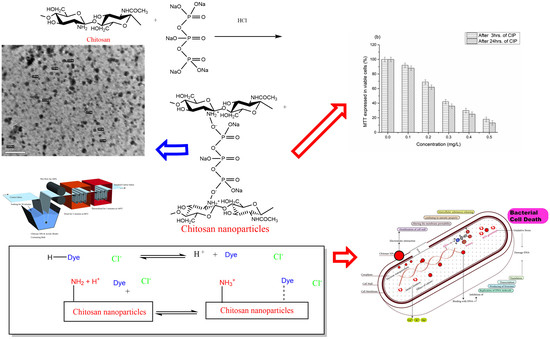
Enhancement of Antimicrobial and Dyeing Properties of Cellulosic Fabrics via Chitosan Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Chitosan Nanoparticles (CSNPs)
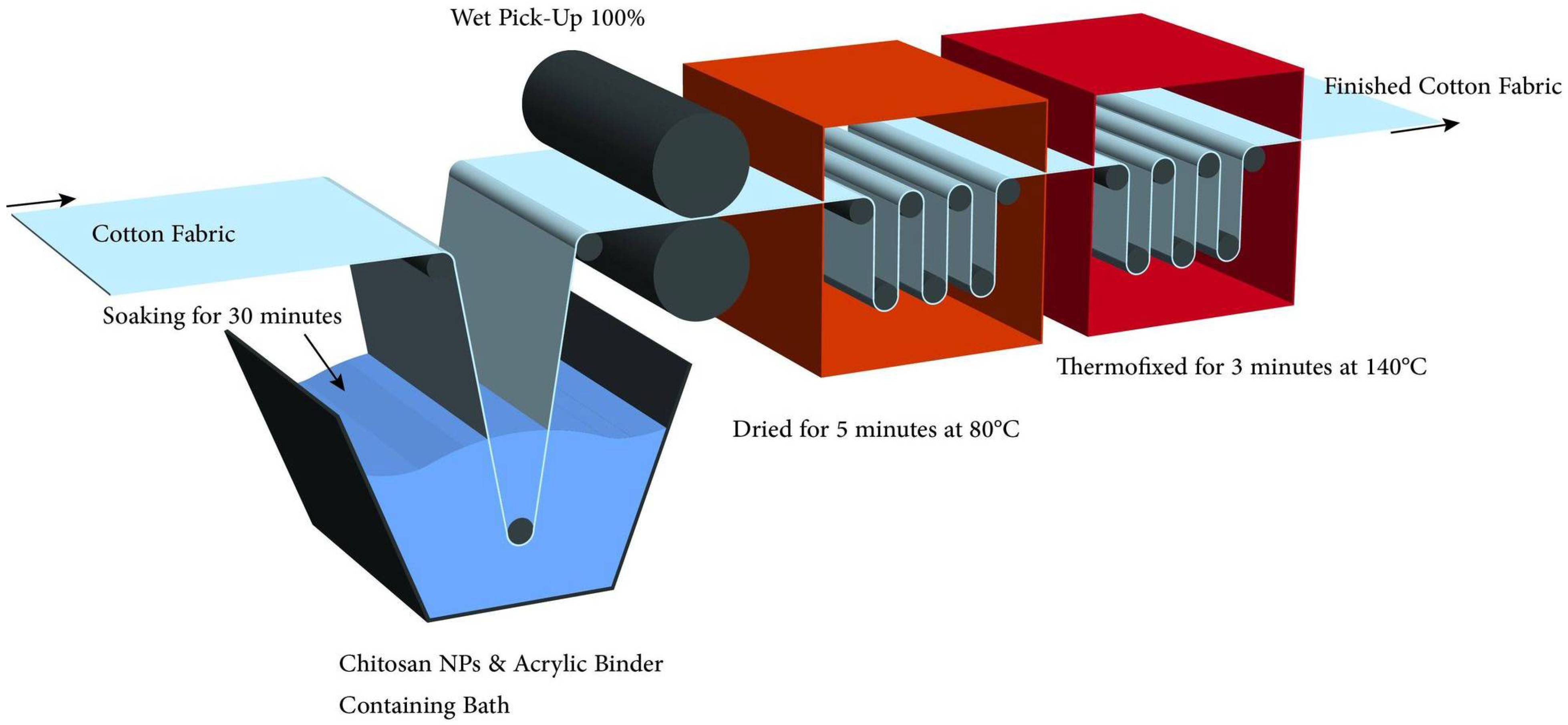
2.2.2. Finishing of Cellulosic Fabrics with Chitosan Nanoparticles
2.2.3. Procedures for Using Two Acid Dyes in Fabric Dyeing
2.3. Characterization
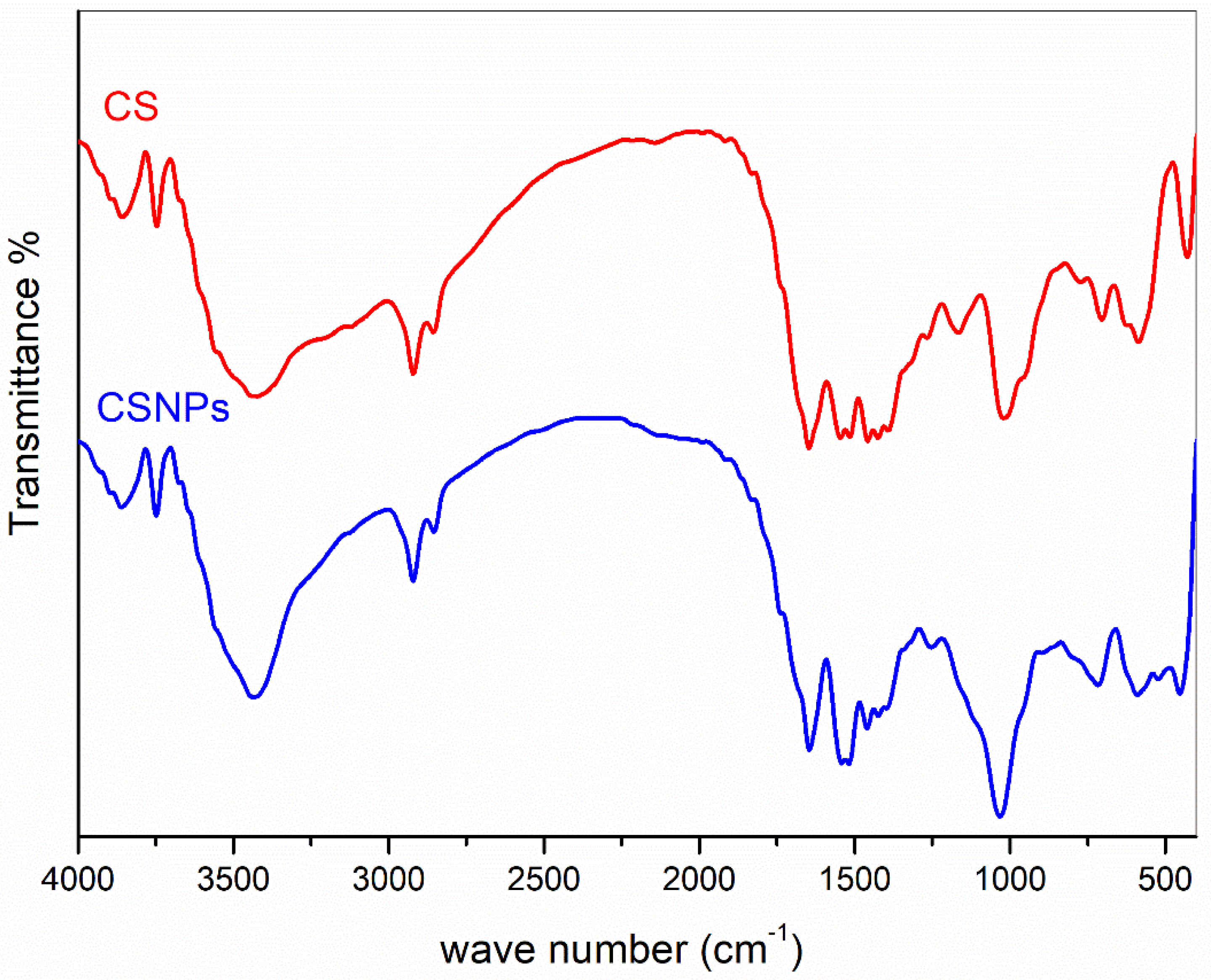
2.3.1. FT-IR Spectra
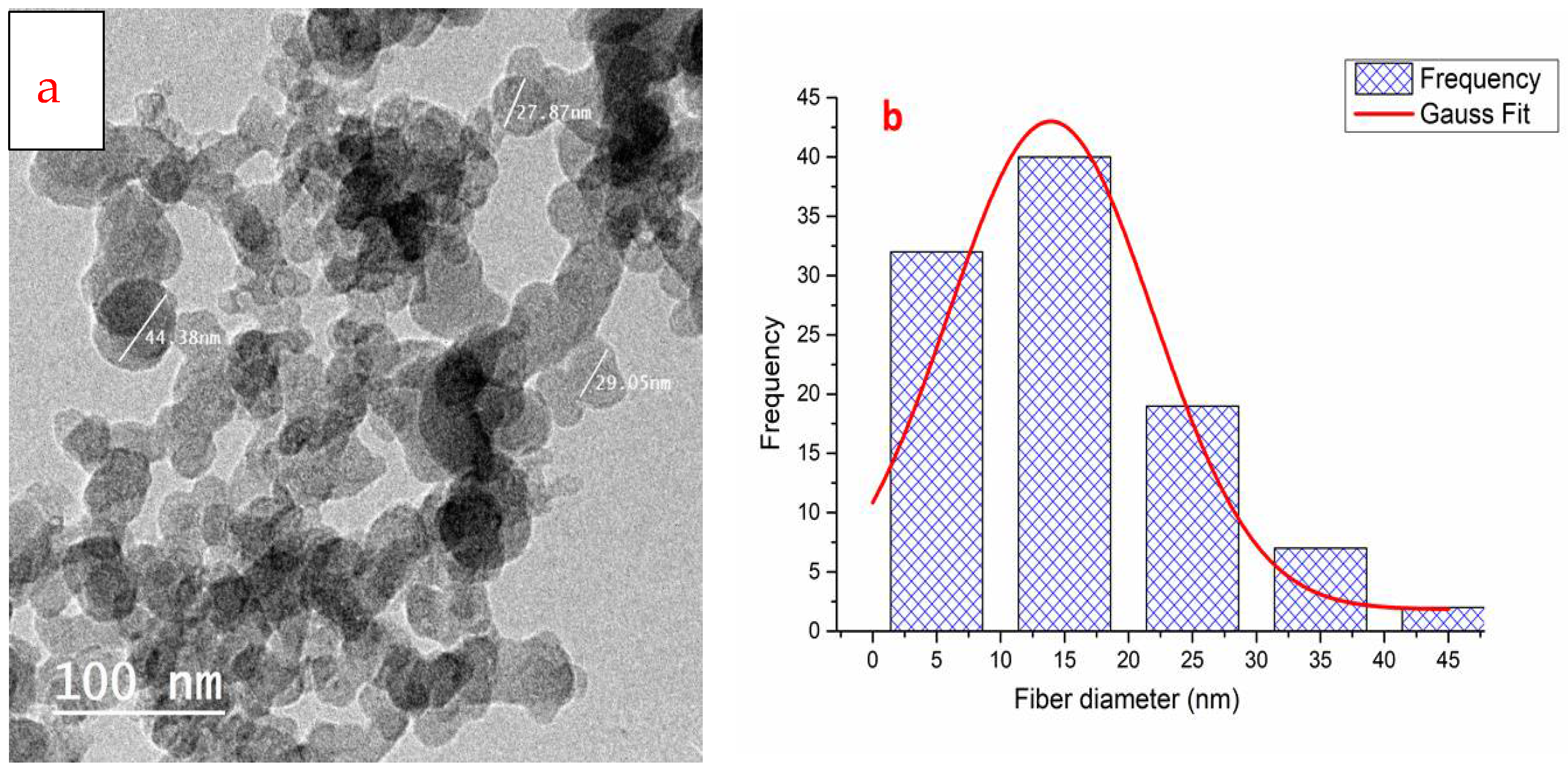
2.3.2. Transmission Electron Microscopy (TEM)
2.3.3. Thermo Gravimetric Analysis (TGA)
2.3.4. Mechanical Properties
2.3.5. Colour Measurements
2.4. Antimicrobial Activity Evaluation
2.4.1. Materials
2.4.2. Test Methods
2.5. Chitosan Nanoparticles Cytotoxicity Test Assay
3. Results
3.1. Synthesis of Chitosan Nanoparticles
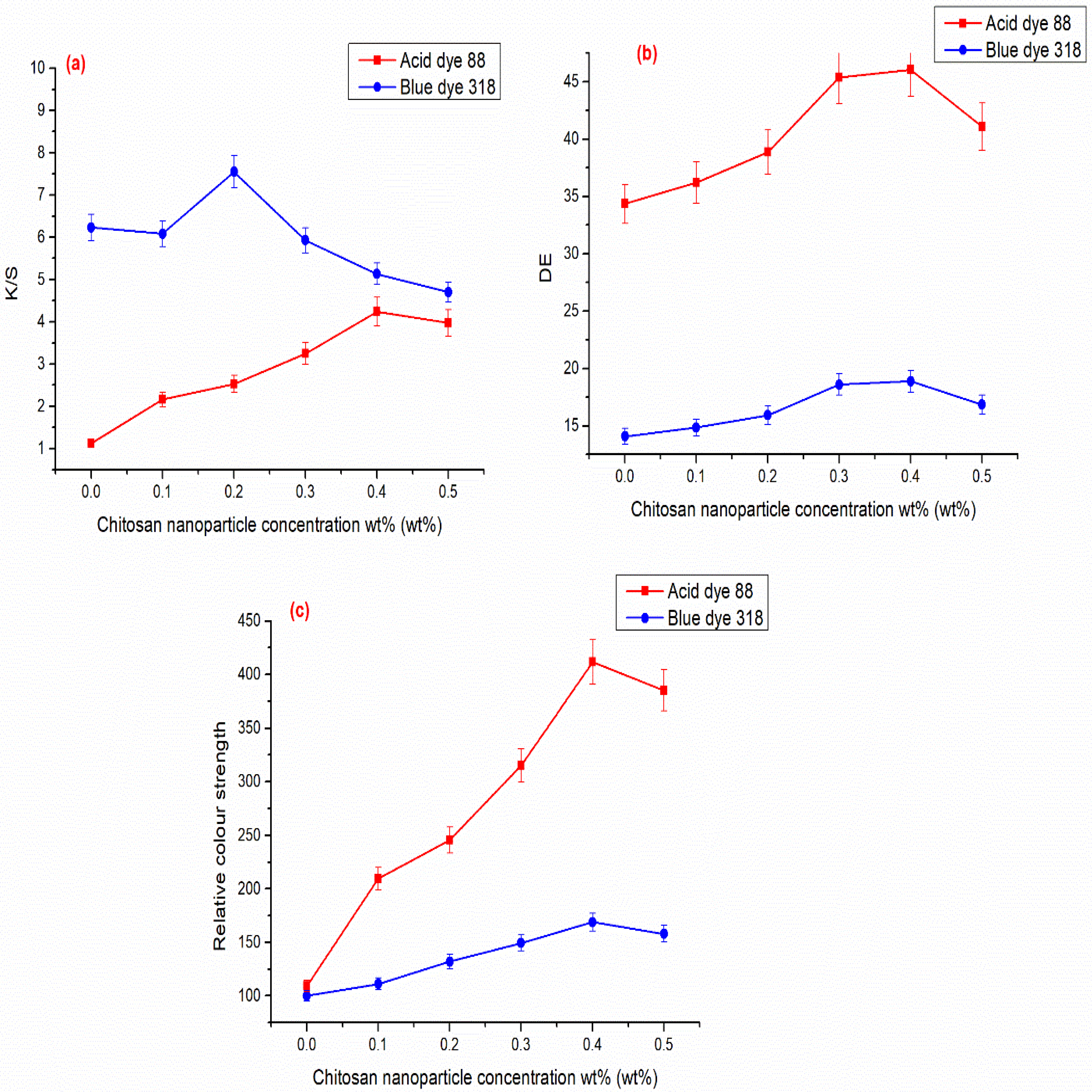
3.2. Chitosan Nanoparticles as a Finishing Agent for Cellulosic Cotton Fabrics and their Dyeing with Two Acid Dyes
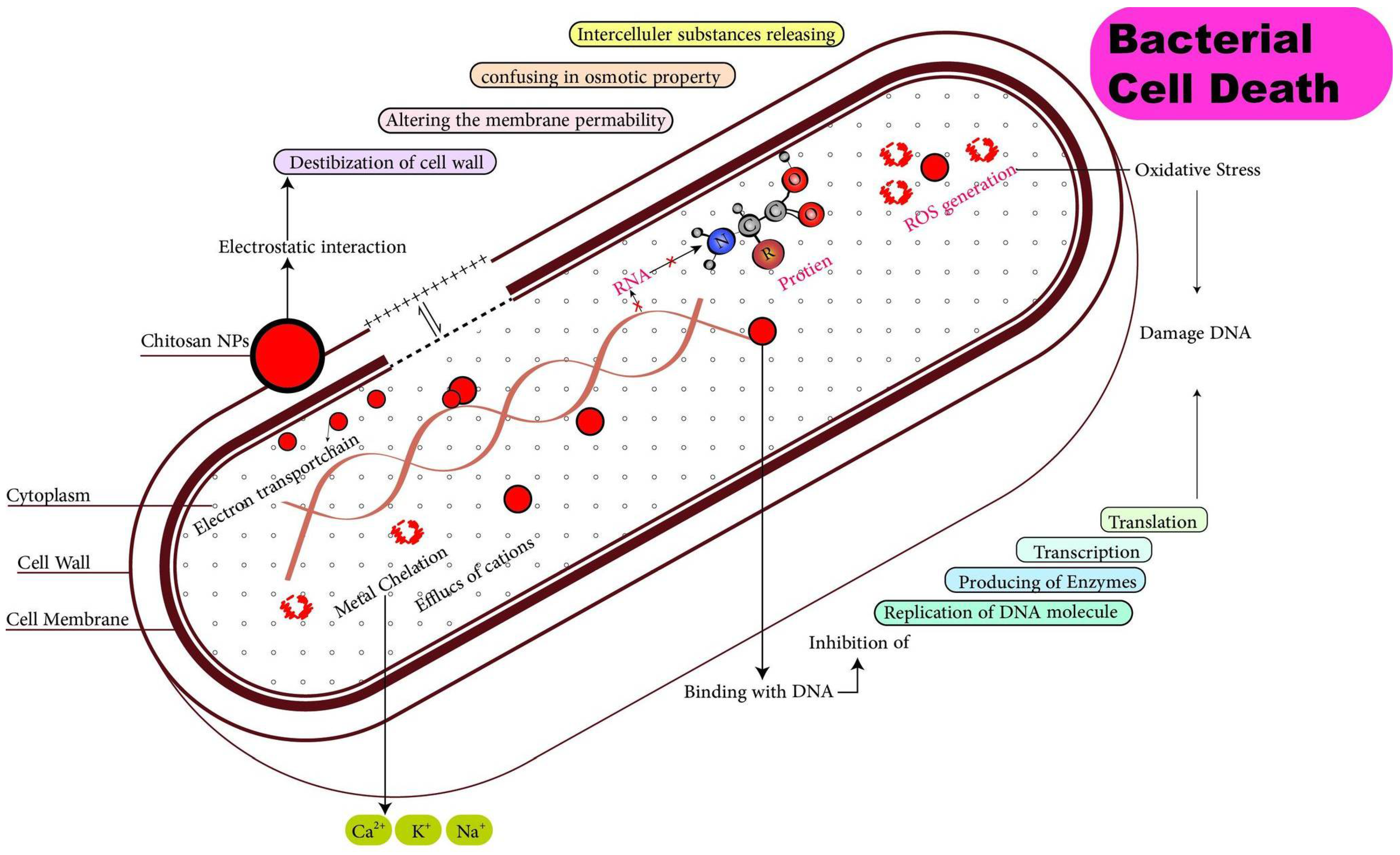
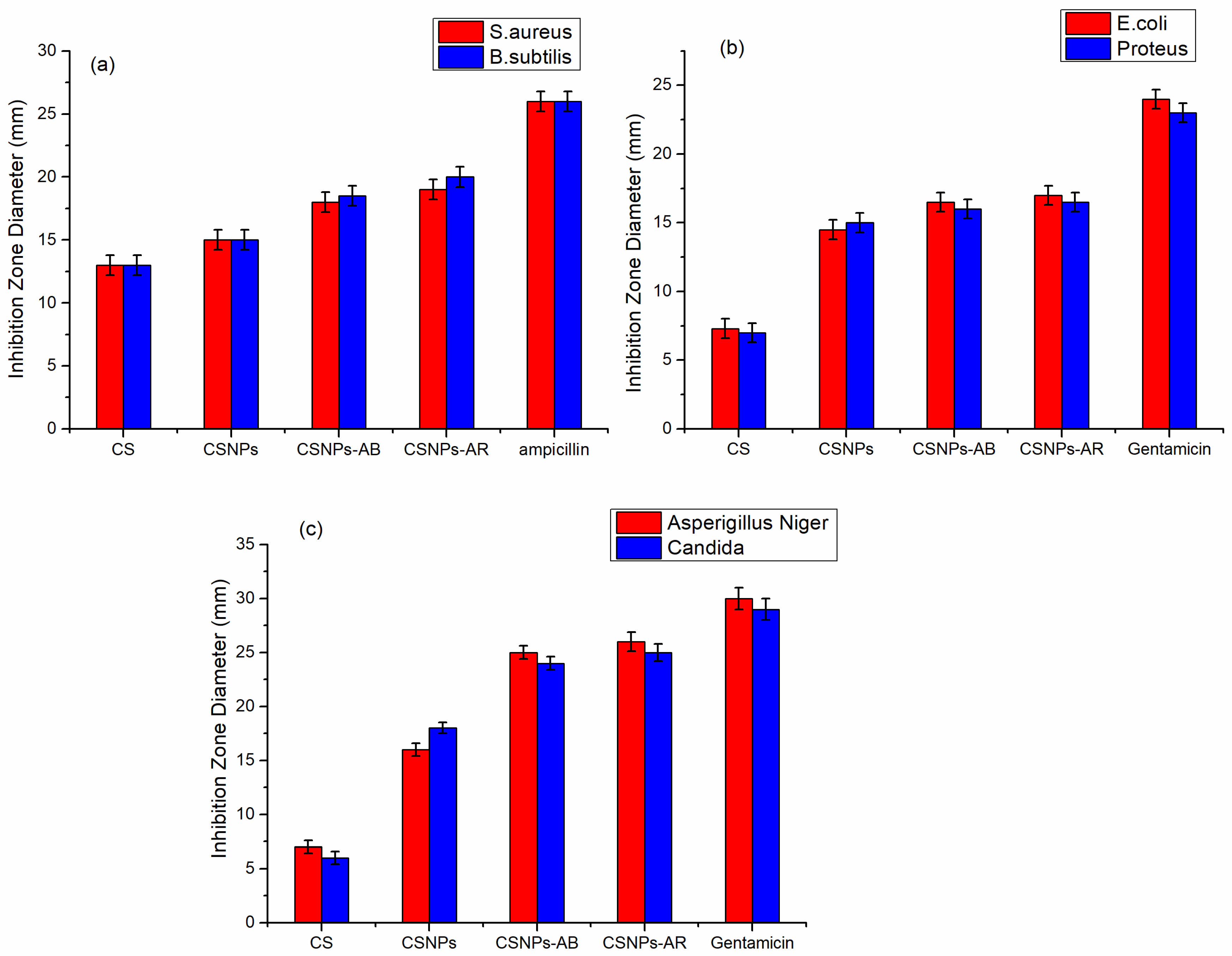
3.3. The Antimicrobial Activity Study
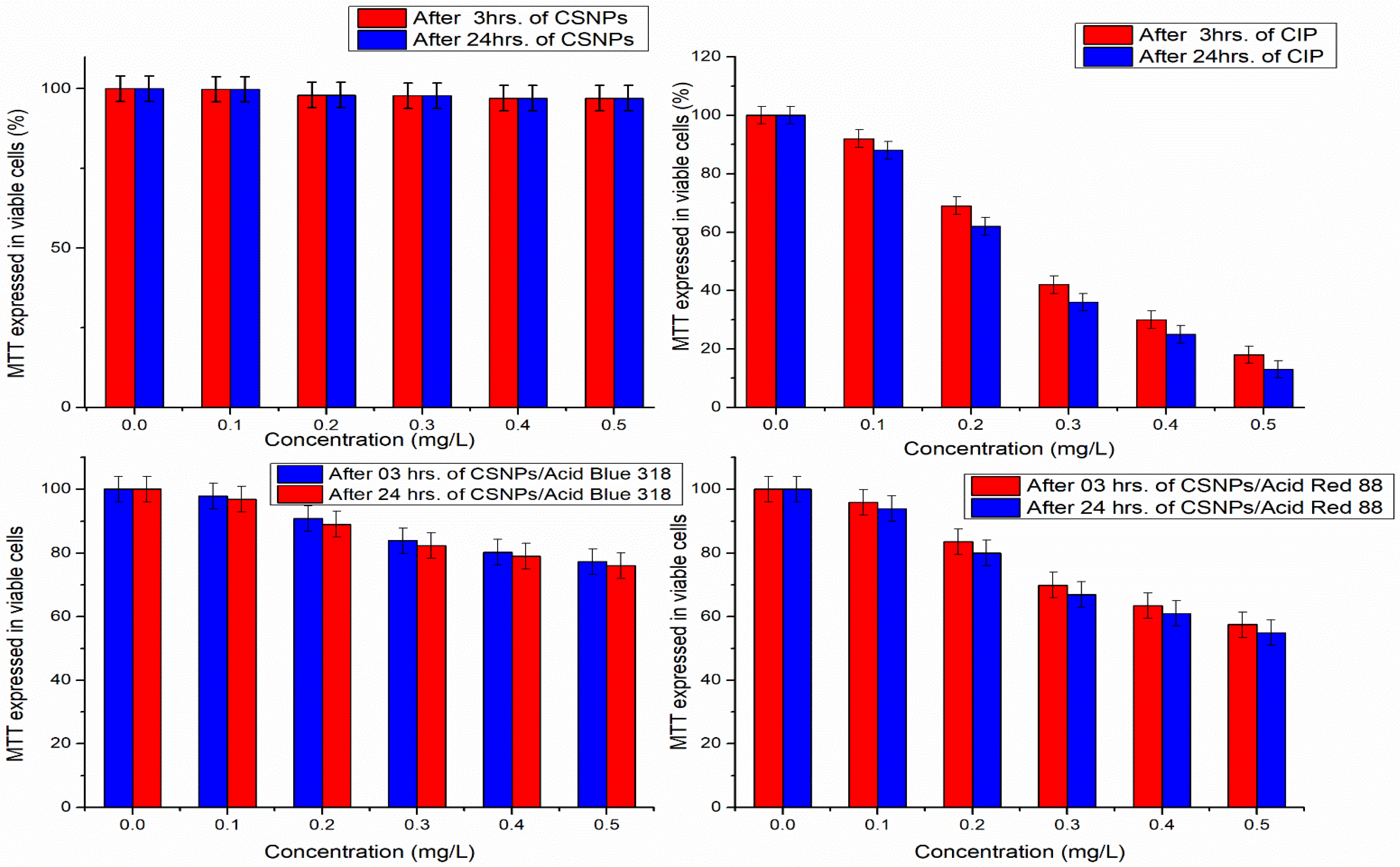
3.4. Cytotoxicity of Chitosan Nanoparticles (CSNPs) via MMT Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibrahim, H.; Emam, E.A.M.; Tawfik, T.M.; El-Aref, A.T. Preparation of cotton gauze coated with carboxymethyl chitosan and its utilization for water filtration. J. Text. Appar. Technol. Manag. 2019, 11, 1–11. [Google Scholar]
- Salama, R.H.; Osman, H.; Ibrahim, H.M. Preparation of biocompatible chitosan nanoparticles loaded with Aloe vera extract for use as a novel drug delivery mechanism to improve the antibacterial characteristics of cellulose-based fabrics. Egypt. J. Chem. 2022, 65, 581–595. [Google Scholar] [CrossRef]
