Film-Forming Polymers for Tooth Erosion Prevention
Abstract
:1. Introduction
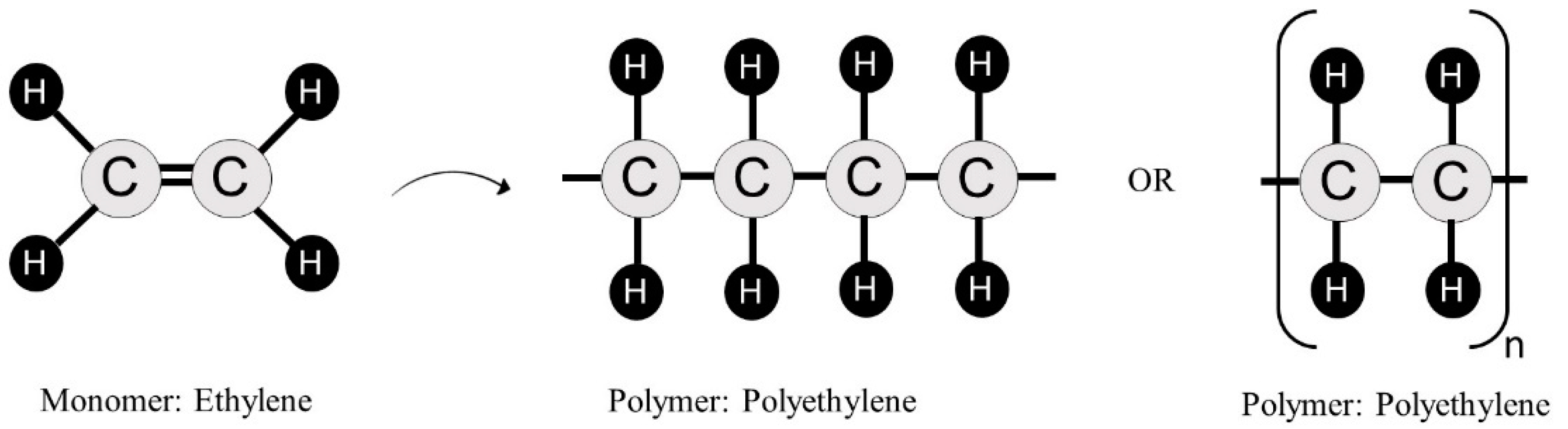
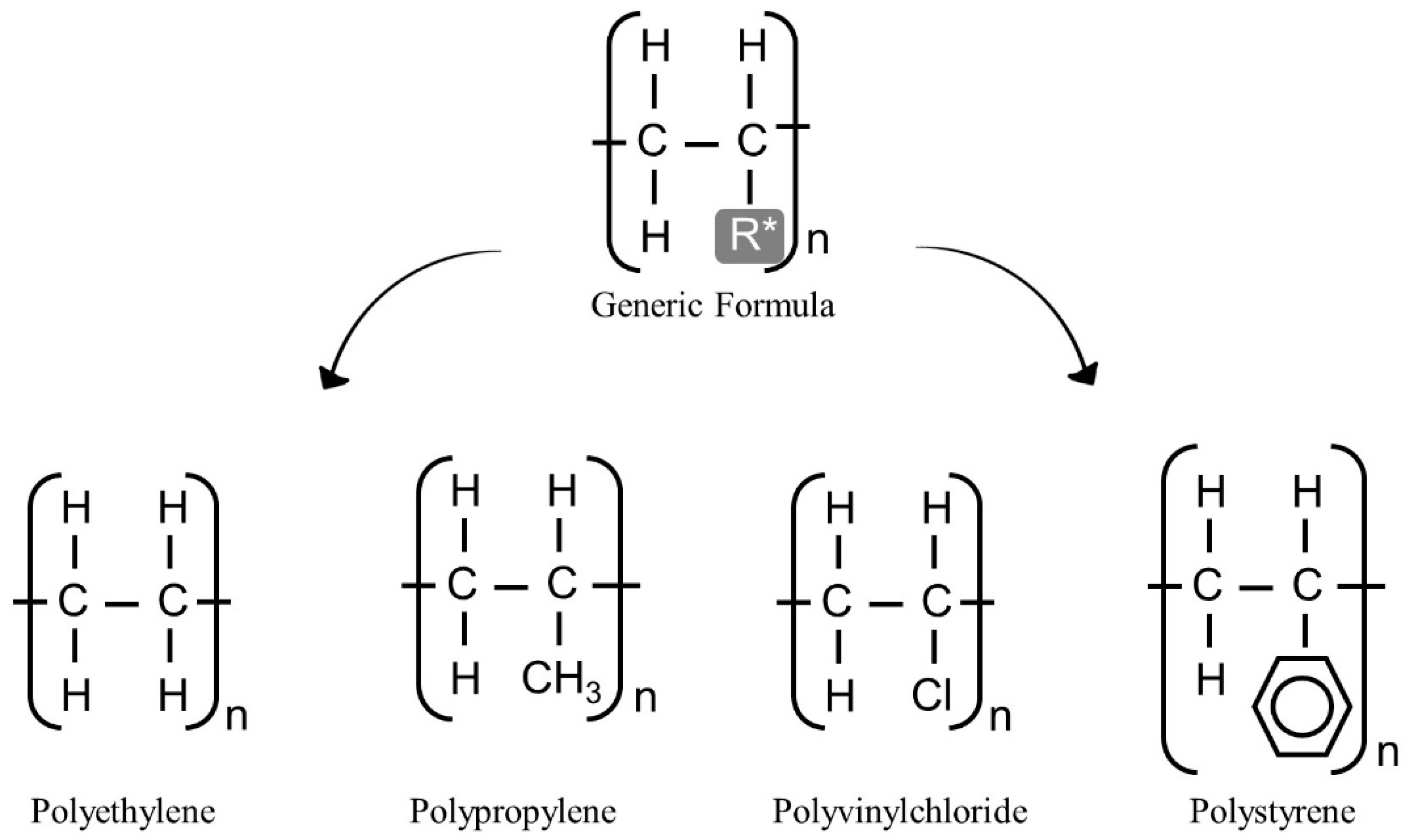
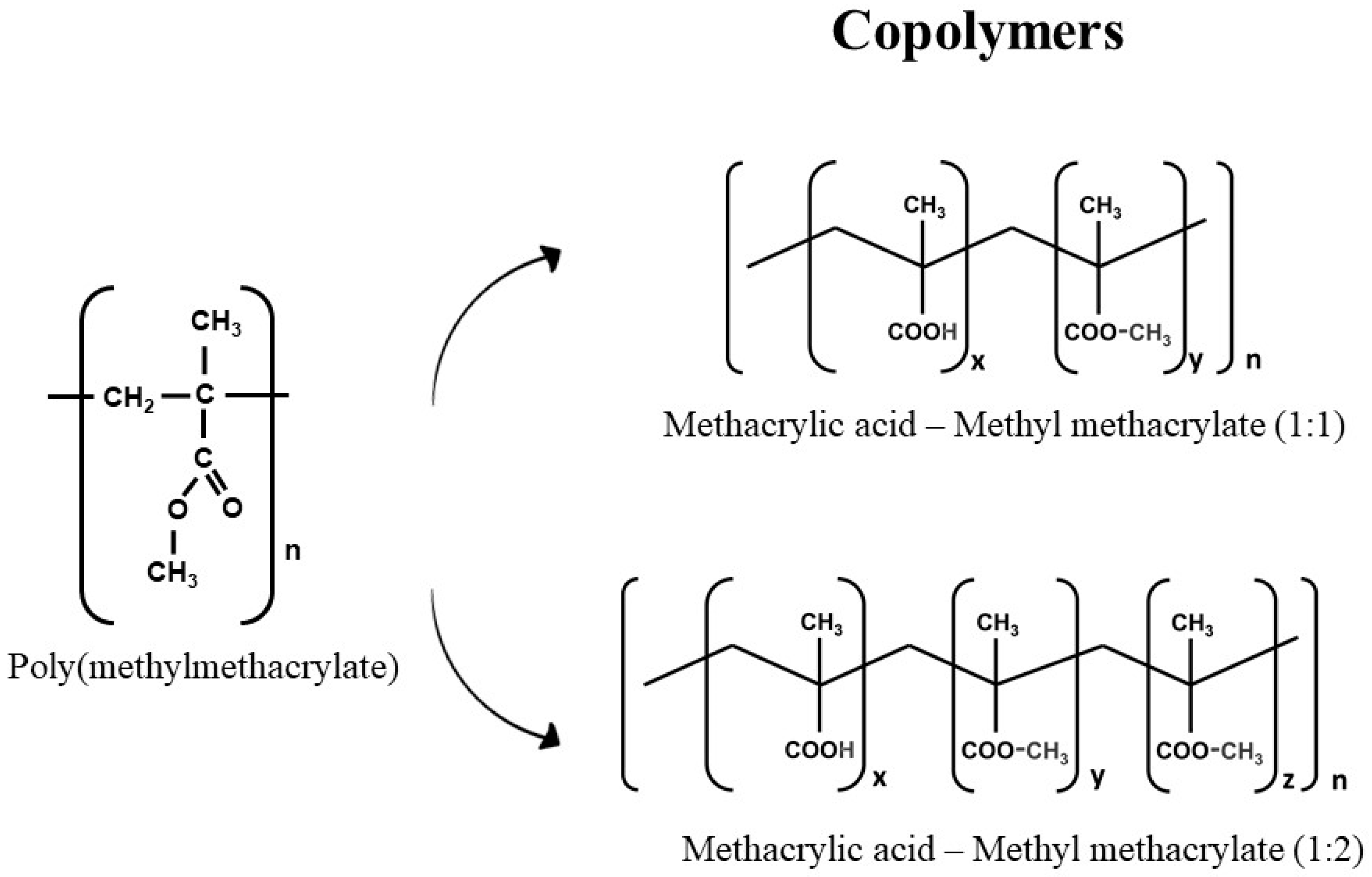
2. Polymers’ Structure
3. Polymers on ETW Prevention
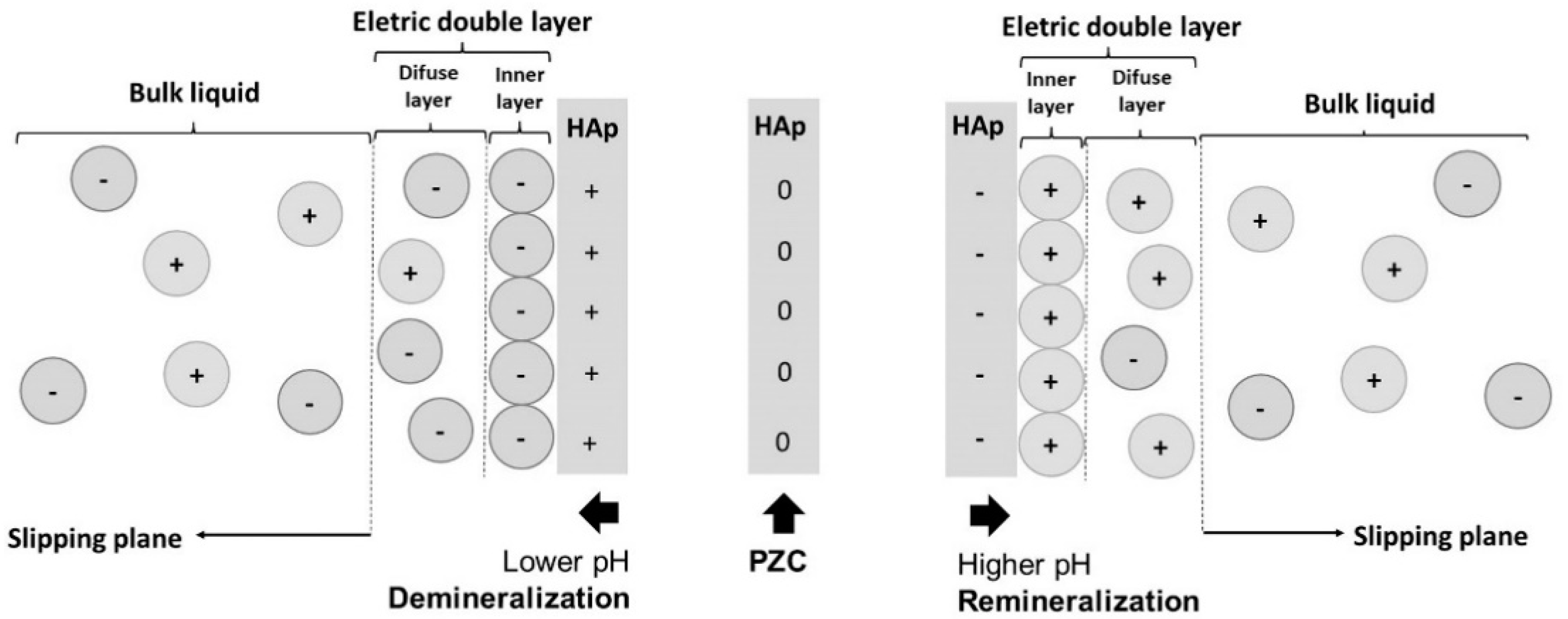
3.1. Mechanism of Action
3.2. Polymers as Active Ingredients in Oral Care Products
| Study | Type | Substrate | Acid Challenge | Intermittent Storage of Samples | Anti-Erosive Treatment | Polymer Effect | |||
|---|---|---|---|---|---|---|---|---|---|
| Polymers Tested | Concentration | Duration | Association with Fluorides | ||||||
| Augusto et al., 2021 [62] | In vitro | Enamel | 0.3% citric acid–pH 2.6 (5 min, 4×/day, 5 days) | Human saliva | Aminomethacrylate copolymer (AMC) | 20 g/L | 2 min, 2×/day, 5 days | 225 ppm F− (NaF); 225 ppm F− (NaF) + 800 ppm Sn2+ (SnCl2) | AMC has potential to enhance the anti-erosive effect of fluoride solutions. |
| Luka et al., 2021 [63] | In vitro | Enamel | 0,5% citric acid–pH 2.4 (2 min, 6×/day, 10 days) | Mineral salt solution | Chitosan with different viscosities (50 mPas, 500 mPas) | 5 g/L | 2 min, 2×/day, 10 days | 500 ppm F− (AmF) + 800 ppm Sn2+ (SnCl2) | Chitosan and F/Sn solution was able to reduce the tissue loss under erosive and under erosive– abrasive conditions. |
| Sakae et al., 2020 [64] | In situ | Enamel | 1% citric acid–pH 2.3 (5 min, 4×/day, 5 days) | Human saliva | Propylene glycol alginate (PGA) | 1 g/L | 2 min, 2×/day, 5 days | 225 ppm F− (NaF); | PGA was not able to improve the protective effect of NaF against erosive enamel wear. |
| Souza et al., 2020 [65] | In vitro | Dentin | 0,1% citric acid–pH 2,5 (90 s, 4×/day, 7 days) | Mineral salt solution | Chitosan with different viscosities (500 mPas, 2000 mPas) | 5 g/L | 30 s, 1×/day, 7 days | 190 ppm F− (NaF); 300 ppm F− (NaF) + 190 ppm Ti4+ (TiF4) | Only chitosan 500 mPas was able to reduce dentin loss compared to the negative control. TiF4/NaF, whether with or without chitosan, had no protective effect. |
| Avila et al., 2020 [39] | In situ | Enamel | 1% citric acid–pH 2.3 (5 min, 4×/day, 5 days) | Human saliva | Carbopol 980 | 1 g/L | 1 min, 2×/day, 5 days | 225 ppm F− (NaF); 225 ppm F− (NaF) + 800 ppm Sn2+ (SnCl2) | The association of Carbopol to fluoride and stannous (FS) significantly protected the enamel against erosive wear, but it was not significantly superior to FS only. |
| Bezerra et al., 2019 [66] | In vitro | Enamel and dentin | 0.3% citric acid–pH 2.6 (5 min, 4×/day, 5 days) | Human saliva | Gantrez MS-955 Plasdone K-29/32 PGA: Propylene glycol alginate CMC: Carboxymethylcellulose | 1 g/L | 2 min, 2×/day, 5 days | 225 ppm F− (NaF); 225 ppm F− (NaF) + 800 ppm Sn2+ (SnCl2) | For enamel, Gantrez, Plasdone, and CMC exhibited an anti-erosive effect, and PGA increased the protection of NaF. For dentin, only Gantrez reduced erosion. |
| Beltrame et al. 2018 [67] | In vitro | Dentin | 0.5% citric acid–pH 2.3 (2 min, 6×/day, 5 days) | Mineral salt solution | Phosphorylated chitosan | 5 g/L | 2 min, 6×/day, 5 days | No | The treatment reduced erosive wear by approximately 32% in neutral and alkaline pH, when compared to the negative control. |
| Avila et al., 2017 [38] | In vitro | Enamel | 0.3% citric acid–pH 2.6 (2 min, 6×/day, 6 days) | Mineral salt solution | Carbopol 980 Carboxymethylcellulose Aristoflex AVC | 1 g/L | 1 min, 6×/day, 5 days | 900 ppm F− (NaF) | Carbopol 980 reduced the erosive wear magnitude to the same extent as the sodium fluoride. |
| João-Souza et al., 2017 [68] | In situ | Enamel | 1% citric acid–pH 2.4 (2 min, 6×/day, 5 days) | Human saliva | LPP: Sodium linear polyphosphate | 20 g/L | 2 min, 2×/day, 5 days | 225 ppm F− (NaF) + 800 ppm Sn2+ (SnCl2) | The presence of LPP did not enhance the anti-erosive effect of the fluoridated solution. |
