Recent Advances in the Removal of Organic Dyes from Aqueous Media with Conducting Polymers, Polyaniline and Polypyrrole, and Their Composites
Abstract
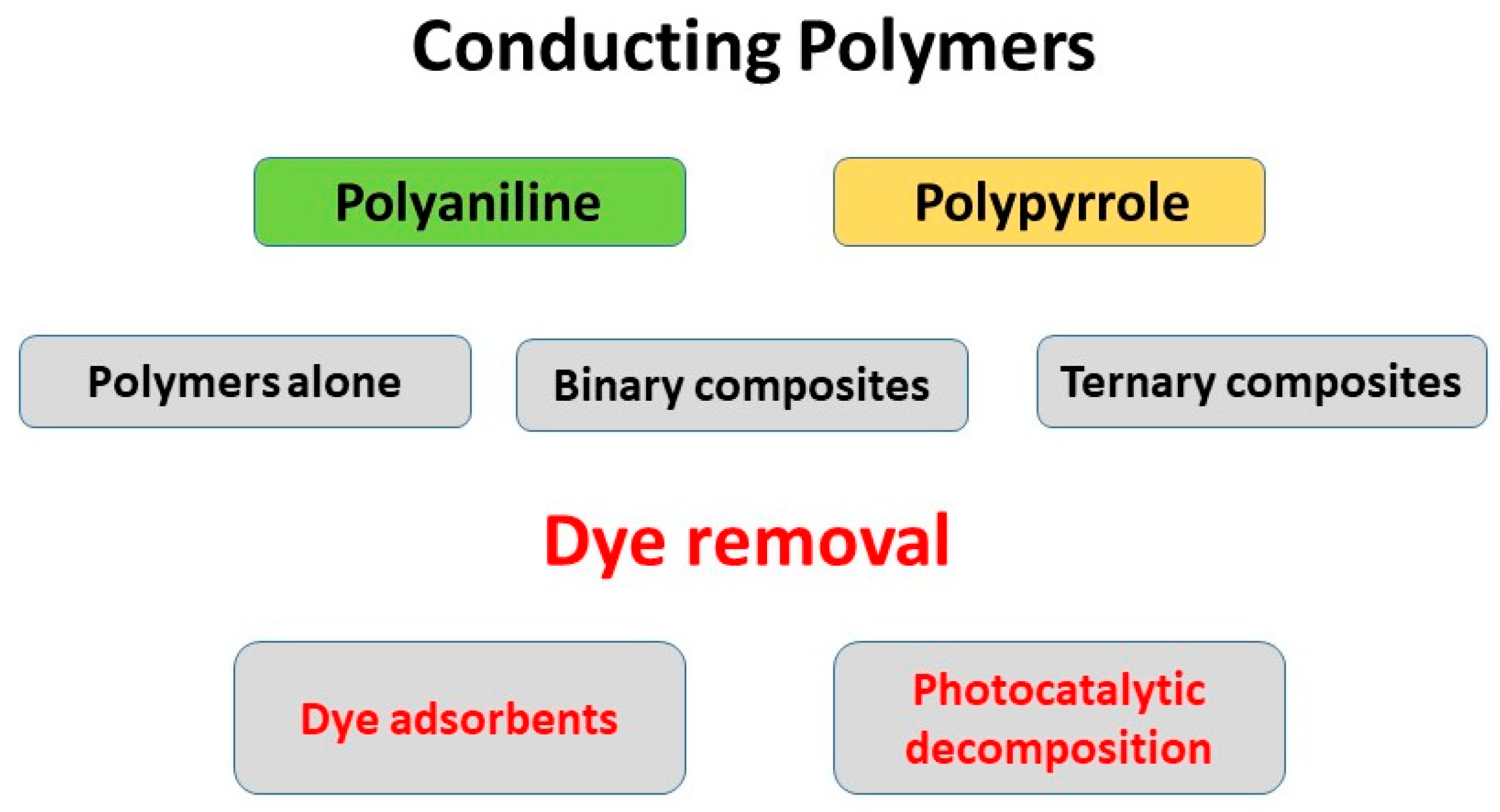
1. Introduction
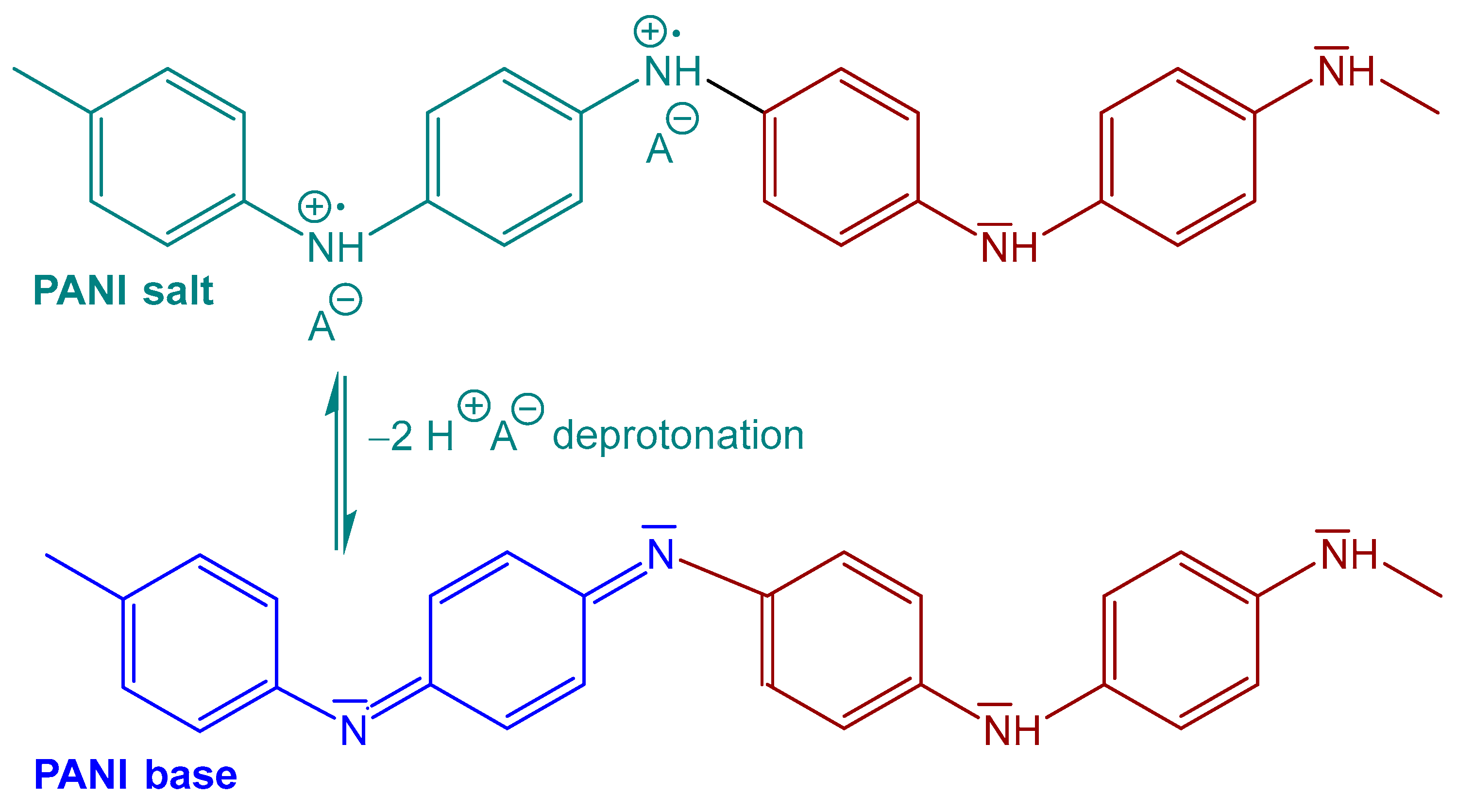
2. Polyaniline Adsorbents
2.1. Binary Polyaniline Composites
2.2. Ternary Polyaniline Composites
3. Polyaniline Photocatalysts
3.1. Binary Polyaniline Composites
3.2. Ternary Polyaniline Composites
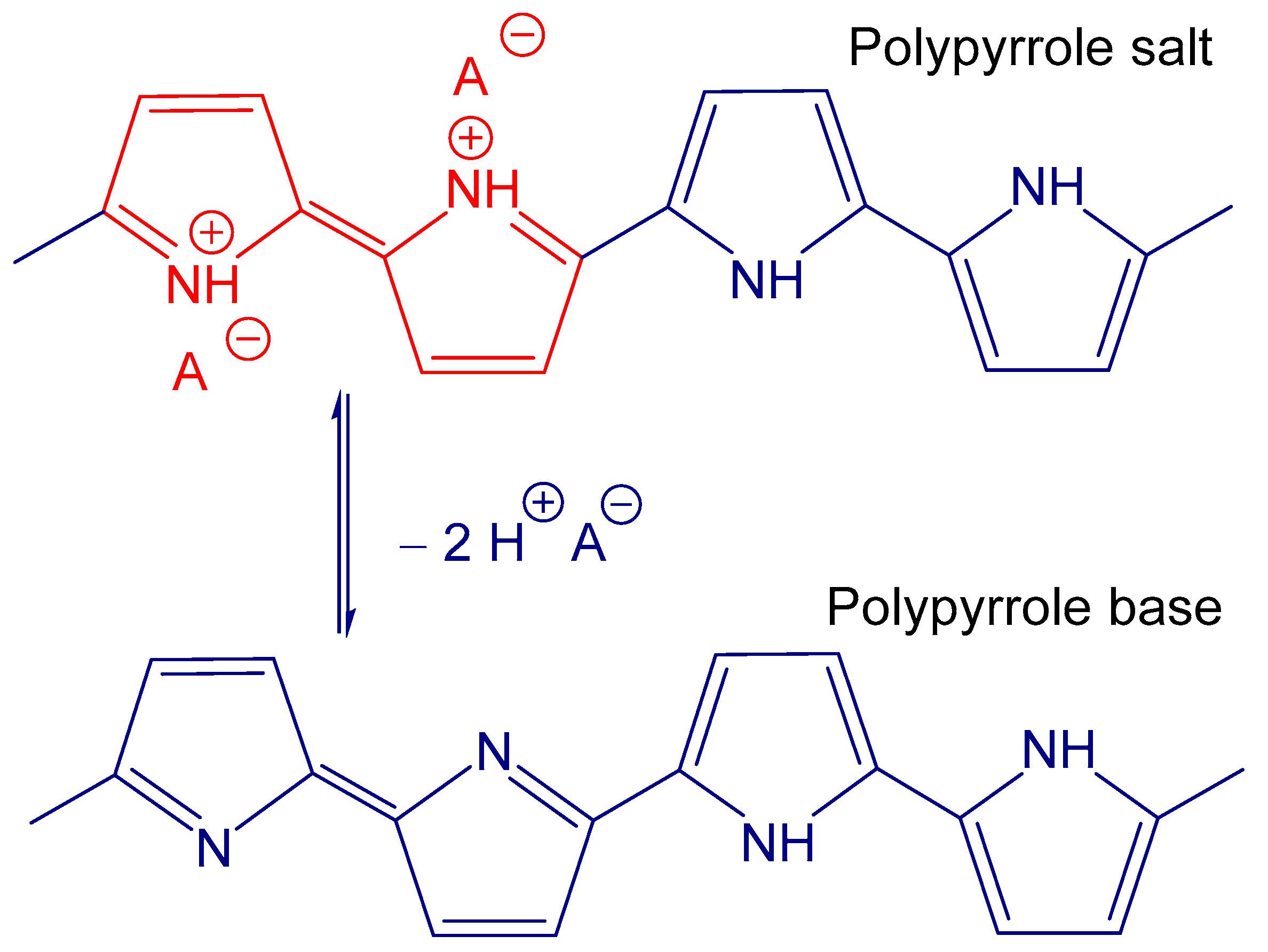
4. Polypyrrole Adsorbents
4.1. Binary Polypyrrole Composites
4.2. Ternary Polypyrrole Composites
5. Polypyrrole Photocatalysts
5.1. Binary Polypyrrole Composites
5.2. Ternary Polypyrrole Composites
6. Polyaniline and Polypyrrole Derivatives
7. Conclusions
8. Future Prospects
Funding
Conflicts of Interest
References
- Stejskal, J. Interaction of conducting polymers, polyaniline and polypyrrole, with organic dyes: Polymer morphology control, dye adsorption and photocatalytic decomposition. Chem. Pap. 2020, 74, 1–54. [Google Scholar] [CrossRef]
- Ambigadevi, J.; Kumar, P.S.; Vo, D.-V.N.; Haran, S.H.; Raghavan, T.S. Recent developments in photocatalytic remediation of textile effluent using semiconductor based nanostructured catalyst: A review. J. Environ. Chem. Eng. 2020, 9, 104881. [Google Scholar] [CrossRef]
- Senguttuvan, S.; Senthilkumar, P.; Janaki, V.; Kamala-Kannan, S. Significance of conducting polyaniline based composites for the removal of dyes and heavy metals from aqueous solution and wastewaters—A review. Chemosphere 2020, 267, 129201. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.W.; Shahadat, M.; Sagadevan, S. Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes. Green Process. Synth. 2022, 11, 617–630. [Google Scholar] [CrossRef]
- Sapurina, I.; Stejskal, J. The mechanism of the oxidative polymerization of aniline and the formation of supramolecular polyaniline structures. Polym. Int. 2008, 57, 1295–1325. [Google Scholar] [CrossRef]
- Stejskal, J.; Gilbert, R.G. Polyaniline. Preparation of a conducting polymer (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 857–867. [Google Scholar] [CrossRef]
- Bekhoukh, A.; Moulefera, I.; Zeggai, F.Z.; Benyoucef, A.; Bachari, K. Anionic methyl orange removal from aqueous solutions by activated carbon reinforced conducting polyaniline as adsorbent: Synthesis, characterization, adsorption behavior, regeneration and kinetics study. J. Polym. Environ. 2021, 30, 886–895. [Google Scholar] [CrossRef]
- Maeda, S.; Armes, S.P. Surface-area measurements on conducting polymer-inorganic oxide nanocomposites. Synth. Met. 1995, 73, 151–155. [Google Scholar] [CrossRef]