- Mohamed, F.A.; Ibrahim, H.M.; Aly, A.A.; El-Alfy, E.A. Improvement of dyeability and antibacterial properties of gelatin treated cotton fabrics with synthesized reactive dye. Biosci. Res. 2018, 15, 4403–4408. [Google Scholar]
- Park, S.; Choi, H.-M.J.C.C.T. Microwave-Mediated Rapid Oxidation and Cationization of Cotton Cellulose. Cell. Chem. Technol. 2018, 52, 311–322. [Google Scholar]
- Ru, J.; Qian, X.; Wang, Y.J.S.r. Low-Salt or Salt-Free Dyeing of Cotton Fibers with Reactive Dyes using Liposomes as Dyeing/Level-Dyeing Promotors. Sci. Rep. 2018, 8, 13045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqua, U.H.; Ali, S.; Hussain, T.; Bhatti, H.N.; Asghar, M. The Dyeing Process and the Environment: Enhanced Dye Fixation on Cellulosic Fabric Using Newly Synthesized Reactive Dye. Pol. J. Environ. Stud. 2017, 26, 2215–2222. [Google Scholar] [CrossRef]
- El-Bisi, M.K.; Othman, R.; Yassin, F.A. Improving antibacterial and ultraviolet properties of cotton fabrics via dual effect of nano-metal oxide and Moringa oleifera extract. Egypt. J. Chem. 2020, 63, 3441–3451. [Google Scholar] [CrossRef]
- Salman, A.; Metwally, F.A.; El-Bisi, M.K.; Emara, G.A.M. Effect of geometrical yarn parameters: Conventional and compact ring spinning on certain functional properties of tio2nps treated woven cotton fabrics. Egypt. J. Chem. 2020, 63, 1757–1766. [Google Scholar] [CrossRef]
- Musa, H.; Abdulmumini, A.; Folashade, M.; Usman, B.; Abba, H. Studies on the Dyeing of Wool and Nylon Fabrics with Some Acid Dyes. IOSR J. Appl. Chem. 2013, 5, 11–17. [Google Scholar] [CrossRef]
- Wang, H.; Lewis, D.J.C.T. Chemical modification of cotton to improve fibre dyeability. Color. Technol. 2002, 118, 159–168. [Google Scholar] [CrossRef]