| Pini et al., 2016 [22] | In vitro | Enamel | 0.5% citric acid–pH 2.8 (2 min, 6×/day, 10 days) | Mineral salt solution | Chitosan with different molecular weight (150, 350, 400, 450 kDa) | 5 g/L | 2 min, 2×/day, 10 days | 500 ppm F− (AmF) + 800 ppm Sn2+ (SnCl2) | Under erosive conditions, the 450 kDa chitosan completely inhibited tissue loss, whereas under abrasive/erosive challenges, the 150 and 350 kDa chitosan showed the best performance, reducing by ~60% the erosive wear compared to the negative control. |
| Scaramucci et al., 2016 [69] | In vitro | Enamel and dentin | 1% citric acid–pH 2.4 (5 min, 6×/day, 5 days) | Human saliva | Sodium linear polyphosphate | 20 g/L | 2 min, 3×/day, 5 days | 225 ppm F− (NaF); 225 ppm F− (NaF) + 800 ppm Sn2+ (SnCl2) | The combination of NaF with SnCl2 and/or LPP can protect the enamel against erosion. For dentin, neither toothbrushing nor the test solutions influenced the development of erosion. |
| Scaramucci et al., 2015 [33] | In vitro | Enamel and dentin | 0.3% citric acid–pH 3.8 and 1% citric acid–pH 2.4 (5 min, 6×/day, 5 days) | Mineral salt solution | Linear sodium polyphosphate Sodium pyrophosphate tetrabasic Sodium tripolyphosphate Sodium caseinate Bovine serum albumin | 2 g/L and 20 g/L | 2 min, 3×/day, 5 days | 225 ppm F− (NaF); 225 ppm F− (NaF) + 800 ppm Sn2+ (SnCl2) | The addition of LPP and/or SnCl2 can improve the fluoride solution’s protection against erosion of enamel but not of dentine. |
| Lei et al., 2014 [70] | In vitro | Enamel | 1% citric acid–pH 3.8 (5 min, 1×, 1 day) | No | Synthetic amphiphilic diblock copolymer | 0.06, 0.12, 0.25, 0.5 and 1 g/L | 5 min, 1× | No | The treatment with the polymer decreased the mineral loss of hydroxyapatite by 36–46% compared to the untreated control and protected the surface morphology of the enamel specimen following exposure to acid. |
| White et al., 2011 [37] | In vitro | Enamel | 0.3% citric acid–pH 3.2 (10 min, 9×, 1 day) | No | Casein CPP: Casein phosphopeptide GMP: Glycomacropeptide | 5 g/L | 10 min, 1× | 300 ppm F− (NaF) | Casein and NaF reduced enamel surface softening compared to the negative control, but CPP and GMP did not. |
| Gracia et al., 2010 [71] | In vitro | Enamel | 1% citric acid–pH 3.8 (5 min, 1×, 1 day) | No | Combination of 0.20% carboxymethylcellulose, 0.010% xanthan gum and 0.75% copovidone | - | 1 min, 1× | 300 mg/L fluoride | The treatment with the polymer significantly reduced the lesion depth and enhanced the delivery of fluoride to the surface of the lesion. |
- Substrate: As the anti-erosive effect of the polymers can vary on enamel and dentin, and dentin is often exposed even at early stages, both substrates should be investigated. Although bovine teeth can be used as a substitute for human teeth, caution must be taken to extrapolate the results, since there are differences regarding susceptibility to demineralization processes and interaction with active agents [72,73]. Thus, if possible and ethically justifiable, human teeth should be preferred.
- Intermittent storage of samples: The efficacy of anti-erosive agents can be affected by protein interactions; thus, the use of human saliva is meaningful if the clinical effect is under investigation [74]. In case of evaluation of the direct interaction between the inorganic compounds of the dental hard tissue and the biopolymer, the inclusion of human saliva might hamper analysis [63].
- Anti-erosive treatment: As the binding affinity seems to be dependent on the polymer’s molecular weight [22], concentration, and pH of the polymer solution or the demineralization solution [70], these parameters should only be changed if it is the intention of the study to investigate their impact; otherwise, these parameters should be kept constant in order to allow comparability.
