High-Transparency and Colorless Polyimide Film Prepared by Inhibiting the Formation of Chromophores
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Measurements
2.3. Preparation of Polyimide Film and End-Capped Polyimide Film
3. Characterization Results and Discussion
3.1. IR Characterization of Polyimide Film
3.2. Performance of Colorless Polyimide Film
3.2.1. Optical Properties of Colorless Polyimide Films
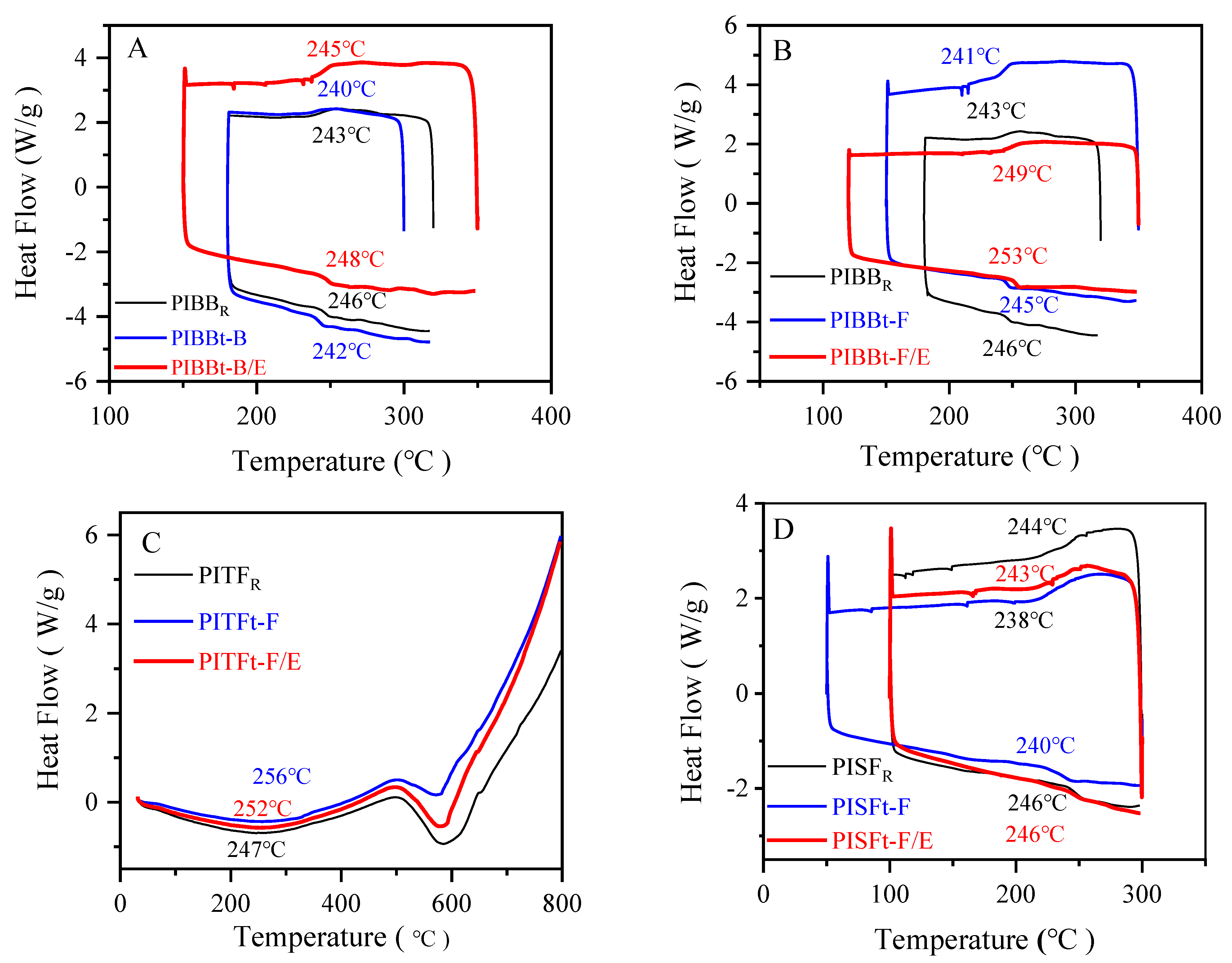
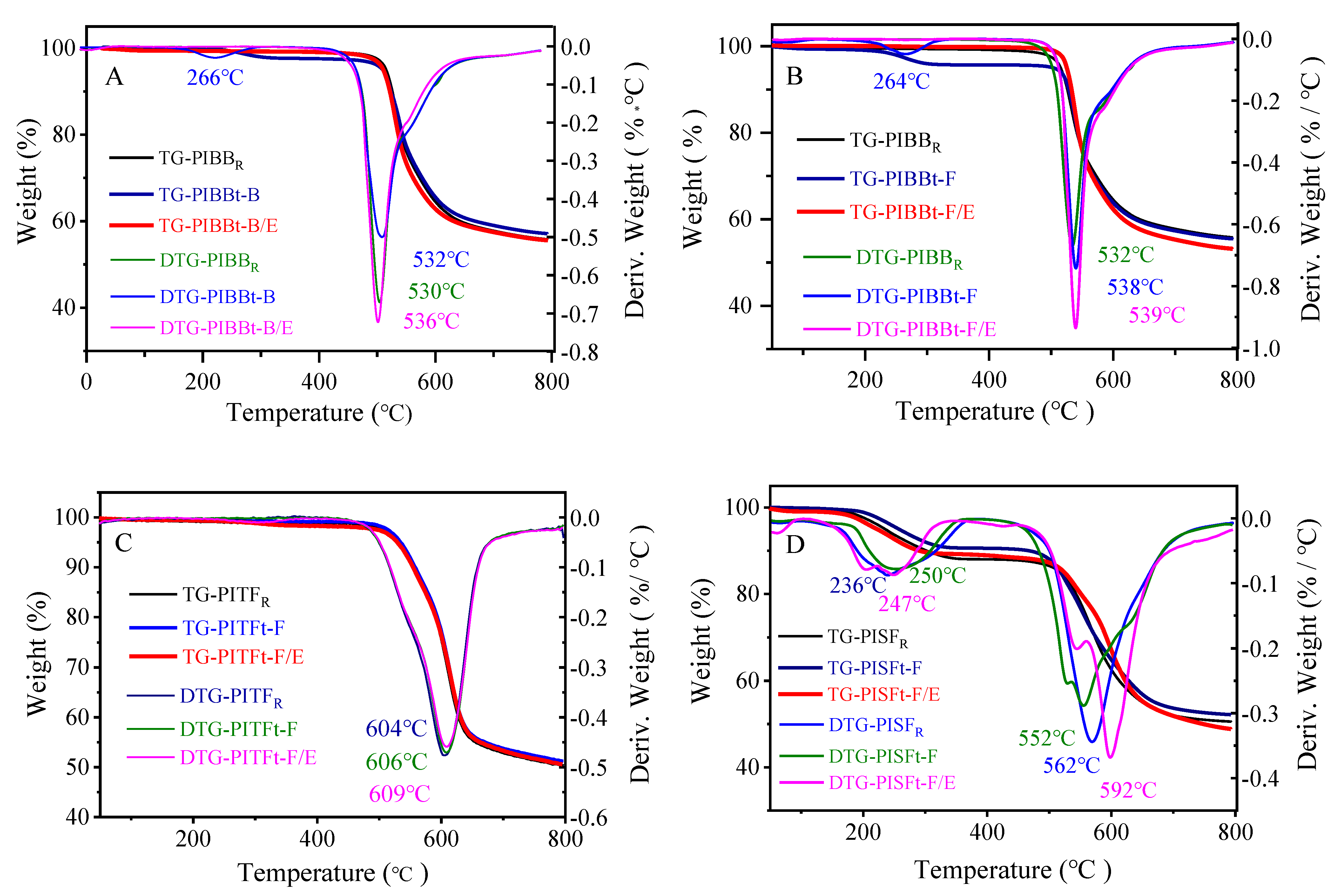
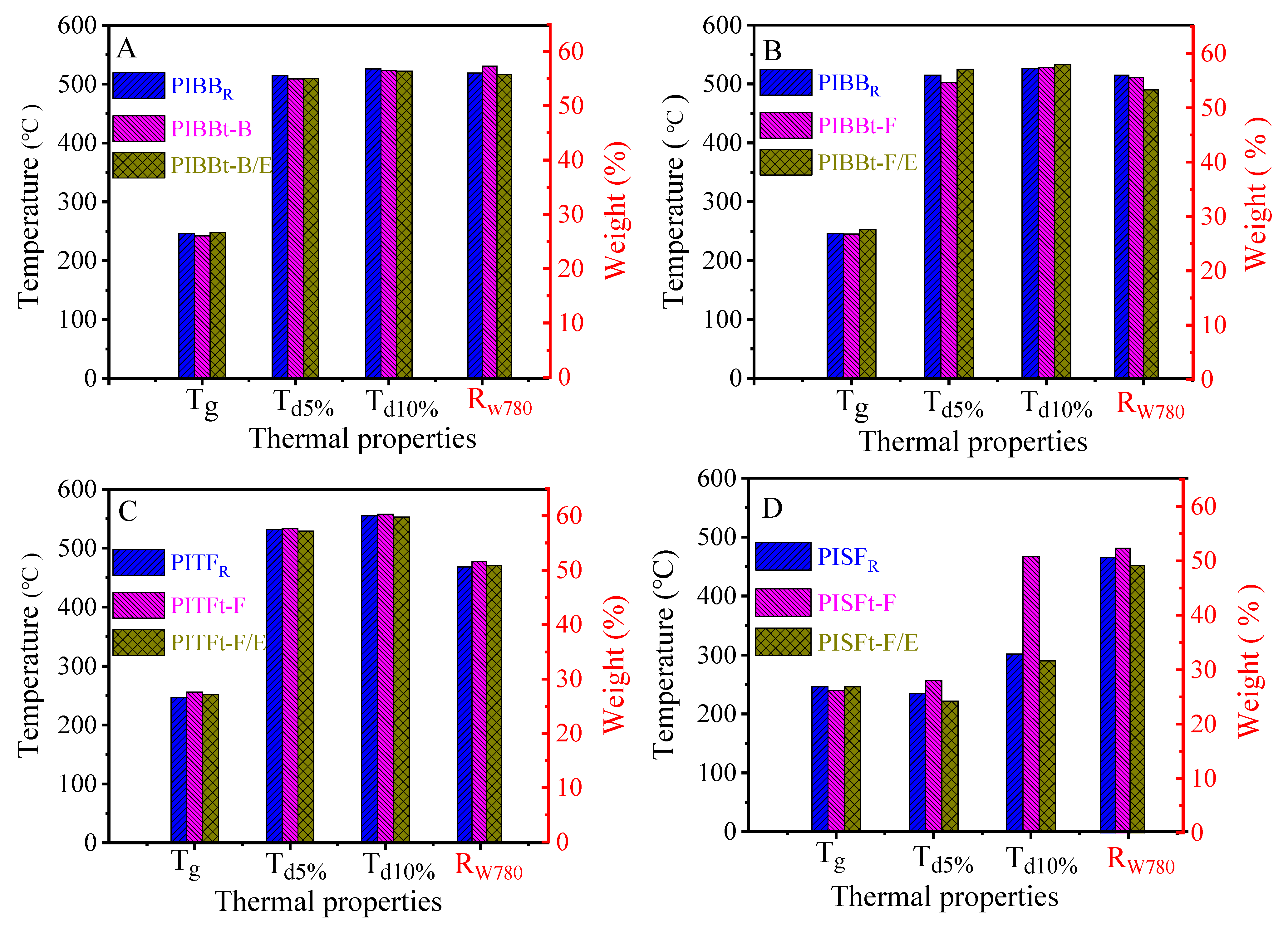
3.2.2. Thermal Properties of Polyamide Acid and Polyimide Films
3.2.3. Mechanical Properties of PI Films
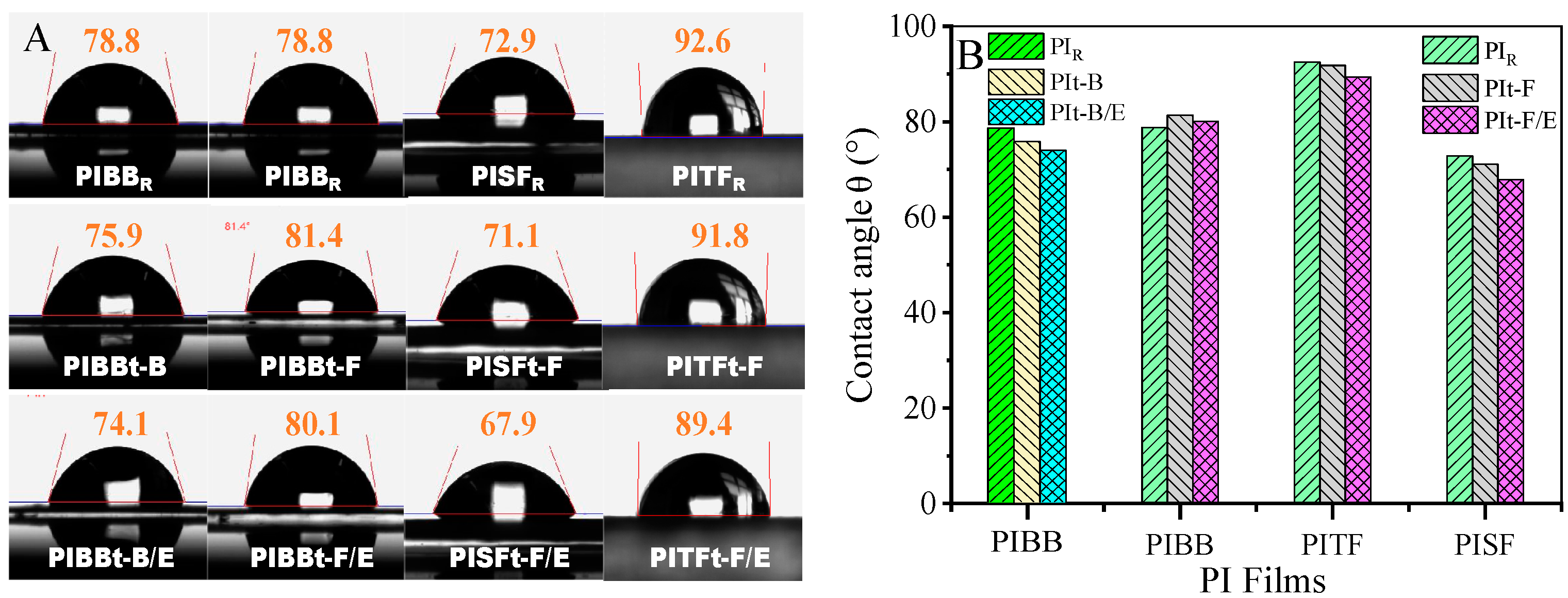
3.2.4. Surface Energy of CPI Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, T.Q.; Zheng, F.; Ma, X.; Ding, T.M.; Chen, S.; Jiang, W.; Zhang, S.Y.; Lu, Q. High heat-resistant polyimide films containing quinoxaline moiety for flexible substrate applications. Polymer 2020, 209, 122963. [Google Scholar] [CrossRef]
- Yang, Z.H.; Guo, H.Q.; Kang, C.Q.; Gao, L.X. Synthesis and characterization of amide-bridged colorless polyimide films with low CTE and high optical performance for flexible OLED displays. Polym. Chem. 2021, 12, 5364–5376. [Google Scholar] [CrossRef]
- Feng, J.B.; Wang, Y.; Qin, X.G.; Lv, Y.D.; Huang, Y.J.; Yang, Q.; Li, G.X.; Kong, M.Q. Property evolution and molecular mechanisms of aluminized colorless transparent polyimide under space ultraviolet irradiation. Polym. Degrad. Stab. 2022, 199, 109915. [Google Scholar] [CrossRef]
- Chang, J.H. Equibiaxially stretchable colorless and transparent polyimides for flexible display substrates. Rev. Adv. Mater. Sci. 2020, 59, 1–9. [Google Scholar] [CrossRef]
- Lian, R.; Lei, X.; Xue, S.; Chen, Y.; Zhang, Q. Janus polyimide films with outstanding AO resistance. good optical transparency and high mechanical strength. Appl. Surf. Sci. 2021, 535, 147654. [Google Scholar] [CrossRef]
