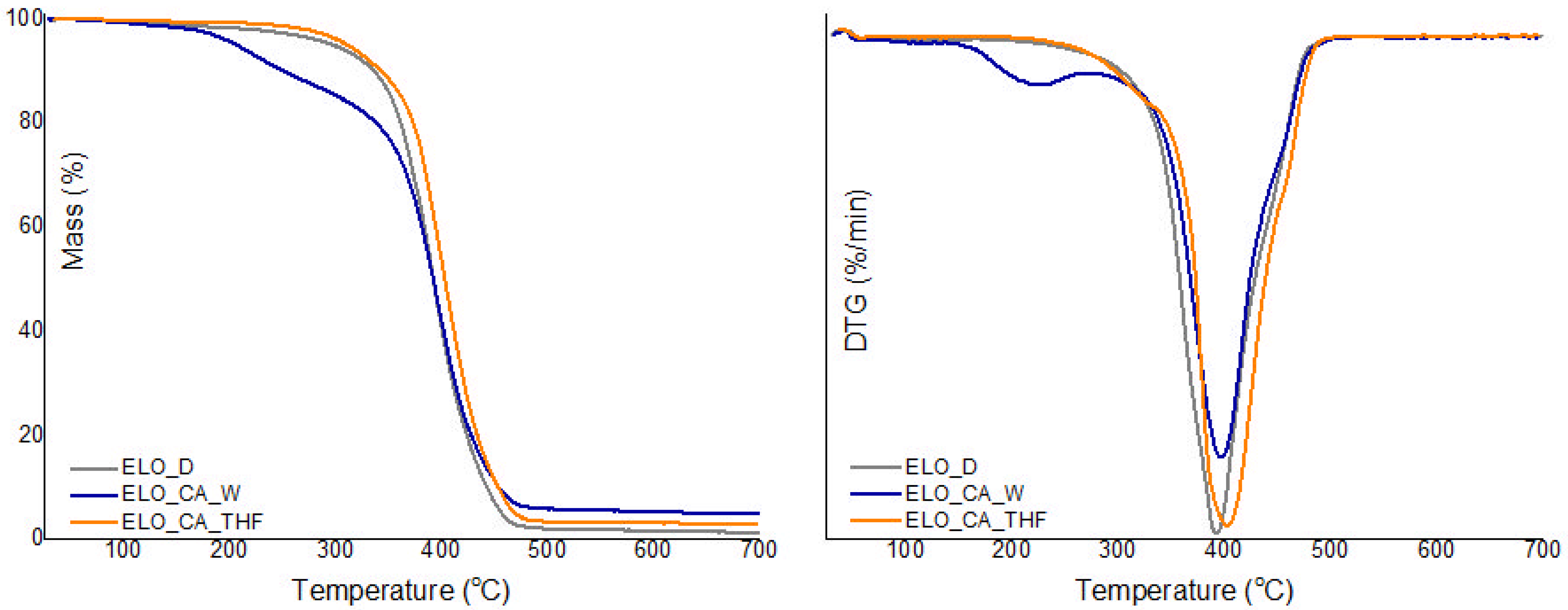
3.5. Thermal Stability
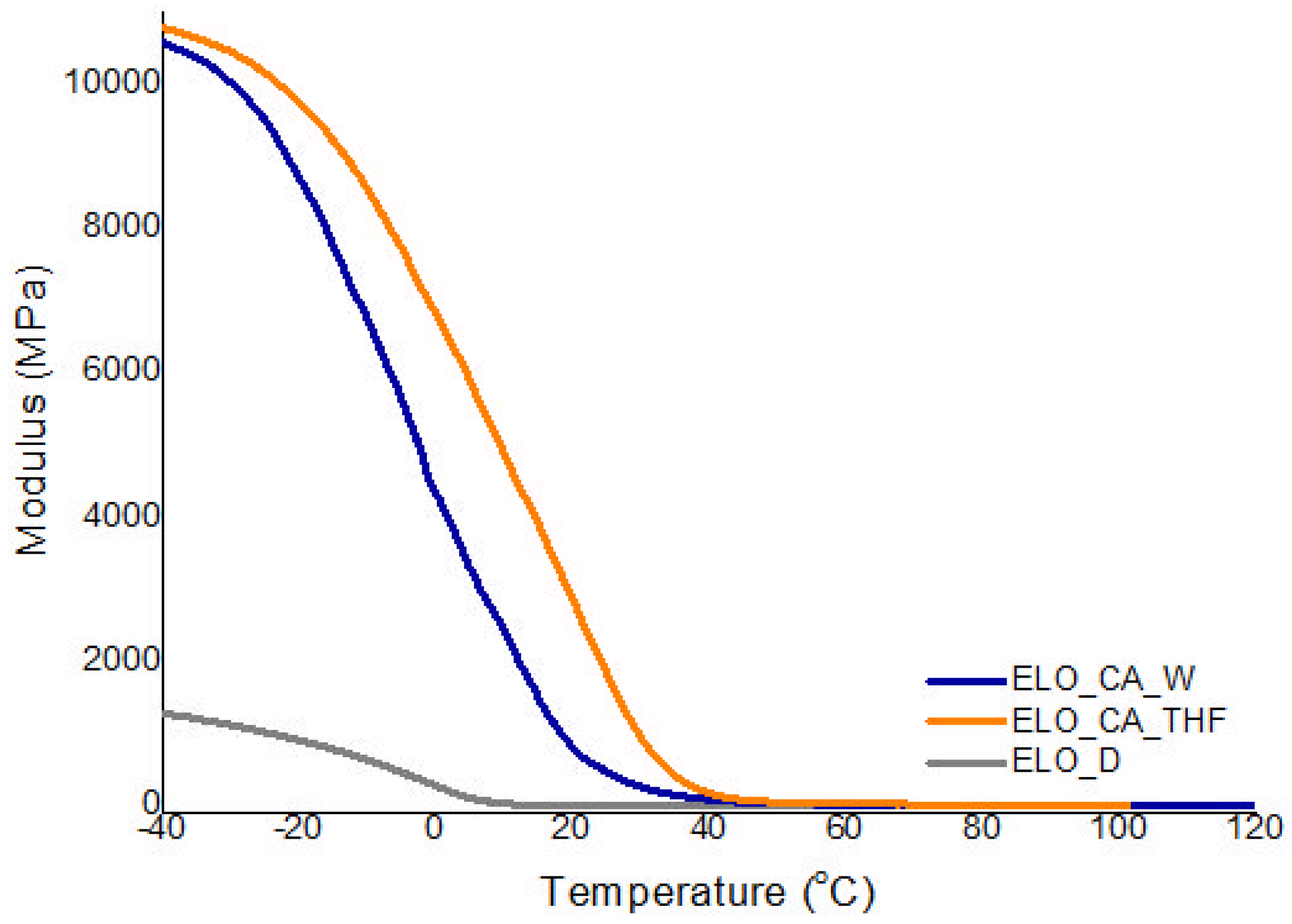
3.6. Dynamic-Mechanical Analysis
3.7. Nanoindentation
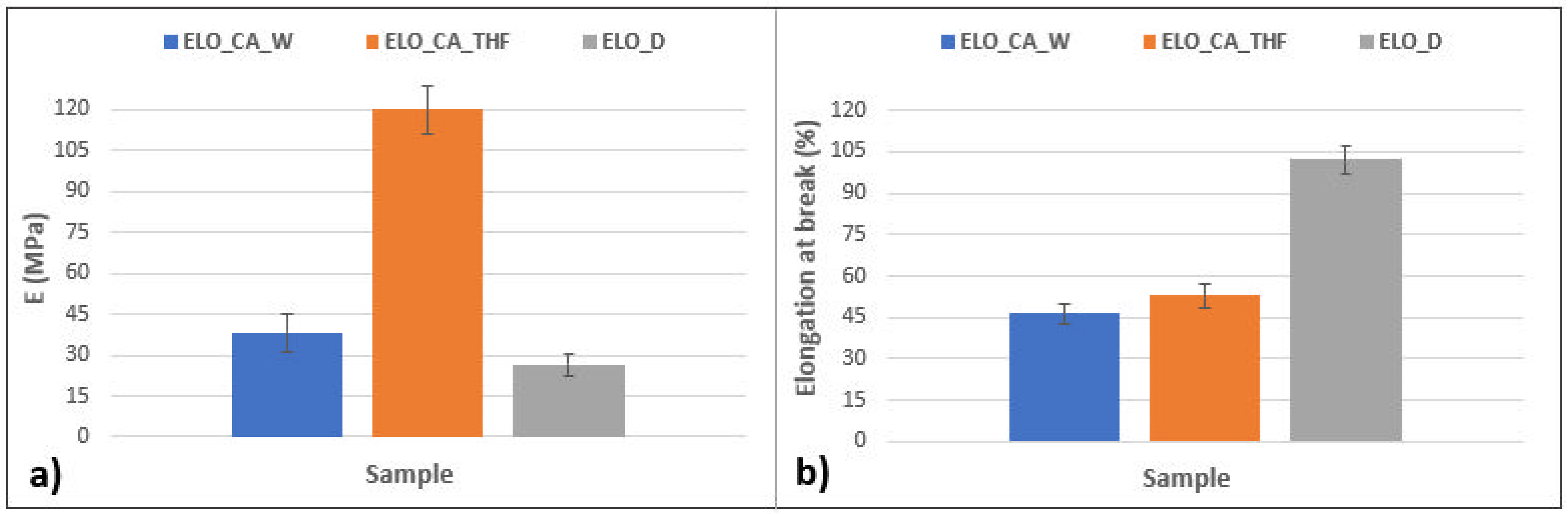
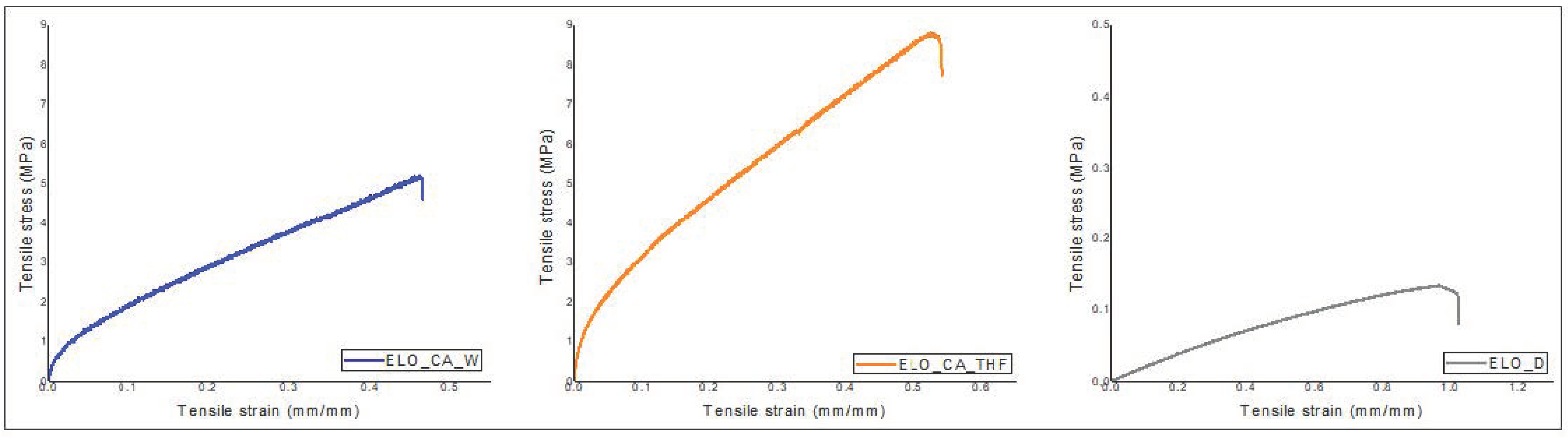
3.8. Mechanical Test
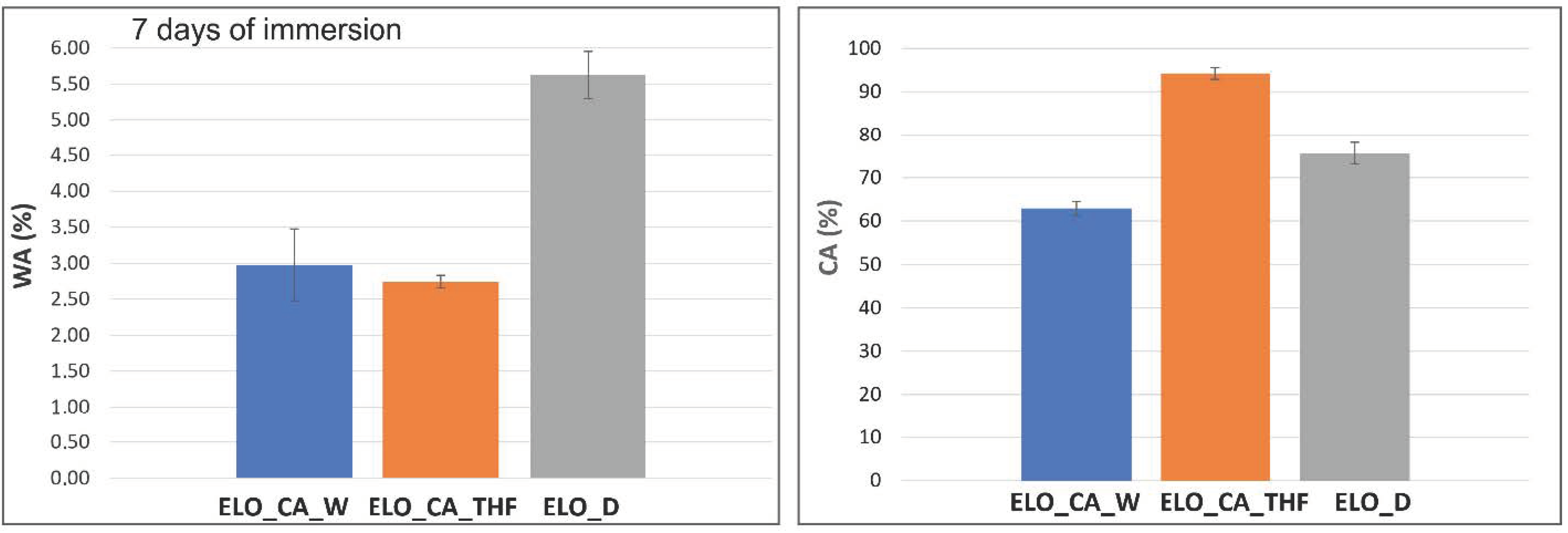
3.9. Degradation Studies
3.10. Wettability Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Manawwer, A.; Deewan, A.; Eram, S.; Fahmina, Z.; Sharif, A. Vegetable oil based eco-friendly coating materials: A review article. Arab. J. Chem. 2021, 7, 469–479. [Google Scholar] [CrossRef]
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater. 2014, 10, 1692–1704. [Google Scholar] [CrossRef]
- Vevere, L.; Fridrihsone, A.; Kirpluks, M.; Cabulis, U. A Review of Wood Biomass-Based Fatty Acids and Rosin Acids Use in Polymeric Materials. Polymers 2020, 12, 2706. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.Z. Epoxidized soybean oil as a potential source of high-temperature lubricants. Ind. Crops Prod. 2002, 15, 247–254. [Google Scholar] [CrossRef]
- Haryono, A.; Triwulandari, E.; Jiang, P. Interaction between vegetable oil based plasticizer molecules and polyvinyl chloride, and their plasticization effect. AIP Conf. Proc. 2017, 1803, 020045. [Google Scholar] [CrossRef]
- Jebrane, M.; Cai, S.; Sandström, C.; Terziev, N. The reactivity of linseed and soybean oil with different epoxidation degree towards vinyl acetate and impact of the resulting copolymer on the wood durability. Express Polym. Lett. 2017, 11, 383–395. [Google Scholar] [CrossRef]