- Association to fluorides: Since fluorides remain the standard in erosion prevention, and polymers added to oral care products may complement the protective effect of fluorides, the interaction between the efficacy of fluorides and polymers should be addressed.
3.3. Polymers as Active Ingredients in Acidic Beverages
3.4. Polymers as Active Ingredients in Antacid Formulations
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Schlueter, N.; Luka, B. Erosive tooth wear—A review on global prevalence and on its prevalence in risk groups. Br. Dent. J. 2018, 224, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, N.; Amaechi, B.T.; Bartlett, D.; Buzalaf, M.A.R.; Carvalho, T.S.; Ganss, C.; Hara, A.T.; Huysmans, M.-C.D.; Lussi, A.; Moazzez, R.; et al. Terminology of Erosive Tooth Wear: Consensus Report of a Workshop Organized by the ORCA and the Cariology Research Group of the IADR. Caries Res. 2019, 54, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Attin, T.; Koidl, U.; Buchalla, W.; Schaller, H.G.; Kielbassa, A.M.; Hellwig, E. Correlation of microhardness and wear in differently eroded bovine dental enamel. Arch. Oral Biol. 1997, 42, 243–250. [Google Scholar] [CrossRef]
- Lussi, A.; Schlueter, N.; Rakhmatullina, E.; Ganss, C. Dental Erosion—An Overview with Emphasis on Chemical and His-topathological Aspects. Caries Res. 2011, 45, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, N.; Jaeggi, T.; Lussi, A. Is Dental Erosion Really a Problem? Adv. Dent. Res. 2012, 24, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.C.; Wiegand, A.; Rios, D.; Buzalaf, M.A.R.; Lussi, A. Fluoride in Dental Erosion. Monogr. Oral. Sci. 2011, 22, 158–170. [Google Scholar] [CrossRef] [Green Version]
- Huysmans, M.-C.; Young, A.; Ganss, C. The Role of Fluoride in Erosion Therapy. Monogr. Oral. Sci. 2014, 25, 230–243. [Google Scholar] [CrossRef]
- Lussi, A.; Carvalho, T.S. The Future of Fluorides and Other Protective Agents in Erosion Prevention. Caries Res. 2015, 49, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Zanatta, R.F.; Caneppele, T.M.F.; Scaramucci, T.; El Dib, R.; Maia, L.C.; Ferreira, D.M.T.P.; Borges, A.B. Protective effect of fluorides on erosion and erosion/abrasion in enamel: A systematic review and meta-analysis of randomized in situ trials. Arch. Oral Biol. 2020, 120, 104945. [Google Scholar] [CrossRef]
- Magalhães, A.C.; Rios, D.; Delbem, A.C.B.; Buzalaf, M.A.R.; Machado, M.A.A.M. Influence of Fluoride Dentifrice on Brushing Abrasion of Eroded Human Enamel: An in situ/ex vivo Study. Caries Res. 2006, 41, 77–79. [Google Scholar] [CrossRef]
- Ganss, C.; Lussi, A.; Grunau, O.; Klimek, J.; Schlueter, N. Conventional and Anti-Erosion Fluoride Toothpastes: Effect on Enamel Erosion and Erosion-Abrasion. Caries Res. 2011, 45, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.C.; dos Santos, M.G.; Comar, L.P.; Buzalaf, M.A.R.; Ganss, C.; Schlueter, N. Effect of a Single Application of TiF4 Varnish versus Daily Use of a Low-Concentrated TiF4/NaF Solution on Tooth Erosion Prevention in vitro. Caries Res. 2016, 50, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Ganss, C.; Hardt, M.; Lussi, A.; Cocks, A.-K.; Klimek, J.; Schlueter, N. Mechanism of action of tin-containing fluoride solutions as anti-erosive agents in dentine—An in vitro tin-uptake, tissue loss, and scanning electron microscopy study. Eur. J. Oral Sci. 2010, 118, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Basting, R.T.; Leme, A.A.; Bridi, E.C.; Amaral, F.L.B.D.; França, F.M.G.; Turssi, C.P.; Bedran-Russo, A.K. Nanomechanical properties, SEM, and EDS microanalysis of dentin treated with 2.5% titanium tetrafluoride, before and after an erosive challenge. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, N.; Klimek, J.; Ganss, C. In vitro efficacy of experimental tin- and fluoride-containing mouth rinses as anti-erosive agents in enamel. J. Dent. 2009, 37, 944–948. [Google Scholar] [CrossRef]