- Chen, C.J.; Yen, H.J.; Hu, Y.C.; Liou, G.S. Novel programmable functional polyimides: Preparation, mechanism of CT induced memory, and ambipolar electrochromic behavior. J. Mater. Chem. C 2013, 1, 7623–7634. [Google Scholar] [CrossRef]
- Koo, D.; Jung, S.; Seo, J.; Jeong, G.; Choi, Y.; Lee, J.; Lee, S.M.; Cho, Y.; Jeong, M.; Lee, J.; et al. Flexible Organic Solar Cells Over 15% Efficiency with Polyimide-Integrated Graphene Electrodes. Joule 2020, 5, 1021–1034. [Google Scholar] [CrossRef]
- Shen, J.L.; Jiang, P.F.; Wang, Y.; Zhang, F.; Li, F.; Tu, G.L. Soluble sulfoxide biphenyl polyimide film with transmittance exceeding 90%. Polymer 2022, 254, 125050. [Google Scholar] [CrossRef]
- Yi, C.H.; Li, W.M.; Shi, S.; He, K.; Ma, P.C.; Chen, M.; Yang, C.L. High-temperature-resistant and colorless polyimide: Preparations. properties, and applications. Sol. Energy 2020, 195, 340–354. [Google Scholar] [CrossRef]
- Nishihara, M.; Christiani, L.; Staykov, A.; Sasaki, K. Experimental and theoretical study of charge-transfer complex hybrid polyimide membranes. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 293–298. [Google Scholar] [CrossRef]
- Ma, P.; Dai, C.; Wang, H.; Li, Z.; Liu, H.; Li, W.; Yang, C. A review on high temperature resistant polyimide films: Heterocyclic structures and nanocomposites. Compos. Commun. 2019, 16, 84–93. [Google Scholar] [CrossRef]
- Lin, D.L.; Liu, Y.Z.; Jia, Z.Q.; Qi, S.L.; Wu, D.Z. Structural evolution of macromolecular chain during pre-Imidization process and its effects on polyimide film properties. J. Phys. Chem. B 2020, 124, 7969–7978. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.C.; Wakita, J.; Ha, C.S.; Ando, S. Highly transparent and refractive polyimides with controlled molecular structure by chlorine side groups. Macromolecules 2009, 42, 5112–5120. [Google Scholar] [CrossRef]
- Song, G.L.; Chen, C.; Wang, X.Y.; Yao, J.N. Synthesis and properties of polyimides derived from 2,2′-dichloro-4,4′,5,5′-biphenyltetracarboxylic dianhydride. Polymer 2019, 183, 121862. [Google Scholar] [CrossRef]
