Silicon-Based Scaffold for Wound Healing Skin Regeneration Applications: A Concise Review
Abstract
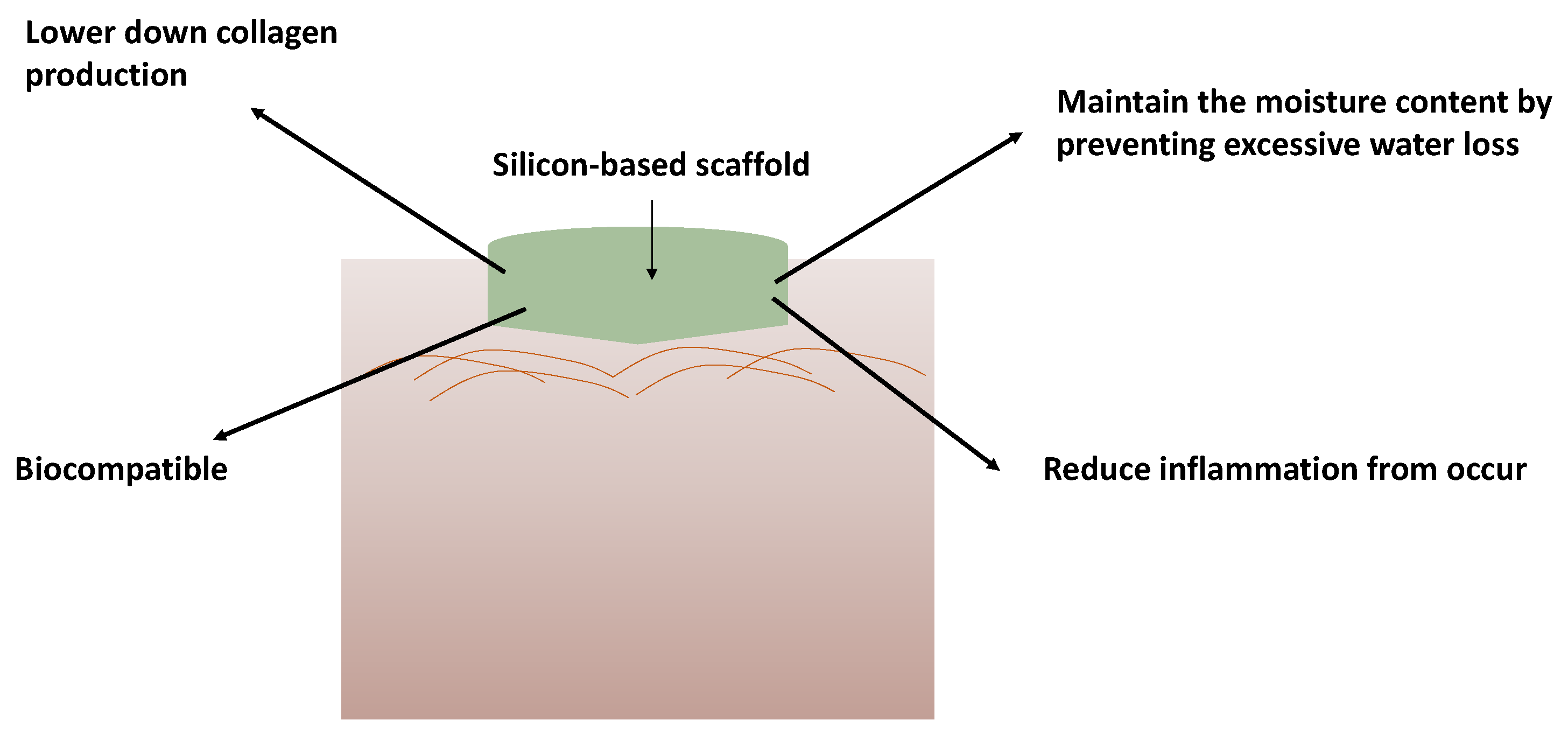
1. Introduction
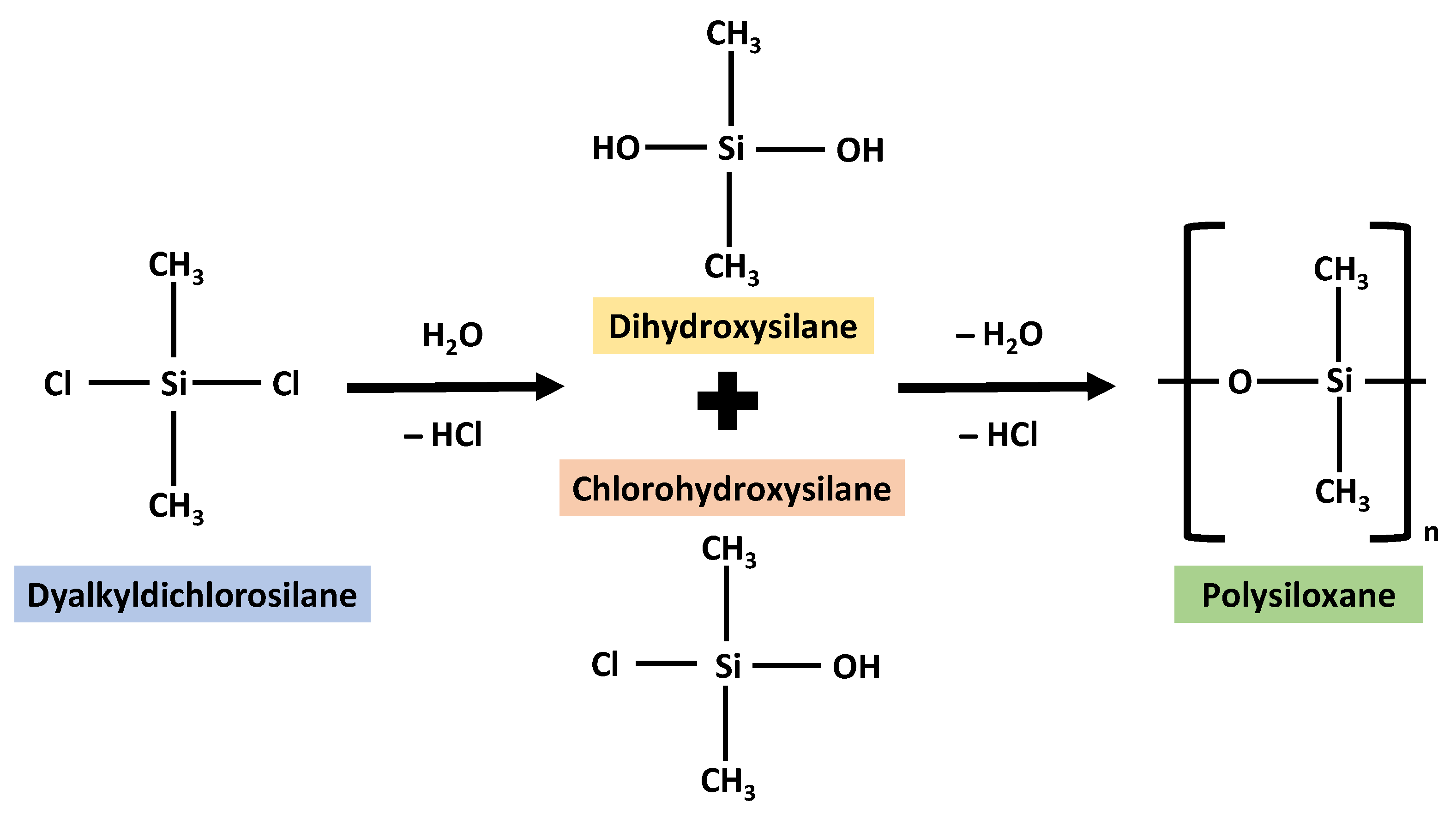
2. Silicon as a Biomaterial
3. Silicon Forms and Applications
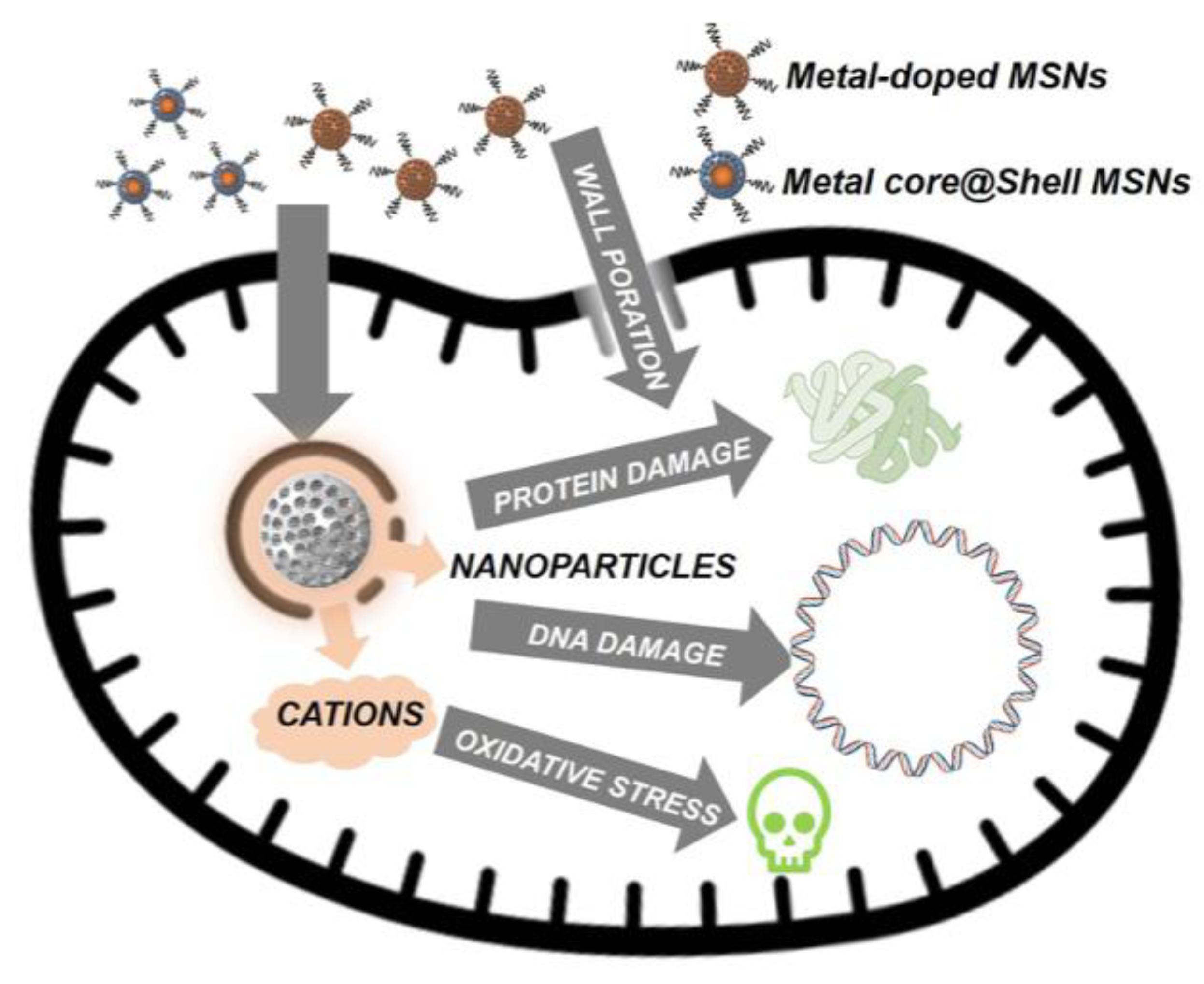
3.1. Nanoparticles
3.2. Fibres
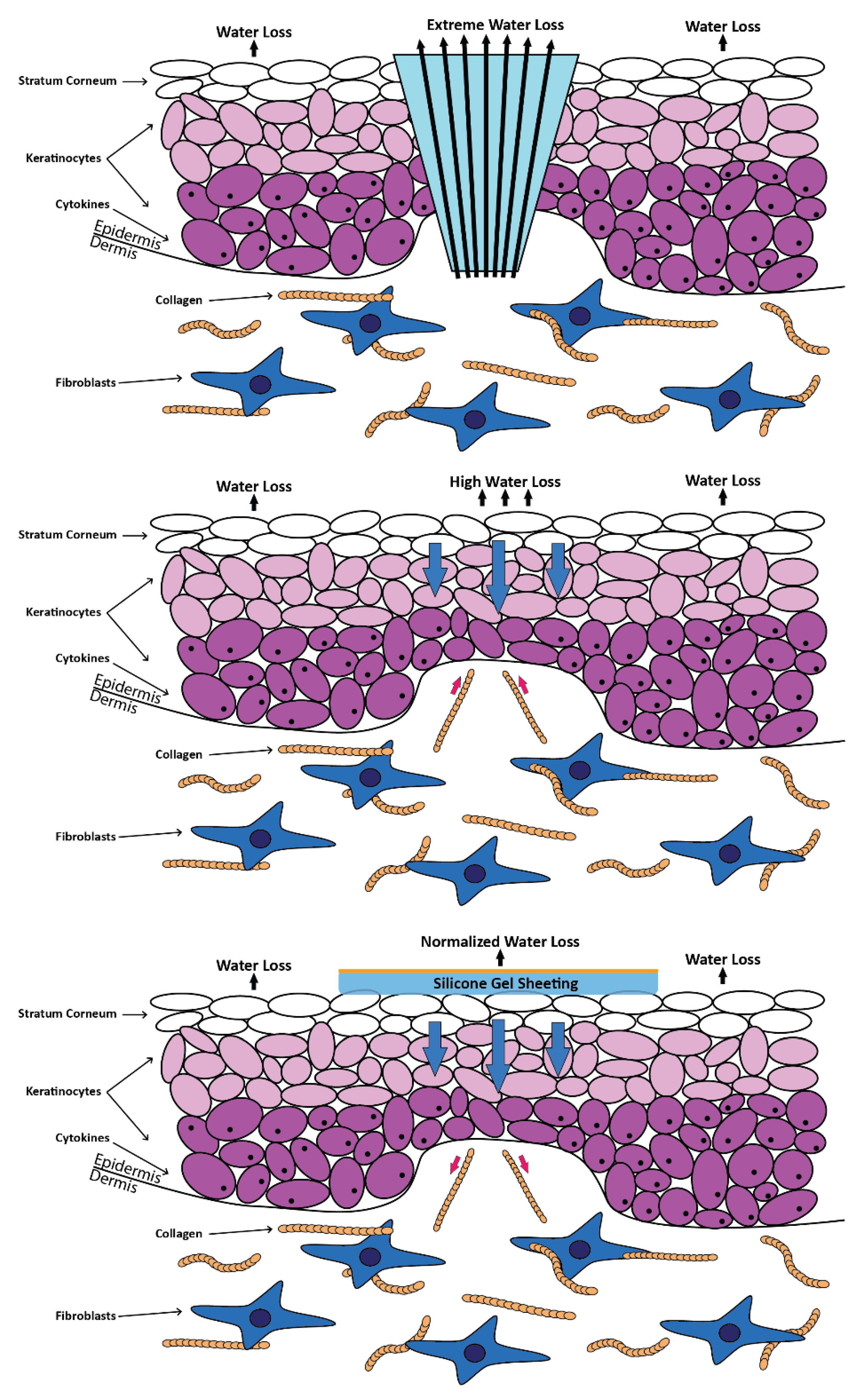
3.3. Hydrogels
3.4. Films
3.5. Porous Sponges
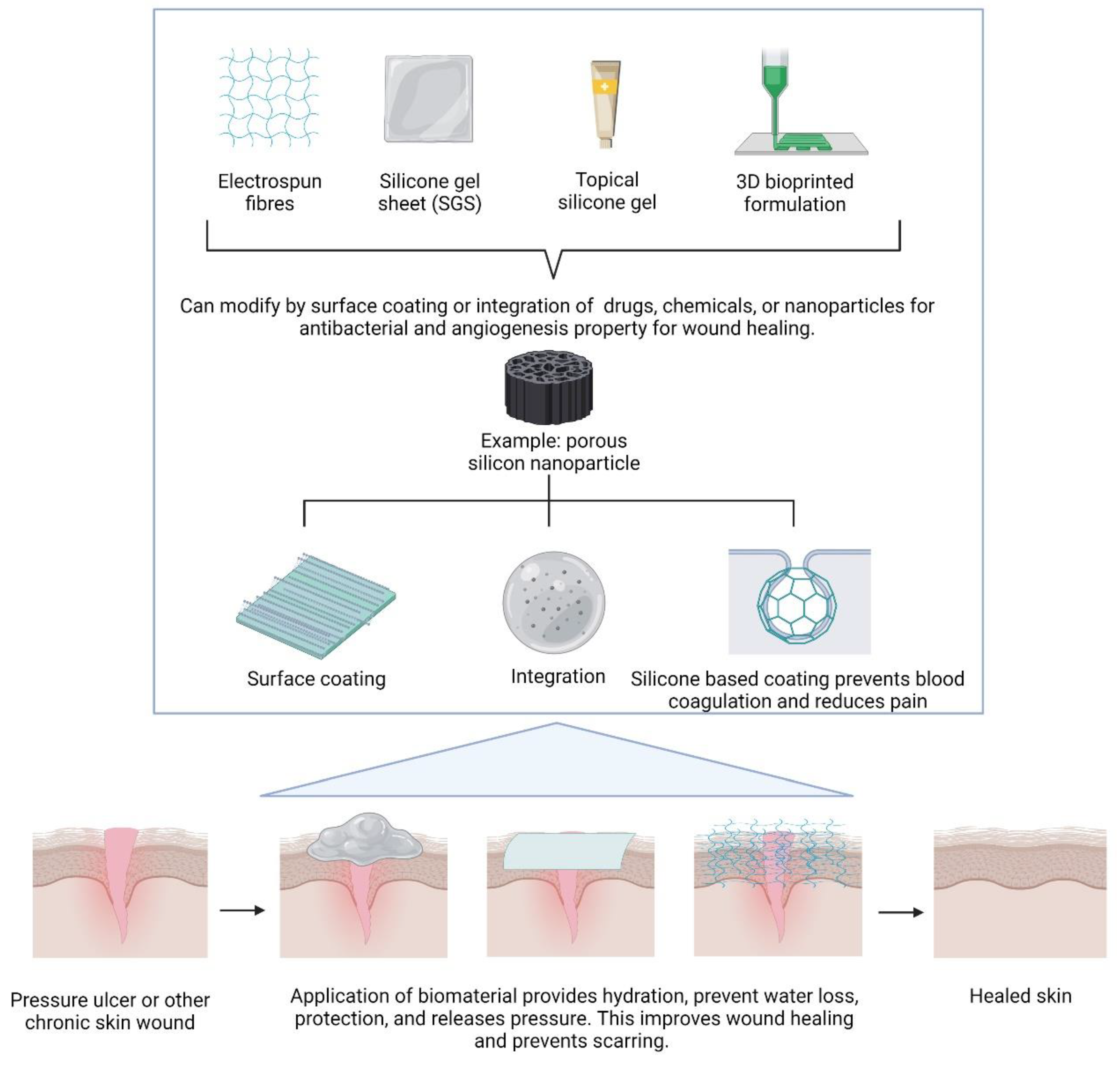
4. Silicon-Based Biomaterials in Wound Healing
4.1. Cell Proliferation
4.2. Cell Viability
5. Limitations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zulkiflee, I.; Fauzi, M.B. Gelatin-polyvinyl alcohol film for tissue engineering: A concise review. Biomedicines 2021, 9, 979. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. Challenges With the Development of Biomaterials for Sustainable Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 127. [Google Scholar] [CrossRef]
- Metcalfe, A.D.; Ferguson, M.W.J. Tissue engineering of replacement skin: The crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J. R. Soc. Interface 2007, 4, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Van Der Veen, V.C.; Boekema, B.K.H.L.; Ulrich, M.M.W.; Middelkoop, E. New dermal substitutes. Wound Repair Regen. 2011, 19 (Suppl. S1), s59–s65. [Google Scholar] [CrossRef] [PubMed]
- Ter Horst, B.; Chouhan, G.; Moiemen, N.S.; Grover, L.M. Advances in keratinocyte delivery in burn wound care. Adv. Drug Deliv. Rev. 2018, 123, 18. [Google Scholar] [CrossRef]
- Andorko, J.I.; Jewell, C.M. Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine. Bioeng. Transl. Med. 2017, 2, 139. [Google Scholar] [CrossRef]