- Zor, S.; Budak, B. Photocatalytic degradation of congo red by using PANI and PANI/ZrO2: Under UV-A light irradiation and dark environmental. Desalination Water Treat. 2020, 201, 420–430. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, J.; Yu, X.; Fu, X.; Zhu, Y.; Zhang, Y. Enhanced removal of organic pollutant by separable and recyclable rGH-PANI/BiOI photocatalyst via the synergism of adsorption and photocatalytic degradation under visible light. J. Mater. Sci. Technol. 2020, 77, 19–27. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M.; Bober, P.; Morávková, Z.; Kopecký, D.; Vrňata, M.; Prokeš, J.; Varga, M.; Watzlová, E. Polypyrrole salts and bases: Superior conductivity of nanotubes and their stability towards the loss of conductivity by deprotonation. RSC Adv. 2016, 6, 88382–88391. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, W.; Yang, H.; Ma, F.; Xu, F. Green synthesis of poly(pyrrole methane) for enhanced adsorption of anionic and cationic dyes from aqueous solution. J. Colloid Interface Sci. 2021, 590, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, J.; Trchová, M. Conducting polypyrrole nanotubes: A review. Chem. Pap. 2018, 72, 1563–1595. [Google Scholar] [CrossRef]
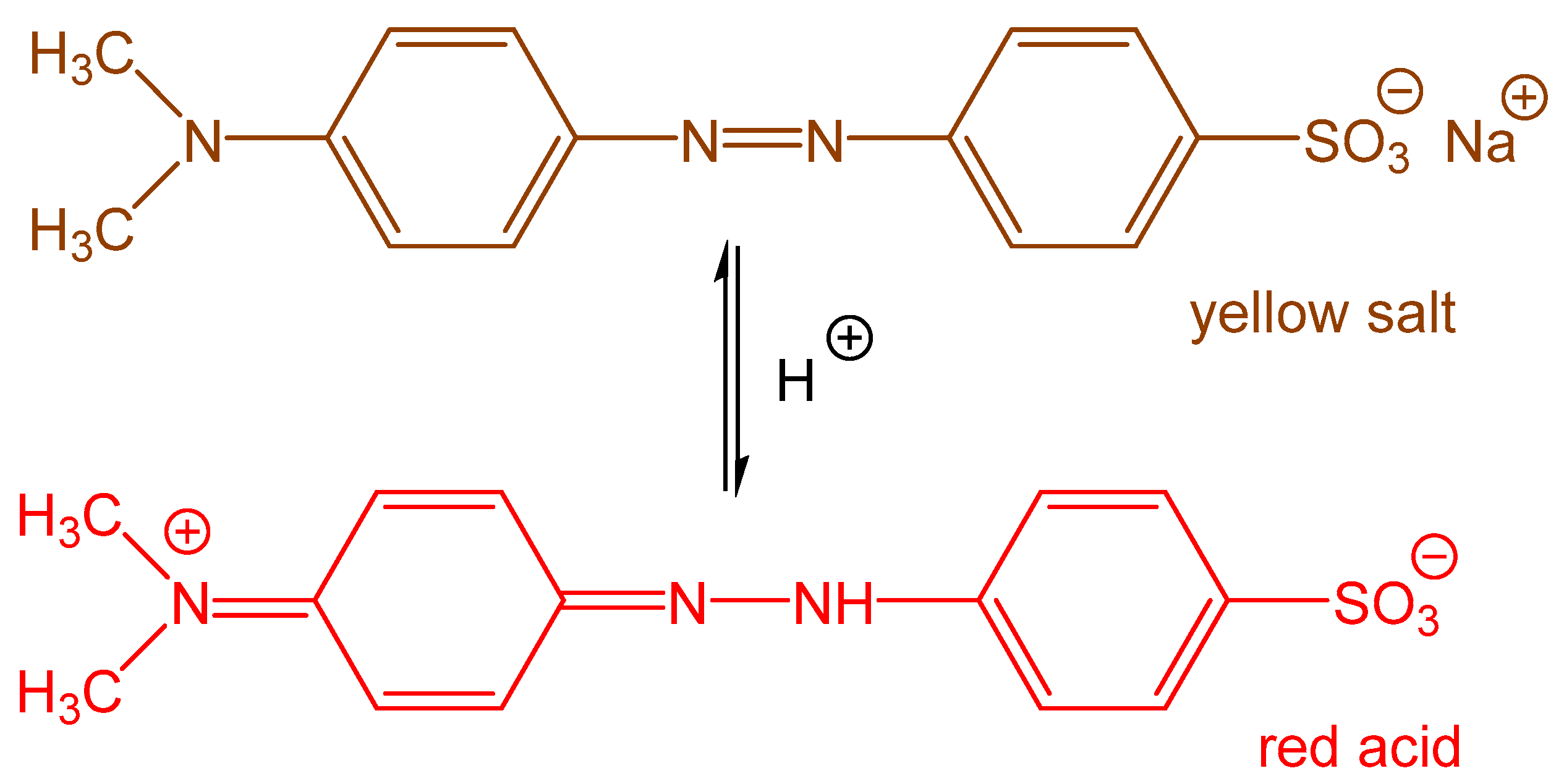
- Stejskal, J.; Prokeš, J. Conductivity and morphology of polyaniline and polypyrrole prepared in the presence of organic dyes. Synth. Met. 2020, 264, 116373. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I.; Vilčáková, J.; Humpolíček, P.; Truong, T.H.; Shishov, M.A.; Trchová, M.; Kopecký, D.; Kolská, Z.; Prokeš, J.; et al. Conducting polypyrrole-coated macroporous melamine sponges: A simple toy or an advanced material? Chem. Pap. 2021, 75, 5035–5055. [Google Scholar] [CrossRef]
- Stejskal, J.; Pekárek, M.; Trchová, M.; Kolská, Z. Adsorption of organic dyes on macroporous melamine sponge incorporating conducting polypyrrole nanotubes. J. Appl. Polym. Sci. 2022, 139, 52156. [Google Scholar] [CrossRef]
- Pete, S.; Kattil, R.A.; Thomas, L. Polyaniline-multiwalled carbon nanotubes (PANI-MWCNTs) composite revisited: An efficient and reusable material for methyl orange dye removal. Diam. Relat. Mater. 2021, 117, 108455. [Google Scholar] [CrossRef]
- Stejskal, J.; Kopecký, D.; Kasparyan, H.; Vilčáková, J.; Prokeš, J.; Křivka, I. Melamine sponges decorated with polypyrrole nanotubes as macroporous conducting pressure sensors. ACS Appl. Nano Mater. 2021, 4, 7513–7519. [Google Scholar] [CrossRef]
- Nayebi, P.; Babamoradi, M. Synthesis of ZnO nanorods/Fe3O4/Polypyrrole nanocomposites for photocatalytic activity under the visible light irradiation. Optik 2021, 244, 167497. [Google Scholar] [CrossRef]
- Jadhav, S.; Jaspal, D. Elimination of cationic azodye from aqueous media using doped polyaniline (PANI): Adsorption optimization and modeling. Can. J. Chem. 2020, 98, 717–724. [Google Scholar] [CrossRef]
