Poly(N-vinylpyrrolidone)–Laponite XLG Nanocomposite Hydrogels: Characterization, Properties and Comparison with Divinyl Monomer-Crosslinked Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrogel Preparation
2.3. Equilibrium Swelling Degree Determination
2.4. Characterizations
3. Results and Discussion
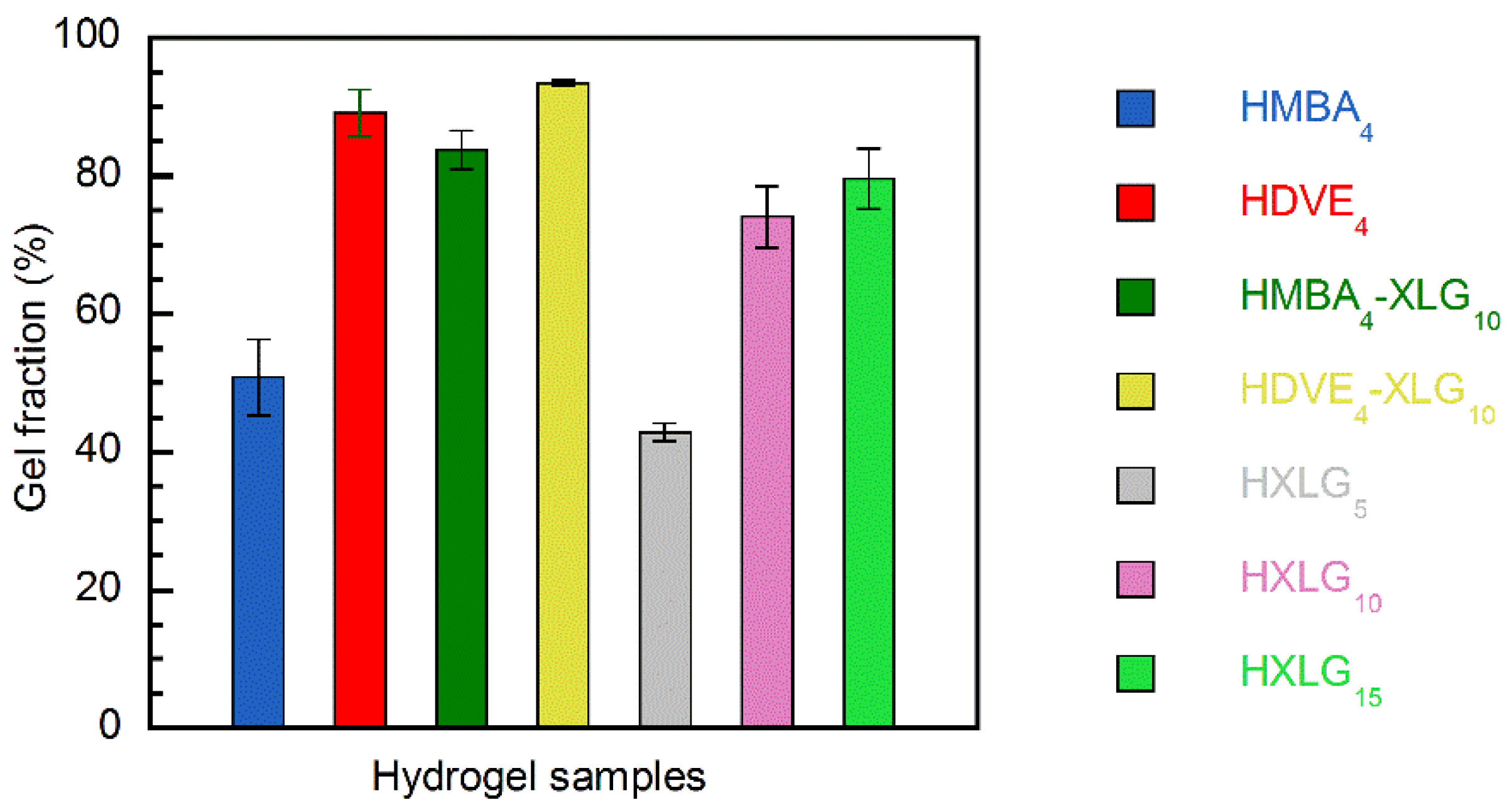
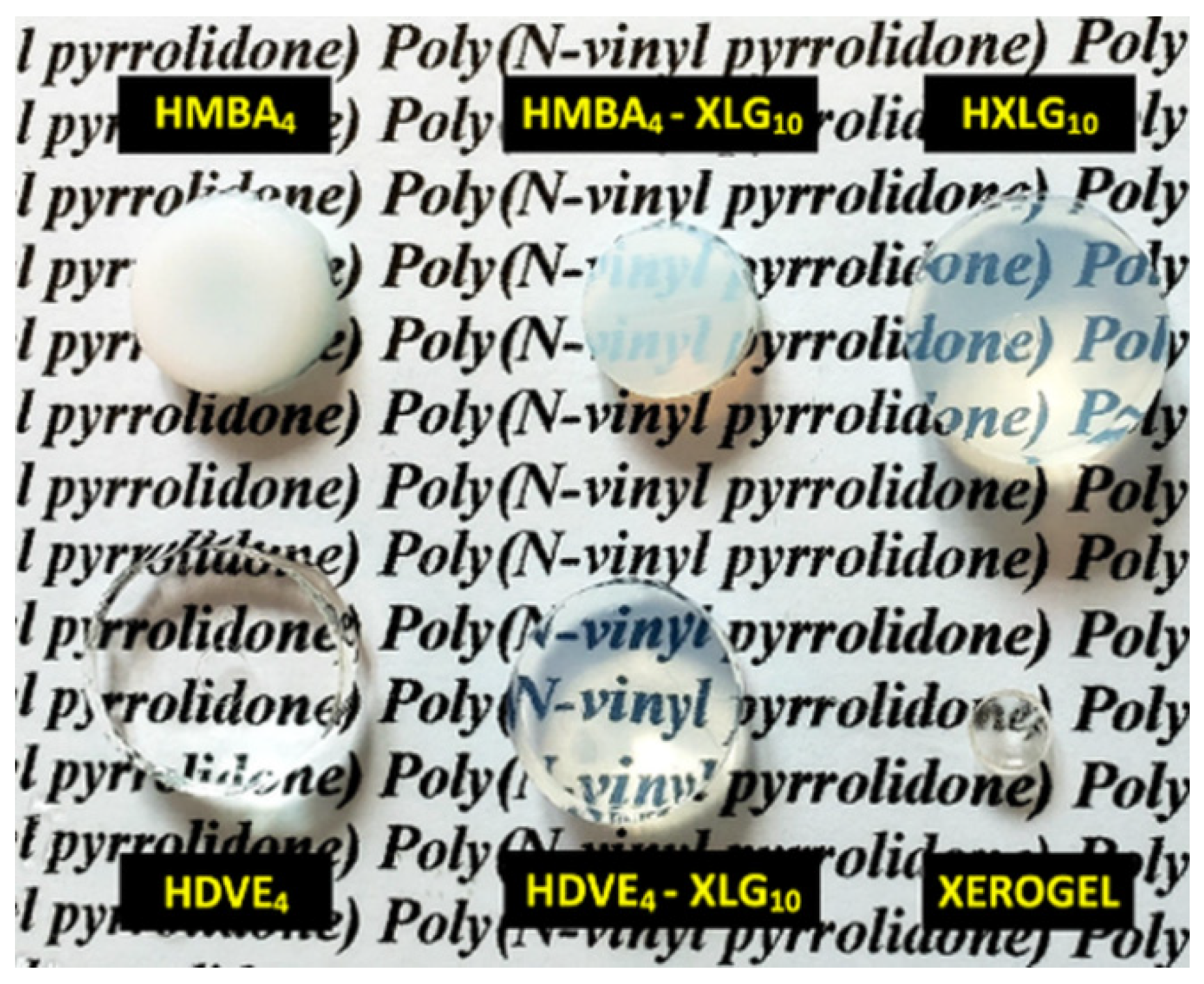
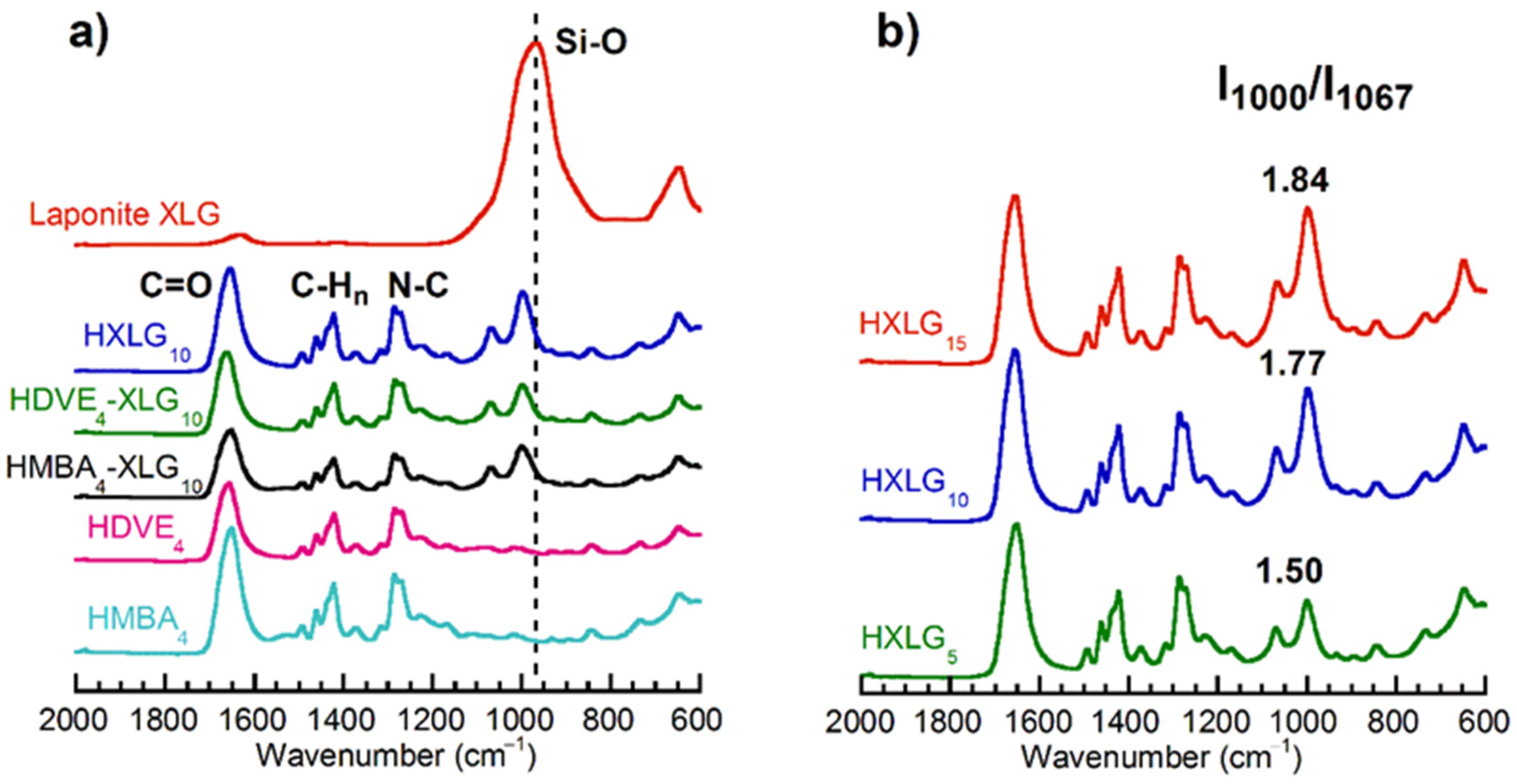
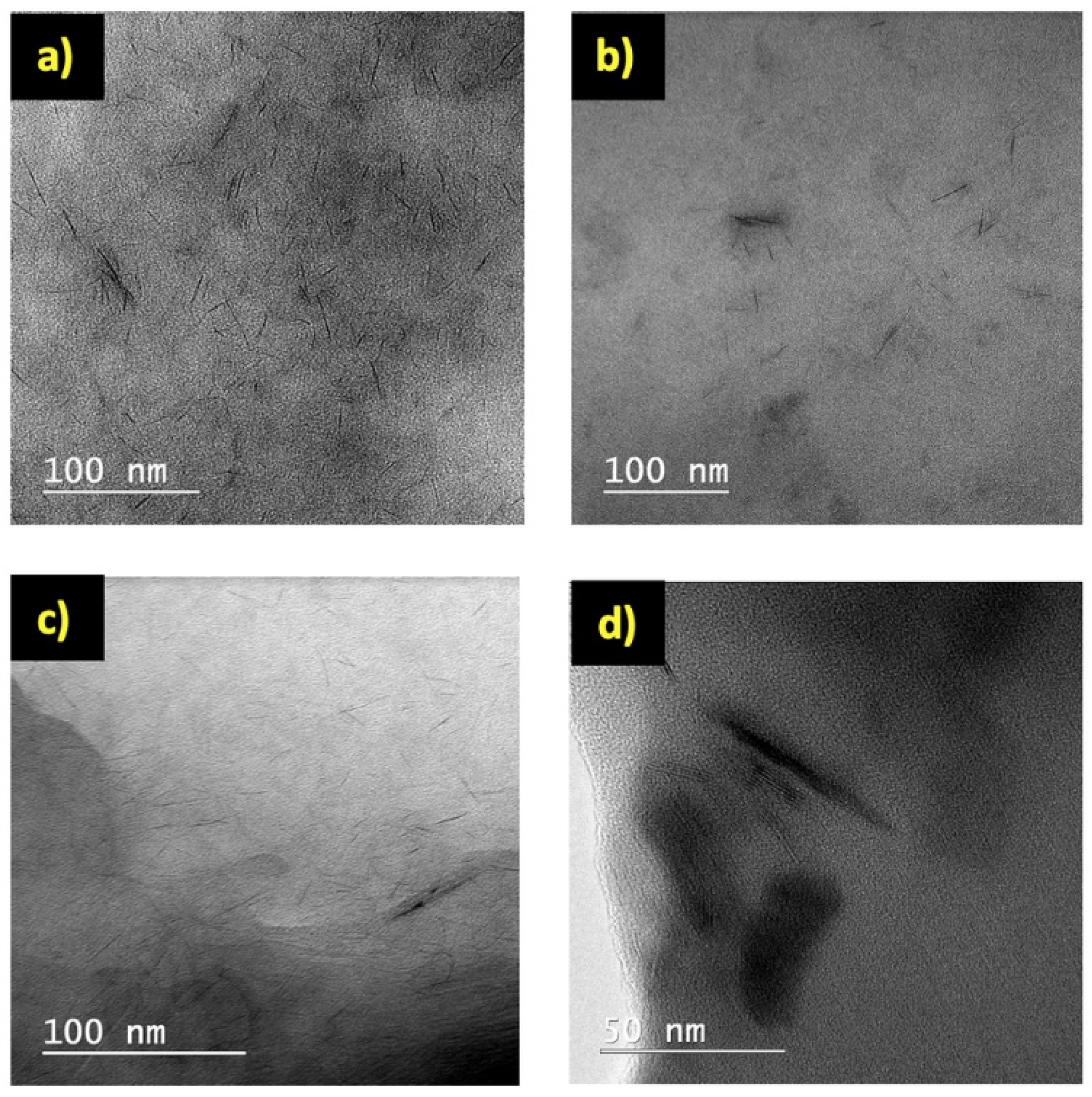
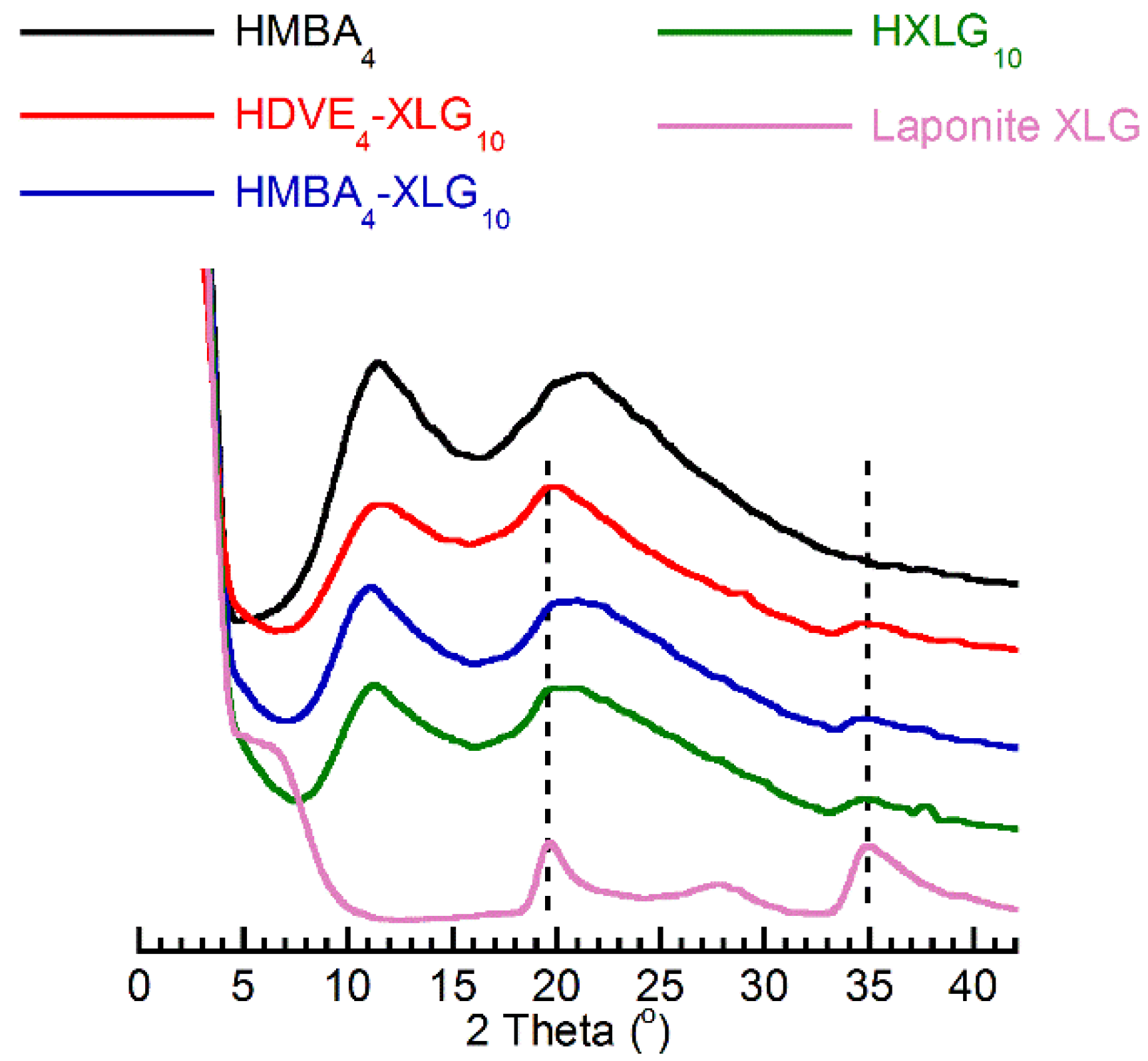
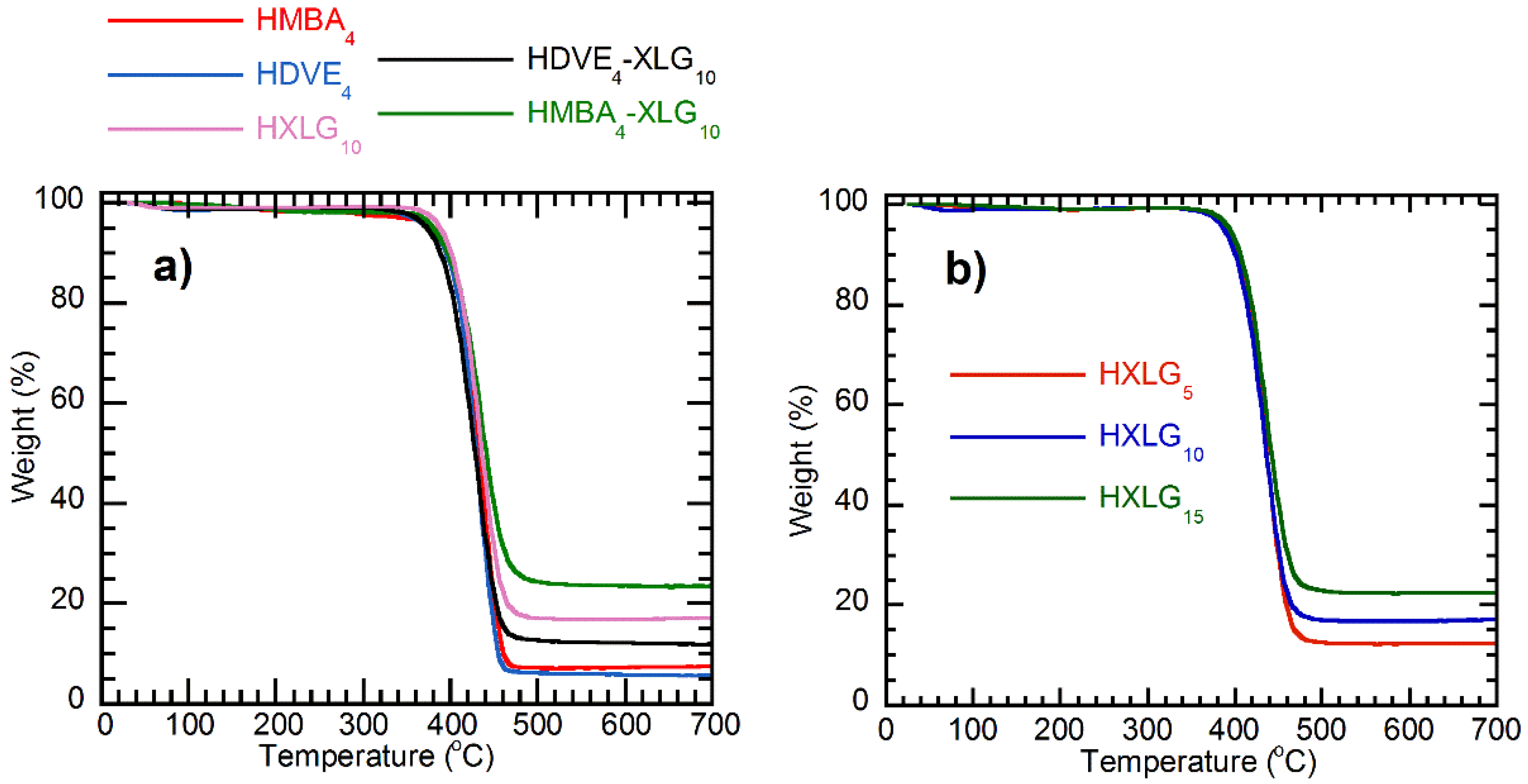
3.1. Synthesis and Structure of Hydrogels
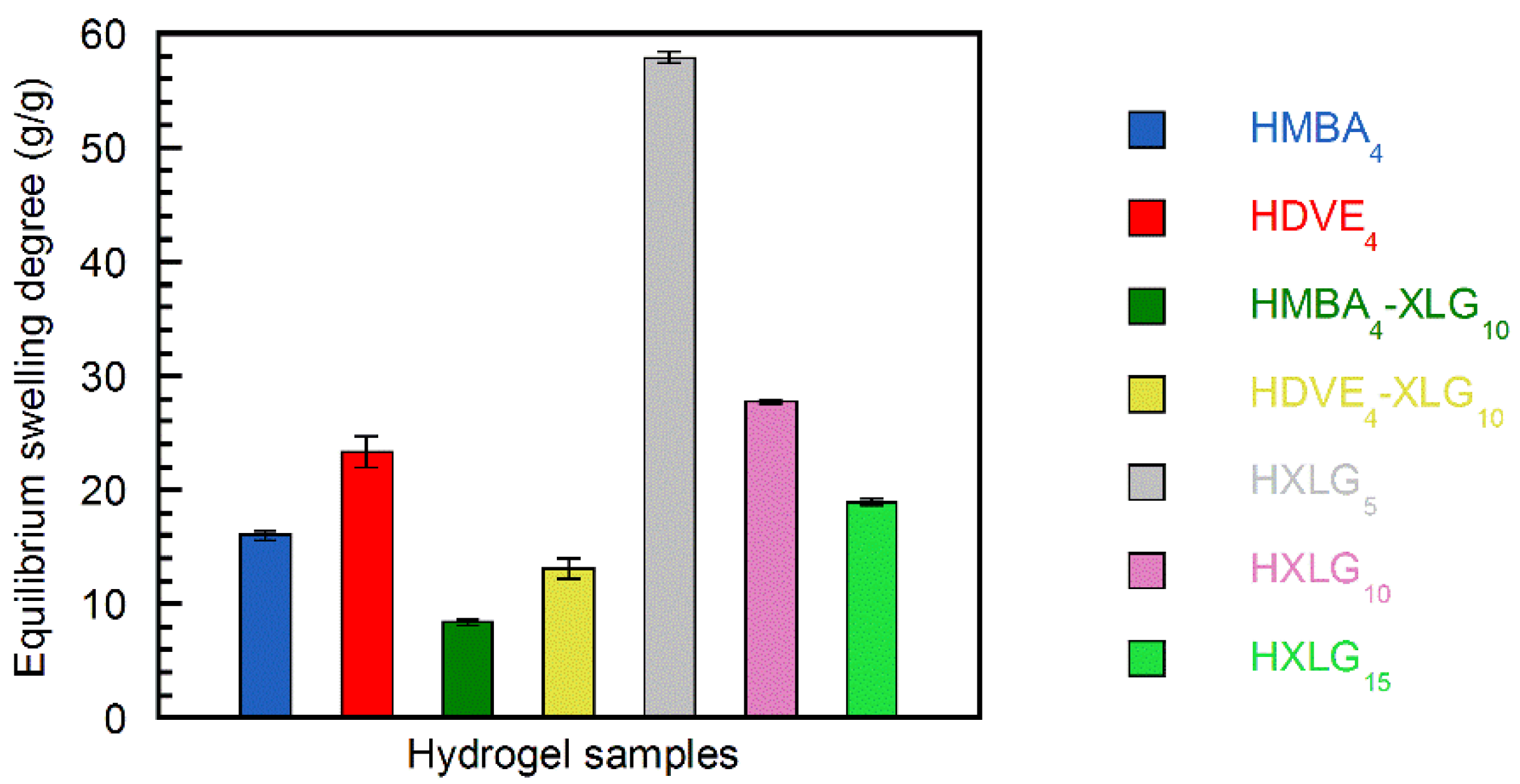
3.2. Equilibrium Swelling Degree
3.3. Viscoelastic Properties
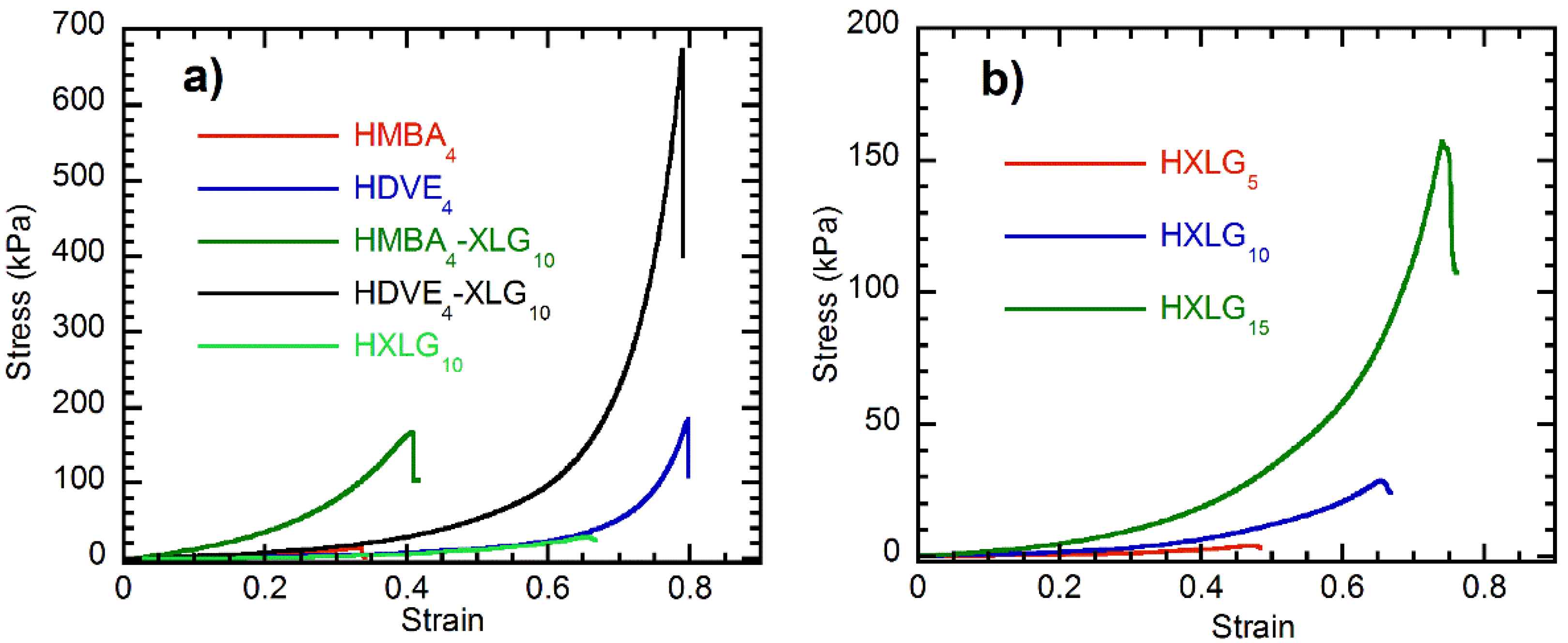
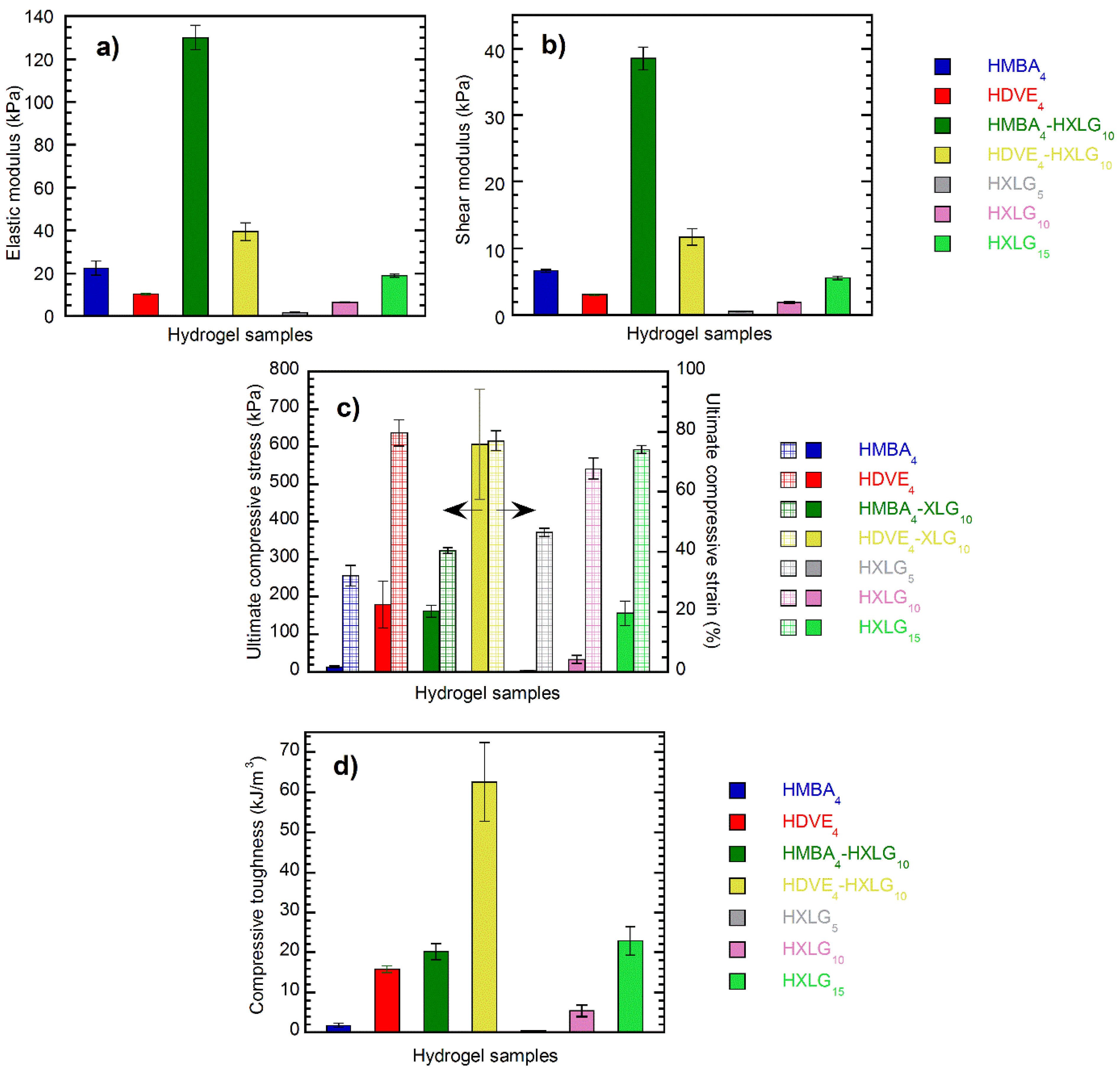
3.4. Compressive Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a hystorical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Yuk, H.; Lin, S.; Liu, X.; Parada, G. Soft materials by design: Unconventional polymer networks give extreme properties. Chem. Rev. 2021, 121, 4309–4372. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T.; Martin, C.; Radecka, I. The production and application of hydrogels for wound management: A review. Eur. Polym. J. 2019, 111, 134–151. [Google Scholar] [CrossRef]
- Onaciu, A.; Munteanu, R.A.; Moldovan, A.I.; Moldovan, C.S.; Berindan-Neagoe, I. Hydrogels based drug delivery synthesis, characterization and administration. Pharmaceutics 2019, 11, 432. [Google Scholar] [CrossRef] [Green Version]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef] [Green Version]
- Peters, J.T.; Wechsler, M.E.; Peppas, N.A. Advanced biomedical hydrogels: Molecular architecture and its impact on medical applications. Regen. Biomater. 2021, 8, rbab060. [Google Scholar] [CrossRef]