- Yeo, H.; Goh, M.; Ku, B.-C.; You, N.-H. Synthesis and characterization of highly-fluorinated colorless polyimides derived from 4,4′-((perfluoro- [1,1′-biphenyl]-4,4′-diyl)bis(oxy))bis(2,6-dimethylaniline) and aromatic dianh-ydrides. Polymer 2015, 76, 280–286. [Google Scholar] [CrossRef]
- Li, F.; Shen, J.; Liu, X.; Cao, Z.; Cai, X.; Li, J.; Ding, K.; Liu, J.; Tu, G. Flexible QLED and OPV based on transparent polyimide substrate with rigid alicyclic asymmetric isomer. Org. Electron. 2017, 51, 54–61. [Google Scholar] [CrossRef]
- Hu, X.; Mu, H.; Wang, Y.; Wang, Z.; Yan, J. Colorless polyimides derived from isomeric dicyclohexyl-tetracarboxylic dianhydrides for optoelectronic applications. Polymer 2018, 134, 8–19. [Google Scholar] [CrossRef]
- Qin, R.; Peng, L.; Deng, H.; Liu, Y.; Liu, X. Enhancing thermal dimensional stability of polyimide composite films through in-situ constructing highly interfacial grafting degree to constrain early chain relaxation. Compos. Part B Eng. 2021, 216, 108829. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Guo, J.; Qi, D.; Li, W.; Shen, K. Novel fluorinated long linear segment hyperbranched polyimides bearing various pendant substituents for applications as optical materials. Polymer 2020, 190, 122216. [Google Scholar] [CrossRef]
- Mi, Z.; Liu, Z.; Yao, J.; Wang, C.; Zhou, C.; Wang, D.; Zhao, X.; Zhou, H.; Zhang, Y.; Chen, C. Transparent and soluble polyimide films from 1,4:3,6-dianhydro- D-mannitol based dianhydride and diamines containing aromatic and semiaromatic units: Preparation. characterization, thermal and mechanical properties. Polym. Degrad. Stab. 2018, 151, 80–89. [Google Scholar] [CrossRef]
- Zhuang, Y.; Seong, J.G.; Lee, Y.M. Polyimides containing aliphatic/alicyclic segments in the main chains. Prog. Polym. Sci. 2019, 92, 35–88. [Google Scholar] [CrossRef]