- Sharma, B.K.; Adhvaryu, A.; Liu, Z.; Erhan, S.Z. Chemical modification of vegetable oils for lubricant applications. J. Am. Oil Chem. Soc. 2006, 83, 129–136. [Google Scholar] [CrossRef]
- Woma, T.Y.; Lawal, S.A.; Abdulrahman, A.S.; Olutoye, M.A.; Ojapah, M.M. Vegetable Oil Based Lubricants: Challenges and Prospects. J. Tribol 2019, 14, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Lewinska, A.; Zebrowski, J.; Duda, M.; Gorka, A.; Wnuk, M. Fatty Acid Profile and Biological Activities of Linseed and Rapeseed Oils. Molecules 2015, 20, 22872–22880. [Google Scholar] [CrossRef] [Green Version]
- Turco, R.; Tesser, R.; Russo, V.; Cogliano, T.; Di Serio, M.; Santacesaria, E. Epoxidation of Linseed Oil by Performic Acid Produced In Situ. Ind. Eng. Chem. Res. 2021, 60, 16607–16618. [Google Scholar]
- Conti Silva, J.A.; Moreira Grilo, L.; Gandini, A.; Martins Lacerda, T. The Prospering of Macromolecular Materials Based on Plant Oils within the Blooming Field of Polymers from Renewable Resources. Polymers 2021, 13, 1722. [Google Scholar] [CrossRef]
- Samper, M.; Ferri, J.; Carbonell, A.; Balart, R.; Gimeno, O. Properties of biobased epoxy resins from epoxidized linseed oil (ELO) crosslinked with a mixture of cyclic anhydride and maleinized linseed oil. Express Polym. Lett. 2019, 13, 407–418. [Google Scholar] [CrossRef]
- Balanuca, B.; Ghebaur, A.; Stan, R.; Vuluga, D.M.; Vasile, E.; Iovu, H. New hybrid materials based on double-functionalized linseed oil and halloysite. Polym. Adv. Technol. 2018, 29, 1744–1752. [Google Scholar] [CrossRef]