- Chakraborty, A.; Roy, A.; Ravi, S.P.; Paul, A. Exploiting the role of nanoparticles for use in hydrogel-based bioprinting applications: Concept, design, and recent advances. Biomater. Sci. 2021, 9, 6337–6354. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Q.; Dai, Z.; Dai, Y.; Xia, F.; Zhang, X. Nanocomposite adhesive hydrogels: From design to application. J. Mater. Chem. 2021, 9, 585–593. [Google Scholar] [CrossRef]
- Park, D.; Kim, J.W.; Shin, K.; Kim, J.W. Bacterial cellulose nanofibrils-reinforced composite hydrogels for mechanical compression-responsive on-demand drug release. Carbohydr. Polym. 2021, 272, 118459. [Google Scholar] [CrossRef]
- Al-Arjan, W.S.; Khan, M.U.A.; Almutairi, H.H.; Alharbi, S.M.; Razak, S.I.A. pH-Responsive PVA/BC-f-GO dressing materials for burn and chronic wound healing with curcumin release kinetics. Polymers 2022, 14, 1949. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Razak, S.I.A.; Haider, S.; Mannan, H.A.; Hussain, J.; Hasan, A. Sodium alginate-f-GO composite hydrogels for tissue regeneration and antitumor applications. Int. J. Biol. Macromol. 2022, 208, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Rudzinski, W.E.; Dave, A.M.; Vaishnav, U.H.; Kumbar, S.G.; Kulkarni, A.R.; Aminabhavi, T.M. Hydrogels as controlled release devices in agriculture. Design. Mon. Polym. 2002, 5, 39–65. [Google Scholar] [CrossRef]
- Ionov, L. Hydrogel-based actuators: Possibilities and limitations. Mater. Today 2014, 17, 494–503. [Google Scholar] [CrossRef]
- Goy, C.B.; Chaile, R.E.; Madrid, R.E. Microfluidics and hydrogel: A powerful combination. React. Func. Polym. 2019, 145, 104314. [Google Scholar] [CrossRef]