- Danu, B.Y.; Agorku, E.S.; Ampong, F.K.; Awudza, J.A.M.; Torve, V.; Danquah, I.M.K.; Ama, O.M.; Osifo, P.O.; Ray, S.S. Iron sulfide functionalized polyaniline nanocomposite for the removal of Eosin Y from water: Equilibrium and kinetic studies. Polym. Sci. Ser. B 2021, 63, 304–313. [Google Scholar] [CrossRef]
- Khairy, M.; Kamar, E.M.; Yehia, M.; Masoud, K.M. High removal efficiency of methyl orange dye by pure and (Cu, N) doped TiO2/polyaniline nanocomposites. Biointerface Res. Appl. Chem. 2021, 12, 893–909. [Google Scholar] [CrossRef]
- Sillanpää, M.; Mahvi, A.H.; Balarak, D.; Khatibi, A.D. Adsorption of Acid Orange 7 dyes from aqueous solution using Polypyrrole/nanosilica composite: Experimental and modelling. Int. J. Environ. Anal. Chem. 2021, 1–18. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nguyen, M.T.; Vuong, B.H.; Le, T.H. Cellulose grafted with polyaniline for simultaneous adsorption of cationic and anionic dyes in wastewater effluent. Cellulose 2022, 29, 7761–7773. [Google Scholar] [CrossRef]
- Anuma, S.; Mishra, P.; Bhat, B.R. Polypyrrole functionalized cobalt oxide graphene (COPYGO) nanocomposite for the efficient removal of dyes and heavy metal pollutants from aqueous effluents. J. Hazard. Mater. 2021, 416, 125929. [Google Scholar] [CrossRef]
- Alsaiari, N.; Amari, A.; Katubi, K.; Alzahrani, F.; Rebah, F.; Tahoon, M. Innovative magnetite based polymeric nanocomposite for simultaneous removal of methyl orange and hexavalent chromium from water. Processes 2021, 9, 576. [Google Scholar] [CrossRef]
- Li, X.; Zhao, X.; Li, X.; Jia, X.; Chang, F.; Zhang, H.; Hu, G. Rapid simultaneous removal of cationic dyes and Cr(VI) by boron cluster polyaniline with a target site. Chem. Commun. 2021, 57, 7569–7572. [Google Scholar] [CrossRef]
- Mohammadi, H.; Ghaedi, M.; Fazeli, M.; Sabzehmeidani, M.M. Removal of hexavalent chromium ions and Acid Red 18 by superparamagnetic CoFe2O4/polyaniline nanocomposites under external ultrasonic fields. Microporous Mesoporous Mater. 2021, 324, 111275. [Google Scholar] [CrossRef]
- Peng, D.-Y.; Zeng, H.-Y.; Xiong, J.; Xu, S.; An, D.S. Improved photocatalytic performance of p-n heterostructure Ag-Ag2MoO4/polyaniline for chromium (VI) reduction and dye degradation. J. Alloy. Compd. 2022, 912, 165063. [Google Scholar] [CrossRef]
- Kuznetsova, T.S.; Burakova, I.V.; Pasko, T.V.; Burakov, A.E.; Melezhik, A.V.; Mkrtchyan, E.S.; Babkin, A.V.; Neskoromnaya, E.A.; Tkachev, A.G. Technology of nanocomposites preparation for sorption purification of aqueous media. Inorg. Mater. Appl. Res. 2022, 13, 434–441. [Google Scholar] [CrossRef]
- Yu, C.; Tan, L.; Shen, S.; Fang, M.; Yang, L.; Fu, X.; Dong, S.; Sun, J. In situ preparation of g-C3N4/polyaniline hybrid composites with enhanced visible-light photocatalytic performance. J. Environ. Sci. 2021, 104, 317–325. [Google Scholar] [CrossRef]