- Balanuca, B.; Stan, R.; Lungu, A.; Vasile, E.; Iovu, H. Hybrid networks based on epoxidized camelina oil. Des. Monomers Polym. 2017, 20, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Balanuca, B.; Stan, R.; Hanganu, A.; Iovu, H. Novel linseed oil-based monomers: Synthesis and characterization. UPB Sci. Bull. B Chem Mater. Sci. 2014, 76, 129–140. [Google Scholar]
- Balanuca, B.; Lungu, A.; Hanganu, A.; Stan, L.R.; Vasile, E.; Iovu, H. Hybrid nanocomposites based on POSS and networks of methacrylated camelina oil and various PEG derivatives. Eur. J. Lipid Sci. Technol. 2014, 116, 458–469. [Google Scholar] [CrossRef]
- Saithai, P.; Lecomte, J.; Dubreucq, E.; Tanrattanakul, V. Effects of different epoxidation methods of soybean oil on the characteristics of acrylated epoxidized soybean oil-co-poly(methyl methacrylate) copolymer. Express Polym. Lett. 2013, 7, 910–992. [Google Scholar] [CrossRef]
- Ma, S.; Webster, D.C. Naturally Occurring Acids as Cross-Linkers to Yield VOC-Free, High-Performance, Fully Bio-Based, Degradable Thermosets. Macromolecules 2015, 48, 7127–7137. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Khandelwal, V.; Manik, G. Development of completely bio-based epoxy networks derived from epoxidized linseed and castor oil cured with citric acid. Polym. Adv. Technol 2018, 29, 2080–2090. [Google Scholar] [CrossRef]
- Lascano Aimacaña, D.S.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Montanes, N. Kinetic Analysis of the Curing of a Partially Biobased Epoxy Resin Using Dynamic Differential Scanning Calorimetry. Polymers 2019, 11, 391. [Google Scholar] [CrossRef] [Green Version]