- Bayat, A.; McGrouther, D.A.; Ferguson, M.W.J. Skin scarring. BMJ Br. Med. J. 2003, 326, 88. [Google Scholar] [CrossRef] [PubMed]
- Simman, R.; Phavixay, L. Split-thickness skin grafts remain the gold standard for the closure of large acute and chronic wounds. J. Am. Col. Certif. Wound Spec. 2011, 3, 55–59. [Google Scholar] [CrossRef][Green Version]
- Shakespeare, P.G. The role of skin substitutes in the treatment of burn injuries. Clin. Dermatol. 2005, 23, 413–418. [Google Scholar] [CrossRef]
- Zare, M.; Ghomi, E.R.; Venkatraman, P.D.; Ramakrishna, S. Silicone-based biomaterials for biomedical applications: Antimicrobial strategies and 3D printing technologies. J. Appl. Polym. Sci. 2021, 138, 50969. [Google Scholar] [CrossRef]
- Mohanty, S.; Alm, M.; Hemmingsen, M.; Dolatshahi-Pirouz, A.; Trifol, J.; Thomsen, P.; Dufva, M.; Wolff, A.; Emnéus, J. 3D Printed Silicone-Hydrogel Scaffold with Enhanced Physicochemical Properties. Biomacromolecules 2016, 17, 1321–1329. [Google Scholar] [CrossRef]
- Durante, C.M. Products & technology Silicone therapy for the treatment and prevention of problematic scars: A practical guideline. Wounds Int. 2020, 11, 64–69. [Google Scholar]
- Odian, G. Step Polymerization. In Principles of Polymerization; John Wiley and Sons: Hoboken, NJ, USA, 2004; pp. 39–197. [Google Scholar] [CrossRef]
- Nayfeh, M.H.; Mitas, L. Silicon nanoparticles: New photonic and electronic material at the transition between solid and molecule. In Nanosilicon; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1–78. [Google Scholar]
- Nishimura, H.; Ritchie, K.; Kasai, R.S.; Goto, M.; Morone, N.; Sugimura, H.; Tanaka, K.; Sase, I.; Yoshimura, A.; Nakano, Y.; et al. Biocompatible fluorescent silicon nanocrystals for single-molecule tracking and fluorescence imaging. J. Cell Biol. 2013, 202, 967–983. [Google Scholar] [CrossRef]