- Ibrahim, H.; El- Zairy, E.M.R.; Emam, E.A.M.; Adel, E. Combined antimicrobial finishing dyeing properties of cotton, polyester fabrics and their blends with acid and disperse dyes. Egypt. J. Chem. 2019, 62, 965–976. [Google Scholar] [CrossRef]
- Mohamed, F.A.; Sheier, M.B.; Reda, M.M.; Ibrahim, H.M. Synthesis, Application, and Antibacterial Activity of New Direct Dyes based on Chromene Derivatives. Curr. Org. Synth. 2022, 19, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.K.R. Coloration of Cationized Cellulosic Fibers–A Review. AATCC J. Res. 2014, 1, 11–19. [Google Scholar] [CrossRef]
- Deeksha, B.; Sadanand, V.; Hariram, N.; Rajulu, A.V. Preparation and properties of cellulose nanocomposite fabrics with in situ generated silver nanoparticles by bioreduction method. J. Bioresour. Bioprod. 2021, 6, 75–81. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloids Surf. B Biointerfaces 2010, 79, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Alves, N.M.; Mano, J.F. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Chen, P. Natural Products with Health Benefits from Marine Biological Resources. Curr. Org. Chem. 2014, 18, 777–792. [Google Scholar] [CrossRef]
- Duttagupta, S.; Varsha, M.J.; Vilasrao, J.K. Chitosan: A Propitious Biopolymer for Drug Delivery. Curr. Drug Deliv. 2015, 12, 369–381. [Google Scholar] [CrossRef]
- Thanou, M.; Verhoef, J.C.; Junginger, H.E. Oral drug absorption enhancement by chitosan and its derivatives. Adv. Drug Deliv. Rev. 2001, 52, 117–126. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- El-Bisi, M.K.; Ibrahim, H.M.; Rabie, A.M.; Elnagar, K.; Taha, G.M.; El-Alfy, E.A. Super hydrophobic cotton fabrics via green techniques. Der Pharma Chem. 2016, 8, 57–69. [Google Scholar]