- Dinu, R.; Mija, A. Bio-based epoxy resins and composites from epoxidized linseed oil crosslinked with different cyclic anhydrides and their combination with lignin. Cellul. Chem. Technol. 2020, 59, 925–938. [Google Scholar] [CrossRef]
- Altuna, F.; Pettarin, V.; Williams, R.J.J. Self-healable polymer networks based on the cross-linking of epoxidised soybean oil by an aqueous citric acid solution. Green Chem. 2013, 15, 3360–3366. [Google Scholar] [CrossRef]
- Ding, C.; Shuttleworth, P.S.; Makin, S.; Clark, J.H.; Matharu, A.S. New insights into the curing of epoxidized linseed oil with dicarboxylic acids. Green Chem. 2015, 17, 4000–4008. [Google Scholar] [CrossRef]
- Corezzi, S.; Fioretto, D.; Santucci, G.; Kenny, J.M. Modeling diffusion-control in the cure kinetics of epoxy-amine thermoset resins: An approach based on configurational entropy. Polymer 2010, 51, 5833–5845. [Google Scholar] [CrossRef]
- Ahmadi, Z. Epoxy in nanotechnology: A short review. Prog. Org. Coat. 2019, 132, 445–448. [Google Scholar] [CrossRef]
- Richaud, E.; Guinault, A.; Baiz, S.; Nizeyimana, F. Epoxidized linseed oils based networks. Case of thermal degradation. Polym. Degrad. Stab. 2019, 166, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Campanella, A.; Zhan, M.; Watt, P.; Grous, A.; Shen, C.; Wool, R. Triglyceride-based Thermosetting Resins with Different Reactive Diluents and Fiber Reinforced Composite Applications. Compos. Part A Appl. Sci. 2015, 72, 192–199. [Google Scholar] [CrossRef]
- Herbert, E.G.; Oliver, W.C.; Pharr, G.M. Nanoindentation and the dynamic characterization of viscoelastic solids. J. Phys. D Appl. Phys. 2008, 41, 074021. [Google Scholar] [CrossRef]