- Gyak, K.-W.; Jeon, S.; Ha, L.; Kim, S.; Kim, J.; Lee, K.-S.; Choi, H.; Kim, D.-P. Biocompatible Microrobots: Magnetically Actuated SiCN-Based Ceramic Microrobot for Guided Cell Delivery. Adv. Healthc. Mater. 2019, 8, 1970085. [Google Scholar] [CrossRef]
- Guo, D.; Ji, X.; Peng, F.; Zhong, Y.; Chu, B.; Su, Y.; He, Y. Photostable and Biocompatible Fluorescent Silicon Nanoparticles for Imaging-Guided Co-Delivery of siRNA and Doxorubicin to Drug-Resistant Cancer Cells. Nano-Micro Lett. 2019, 11, 27. [Google Scholar] [CrossRef]
- Liu, H.; Yuan, M.; Liu, Y.; Guo, Y.; Xiao, H.; Guo, L.; Liu, F. Self-Monitoring and Self-Delivery of Self-Assembled Fluorescent Nanoparticles in Cancer Therapy. Int. J. Nanomed. 2021, 16, 2487. [Google Scholar] [CrossRef]
- Peng, F.; Su, Y.; Zhong, Y.; Fan, C.; Lee, S.T.; He, Y. Silicon nanomaterials platform for bioimaging, biosensing, and cancer therapy. Acc. Chem. Res. 2014, 47, 612–623. [Google Scholar] [CrossRef]
- Su, Y.; Ji, X.; He, Y. Water-Dispersible Fluorescent Silicon Nanoparticles and their Optical Applications. Adv. Mater. 2016, 28, 10567–10574. [Google Scholar] [CrossRef]
- Wang, H.; He, Y. Recent advances in silicon nanomaterial-based fluorescent sensors. Sensors 2017, 17, 268. [Google Scholar] [CrossRef] [PubMed]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica Nanoparticles—A Versatile Tool for the Treatment of Bacterial Infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.R.; Vallet-Regí, M. Recent Advances Toward the Use of Mesoporous Silica Nanoparticles for the Treatment of Bacterial Infections. Int. J. Nanomed. 