- Addy, M. Oral hygiene products: Potential for harm to oral and systemic health? Periodontology 2000 2008, 48, 54–65. [Google Scholar] [CrossRef]
- Frese, C.; Wohlrab, T.; Sheng, L.; Kieser, M.; Krisam, J.; Wolff, D. Clinical effect of stannous fluoride and amine fluoride containing oral hygiene products: A 4-year randomized controlled pilot study. Sci. Rep. 2019, 9, 7681. [Google Scholar] [CrossRef] [Green Version]
- Wiegand, A.; Waldheim, E.; Sener, B.; Magalhães, A.C.; Attin, T. Comparison of the Effects of TiF4 and NaF Solutions at pH 1.2 and 3.5 on Enamel Erosion in vitro. Caries Res. 2009, 43, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Buzalaf, M.A.R.; Magalhães, A.C.; Wiegand, A. Alternatives to Fluoride in the Prevention and Treatment of Dental Erosion. Monogr. Oral. Sci. 2014, 25, 244–252. [Google Scholar] [CrossRef]
- Callister, W.D.C., Jr. Materials Science and Engineering—An Introduction. In Anti-Corrosion Methods and Materials, 5th ed.; Wiley: London, UK, 2000. [Google Scholar] [CrossRef]
- Canevarolo, S.V., Jr. Polymer Science: A Textbook for Engineers and Technologists; Carl Hanser Verlag GmbH Co KG: Munich, Germany, 2019. [Google Scholar]
- Pini, N.I.P.; Lima, D.A.N.L.; Lovadino, J.R.; Ganss, C.; Schlueter, N. In vitro Efficacy of Experimental Chitosan-Containing Solutions as Anti-Erosive Agents in Enamel. Caries Res. 2016, 50, 337–345. [Google Scholar] [CrossRef]
- Hannig, M.; Joiner, A. The Structure, Function and Properties of the Acquired Pellicle. Monogr. Oral Sci. 2005, 19, 29–64. [Google Scholar] [CrossRef]
- Hannig, M.; Hannig, C. The Pellicle and Erosion. Monogr. Oral Sci. 2014, 25, 206–214. [Google Scholar] [CrossRef]
- Vukosavljevic, D.; Custodio, W.; Buzalaf, M.A.; Hara, A.T.; Siqueira, W.L. Acquired pellicle as a modulator for dental erosion. Arch. Oral Biol. 2014, 59, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Arends, J. Zeta potentials of enamel and apatites J. Arends. J. Dent. 1979, 7, 246–253. [Google Scholar] [CrossRef]
- Young, A.; Smistad, G.; Karlsen, J.; Rölla, G.; Rykke, M. Zeta Potentials of Human Enamel and Hydroxyapatite as Measured by the Coulter® DELSA 440. Adv. Dent. Res. 1997, 11, 560–565. [Google Scholar] [CrossRef]
- Shaw, D.J. Introduction to Colloid and Surface Chemistry: Fourth Edition; Butterworth-Heinemann: Oxford, UK, 2013; ISBN 0750611820. [Google Scholar]
- Grossman, L.I.; Brickman, B.M. Some Observations on the ph of Saliva. J. Dent. Res. 1937, 16, 409–416. [Google Scholar] [CrossRef]
- Elliott, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates. In Studies in Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar] [CrossRef]
- Yin, G.; Liu, Z.; Zhan, J.; Ding, F.; Yuan, N. Impacts of the surface charge property on protein adsorption on hydroxyapatite. Chem. Eng. J. 2002, 87, 181–186. [Google Scholar] [CrossRef]
- Matsumoto, M.; Miyake, T.; Noshi, H.; Kambara, M.; Konishi, K. Zeta potential studies on the adsorption of proteins on a synthetic hydroxyapatite. Colloids Surf. 1989, 40, 77–84. [Google Scholar] [CrossRef]
- Scaramucci, T.; Borges, A.B.; Lippert, F.; Zero, D.T.; Aoki, I.V.; Hara, A.T. Anti-erosive properties of solutions containing fluoride and different film-forming agents. J. Dent. 2015, 43, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Beyer, M.; Reichert, J.; Sigusch, B.W.; Watts, D.C.; Jandt, K.D. Morphology and structure of polymer layers protecting dental enamel against erosion. Dent. Mater. 2012, 28, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Sodata, P.; Juntavee, A.; Juntavee, N.; Peerapattana, J. Optimization of Adhesive Pastes for Dental Caries Prevention. AAPS PharmSciTech 2017, 18, 3087–3096. [Google Scholar] [CrossRef] [PubMed]