- Mohamed, F.A.; Shaban, E.; Ibrahim, H.M. Synthesis and antibacterial activity of some novel nucleus N-aminorhodanine based bis monofunctional and bifunctional reactive dyes and their application on wool and cotton fabrics. Egypt. J. Chem. 2022, 65, 597–608. [Google Scholar] [CrossRef]
- Ali, M.A.; Bydoon, E.A.; Ibrahim, H.M. Bioactive Composite Nonwoven Surgical Dressing based on Cellulose Coated with Nanofiber Membrane using the layer-by-layer technique. Egypt. J. Chem. 2022, 65, 525–542. [Google Scholar] [CrossRef]
- Abou-Okeil, A.; Refaei, R.; Khalil, E.M.; Refaei, R.; Moustafa, S.E.; Ibrahim, H.M. Fabrication of novel green magnetic electrospun nanofibers based on Fe3O4 nanoparticles/PVA/chitosan/collagen. Egypt. J. Chem. 2022, 65, 283–299. [Google Scholar] [CrossRef]
- Li, H.-P.; Qin, L.; Wang, Z.-D.; Li, S. Synthesis and characterization of ramose tetralactosyl-lysyl-chitosan-5-fluorouracil and its in vitro release. Res. Chem. Intermed. 2012, 38, 1421–1429. [Google Scholar] [CrossRef]
- Gupta, N.K.; Tomar, P.; Sharma, V.; Dixit, V.K. Development and characterization of chitosan coated poly-(ɛ-caprolactone) nanoparticulate system for effective immunization against influenza. Vaccine 2011, 29, 9026–9037. [Google Scholar] [CrossRef] [PubMed]
- Borges, O.; Tavares, J.; de Sousa, A.; Borchard, G.; Junginger, H.E.; Cordeiro-da-Silva, A. Evaluation of the immune response following a short oral vaccination schedule with hepatitis B antigen encapsulated into alginate-coated chitosan nanoparticles. Eur. J. Pharm. Sci. 2007, 32, 278–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surf. B Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef]