2021, 16, 4409–4430. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pan, J.; Yang, Y.; Cui, X.; Qu, Y. Production of Minor Ginenosides from Panax notoginseng by Microwave Processing Method and Evaluation of Their Blood-Enriching and Hemostatic Activity. Molecules 2018, 23, 1243. [Google Scholar] [CrossRef] [PubMed]
- Grotheer, V.; Windolf, J.; Suschek, C.V. Evaluation of pro-angiogenic properties of an inorganic silica gel fibre fleece. J. Wound Care 2021, 30, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Grotheer, V.; Goergens, M.; Fuchs, P.C.; Dunda, S.; Pallua, N.; Windolf, J.; Suschek, C.V. The performance of an orthosilicic acid-releasing silica gel fiber fleece in wound healing. Biomaterials 2013, 34, 7314–7327. [Google Scholar] [CrossRef] [PubMed]
- Zawani, M.; Fauzi, M.B. Injectable hydrogels for chronic skin wound management: A concise review. Biomedicines 2021, 9, 527. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.; Yong, A. The use of silicone gel to enhance skin wound healing by secondary intention following tumor excision on the scalp and extremities: A descriptive study. J. Am. Acad. Dermatol. 2018, 79, AB294. [Google Scholar] [CrossRef]
- Wang, F.; Li, X.; Wang, X.; Jiang, X. Efficacy of topical silicone gel in scar management: A systematic review and meta-analysis of randomised controlled trials. Int. Wound J. 2020, 17, 765. [Google Scholar] [CrossRef]
- Zappi, E.; Barnett, J.G.; Zappi, M.; Barnett, C.R. The long-term host response to liquid silicone injected during soft tissue augmentation procedures: A microscopic appraisal. Dermatol. Surg. 2007, 33 (Suppl. S2), S186–S192. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-S.; Yeh, C.-H.; Tung, C.-L.; Jiang, C.-H.; Yeh, M.-L. Mechanical Evaluation of Silicone Gel on Wound Healing of Rat Skin. Wounds 2014, 26, E7–E14. [Google Scholar]