- Yazdi, M.K.; Vatanpour, V.; Taghizadeha, A.; Taghizadeha, M.; Ganjalia, M.R.; Munird, M.T.; Habibzadehf, S.; Saeb, M.R.; Ghaedih, M. Hydrogel membranes: A review. Mater. Sci. Eng. C 2020, 114, 111023. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel machines. Mater. Today 2020, 36, 102–124. [Google Scholar] [CrossRef]
- Khan, S.A.; Khan, T.A. Clay-hydrogel nanocomposites for adsorptive amputation of environmental contaminants from aqueous phase: A review. J. Environ. Chem. Eng. 2021, 9, 105575. [Google Scholar] [CrossRef]
- Pereira, A.G.B.; Rodrigues, F.H.A.; Paulino, A.T.; Martins, A.F.; Fajardo, A.R. Recent advances on composite hydrogels designed for the remediation of dye-contaminated water and wastewater: A review. J. Clean. Prod. 2021, 284, 124703. [Google Scholar] [CrossRef]
- Schexnailder, P.; Schmidt, G. Nanocomposite polymer hydrogels. Colloid Polym. Sci. 2009, 287, 1–11. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haraguchi, K. Nanocomposite hydrogels. Curr. Opin. Solid State Mater. Sci. 2007, 11, 47–54. [Google Scholar] [CrossRef]
- Peak, C.W.; Wilker, J.J.; Schmidt, G. A review on tough and sticky hydrogels. Colloid Polym. Sci. 2013, 291, 2031–2047. [Google Scholar] [CrossRef]
- Haraguchi, K.; Takehisa, T. Nanocomposite hydrogels: A unique organic-inorganic network structure with extraordinary mechanical, optical, and swelling/de-swelling properties. Adv. Mater. 2002, 14, 1120–1124. [Google Scholar] [CrossRef]
- Haraguchi, K. Synthesis and properties of soft nanocomposite materials with novel organic/inorganic network structures. Polym. J. 2011, 43, 223–241. [Google Scholar] [CrossRef]
- Miyazaki, S.; Endo, H.; Karino, T.; Haraguchi, K.; Shibayama, M. Gelation mechanism of poly(N-isopropylacrylamide)-clay nanocomposite gels. Macromolecules 2007, 40, 4287–4295. [Google Scholar] [CrossRef]