- Yuan, C.; Li, D.; Yuan, X.; Liu, L.; Huang, Y. Preparation of semi-aliphatic polyimide for organic-solvent-free sizing agent in CF/PEEK composites. Compos. Sci. Technol. 2021, 201, 108490. [Google Scholar] [CrossRef]
- Tapaswi, P.K.; Choi, M.C.; Jeong, K.M.; Ando, S.; Ha, C.S. Transparent aromatic polyimides derived from thiophenyl-substituted benzidines with high refractive index and small birefringence. Macromolecules 2015, 48, 3462–3474. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, X.R.; Zheng, F.; Lu, X.M.; Lu, Q.H. High performance transparent polyimides by controlling steric hindrance of methyl side groups. Eur. Polym. J. 2019, 120, 109235. [Google Scholar] [CrossRef]
- Shin, H.I.; Kwark, Y.-J.; Chang, J.-H. Colorless and transparent copolyimides and their nanocomposites: Thermo-optical properties, morphologies, and gas permeabilities. Polymers 2019, 11, 585. [Google Scholar]
- Min, H.; Kang, B.; Shin, Y.S.; Kim, B.; Lee, S.W.; Cho, J.H. Transparent and Colorless Polyimides Containing Multiple Trifluoromethyl Groups as Gate Insulators for Flexible Organic Transistors with Superior Electrical Stability. ACS Appl. Mater Interfaces 2020, 12, 18739–18747. [Google Scholar] [CrossRef]
- Tsai, C.L.; Yen, H.J.; Liou, G.S. Highly transparent polyimide hybrids for optoelectronic applications. React. Funct. Polym. 2016, 108, 2–30. [Google Scholar] [CrossRef]
- Nam, K.H.; Kim, S.; Song, J.; Baek, S.; Paek, S.H.; Ku, B.C.; Han, H. Dimensionally stable and light-colored polyimide hybrid reinforced with layered silicate. Macromol. Res. 2016, 24, 104–113. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, F.; Leng, J. Metal mesh embedded in colorless shape memory polyimide for flexible transparent electric-heater and actuators. Appl. Mater. Today 2020, 21, 100797. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Xu, J.; Huang, J.; Yang, Y.; Tan, R.; Chen, G.; Fang, X.; Zhao, Y.; Song, W. Robust ultrathin and transparent AZO/Ag-SnO/AZO on polyimide substrate for flexible thin film heater with temperature over 400 °C. J. Mater. Sci. Technol. 