- Sun, D.; Guo, S.-Y.; Yang, L.; Wang, Y.-R.; Wei, X.-H.; Song, S.; Yang, Y.-W.; Gan, Y.; Wang, Z.-T. Silicone elastomer gel impregnated with 20(S)-protopanaxadiol-loaded nanostructured lipid carriers for ordered diabetic ulcer recovery. Acta Pharmacol. Sin. 2020, 41, 119–128. [Google Scholar] [CrossRef]
- Tang, H.; Lv, G.; Fu, J.; Niu, X.; Li, Y.; Zhang, M.; Zhang, G.; Hu, D.; Chen, X.; Lei, J.; et al. An open, parallel, randomized, comparative, multicenter investigation evaluating the efficacy and tolerability of Mepilex Ag versus silver sulfadiazine in the treatment of deep partial-thickness burn injuries. J. Trauma Acute Care Surg. 2015, 78, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Dalziel, S.R.; Herd, E.; Johnson, K.; Wong She, R.; Shepherd, M. A Randomized Controlled Study of Silver-Based Burns Dressing in a Pediatric Emergency Department. J. Burn Care Res. 2016, 37, e340–e347. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.W.G.; See, J.L.; Yang, S.-H.; Tan, B.K. Early Experience with Biobranetm for Definitive Coverage of Tangentially Excised Partial-Thickness Thermal Burns. World J. Plast. Surg. 2021, 10, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Woodroof, E.A. The Search for an Ideal Temporary Skin Substitute: AWBAT. Eplasty 2009, 9, e10. [Google Scholar] [PubMed]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C 2015, 48, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Westra, I.; Niessen, F.B. Topical Silicone Sheet Application in the Treatment of Hypertrophic Scarsand Keloids. J. Clin. Aesthet. Dermatol. 2016, 9, 28. [Google Scholar] [PubMed]