- Ferse, B.; Richter, S.; Eckert, F.; Kulkarni, A.; Papadakis, C.M.; Arndt, K.F. Gelation mechanism of poly(N-isopropylacrylamide)-clay nanocomposite hydrogels synthesized by photopolymerization. Langmuir 2008, 24, 12627–12635. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, L.; Li, Z.; Liang, B. Preparation and characterization of porous poly (N-isopropylacrylamide)/clay nanocomposite hydrogels. Polym. Bull. 2008, 61, 593–602. [Google Scholar] [CrossRef]
- Can, V.; Abdurrahmanoglu, S.; Okay, O. Unusual swelling behavior of polymer-clay nanocomposite hydrogels. Polymer 2007, 48, 5016–5023. [Google Scholar] [CrossRef]
- Okay, O.; Oppermann, W. Polyacrylamide-clay nanocomposite hydrogels: Rheological and light scattering characterization. Macromolecules 2007, 40, 3378–3387. [Google Scholar] [CrossRef]
- Xiong, L.; Hu, X.; Liu, X.; Tong, Z. Network chain density and relaxation of in situ synthesized polyacrylamide/hectorite clay nanocomposite hydrogels with ultrahigh tensibility. Polymer 2008, 49, 5064–5071. [Google Scholar] [CrossRef]
- Haraguchi, K.; Farnworth, R.; Ohbayashi, A.; Takehisa, T. Compositional effects on mechanical properties of nanocomposite hydrogels composed of poly(N,N-dimethylacrylamide) and clay. Macromolecules 2003, 36, 5732–5741. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Yang, S.; Xu, J. PDMAA/Clay nanocomposite hydrogels based on two different initiations. Colloid Surf. A Physicochem. Eng. Asp. 2011, 390, 20–24. [Google Scholar] [CrossRef]
- Li, H.; Wu, R.; Zhu, J.; Guo, P.; Ren, W.; Xu, S.; Wang, J. pH/temperature double responsive behaviors and mechanical strength of Laponite-crosslinked poly(DEA-co-DMAEMA) nanocomposite hydrogels. J. Polym. Sci. B. Polym. Phys. 2015, 53, 876–884. [Google Scholar] [CrossRef]
- Zhai, X.; Ma, Y.; Hou, C.; Gao, F.; Zhang, Y.; Ruan, C.; Pan, H.; Lu, W.W.; Liu, W. 3D-Printed high strength bioactive supramolecular polymer/clay nanocomposite hydrogel scaffold for bone regeneration. ACS Biomater. Sci. Eng. 2017, 3, 1109–1118. [Google Scholar] [CrossRef]