2020, 48, 156–162. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, X.; Wang, J.; He, W.; Silberschmidt, V.V.; Wang, S.; Tao, Z.; Xu, H. Properties and application of polyimide-based composites by blending surface functionalized boron nitride nanoplates. J. Appl. Polym. Sci. 2015, 132, 41889. [Google Scholar] [CrossRef]
- Lu, Y.H.; Hao, J.C.; Xiao, G.Y.; Chen, L.; Wang, T.H.; Hu, Z.Z. Preparation and properties of in situ amino−functionalized graphene oxide/polyimide composite films. Appl. Surf. Sci. 2017, 422, 710–719. [Google Scholar] [CrossRef]
- Niu, Y.; Fang, Q.; Zhang, X.; Zhao, J.; Li, Y. Structural evolution, induced effects and graphitization mechanism of reduced graphene oxide sheets/polyimide composites. Compos. Part B Eng. 2018, 134, 127–132. [Google Scholar] [CrossRef]
- Lee, D.H.; Yun, H.D.; Jung, E.D.; Chu, J.H.; Nam, Y.S.; Song, S.; Seok, S.H.; Song, M.H.; Kwon, S.Y. Ultrathin graphene intercalation in PEDOT: PSS/colorless polyimide-based transparent electrodes for enhancement of optoelectronic performance and operational stability of organic devices. ACS Appl. Mater Interfaces 2019, 11, 21069–21077. [Google Scholar] [CrossRef]
- Su, B.; Zhou, Y.-G. Improvement of transparencies and mechanical properties of poly(cyclohexylene dimethylene cyclohexanedicarboxylate) parts using a compounding nucleating agent to control crystallization. Materials 2019, 12, 563. [Google Scholar] [CrossRef] [Green Version]
- Nam, K.H.; Jin, J.U.; Lee, D.H.; Han, H.; Goh, M.; Yu, J.; Ku, B.C.; You, N.H. Towards solution-processable, thermally robust, transparent polyimide-chain-end tethered organosilicate nanohybrids. Compos. B Eng. 2019, 163, 290–296. [Google Scholar] [CrossRef]
- Yang, S.Y.; Park, C.E.; Jung, M.S. Effects of reactive end-capper on mechanical properties of chemical amplified photosensitive polyimide. Polymer 2003, 44, 3243–3249. [Google Scholar] [CrossRef]