- Augusto, M.G.; Santos, T.M.d.A.; Scaramucci, T.; Aoki, I.V.; Torres, C.R.G.; Hara, A.T.; Borges, A.B. Protective Effect of Solutions Containing Polymers Associated with Fluoride and Stannous Chloride on Hydroxyapatite Dissolution. Caries Res. 2021, 55, 122–129. [Google Scholar] [CrossRef] [PubMed]
- White, A.J.; Gracia, L.H.; Barbour, M.E. Inhibition of Dental Erosion by Casein and Casein-Derived Proteins. Caries Res. 2011, 45, 13–20. [Google Scholar] [CrossRef]
- Ávila, D.M.D.S.; Zanatta, R.F.; Scaramucci, T.; Aoki, I.V.; Torres, C.R.G.; Borges, A.B. Influence of bioadhesive polymers on the protective effect of fluoride against erosion. J. Dent. 2017, 56, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ávila, D.M.D.S.; Zanatta, R.F.; Scaramucci, T.; Aoki, I.V.; Torres, C.R.G.; Borges, A.B. Randomized in situ trial on the efficacy of Carbopol in enhancing fluoride / stannous anti-erosive properties. J. Dent. 2020, 101, 103347. [Google Scholar] [CrossRef] [PubMed]
- Beyer, M.; Reichert, J.; Heurich, E.; Jandt, K.D.; Sigusch, B.W. Pectin, alginate and gum arabic polymers reduce citric acid erosion effects on human enamel. Dent. Mater. 2010, 26, 831–839. [Google Scholar] [CrossRef]
- Dalmoro, A.; Barba, A.A.; Lamberti, G.; Grassi, M.; D’Amore, M. Pharmaceutical applications of biocompatible polymer blends containing sodium alginate. Adv. Polym. Technol. 2012, 31, 219–230. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Jardim, J.J.; Alves, L.S.; Maltz, M. The history and global market of oral home-care products. Braz. Oral Res. 2009, 23, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Zaman, M.A.; Martin, G.P.; Rees, G.D. Bioadhesion and retention of non-aqueous delivery systems in a dental hard tissue model. J. Dent. 2010, 38, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.S.; Lussi, A. Combined effect of a fluoride-, stannous- and chitosan-containing toothpaste and stannous-containing rinse on the prevention of initial enamel erosion–abrasion. J. Dent. 2014, 42, 450–459. [Google Scholar] [CrossRef]
- Ganss, C.; von Hinckeldey, J.; Tolle, A.; Schulze, K.; Klimek, J.; Schlueter, N. Efficacy of the stannous ion and a biopolymer in toothpastes on enamel erosion/abrasion. J. Dent. 2012, 40, 1036–1043. [Google Scholar] [CrossRef]
- Schlueter, N.; Klimek, J.; Ganss, C. Effect of a chitosan additive to a Sn2+-containing toothpaste on its anti-erosive/anti-abrasive efficacy—a controlled randomised in situ trial. Clin. Oral Investig. 2014, 18, 107–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, C.E.; Creeth, J.E.; Paul, A.J.; Carson, C.; Tadesse, G.; Brown, A. The effect of dentifrice ingredients on enamel erosion prevention and repair. Surf. Interface Anal. 2021, 53, 528–539. [Google Scholar] [CrossRef]
- Hooper, S.M.; Newcombe, R.G.; Faller, R.; Eversole, S.; Addy, M.; West, N.X. The protective effects of toothpaste against erosion by orange juice: Studies in situ and in vitro. J. Dent. 2007, 35, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Cruz, N.V.S.; Pessan, J.P.; Manarelli, M.M.; de Souza, M.D.B.; Delbem, A.C.B. In vitro effect of low-fluoride toothpastes containing sodium trimetaphosphate on enamel erosion. Arch. Oral Biol. 2015, 60, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Danelon, M.; Pessan, J.P.; dos Santos, V.R.; Chiba, E.K.; Garcia, L.S.G.; de Camargo, E.R.; Delbem, A.C.B. Fluoride toothpastes containing micrometric or nano-sized sodium trimetaphosphate reduce enamel erosion in vitro. Acta Odontol. Scand. 