- Zhai, X.; Ruan, C.; Shen, J.; Zheng, C.; Zhao, X.; Pan, H.; Lu, W.W. Clay-based nanocomposite hydrogel with attractive mechanical properties and sustained bioactive ion release for bone defect repair. J. Mater. Chem. B. 2021, 9, 2394–2406. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, K.; Murata, K.; Takehisa, T. Stimuli-responsive nanocomposite gels and soft nanocomposites consisting of inorganic clays and copolymers with different chemical affinities. Macromolecules 2012, 45, 385–391. [Google Scholar] [CrossRef]
- Mujumdar, S.K.; Siegel, R.A. Introduction of pH-sensitivity into mechanically strong nanoclay composite hydrogels based on N-isopropylacrylamide. J. Polym. Sci. A. Polym. Chem. 2008, 46, 6630–6640. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Xiong, L.; Wang, T.; Lin, Z.; Liu, X.; Tong, Z. Synthesis and dual response of ionic nanocomposite hydrogels with ultrahigh tensibility and transparence. Polymer 2009, 50, 1933–1938. [Google Scholar] [CrossRef]
- Strachota, B.; Strachota, A.; Horodecka, S.; Slouf, M.; Dybal, J. Monolithic nanocomposite hydrogels with fast dual T- and pH- stimuli responsiveness combined with high mechanical properties. J. Mater. Res. Technol. 2021, 15, 6079–6097. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, M.; Hu, X.; Liu, X.; Tong, Z. Ultrahigh deformability and transparence of hectorite clay nanocomposite hydrogels with nimble pH response. Macromolecules 2009, 42, 3811–3817. [Google Scholar] [CrossRef]