- Ramyadevi, J.; Jeyasubramanian, K.; Marikani, A.; Rajakumar, G.; Rahuman, A.A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 2012, 71, 114–116. [Google Scholar] [CrossRef]
- Stanić, V.; Dimitrijević, S.; Antić-Stanković, J.; Mitrić, M.; Jokić, B.; Plećaš, I.B.; Raičević, S. Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Appl. Surf. Sci. 2010, 256, 6083–6089. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, F.; Olive, P.L.; Banuelos, A.; Orrantia, E.; Nino, N.; Sanchez, E.M.; Ruiz, F.; Bach, H.; Av-Gay, Y. Synthesis, characterization, and evaluation of antimicrobial and cytotoxic effect of silver and titanium nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Lellouche, J.; Kahana, E.; Elias, S.; Gedanken, A.; Banin, E. Antibiofilm activity of nanosized magnesium fluoride. Biomaterials 2009, 30, 5969–5978. [Google Scholar] [CrossRef]
- Tiyaboonchai, W. Chitosan nanoparticles: A promising system for drug delivery. Naresuan Univ. J. 2013, 11, 51–66. [Google Scholar]
- Elgadir, M.A.; Uddin, M.S.; Ferdosh, S.; Adam, A.; Chowdhury, A.J.K.; Sarker, M.Z.I. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J. Food Drug Anal. 2015, 23, 619–629. [Google Scholar] [CrossRef]
- Piras, A.M.; Maisetta, G.; Sandreschi, S.; Esin, S.; Gazzarri, M.; Batoni, G.; Chiellini, F. Preparation, physical–chemical and biological characterization of chitosan nanoparticles loaded with lysozyme. Int. J. Biol. Macromol. 2014, 67, 124–131. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Saad, M.M.; Aly, N.M. Preparation of single layer nonwoven fabric treated with chitosan nanoparticles and its utilization in gas filtration. Int. J. ChemTech Res. 2016, 9, 1–16. [Google Scholar]