- Jung, M.S.; Joo, W.J.; Kwon, O.; Sohn, B.H.; Jung, H.T. A high-performance positive-working photosensitive. polyimide: Effects of reactive end groups on the physical properties of the films. J. Appl. Polym. Sci. 2006, 102, 2180–2188. [Google Scholar] [CrossRef]
- Liu, C.Z.; Sun, M.M.; Zhang, B.; Zhang, X.G.; Li, J.H.; Xue, G.; Zhang, X.W.; Zhou, H. Synthesis and characterization of bisphthalonitrile-terminated polyimide precursors with unique advantages in processing and adhesive properties. Polymer 2021, 212, 123290. [Google Scholar] [CrossRef]
- Wang, K.; Yuan, X.J.; Zhan, M.S. Comparison between microwave and thermal curing of a polyimide adhesive end-caped with phenylethynyl groups. Int. J. Adhes. Adhes. 2017, 74, 28–34. [Google Scholar] [CrossRef]
- Chen, Z.W.; Wang, L.Q.; Lin, J.P.; Du, L. Thermal curing mechanism of acetylene-terminated polyimides: A combination of density functional theory computation and microkinetic analysis. Polymer 2021, 212, 123290. [Google Scholar] [CrossRef]
- Song, Y.; Yao, H.Y.; Tan, H.W.; Zhu, S.Y.; Dong, B.; Guan, S.W. Changing the memory behaviors from volatile to nonvolatile via end-capping of hyperbranched polyimides with polycyclic arenes. Dye. Pigment. 2017, 139, 730–736. [Google Scholar] [CrossRef]
- Wang, C.O.; Gao, M.Y.; Jia, Y.; Zhai, L.; He, M.H.; Mo, S.; Fan, L. End-capped transparent polyimide films with improved thermal durability for optoelectronic application. Int. Conf. Disp. Technol. 2021, 52, 861–863. [Google Scholar] [CrossRef]

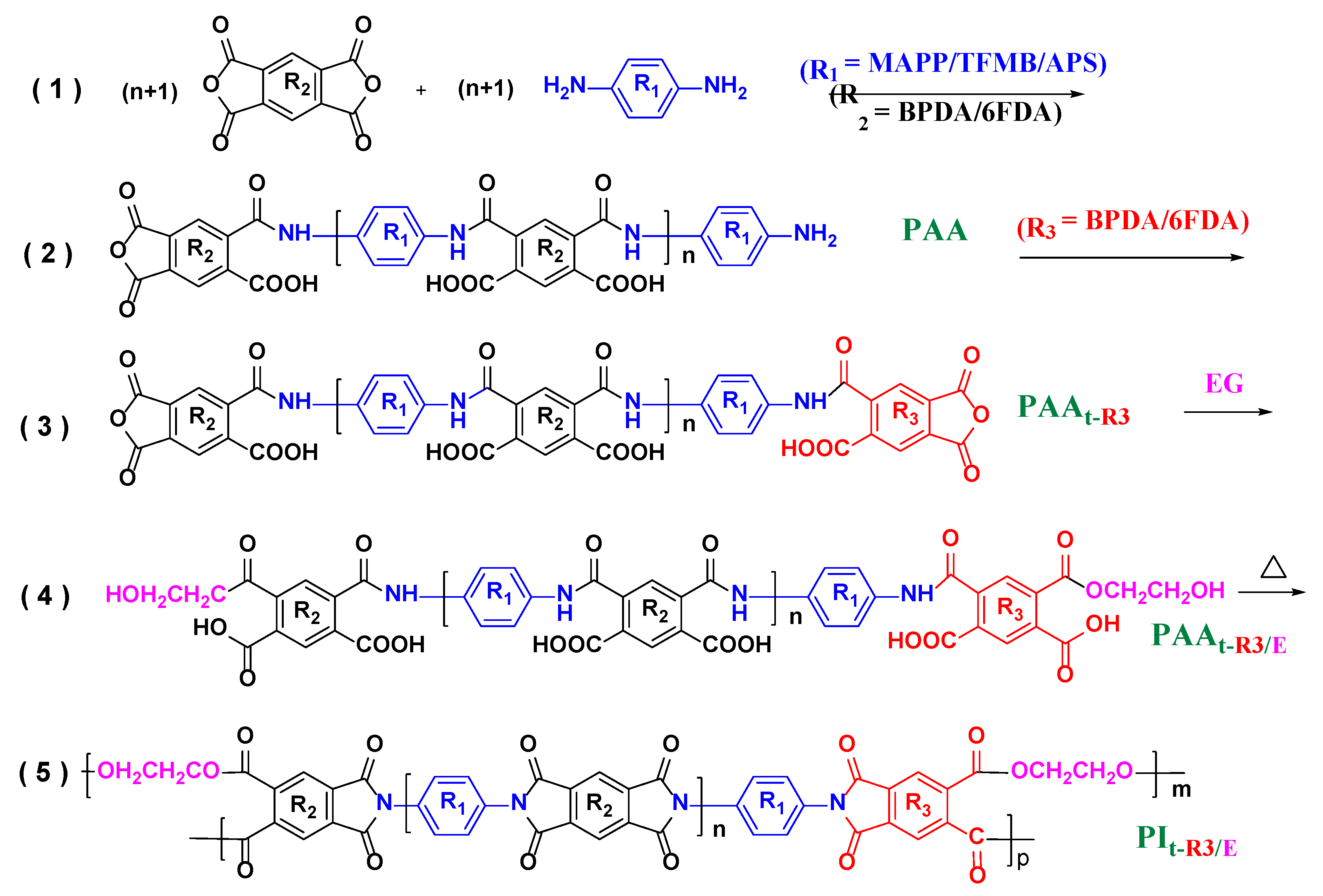
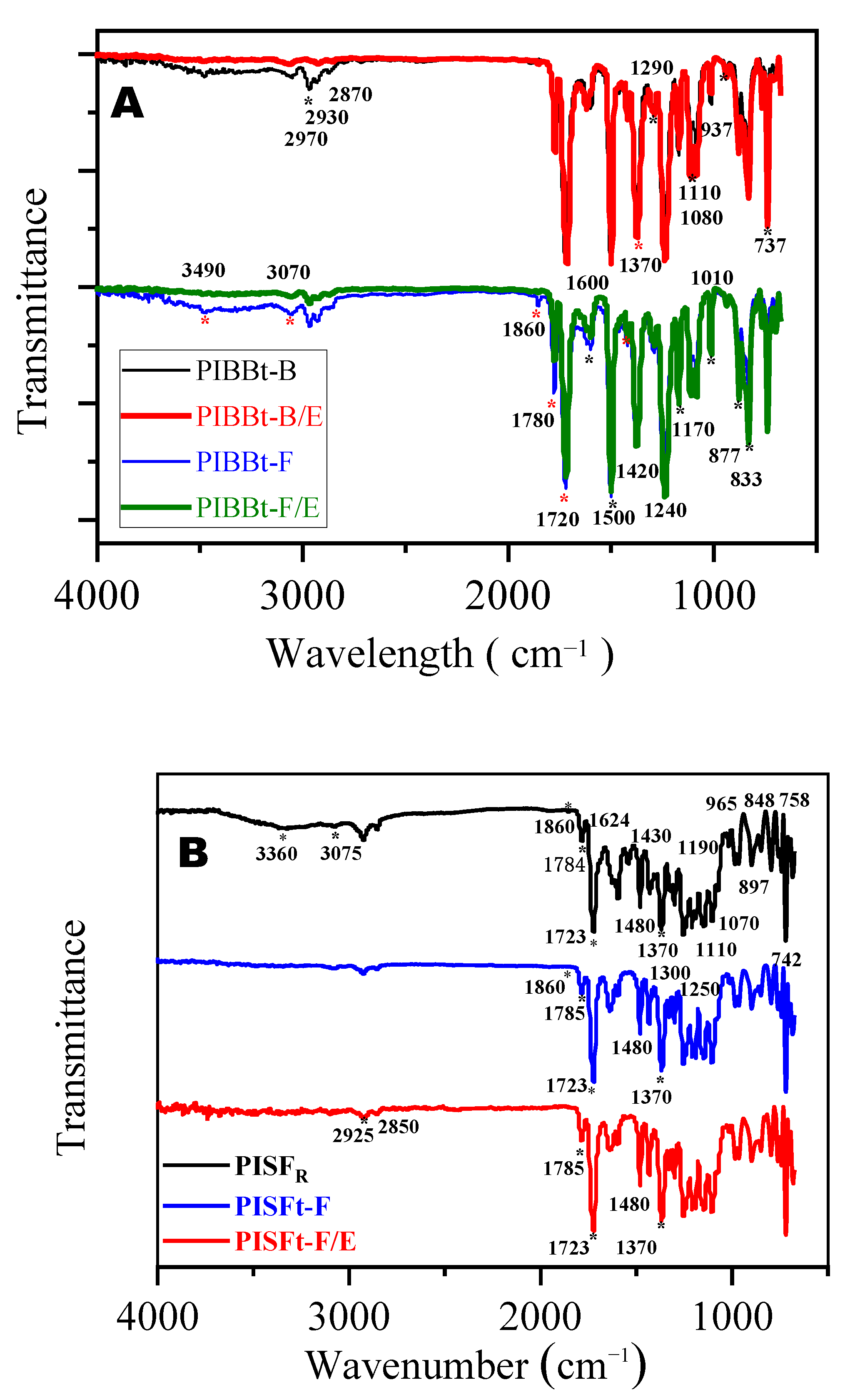
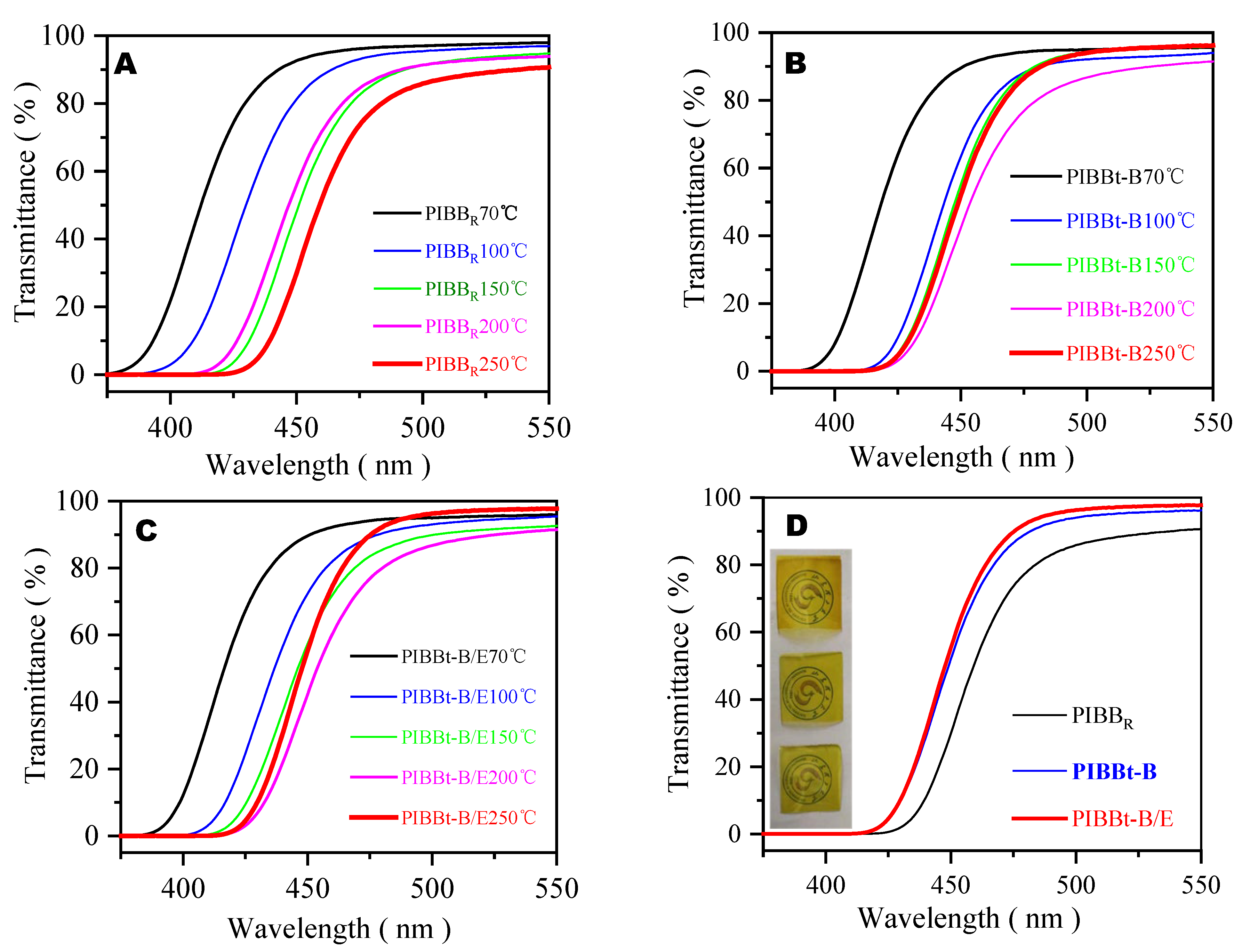
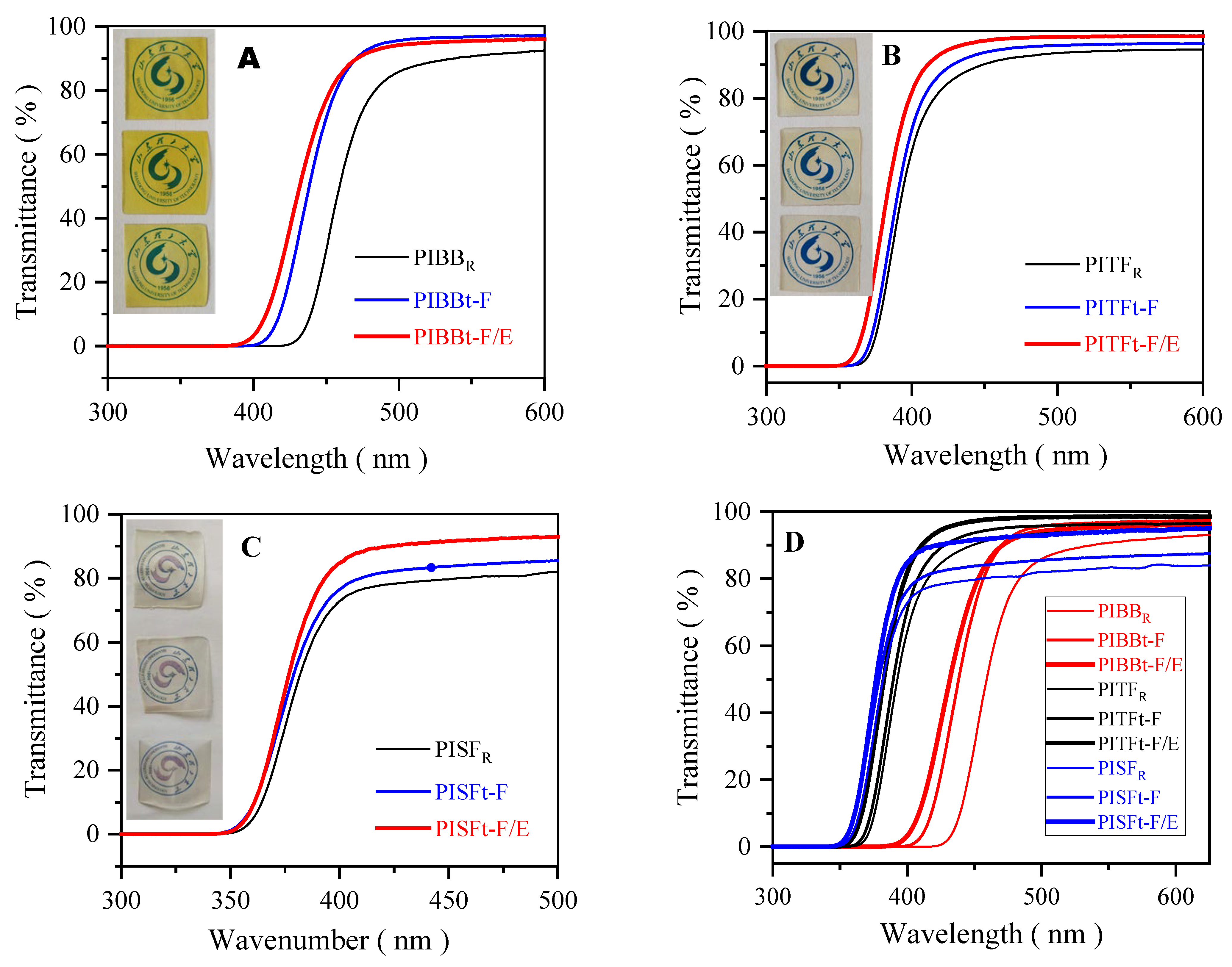


| Films | Temp. (°C) | 70.0 | 100 | 150 | 200 | 250 |
|---|---|---|---|---|---|---|
| PIBBR | λcut (nm) | 362 | 370 | 398 | 393 | 414 |
| PIBBt-B | λcut (nm) | 373 | 395 | 400 | 402 | 402 |
| PIBBt-B/E | λcut (nm) | 371 | 386 | 401 | 405 | 400 |
| PIBBR | T450 (%) | 92.6 | 81.2 | 48.0 | 55.9 | 29.9 |