- Zhu, M.; Xiong, L.; Wang, T.; Liu, X.; Wang, C.; Tong, Z. High tensibility and pH-responsive swelling of nanocomposite hydrogels containing the positively chargeable 2-(dimethylamino)ethyl methacrylate monomer. React. Funct. Polym. 2010, 70, 267–271. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, J.; Shen, K.; Wang, R.; Du, J.; Zhao, X.; Yang, Y.; Xu, K.; Zhang, Q.; Zhang, Y.; et al. Highly stretchable nanocomposite hydrogels with outstanding antifatigue fracture based on robust noncovalent interactions for wound healing. Chem. Mater. 2021, 33, 6453–6463. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, D.; Niu, Z. Removal of heavy metals by poly(vinyl pyrrolidone)/Laponite nanocomposite hydrogels. Adv. Mater. Res. 2013, 631–632, 291–297. [Google Scholar]
- Zhang, J.J.; Li, W.D.; Rong, J.H. Preparation and characterization of PVP/clay nanocomposite hydrogels. Chem. J. Chinese U. 2010, 31, 2081–2087. [Google Scholar]
- Chen, Y.; Xu, W.; Xiong, Y.; Peng, C.; Liu, W.; Zeng, G.; Peng, Y. Synthesis and characterization of pH and temperature double-sensitive nanocomposite hydrogels consisting of poly(dimethylaminoethyl methacrylate) and clay. J. Mater. Res. 2013, 28, 1394–1404. [Google Scholar] [CrossRef]
- Xia, M.; Wu, W.; Liu, F.; Theato, P.; Zhu, M. Swelling behavior of thermosensitive nanocomposite hydrogels composed of oligo(ethylene glycol) methacrylates and clay. Eur. Polym. J. 2015, 69, 472–482. [Google Scholar] [CrossRef]
- Tomas, H.; Alves, C.S.; Rodrigues, J. Laponite®: A key nanoplatform for biomedical applications? Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2407–2420. [Google Scholar] [CrossRef]
- Mongondry, P.; Tassin, J.F.; Nicolai, T. Revised state diagram of Laponite dispersions. J. Colloid Interface Sci. 2005, 283, 397–405. [Google Scholar] [CrossRef]
- Haaf, F.; Sanner, A.; Straub, F. Polymers of N-vinylpyrrolidone: Synthesis, characterization and uses. Polym. J. 1985, 17, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Fink, J.K. Handbook of Engineering and Specialty Thermoplastics, Volume 2: Water Soluble Polymers; John Wiley and Sons Scrivener Publishing LLC: Salem, MA, USA, 2011; pp. 293–342. [Google Scholar]