- Ibrahim, N.A.; Kadry, G.A.; Eid, B.M.; Ibrahim, H.M. Enhanced antibacterial properties of polyester and polyacrylonitrile fabrics using Ag-Np dispersion/microwave treatment. AATCC J. Res. 2014, 1, 13–19. [Google Scholar] [CrossRef]
- Fatima, A.; Yasir, S.; Khan, M.S.; Manan, S.; Ullah, M.W.; Ul-Islam, M. Plant extract-loaded bacterial cellulose composite membrane for potential biomedical applications. J. Bioresour. Bioprod. 2021, 6, 26–32. [Google Scholar] [CrossRef]
- Zou, Y.; Zhao, J.; Zhu, J.; Guo, X.; Chen, P.; Duan, G.; Liu, X.; Li, Y. A mussel-inspired polydopamine-filled cellulose aerogel for solar-enabled water remediation. ACS Appl. Mater. Interfaces 2021, 13, 7617–7624. [Google Scholar] [CrossRef]
- Mohamed, F.A.; Abd El-Megied, S.A.; Bashandy, M.S.; Ibrahim, H.M. Synthesis, application and antibacterial activity of new reactive dyes based on thiazole moiety. Pigment. Resin Technol. 2018, 47, 246–254. [Google Scholar] [CrossRef]
- Maneerung, T.; Tokura, S.; Rujiravanit, R.J.C.p. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 2008, 72, 43–51. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; Hudson, S.M. Chitosan nanoparticles: Polyphosphates cross-linking and protein delivery properties. Int. J. Biol. Macromol. 2019, 136, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh Kafshgari, M.; Khorram, M.; Khodadoost, M.; Khavari, S. Reinforcement of chitosan nanoparticles obtained by an ionic cross-linking process. Iran. Polym. J. 2011, 20, 445–456. [Google Scholar]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Naskar, S.; Kuotsu, K.; Sharma, S. Chitosan-based nanoparticles as drug delivery systems: A review on two decades of research. J. Drug Target. 2019, 27, 379–393. [Google Scholar] [CrossRef]