- Abinaya, M.; Muthuraj, V. Bi-functional catalytic performance of silver manganite/polypyrrole nanocomposite for electrocatalytic sensing and photocatalytic degradation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125321. [Google Scholar] [CrossRef]
- Motamedi, M.; Mollahosseini, A.; Negarestani, M. Ultrasonic-assisted batch operation for the adsorption of rifampin and reactive orange 5 onto engineered zeolite–polypyrrole/TiO2 nanocomposite. Int. J. Environ. Sci. Technol. 2022, 19, 7547–7564. [Google Scholar] [CrossRef]
- Sapurina, I.; Bubulinca, C.; Trchová, M.; Prokeš, J.; Stejskal, J. Solid manganese dioxide as heterogeneous oxidant of aniline in the preparation of conducting polyaniline or polyaniline/manganese dioxide composites. Colloids Surf. A Physicochem. Eng. Asp. 2022, 638, 128298. [Google Scholar] [CrossRef]
- Lyu, W.; Yu, M.; Li, J.; Feng, J.; Yan, W. Adsorption of anionic acid red G dye on polyaniline nanofibers synthesized by FeCl3 oxidant: Unravelling the role of synthetic conditions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129203. [Google Scholar] [CrossRef]
- Myasoedova, T.N.; Gadzhieva, V.A.; Miroshnichenko, Y.S. Properties of mesoporous pani nanorods obtained by facile acid-free synthesis as a sorbent for methylene blue and indigo carmine removal. J. Polym. Res. 2022, 29, 1–13. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, Y.; Xu, L.; Qiu, Z.; Wang, Z.; Luo, Y.; Du, K. Amino acid-doped polyaniline nanotubes as efficient adsorbent for wastewater treatment. J. Chem. 2022, 2022, 2041512. [Google Scholar] [CrossRef]
- Wu, Y.; Chang, H.; Peng, J.; Liu, Y.; Sun, B.; Yang, Z.; Gao, S.; Liu, F. A facile strategy to fabricate hollow spherical polyaniline and its application to dyes removal. Polym. Bull. 2022, 1–14. [Google Scholar] [CrossRef]
- Duhan, M.; Kaur, R. Nano-structured polyaniline as a potential adsorbent for methylene blue dye removal from effluent. J. Compos. Sci. 2021, 5, 7. [Google Scholar] [CrossRef]
- Ali, L.I.A.; Ismail, H.K.; Alesary, H.F.; Aboul-Enein, H.Y. A nanocomposite based on polyaniline, nickel and manganese oxides for dye removal from aqueous solutions. Int. J. Environ. Sci. Technol. 2020, 18, 2031–2050. [Google Scholar] [CrossRef]
- Zou, Z.J.; Li, Y.L.; Ma, Z.W.; Jin, Y.Q.; Lü, Q.F. Preparation and dye adsorption of low-cost polyaniline-tea saponin nanocomposites. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2021, 36, 546–556. [Google Scholar] [CrossRef]
- Bober, P.; Minisy, I.; Acharya, U.; Pfleger, J.; Babayan, V.; Kazantseva, N.; Hodan, J.; Stejskal, J. Conducting polymer composite aerogel with magnetic properties for organic dye removal. Synth. Met. 2019, 260, 116266. [Google Scholar] [CrossRef]