- Olăreț, E.; Bălănucă, B.; Onaș, A.M.; Ghițman, J.; Iovu, H.; Stancu, I.-C.; Serafim, A. Double-Cross-Linked Networks Based on Methacryloyl Mucin. Polymers 2021, 13, 1706. [Google Scholar] [CrossRef]
- Olăreț, E.; Steinmüller-Nethl, D.; Iovu, H.; Stancu, I.C. Nanodiamond loaded fish gelatin enzymatically crosslinked hydrogels. U.P.B. Sci. Bull. Ser. B 2022, 84, 27–40. [Google Scholar]
- Abdus Samad, U.; Alam, A.; Chafidz, A.; Al-Zahrani, S.; Alharthy, N. Enhancing mechanical properties of epoxy/polyaniline coating with addition of ZnO nanoparticles: Nanoindentation characterization. Prog. Org. Coat. 2018, 119, 109–115. [Google Scholar] [CrossRef]
- Kalita, D.J.; Tarnavchyk, I.; Chisholm, B.J.; Webster, D.C. Novel bio-based epoxy resins from eugenol as an alternative to BPA epoxy and high throughput screening of the cured coatings. Polymer 2021, 223, 124191. [Google Scholar] [CrossRef]
- Stefanidis, D.; Jencks, W.P. General base catalysis of ester hydrolysis. J. Am. Chem. Soc. 1993, 115, 6045–6050. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Z.; Shi, J.; Chen, G.; Zhang, Q.; Weng, Z.; Wu, K.; Lu, M. Green synthesis of graphene with the assistance of modified lignin and its application in anticorrosive waterborne epoxy coatings. Appl. Surf. Sci. 2019, 484, 759–770. [Google Scholar] [CrossRef]


| Polymer Matrix | |||||
|---|---|---|---|---|---|
| System Code | Components | Curing Temperatures | |||
| ELO | CA | D230 | |||
| H2O | THF | ||||
| ELO_CA_W | x | x | - | - | 80 °C, 3 h 120 °C, 1 h 150 °C, 1 h |
| ELO_CA_THF | x | - | x | - | 80 °C, 3 h 120 °C, 1 h 150 °C, 1 h |