- Bleasdale, B.; Finnegan, S.; Murray, K.; Kelly, S.; Percival, S.L. The Use of Silicone Adhesives for Scar Reduction. Adv. Wound Care 2015, 4, 422–430. [Google Scholar] [CrossRef]
- Chen, X.; Wo, F.; Jin, Y.; Tan, J.; Lai, Y.; Wu, J. Drug-Porous Silicon Dual Luminescent System for Monitoring and Inhibition of Wound Infection. ACS Nano 2017, 11, 7938–7949. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Xiao, S.J.; Wang, J.; Guo, D.J. Stability improvement of porous silicon surface structures by grafting polydimethylsiloxane polymer monolayers. Thin Solid Films 2005, 474, 306–309. [Google Scholar] [CrossRef]
- Gu, L.; Hall, D.J.; Qin, Z.; Anglin, E.; Joo, J.; Mooney, D.J.; Howell, S.B.; Sailor, M.J. In vivo time-gated fluorescence imaging with biodegradable luminescent porous silicon nanoparticles. Nat Commun. 2013, 4, 2326. [Google Scholar] [CrossRef]
- Jenie, S.N.A.; Plush, S.E.; Voelcker, N.H. Recent Advances on Luminescent Enhancement-Based Porous Silicon Biosensors. Pharm. Res. 2016, 33, 2314–2336. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jia, Z.; Trush, M.A. Defining ROS in Biology and Medicine. React. Oxyg. Species 2016, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Loni, A.; Defforge, T.; Caffull, E.; Gautier, G.; Canham, L.T. Porous silicon fabrication by anodisation: Progress towards the realisation of layers and powders with high surface area and micropore content. Microporous Mesoporous Mater. 2015, 213, 188–191. [Google Scholar] [CrossRef]