- Rayment, P.; Butler, M.F. Investigation of ionically crosslinked chitosan and chitosan–bovine serum albumin beads for novel gastrointestinal functionality. J. Appl. Polym. Sci. 2008, 108, 2876–2885. [Google Scholar] [CrossRef]
- Kolesov, S.V.; Gurina, M.S.; Mudarisova, R.K. On the Stability of Aqueous Nanodispersions of Polyelectrolyte Complexes Based on Chitosan and N-Succinyl-Chitosan. Polym. Sci. Ser. A 2019, 61, 253–259. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, Z.; Du, Y.; Kennedy, J.F. Controlled release of ciprofloxacin hydrochloride from chitosan/polyethylene glycol blend films. Carbohydr. Polym. 2007, 69, 336–343. [Google Scholar] [CrossRef]
- Xu, Y.; Du, Y. Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int. J. Pharm. 2003, 250, 215–226. [Google Scholar] [CrossRef]
- El-Alfy, E.A.; El-Bisi, M.K.; Taha, G.M.; Ibrahim, H.M. Preparation of biocompatible chitosan nanoparticles loaded by tetracycline, gentamycin and ciprofloxacin as novel drug delivery system for improvement the antibacterial properties of cellulose based fabrics. Int. J. Biol. Macromol. 2020, 161, 1247–1260. [Google Scholar] [CrossRef]
- Tian, M.; Qu, L.; Zhang, X.; Zhang, K.; Zhu, S.; Guo, X.; Han, G.; Tang, X.; Sun, Y. Enhanced mechanical and thermal properties of regenerated cellulose/graphene composite fibers. Carbohydr. Polym. 2014, 111, 456–462. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, P.; Kieft, T.L.; Ryan, S.J.; Baker, S.M.; Wiesmann, W.P.; Rogelj, S. Antibacterial action of a novel functionalized chitosan-arginine against Gram-negative bacteria. Acta Biomater. 2010, 6, 2562–2571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]




| Fabrics | Cotton Fabrics Treated with CSNPs | ||||
|---|---|---|---|---|---|
| N % | E % | TS kg | YI | WI | |