- Lyu, W.; Li, J.; Trchová, M.; Wang, G.; Liao, Y.; Bober, P.; Stejskal, J. Fabrication of polyaniline/poly(vinyl alcohol)/montmorillonite hybrid aerogels toward efficient adsorption of organic dye pollutants. J. Hazard. Mater. 2022, 435, 129004. [Google Scholar] [CrossRef] [PubMed]
- Riede, A.; Helmstedt, M.; Sapurina, I.; Stejskal, J. In situ polymerized polyaniline films. Film formation in dispersion polymerization of aniline. J. Colloid Interface Sci. 2002, 248, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Perween, S.; Ranjan, A. Dramatic enhancement in adsorption of Congo red dye in polymer-nanoparticle composite of polyaniline-zinc titanate. J. Environ. Chem. Eng. 2021, 9, 105149. [Google Scholar] [CrossRef]
- Das, M.; Ray, P.G.; Dhara, S.; Roy, S. Symbiotically Augmented removal of Congo red by polyaniline/cobalt sulfide/graphite composites. Mater. Chem. Phys. 2021, 278, 125487. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, H.; Zhang, W.; Lu, Z.; Shen, M.; Liu, T.; Bai, J.; Yang, Y.; Zhang, J. Free-standing hybrid film for separation of dye pollutant with self-cleaning ability under visible light. Chemosphere 2021, 291, 132725. [Google Scholar] [CrossRef] [PubMed]
- Li, D.W.; Tao, Y.L.; Li, S.; Wu, Y.N.; Wang, C.R.; Lv, Y.R.; Zhu, G.S.; Qiu, H.F.; Liu, X.; Chen, C. Porous cage-like microfiber of fly ash magnetic powder (CMS)/polyaniline (PANI) composites with absorption properties. Phys. Scripta 2022, 97, 085817. [Google Scholar] [CrossRef]
- Xiong, H.; Zhang, B.; Cui, C.; Xu, Y. Polyaniline/FeOOH composite for removal of Acid Orange II from aqueous solutions. Mater. Chem. Phys. 2022, 278, 125701. [Google Scholar] [CrossRef]
- Momina; Ahmad, K. Remediation of anionic dye from aqueous solution through adsorption on polyaniline/FO nanocomposite-modelling by artificial neural network (ANN). J. Mol. Liq. 2022, 360, 119497. [Google Scholar] [CrossRef]
- Das, P.; Nisa, S.; Debnath, A.; Saha, B. Enhanced adsorptive removal of toxic anionic dye by novel magnetic polymeric nanocomposite: Optimization of process parameters. J. Dispers. Sci. Technol. 2020, 43, 880–895. [Google Scholar] [CrossRef]
- Das, P.; Debnath, A. Fabrication of MgFe2O4/polyaniline nanocomposite for amputation of methyl red dye from water: Isotherm modeling, kinetic and cost analysis. J. Dispers. Sci. Technol. 2022, 1–12. [Google Scholar] [CrossRef]
- Fanourakis, S.K.; Barroga, S.Q.; Perez, J.V.D.; He, L.; Rodrigues, D.F. In situ polymerization of polypyrrole and polyaniline on the surface of magnetic molybdenum trioxide nanoparticles: Implications for water treatment. ACS Appl. Nano Mater. 2021, 4, 12415–12428. [Google Scholar] [CrossRef]
- Peng, L.-G.; Zhao, P.; Cheng, H.-Q.; He, Q.-R.; Wang, X.-H.; Liu, J.-X.; Wang, J.-L. Adsorption Studies of Reactive Green 19 from Aqueous Solutions by Polyaniline/Montmorillonite Nanocomposite. Sci. Adv. Mater. 2022, 14, 535–544. [Google Scholar] [CrossRef]