- Bimbo, L.M.; Sarparanta, M.; Santos, H.A.; Airaksinen, A.J.; Mäkilä, E.; Laaksonen, T.; Peltonen, L.; Lehto, V.-P.; Hirvonen, J.; Salonen, J. Biocompatibility of thermally hydrocarbonized porous silicon nanoparticles and their biodistribution in rats. ACS Nano 2010, 4, 3023–3032. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Bai, Y.; Zhao, J.; Xia, H.; Kong, Y.; Yao, Z.; Yan, R.; Zhang, X.; Hu, X.; Liu, M.; et al. Silicone rubber membrane with specific pore size enhances wound regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e905–e917. [Google Scholar] [CrossRef]
- Bianchera, A.; Catanzano, O.; Boateng, J.; Elviri, L. The Place of Biomaterials in Wound Healing. In Therapeutic Dressings and Wound Healing Applications; John Wiley and Sons: Hoboken, NJ, USA, 2020; pp. 337–366. [Google Scholar] [CrossRef]
- Tolksdorf, J.; Horch, R.E.; Grüner, J.S.; Schmid, R.; Kengelbach-Weigand, A.; Schubert, D.W.; Werner, S.; Schneidereit, D.; Friedrich, O.; Ludolph, I. Size matters—In vitro behaviour of human fibroblasts on textured silicone surfaces with different pore sizes. J. Mater. Sci. Mater. Med. 2020, 31, 23. [Google Scholar] [CrossRef]
- Grotheer, V.; Eckhardt, D.; Schulz, J.; Messel, O.; Windolf, J.; Suschek, C.V. The Effect of Aging and Culture Senescence on Fibroblast Proliferation and Osteogenic Differentiation. J. Tissue Sci. Eng. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Han, P.; Wu, C.; Xiao, Y. The effect of silicate ions on proliferation, osteogenic differentiation and cell signalling pathways (WNT and SHH) of bone marrow stromal cells. Biomater. Sci. 2013, 1, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.Y.; Ding, S.J.; Chang, H.C. The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater. 2011, 7, 2604–2614. [Google Scholar] [CrossRef]