| Untreated cotton fabrics | 0.0 | 11 | 48 | 1.59 | 54.37 |
| Cotton fabrics treated with CSNPs | 0.18 | 13 | 50 | 1.39 | 54.48 |
| Acid Blue dyed cotton treated with CSNPs 317 | 0.22 | 14 | 52 | 1.25 | 54.97 |
| Cotton treated with CSNPs and Acid Red 88 dyed | 0.24 | 16 | 54 | 1.19 | 55.34 |
| CSNPs (wt.%) | Light Fastness | Washing Fastness | Perspiration Fastness | Rubbing Fastness | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkaline | Acidic | ||||||||||||
| Alt | SC | SW | Alt | SC | SW | Alt | SC | SW | Dry | Wet | |||
| 0.1 | AB | 4–5 | 4–5 | 4 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 |
| AR | 4–5 | 4–5 | 4 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | |
| 0.3 | AB | 5 | 4 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 |
| AR | 5 | 4 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | |
| 0.5 | AB | 5–6 | 4 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 |
| AR | 5–6 | 4 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosaad, R.M.; Alhalafi, M.H.; Emam, E.-A.M.; Ibrahim, M.A.; Ibrahim, H. Enhancement of Antimicrobial and Dyeing Properties of Cellulosic Fabrics via Chitosan Nanoparticles. Polymers 2022, 14, 4211. https://doi.org/10.3390/polym14194211
Mosaad RM, Alhalafi MH, Emam E-AM, Ibrahim MA, Ibrahim H. Enhancement of Antimicrobial and Dyeing Properties of Cellulosic Fabrics via Chitosan Nanoparticles. Polymers. 2022; 14(19):4211. https://doi.org/10.3390/polym14194211
Chicago/Turabian StyleMosaad, Rehab M., Mona H. Alhalafi, El-Amir M. Emam, Marwan A. Ibrahim, and Hassan Ibrahim. 2022. "Enhancement of Antimicrobial and Dyeing Properties of Cellulosic Fabrics via Chitosan Nanoparticles" Polymers 14, no. 19: 4211. https://doi.org/10.3390/polym14194211