- Teodorescu, M.; Bercea, M. Poly(vinylpyrrolidone)—A versatile polymer for biomedical and beyond medical applications. Polym. Plast. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- D’Errico, G.; De Lellis, M.; Mangiapia, G.; Tedeschi, A.; Ortona, O.; Fusco, S.; Borzacchiello, A.; Ambrosio, L. Structural and mechanical properties of UV-photo-cross-linked poly(N-vinyl-2-pyrrolidone) hydrogels. Biomacromolecules 2008, 9, 231–240. [Google Scholar] [CrossRef]
- Thurmer, M.B.; Diehl, C.E.; Brum, F.J.B.; dos Santos, L.A. Preparation and characterization of hydrogels with potential for use as biomaterials. Mater. Res. 2014, 17 (Suppl. S1), 109–113. [Google Scholar] [CrossRef] [Green Version]
- Bühler, V. Polyvinylpyrrolidone Excipients for Pharmaceuticals: Povidone, Crospovidone and Copovidone; Springer: Berlin/Heidelberg, Germany, 2005; pp. 150–168. [Google Scholar]
- Hajikarimi, A.; Sadeghi, M. Free radical synthesis of cross-linking gelatin base poly NVP/acrylic acid hydrogel and nanoclay hydrogel as cephalexin drug deliver. J. Polym. Res. 2020, 27, 57. [Google Scholar] [CrossRef]
- Wang, D.; Hill, D.J.T.; Rasoul, F.; Whittaker, A.K. A study of the swelling and model protein release behaviours of radiation formed poly(n-vinyl-2-pyrrolidone-co-acrylic acid) hydrogels. Radiat. Phys. Chem. 2011, 80, 207–212. [Google Scholar] [CrossRef]
- Senkal, B.F.; Erkal, D.; Yavuz, E. Removal of dyes from water by poly(vinyl pyrrolidone) hydrogel. Polym. Adv. Technol. 2006, 17, 924–927. [Google Scholar] [CrossRef]
- PubChem. Laponite. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Laponite (accessed on 5 August 2022).