- Tanweer, M.S.; Iqbal, Z.; Alam, M. Experimental insights into mesoporous polyaniline-based nanocomposites for anionic and cationic dye removal. Langmuir 2022, 38, 8837–8853. [Google Scholar] [CrossRef]
- Rajaji, U.; Rani, S.E.G.D.; Chen, S.M.; Rajakumar, K.; Govindasamy, M.; Alzahrani, F.M.; Alsaiari, N.S.; Ouladsmane, M.; Lydia, I.S. Synergistic photocatalytic activity of SnO2/PANI nanocomposite for the removal of direct blue 15 under UV light irradiation. Ceramics Int. 2021, 47, 29225–29231. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Lee, H.S.; Lee, C.-G.; Park, S.-J.; Lee, J.; Jung, S.; Shin, G.-A. Application of PANI/TiO2 composite for photocatalytic degradation of contaminants from aqueous solution. Appl. Sci. 2020, 10, 6710. [Google Scholar] [CrossRef]
- Maldonado-Larios, L.; Mayen-Mondragón, R.; Martínez-Orozco, R.; Páramo-García, U.; Gallardo-Rivas, N.; García-Alamilla, R. Electrochemically-assisted fabrication of titanium-dioxide/polyaniline nanocomposite films for the electroremediation of congo red in aqueous effluents. Synth. Met. 2020, 268, 116464. [Google Scholar] [CrossRef]
- Imgharn, A.; Anchoum, L.; Hsini, A.; Naciri, Y.; Laabd, M.; Mobarak, M.; Aarab, N.; Bouziani, A.; Szunerits, S.; Boukherroub, R.; et al. Effectiveness of a novel polyaniline@Fe-ZSM-5 hybrid composite for Orange G dye removal from aqueous media: Experimental study and advanced statistical physics insights. Chemosphere 2022, 295, 133786. [Google Scholar] [CrossRef]
- Toumi, I.; Djelad, H.; Chouli, F.; Benyoucef, A. Synthesis of PANI@ZnO hybrid material and evaluations in adsorption of Congo red and methylene blue dyes: Structural characterization and adsorption performance. J. Inorg. Organomet. Polym. Mater. 2021, 32, 112–121. [Google Scholar] [CrossRef]
- Turkten, N.; Karatas, Y.; Bekbolet, M. Preparation of PANI Modified ZnO Composites via different methods: Structural, morphological and photocatalytic properties. Water 2021, 13, 1025. [Google Scholar] [CrossRef]
- Benchikh, I.; Dahou, F.Z.; Lahreche, S.; Sabantina, L.; Benmimoun, Y.; Benyoucef, A. Development and characterisation of novel hybrid materials of modified ZnO-SiO2 and polyaniline for adsorption of organic dyes. Int. J. Environ. Anal. Chem. 2022, 1–20. [Google Scholar] [CrossRef]
- Kumar, N.; Bahl, T.; Kumar, R. Study of the methylene blue adsorption mechanism using ZrO2/Polyaniline nanocomposite. Nano Express 2020, 1, 030025. [Google Scholar] [CrossRef]
- Eisazadeh, N.; Eisazadeh, H.; Ghadakpour, M. Comparison between various adsorbents for Direct Blue dye 14 removal from aqueous solution. Fibers Polym. 2021, 22, 149–158. [Google Scholar] [CrossRef]