2018, 76, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, N.; Lussi, A.; Tolle, A.; Ganss, C. Effects of Erosion Protocol Design on Erosion/Abrasion Study Outcome and on Active Agent (NaF and SnF2) Efficacy. Caries Res. 2016, 50, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Somani, R.; Jaidka, S.; Singh, D.J.; Arora, V. Remineralizing potential of various agents on dental erosion. J. Oral Biol. Craniofacial Res. 2014, 4, 104–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceci, M.; Mirando, M.; Beltrami, R.; Chiesa, M.; Poggio, C. Protective effect of casein phosphopeptide-amorphous calcium phosphate on enamel erosion: Atomic force microscopy studies. Scanning 2015, 37, 327–334. [Google Scholar] [CrossRef]
- de Alencar, C.R.B.; Magalhães, A.C.; Machado, M.A.D.A.M.; de Oliveira, T.M.; Honório, H.M.; Rios, D. In situ effect of a commercial CPP-ACP chewing gum on the human enamel initial erosion. J. Dent. 2014, 42, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- De Alencar, C.R.B.; De Oliveira, G.C.; Magalhães, A.C.; Buzalaf, M.A.R.; Machado, M.A.D.A.M.; Honório, H.M.; Rios, D. In situ effect of CPP-ACP chewing gum upon erosive enamel loss. J. Appl. Oral Sci. 2017, 25, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Bayrak, S.; Tuloglu, N.; Bicer, H.; Tunc, E.S. Effect of Fluoride Varnish Containing CPP-ACP on Preventing Enamel Erosion. Scanning 2017, 2017, 1897825. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, E.C. Remineralization of Enamel Subsurface Lesions by Casein Phosphopeptide-stabilized Calcium Phosphate Solutions. J. Dent. Res. 1997, 76, 1587–1595. [Google Scholar] [CrossRef]
- Wegehaupt, F.J.; Attin, T. The Role of Fluoride and Casein Phosphopeptide/Amorphous Calcium Phosphate in the Prevention of Erosive/Abrasive Wear in an in vitro Model Using Hydrochloric Acid. Caries Res. 2010, 44, 358–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiegand, A.; Attin, T. Randomised in situ trial on the effect of milk and CPP-ACP on dental erosion. J. Dent. 2014, 42, 1210–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augusto, M.G.; da Silva, L.F.O.; Scaramucci, T.; Aoki, I.V.; Torres, C.R.G.; Borges, A.B. Protective effect of anti-erosive solutions enhanced by an aminomethacrylate copolymer. J. Dent. 2021, 105, 103540. [Google Scholar] [CrossRef]
- Luka, B.; Arbter, V.; Sander, K.; Duerrschnabel, A.; Schlueter, N. Impact of mucin on the anti-erosive/anti-abrasive efficacy of chitosan and/or F/Sn in enamel in vitro. Sci. Rep. 2021, 11, 5285. [Google Scholar] [CrossRef] [PubMed]
- Sakae, L.O.; Niemeyer, S.H.; Bezerra, S.J.C.; Borges, A.B.; Turssi, C.P.; Scaramucci, T. The Addition of Propylene Glycol Alginate to a Fluoride Solution to Control Enamel Wear: An in situ Study. Caries Res. 2020, 54, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.M.; Machado, P.F.; Vecchia, L.R.; Magalhães, A.C. Effect of chitosan solutions with or without fluoride on the protection against dentin erosion in vitro. Eur. J. Oral Sci. 2020, 128, 495–500. [Google Scholar] [CrossRef]
- Bezerra, S.J.; João-Souza, S.H.; Aoki, I.V.; Borges, A.B.; Hara, A.T.; Scaramucci, T. Anti-Erosive Effect of Solutions Containing Sodium Fluoride, Stannous Chloride, and Selected Film-Forming Polymers. Caries Res. 2019, 53, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, A.P.C.A.; Suchyta, D.; Alraheam, I.A.; Mohammed, A.; Schoenfisch, M.; Walter, R.; Almeida, I.C.S.; Souza, L.C.; Miguez, P.A. Effect of Phosphorylated Chitosan on Dentin Erosion: An in vitro Study. Caries Res. 2018, 52, 378–386. [Google Scholar] [CrossRef] [PubMed]