| PIBBt-B | T450 (%) | 89.6 | 63.4 | 55.2 | 42.3 | 52.0 |
| PIBBt-B/E | T450 (%) | 89.6 | 72.4 | 57.4 | 43.3 | 55.4 |
| PIBBR | Tmax (%) | 99.2 | 99.4 | 97.6 | 96.4 | 93.7 |
| PIBBt-B | Tmax (%) | 96.5 | 96.4 | 96.2 | 93.9 | 97.8 |
| PIBBt-B/E | Tmax (%) | 96.6 | 96.7 | 93.8 | 94.7 | 98.9 |
| PIs | Diamine/Dianhydride | Blockers | ηinh | λcut (nm) | T450 (%) | Tmax (%) |
|---|---|---|---|---|---|---|
| PIBBR | 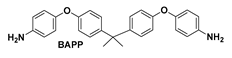 | None | 0.5613 | 414 | 29.9 | 93.7 |
| PIBBt-F | 6FDA | 0.4875 | 388 | 71.3 | 97.0 | |
| PIBBt-F/E | 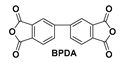 | 6FDA/EG | 0.3425 | 370 | 77.0 | 97.5 |
| PITFR | 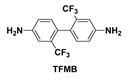 | None | 0.1599 | 345 | 90.2 | 94.7 |
| PITFt-F | 6FDA | 0.1561 | 340 | 93.6 | 96.3 | |
| PITFt-F/E | 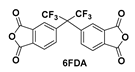 | 6FDA/EG | 0.2423 | 335 | 97.1 | 98.2 |
| PISFR | 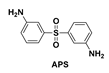 | None | 0.1643 | 341 | 79.6 | 84.2 |
| PISFt-F | 6FDA | 0.1431 | 336 | 83.8 | 87.1 | |
| PISFt-F/E | 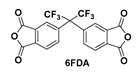 | 6FDA/EG | 0.2437 | 334 | 91.6 | 94.8 |
| Stage | No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | No. 6 |
|---|---|---|---|---|---|---|
| Temp. (°C) | 25–103 | 103–163 | 163–278 | 278–453 | 453–643 | 653–791 |
| Weight loss % | 100–98 | 98–97 | 97–91 | 91–90 | 90–58 | 58–51 |
| Lost groups | H2O | H2O | H2O | CO2 | −CONCO− | H2 |
| Number | Many | Few | Many | Few | Many | Many |
| Speed | Quick | Slowly | Quick | Slowly | Quick | Slowly |
| Thermo- reaction | Solvent evaporation | Morkit | Imidization | Anhydride pyrolysis | Imide ring pyrolysis | Carbonation |
| Parameters | PIBB R | PIBB t-B | PIBB t-B/E | PIBB t-F | PIBB t-F/E | PITF R | PITF t-F | PITF t-F/E | PISF R | PISF t-F | PISF t-F/E |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tg | 246 | 242 | 248 | 245 | 253 | 247 | 256 | 252 | 246 | 240 | 246 |
| Td5% | 515 | 509 | 510 | 503 | 525 | 532 | 534 | 529 | 235 | 257 | 222 |