- Gohoho, H.D.; Noby, H.; Hayashi, J.; El-shazlyShazly, A.H. Various acids functionalized polyaniline-peanut shell activated carbon composites for dye removal. J. Mater. Cycles Waste Manag. 2022, 24, 1508–1523. [Google Scholar] [CrossRef]
- Lahreche, S.; Moulefera, I.; El Kebir, A.; Sabantina, L.; Kaid, M.; Benyoucef, A. Application of activated carbon adsorbents prepared from prickly pear fruit seeds and a conductive polymer matrix to remove congo red from aqueous solutions. Fibers 2022, 10, 7. [Google Scholar] [CrossRef]
- Meena, P.L.; Saini, J.K.; Surela, A.K.; Poswal, K.; Chhachhia, L.K. Fabrication of polyaniline-coated porous and fibrous nanocomposite with granular morphology using tea waste carbon for effective removal of rhodamine B dye from water samples. Biomass Convers. Biorefinery 2022, 1–20. [Google Scholar] [CrossRef]
- Li, R.; Li, T.; Wan, Y.; Zhang, X.; Liu, X.; Li, R.; Pu, H.; Gao, T.; Wang, X.; Zhou, Q. Efficient decolorization of azo dye wastewater with polyaniline/graphene modified anode in microbial electrochemical systems. J. Hazard. Mater. 2021, 421, 126740. [Google Scholar] [CrossRef]
- Khan, M.A.; Govindasamy, R.; Ahmad, A.; Siddiqui, M.; Alshareef, S.; Hakami, A.; Rafatullah, M. Carbon based polymeric nanocomposites for dye adsorption: Synthesis, characterization, and application. Polymers 2021, 13, 419. [Google Scholar] [CrossRef]
- Razzaq, S.; Akhtar, M.; Zulfiqar, S.; Zafar, S.; Shakir, I.; Agboola, P.O.; Haider, S.; Warsi, M.F. Adsorption removal of Congo red onto L-cysteine/rGO/PANI nanocomposite: Equilibrium, kinetics and thermodynamic studies. J. Taibah Univ. Sci. 2021, 15, 50–62. [Google Scholar] [CrossRef]
- Katowah, D.F.; Saleh, S.M.; Alqarni, S.A.; Ali, R.; Mohammed, G.I.; Hussein, M.A. Network structure-based decorated CPA@CuO hybrid nanocomposite for methyl orange environmental remediation. Sci. Rep. 2021, 11, 1–21. [Google Scholar] [CrossRef]
- Singh, A.R.; Dhumal, P.S.; Bhakare, M.A.; Lokhande, K.D.; Bondarde, M.P.; Some, S. In-situ synthesis of metal oxide and polymer decorated activated carbon-based photocatalyst for organic pollutants degradation. Sep. Purif. Technol. 2022, 286, 120380. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, F.; Tian, X.; Hu, X.; Liu, Y.; Zhao, X.; Qu, R.; Ji, C.; Niu, Y. Polyaniline dispersed by Kevlar fiber for uptake of organic dye. Pigment Resin Technol. 2020, 50, 346–355. [Google Scholar] [CrossRef]
- Liu, M.-L.; Li, L.; Sun, Y.-X.; Fu, Z.-J.; Cao, X.-L.; Sun, S.-P. Scalable conductive polymer membranes for ultrafast organic pollutants removal. J. Membr. Sci. 2021, 617, 118644. [Google Scholar] [CrossRef]
- Jahan, K.; Tyeb, S.; Kumar, N.; Verma, V. Bacterial cellulose-polyaniline porous mat for removal pf methyl orange and bacterial pathogens from potable water. J. Polym. Environ. 2021, 29, 1257–1270. [Google Scholar] [CrossRef]