- João-Souza, S.H.; Bezerra, S.; de Freitas, P.M.; de Lima, N.B.; Aranha, A.C.C.; Hara, A.T.; Scaramucci, T. In situ evaluation of fluoride-, stannous- and polyphosphate-containing solutions against enamel erosion. J. Dent. 2017, 63, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaramucci, T.; João-Souza, S.H.; Lippert, F.; Eckert, G.J.; Aoki, I.V.; Hara, A.T. Influence of Toothbrushing on the Antierosive Effect of Film-Forming Agents. Caries Res. 2016, 50, 104–110. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, T.; Mitchell, J.W.; Zaidel, L.; Qiu, J.; Kilpatrick-Liverman, L. Bioinspired amphiphilic phosphate block copolymers as non-fluoride materials to prevent dental erosion. RSC Adv. 2014, 4, 49053–49060. [Google Scholar] [CrossRef] [Green Version]
- Gracia, L.H.; Brown, A.; Rees, G.D.; Fowler, C.E. Studies on a novel combination polymer system: In vitro erosion prevention and promotion of fluoride uptake in human enamel. J. Dent. 2010, 38, S4–S11. [Google Scholar] [CrossRef]
- Attin, T.; Wegehaupt, F.; Gries, D.; Wiegand, A. The potential of deciduous and permanent bovine enamel as substitute for deciduous and permanent human enamel: Erosion–abrasion experiments. J. Dent. 2007, 35, 773–777. [Google Scholar] [CrossRef]
- White, A.J.; Yorath, C.; Hengel, V.T.; Leary, S.D.; Huysmans, M.-C.D.N.J.M.; Barbour, M.E. Human and bovine enamel erosion under ‘single-drink’ conditions. Eur. J. Oral Sci. 2010, 118, 604–609. [Google Scholar] [CrossRef]
- Cheaib, Z.; Lussi, A. Impact of Acquired Enamel Pellicle Modification on Initial Dental Erosion. Caries Res. 2011, 45, 107–112. [Google Scholar] [CrossRef]
- Schlueter, N.; Neutard, L.; Von Hinckeldey, J.; Klimek, J.; Ganss, C. Tin and fluoride as anti-erosive agents in enamel and dentine in vitro. Acta Odontol. Scand. 2010, 68, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Ganss, C.; Neutard, L.; Von Hinckeldey, J.; Klimek, J.; Schlueter, N. Efficacy of a Tin/Fluoride Rinse: A Randomized In Situ Trial on Erosion. J. Dent. Res. 2010, 89, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lussi, A. Functional foods/ingredients on dental erosion. Eur. J. Nutr. 2012, 51, 39–48. [Google Scholar] [CrossRef]
- Aldosari, M.A.; Scaramucci, T.; Liu, S.S.Y.; Warrick-Polackoff, J.M.; Eckert, G.J.; Hara, A.T. Susceptibility of partially desalivated rats to erosive tooth wear by calcium-supplemented beverages. Oral Dis. 2018, 24, 355–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, C.S.; Kato, M.T.; Buzalaf, M.A.R. Effect of supplementation of soft drinks with green tea extract on their erosive potential against dentine. Aust. Dent. J. 2011, 56, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.C.; Moraes, S.M.; Rios, D.; Buzalaf, M.A.R. Effect of ion supplementation of a commercial soft drink on tooth enamel erosion. Food Addit. Contam. Part A 2009, 26, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.S.; Montagnolli, L.G.; Kato, M.T.; Sampaio, F.C.; Buzalaf, M.A.R. Calcium glycerophosphate supplemented to soft drinks reduces bovine enamel erosion. J. Appl. Oral Sci. 2012, 20, 410–413. [Google Scholar] [CrossRef] [Green Version]
- Barbour, M.E.; Shellis, R.P.; Parker, D.M.; Allen, G.C.; Addy, M. An investigation of some food-approved polymers as agents to inhibit hydroxyapatite dissolution. Eur. J. Oral Sci. 2005, 113, 457–461. [Google Scholar] [CrossRef]
- Hara, A.; Carvalho, J.; Zero, D. Causes of Dental Erosion: Extrinsic Factors. In Dental Erosion and its Clinical Management; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Scaramucci, T.; Sobral, M.A.P.; Eckert, G.J.; Zero, D.T.; Hara, A.T. In situ Evaluation of the Erosive Potential of Orange Juice Modified by Food Additives. Caries Res. 2012, 46, 55–61. [Google Scholar] [CrossRef]
- Hooper, S.; Hughes, J.; Parker, D.; Finke, M.; Newcombe, R.G.; Addy, M.; West, N. A clinical study in situ to assess the effect of a food approved polymer on the erosion potential of drinks. J. Dent. 2007, 35, 541–546. [Google Scholar] [CrossRef]