- Kubaski, F.; Osago, H.; Mason, R.W.; Yamaguchi, S.; Kobayashi, H.; Tsuchiya, M.; Orii, T.; Tomatsu, S. Glycosaminoglycans detection methods: Applications of mass spectrometry. Mol. Genet. Metab. 2017, 120, 67. [Google Scholar] [CrossRef]
- Schwarz, K. A bound form of silicon in glycosaminoglycans and polyuronides. Proc. Natl. Acad. Sci. USA 1973, 70, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, M.K.; Rodriguez-Devora, J.I.; Fraser, K.; Tseng, T.L.B. Silicon substrate as a novel cell culture device for myoblast cells. J. Biomed. Sci. 2014, 21, 47. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulkiflee, I.; Masri, S.; Zawani, M.; Salleh, A.; Amirrah, I.N.; Wee, M.F.M.R.; Yusop, S.M.; Fauzi, M.B. Silicon-Based Scaffold for Wound Healing Skin Regeneration Applications: A Concise Review. Polymers 2022, 14, 4219. https://doi.org/10.3390/polym14194219
Zulkiflee I, Masri S, Zawani M, Salleh A, Amirrah IN, Wee MFMR, Yusop SM, Fauzi MB. Silicon-Based Scaffold for Wound Healing Skin Regeneration Applications: A Concise Review. Polymers. 2022; 14(19):4219. https://doi.org/10.3390/polym14194219
Chicago/Turabian StyleZulkiflee, Izzat, Syafira Masri, Mazlan Zawani, Atiqah Salleh, Ibrahim Nor Amirrah, Mohd Farhanulhakim Mohd Razip Wee, Salma Mohamad Yusop, and Mh Busra Fauzi. 2022. "Silicon-Based Scaffold for Wound Healing Skin Regeneration Applications: A Concise Review" Polymers 14, no. 19: 4219. https://doi.org/10.3390/polym14194219
APA StyleZulkiflee, I., Masri, S., Zawani, M., Salleh, A., Amirrah, I. N., Wee, M. F. M. R., Yusop, S. M., & Fauzi, M. B. (2022). Silicon-Based Scaffold for Wound Healing Skin Regeneration Applications: A Concise Review. Polymers, 14(19), 4219. https://doi.org/10.3390/polym14194219