| Td10% | 526 | 523 | 522 | 528 | 533 | 555 | 558 | 553 | 302 | 467 | 290 |
| Rw780 | 56.0 | 57.3 | 55.7 | 55.6 | 53.3 | 50.6 | 51.6 | 50.9 | 50.6 | 52.3 | 49.1 |
| Parameters | PIBB R | PIBB t-B | PIBB t-B/E | PIBB t-F | PIBB t-F/E | PITF R | PITF t-F | PITF t-F/E | PISF R | PISF t-F | PISF t-F/E |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rm (MPa) | 66.9 | 70.5 | 75.8 | 73.5 | 80.7 | 86.0 | 76.8 | 82.0 | 48.3 | 55.6 | 61.5 |
| E (MPa) | 256 | 214 | 285 | 281 | 295 | 323 | 303 | 335 | 208 | 223 | 235 |
| e (%) | 35 | 29 | 31 | 34 | 32 | 18 | 22 | 23 | 23 | 25 | 27 |
| Contact Angle | PIBB R | PIBB t-B | PIBB t-B/E | PIBB t-F | PIBB t-F/E | PITF R | PITF t-F | PITF t-F/E | PISF R | PISF t-F | PISF t-F/E |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ( ͦ ) | 78.8 | 75.9 | 74.1 | 81.4 | 80.1 | 92.5 | 91.8 | 89.4 | 72.9 | 71.1 | 67.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.; Liu, P.; Yue, J.; Huan, H.; Yang, Z.; Yang, K.; Guo, H.; Zhao, J. High-Transparency and Colorless Polyimide Film Prepared by Inhibiting the Formation of Chromophores. Polymers 2022, 14, 4242. https://doi.org/10.3390/polym14194242
Su C, Liu P, Yue J, Huan H, Yang Z, Yang K, Guo H, Zhao J. High-Transparency and Colorless Polyimide Film Prepared by Inhibiting the Formation of Chromophores. Polymers. 2022; 14(19):4242. https://doi.org/10.3390/polym14194242
Chicago/Turabian StyleSu, Chuanxiang, Pengjia Liu, Jingyu Yue, Hengjian Huan, Zhenghui Yang, Kai Yang, Haiquan Guo, and Jianying Zhao. 2022. "High-Transparency and Colorless Polyimide Film Prepared by Inhibiting the Formation of Chromophores" Polymers 14, no. 19: 4242. https://doi.org/10.3390/polym14194242
APA StyleSu, C., Liu, P., Yue, J., Huan, H., Yang, Z., Yang, K., Guo, H., & Zhao, J. (2022). High-Transparency and Colorless Polyimide Film Prepared by Inhibiting the Formation of Chromophores. Polymers, 14(19), 4242. https://doi.org/10.3390/polym14194242