- West, N.X.; Hughes, J.A.; Parker, D.; Weaver, L.J.; Moohan, M.; De’Ath, J.; Addy, M. Modification of soft drinks with xanthan gum to minimise erosion: A study in situ. Br. Dent. J. 2004, 196, 478–481. [Google Scholar] [CrossRef] [Green Version]
- Numata, K. How to define and study structural proteins as biopolymer materials. Polym. J. 2020, 52, 1043–1056. [Google Scholar] [CrossRef]
- Reynolds, E.; Riley, P.F.; Storey, E. Phosphoprotein inhibition of hydroxyapatite dissolution. Calcif. Tissue Res. 1982, 34 (Suppl. 2), S52–S56. [Google Scholar]
- Kawasaki, K.; Kambara, M.; Matsumura, H.; Norde, W. Measurements of the Wettability of Protein–Covered Hydroxyapatite Surfaces. Caries Res. 1999, 33, 473–478. [Google Scholar] [CrossRef]
- Hemingway, C.A.; White, A.J.; Shellis, R.P.; Addy, M.; Parker, D.M.; Barbour, M.E. Enamel Erosion in Dietary Acids: Inhibition by Food Proteins in vitro. Caries Res. 2010, 44, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Barbour, M.E.; Shellis, R.P.; Parker, D.M.; Allen, G.C.; Addy, M. Inhibition of hydroxyapatite dissolution by whole casein: The effects of pH, protein concentration, calcium, and ionic strength. Eur. J. Oral Sci. 2008, 116, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Scaramucci, T.; Hara, A.T.; Aoki, I.V.; Sobral, M.A.P. Supplementation of an Orange Juice with Dietary Proteins to Prevent Enamel and Dentin Erosion. Braz. Dent. J. 2015, 26, 263–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, E.; Wade, A.; Crawford, G.; Jenner, B.; Levinson, N.; Wilkinson, J. Randomised clinical trial: Relief of upper gastrointestinal symptoms by an acid pocket-targeting alginate-antacid (Gaviscon Double Action)—A double-blind, placebo-controlled, pilot study in gastro-oesophageal reflux disease. Aliment. Pharmacol. Ther. 2014, 39, 595–602. [Google Scholar] [CrossRef]
- Burdick, J.A.; Stevens, M.M. Biomedical hydrogels. In Biomaterials, Artificial Organs and Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2005; Volume 107–115. [Google Scholar] [CrossRef]
- Kwiatek, M.A.; Roman, S.; Fareeduddin, A.; Pandolfino, J.E.; Kahrilas, P.J. An alginate-antacid formulation (Gaviscon Double Action Liquid) can eliminate or displace the postprandial ‘acid pocket’ in symptomatic GERD patients. Aliment. Pharmacol. Ther. 2011, 34, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, M.D.S.C.; Mantilla, T.F.; Bridi, E.C.; Basting, R.T.; França, F.M.G.; Amaral, F.L.B.; Turssi, C.P. Rinsing with antacid suspension reduces hydrochloric acid-induced erosion. Arch. Oral Biol. 2016, 61, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Turssi, C.P.; Vianna, L.M.F.F.; Hara, A.T.; Amaral, F.L.B.D.; França, F.M.G.; Basting, R.T. Counteractive effect of antacid suspensions on intrinsic dental erosion. Eur. J. Oral Sci. 2012, 120, 349–352. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Augusto, M.G.; Scaramucci, T.; Campos, T.M.B.; Aoki, I.V.; Schlueter, N.; Borges, A.B. Film-Forming Polymers for Tooth Erosion Prevention. Polymers 2022, 14, 4225. https://doi.org/10.3390/polym14194225
Augusto MG, Scaramucci T, Campos TMB, Aoki IV, Schlueter N, Borges AB. Film-Forming Polymers for Tooth Erosion Prevention. Polymers. 2022; 14(19):4225. https://doi.org/10.3390/polym14194225
Chicago/Turabian StyleAugusto, Marina Gullo, Tais Scaramucci, Tiago Moreira Bastos Campos, Idalina Vieira Aoki, Nadine Schlueter, and Alessandra Bühler Borges. 2022. "Film-Forming Polymers for Tooth Erosion Prevention" Polymers 14, no. 19: 4225. https://doi.org/10.3390/polym14194225
APA StyleAugusto, M. G., Scaramucci, T., Campos, T. M. B., Aoki, I. V., Schlueter, N., & Borges, A. B. (2022). Film-Forming Polymers for Tooth Erosion Prevention. Polymers, 14(19), 4225. https://doi.org/10.3390/polym14194225