| ELO_D | x | - | - | x | 130 °C, 2 h 160 °C, 3 h |
| Sample | ∆H a (J/g) | Tp b (°C) | Ea c (KJ/mol) |
|---|---|---|---|
| ELO_CA_W | 125.5 | 100.4 | 39.27 |
| ELO_CA_THF | 174.1 | 108.0 | 69.84 |
| ELO_D | 170.7 | 218.4 | 20.04 |
| Sample | TGA | DMA | ||||
|---|---|---|---|---|---|---|
| Td5% a (°C) | Td10% b (°C) | Residual Mass (%, at 700 °C) | Tmax (°C) | Tg c (°C) | Crosslink Density (mol/ m3) | |
| ELO_CA_W | 206.9 | 250.7 | 4.94 | 395.8 | 39 | 1414 |
| ELO_CA_THF | 311.4 | 343.0 | 3.07 | 402.4 | 41 | 2477 |
| ELO_D | 298.2 | 336.2 | 1.36 | 394.5 | 29 | 417 |
| Sample | θ (°, water) | θ (°, EG) a | Surface Free Energy (Nm/m) |
|---|---|---|---|
| ELO_CA_W | 62.89 ± 1.65 | 52.37 ± 2.54 | 41.50 |
| ELO_CA_THF | 94.25 ± 1.36 | 67.49 ± 1.53 | 29.50 |
| ELO_D | 75.65 ± 2.45 | 61.73 ± 2.48 | 28.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Necolau, M.I.; Damian, C.M.; Olaret, E.; Iovu, H.; Balanuca, B. Comparative Thermo-Mechanical Properties of Sustainable Epoxy Polymer Networks Derived from Linseed Oil. Polymers 2022, 14, 4212. https://doi.org/10.3390/polym14194212
Necolau MI, Damian CM, Olaret E, Iovu H, Balanuca B. Comparative Thermo-Mechanical Properties of Sustainable Epoxy Polymer Networks Derived from Linseed Oil. Polymers. 2022; 14(19):4212. https://doi.org/10.3390/polym14194212
Chicago/Turabian StyleNecolau, Madalina Ioana, Celina Maria Damian, Elena Olaret, Horia Iovu, and Brindusa Balanuca. 2022. "Comparative Thermo-Mechanical Properties of Sustainable Epoxy Polymer Networks Derived from Linseed Oil" Polymers 14, no. 19: 4212. https://doi.org/10.3390/polym14194212