- Cursaru, B.; Stanescu, P.; Teodorescu, M. Compression properties of hydrogels synthesized from diepoxy-terminated poly(ethylene glycol)s and aliphatic polyamines. Mater. Plast. 2008, 45, 314–319. [Google Scholar]
- Norioka, C.; Inamoto, Y.; Hajime, C.; Kawamura, A.; Miyata, T. A universal method to easily design tough and stretchable hydrogels. NPG Asia Mater. 2021, 13, 34. [Google Scholar] [CrossRef]
- Bui, H.L.; Huang, C.J. Tough polyelectrolyte hydrogels with antimicrobial property via incorporation of natural multivalent phytic acid. Polymers 2019, 11, 1721. [Google Scholar] [CrossRef] [Green Version]
- Haraguchi, K.; Li, H.J.; Matsuda, K.; Takehisa, T.; Elliott, E. Mechanism of forming organic/inorganic network structures during in-situ free-radical polymerization in PNIPA-clay nanocomposite hydrogels. Macromolecules 2005, 38, 3482–3490. [Google Scholar] [CrossRef]
- Chatterjee, A.M.; Burns, C.M. Solvent effects in free radical copolymerization. Can. J. Chem. 1971, 49, 3249–3251. [Google Scholar] [CrossRef]
- Singh, P.; Sawhney, H.K. Studies on copolymerization of acrylamide and N-vinylpyrrolidone. J. Macromol. Sci. Part A Pure Appl. Chem. 2008, 31, 613–627. [Google Scholar] [CrossRef]
- Tapdiqov, S.; Zeynalov, N.; Babayeva, D.; Nasiyyati, E.; Humbatova, S. Copolymerization of N-vinylpyrrolidone with N,N′-methylene-bis-acrylamide: Properties and structure. Am. J. Polym. Sci. 2015, 5, 18–23. [Google Scholar] [CrossRef]
- Zhunuspayev, D.E.; Mun, G.A.; Khutoryanskiy, V.V. Temperature-responsive properties and drug stabilization capacity of amphiphilic copolymers based on N-vinylpyrrolidone and vinyl propyl ether. Langmuir 2010, 26, 7590–7597. [Google Scholar] [CrossRef] [PubMed]
- Odian, G. Principles of Polymerization, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004; p. 200. [Google Scholar]
- Srivastava, S.; Srivastava, A.K. Synthesis and characterization of poly(N-vinyl pyrrolidone) initiated by stibonium ylide. Design. Mon. Polym. 2006, 9, 29–39. [Google Scholar] [CrossRef]
- Mireles, L.K.; Wu, M.R.; Saadeh, N.; Yahia, L.; Sacher, E. Physicochemical characterization of polyvinyl pyrrolidone: A tale of two polyvinyl pyrrolidones. ACS Omega 2020, 5, 30461–30467. [Google Scholar] [CrossRef]
- Ninciuleanu, C.; Ianchis, R.; Alexandrescu, E.; Mihaescu, C.; Trica, B.; Scomoroscenco, C.; Nistor, C.; Petcu, C.; Preda, S.; Teodorescu, M. Nanocomposite hydrogels based on poly(methacrylic acid) and Laponite XLG. UPB Sci. Bull. Series B 2021, 83, 44–58. [Google Scholar]
- Timaeva, O.; Pashkin, I.; Mulakov, S.; Kuzmicheva, G.; Konarev, P.; Terekhova, R.; Sadovskaya, N.; Czakkel, O.; Prevost, S. Synthesis and physico-chemical properties of poly(N-vinyl pyrrolidone)-based hydrogels with titania nanoparticles. J. Mater. Sci. 2020, 55, 3005–3021. [Google Scholar] [CrossRef]
- Ninciuleanu, C.M.; Ianchis, R.; Alexandrescu, E.; Mihaescu, C.I.; Scomoroscenco, C.; Nistor, C.L.; Preda, S.; Petcu, C.; Teodorescu, M. The effects of monomer, crosslinking agent, and filler concentrations on the viscoelastic and swelling properties of poly(methacrylic acid) hydrogels: A comparison. Materials 2021, 14, 2305. [Google Scholar] [CrossRef]
- Hedden, R.C.; Saxena, H.; Cohen, C. Mechanical properties and swelling behaviour of end-linked poly(diethylsiloxane) networks. Macromolecules 2000, 33, 8676–8684. [Google Scholar] [CrossRef]
- Teodorescu, M.; Cursaru, B.; Stanescu, P.O. Swelling and diffusion characteristics of hydrogels synthesized from diepoxy-terminated poly(ethylene glycol)s and aliphatic polyamines. Soft Mater. 2010, 8, 288–306. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Peppas, N.A. The Swollen Polymer Network Hypothesis: Quantitative Models of Hydrogel Swelling, Stiffness, and Solute Transport. Prog. Polym. Sci. 2020, 105, 101243. [Google Scholar] [CrossRef]
- Anseth, K.S.; Bowman, C.N.; Brannon-Peppas, L. Mechanical properties of hydrogels and their experimental determination. Biomaterials 1996, 17, 1647–1657. [Google Scholar] [CrossRef]
- Subramani, R.; Izquierdo-Alvarez, A.; Bhattacharya, P.; Meerts, M.; Moldenaers, P.; Ramon, H.; Van Oosterwyck, H. The influence of swelling on elastic properties of polyacrylamide hydrogels. Front. Mater. 2020, 7, 212. [Google Scholar] [CrossRef]
- Huglin, M.B.; Rehab, M.M.A.M.; Zakaria, M.B. Thermodynamic interactions in copolymeric hydrogels. Macromolecules 1986, 19, 2986–2991. [Google Scholar] [CrossRef]
- Yan, J.; Li, J.; Runge, M.B.; Dadsetan, M.; Chen, Q.; Lu, L.; Yaszemski, M.J. Crosslinking characteristics and mechanical properties of an injectable biomaterial composed of polypropylene fumarate and polycaprolactone copolymer. J. Biomater. Sci. Polym. Ed. 2011, 22, 489–504. [Google Scholar] [CrossRef] [PubMed]



| Sample | NVP(g) | MBA 2 (g) | DVE 2 (g) | AIBN 3 (g) | XLG 4,5 (g) | DW (g) |
|---|---|---|---|---|---|---|
| HMBA4 | 2.0 | 0.111 | - | 0.007 | - | 7.88 |
| HDVE4 | 2.0 | - | 0.146 | 0.007 | - | 7.85 |
| HMBA4-XLG10 | 2.0 | 0.111 | - | 0.007 | 0.200 | 7.68 |
| HDVE4-XLG10 | 2.0 | - | 0.146 | 0.007 | 0.200 | 7.65 |
| HXLG5 | 2.0 | - | - | 0.007 | 0.100 | 7.89 |
| HXLG10 | 2.0 | - | - | 0.007 | 0.200 | 7.79 |
| HXLG15 | 2.0 | - | - | 0.007 | 0.300 | 7.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podaru, I.A.; Stănescu, P.O.; Ginghină, R.; Stoleriu, Ş.; Trică, B.; Şomoghi, R.; Teodorescu, M. Poly(N-vinylpyrrolidone)–Laponite XLG Nanocomposite Hydrogels: Characterization, Properties and Comparison with Divinyl Monomer-Crosslinked Hydrogels. Polymers 2022, 14, 4216. https://doi.org/10.3390/polym14194216
Podaru IA, Stănescu PO, Ginghină R, Stoleriu Ş, Trică B, Şomoghi R, Teodorescu M. Poly(N-vinylpyrrolidone)–Laponite XLG Nanocomposite Hydrogels: Characterization, Properties and Comparison with Divinyl Monomer-Crosslinked Hydrogels. Polymers. 2022; 14(19):4216. https://doi.org/10.3390/polym14194216
Chicago/Turabian StylePodaru, Ionela Alice, Paul O. Stănescu, Raluca Ginghină, Ştefania Stoleriu, Bogdan Trică, Raluca Şomoghi, and Mircea Teodorescu. 2022. "Poly(N-vinylpyrrolidone)–Laponite XLG Nanocomposite Hydrogels: Characterization, Properties and Comparison with Divinyl Monomer-Crosslinked Hydrogels" Polymers 14, no. 19: 4216. https://doi.org/10.3390/polym14194216
APA StylePodaru, I. A., Stănescu, P. O., Ginghină, R., Stoleriu, Ş., Trică, B., Şomoghi, R., & Teodorescu, M. (2022). Poly(N-vinylpyrrolidone)–Laponite XLG Nanocomposite Hydrogels: Characterization, Properties and Comparison with Divinyl Monomer-Crosslinked Hydrogels. Polymers, 14(19), 4216. https://doi.org/10.3390/polym14194216