- Mansor, E.S.; Ali, H.; Abdel-Karim, A. Efficient and reusable polyethylene oxide/polyaniline composite membrane for dye adsorption and filtration. Colloids Interface Sci. Commun. 2020, 39, 100314. [Google Scholar] [CrossRef]
- Mendieta-Rodríguez, L.S.; González-Rodríguez, L.M.; Alcaraz-Espinoza, J.J.; Chávez-Guajardo, A.E.; Medina-Llamas, J.C. Synthesis and characterization of a polyurethane-polyaniline macroporous foam material for methyl orange removal in aqueous media. Mater. Today Commun. 2021, 26, 102–155. [Google Scholar] [CrossRef]
- Bagheri, N.; Lakouraj, M.M.; Hasantabar, V.; Mohseni, M. Biodegradable macro-porous CMC-polyaniline hydrogel: Synthesis, characterization and study of microbial elimination and sorption capacity of dyes from waste water. J. Hazard. Mater. 2021, 403, 123631. [Google Scholar] [CrossRef]
- Alam, J.; Shukla, A.K.; Ansari, M.A.; Ali, F.A.A.; Alhoshan, M. Dye separation and antibacterial activities of polyaniline thin film-coated poly(phenyl sulfone) membranes. Membranes 2021, 11, 25. [Google Scholar] [CrossRef]
- Mahi, O.; Khaldi, K.; Belardja, M.S.; Belmokhtar, A.; Benyoucef, A. Development of a New Hybrid Adsorbent from Opuntia Ficus Indica NaOH-Activated with PANI-Reinforced and Its Potential Use in Orange-G Dye Removal. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2095–2104. [Google Scholar] [CrossRef]
- Imgharn, A.; Ighnih, H.; Hsini, A.; Naciri, Y.; Laabd, M.; Kabli, H.; Elamine, M.; Lakhmiri, R.; Souhail, B.; Albourine, A. Synthesis and characterization of polyaniline-based biocomposites for effective removal of Orange G dye adsorption in dynamic regime. Chem. Phys. Lett. 2021, 778, 138811. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A. Facile synthesis of polypyrrole decorated chitosan-based magsorbent: Characterizations, performance, and applications in removing cationic and anionic dyes from aqueous medium. Int. J. Biol. Macromol. 2020, 161, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, J.; Zhu, L.; Liu, H.; Yu, S.; Xue, J.; Zhu, X.; Xue, Q. Reusable membrane with multifunctional skin layer for effective removal of insoluble emulsified oils and soluble dyes. J. Hazard. Mater. 2021, 415, 125677. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, H.; Umar, M.; Nawaz, I.; Zia, Q.; Tabassum, M.; Razzaq, H.; Gong, H.; Zhao, X.; Liu, X. Photodegradation of textile pollutants by nanocomposite membranes of polyvinylidene fluoride integrated with polyaniline–titanium dioxide nanotubes. Chem. Eng. J. 2021, 419, 129542. [Google Scholar] [CrossRef]
- Nawaz, H.; Umar, M.; Nawaz, I.; Ullah, A.; Khawar, M.T.; Nikiel, M.; Razzaq, H.; Siddiq, M.; Liu, X. Hybrid PVDF/PANI membrane for removal of dyes from textile wastewater. Adv. Eng. Mater. 2021, 24, 2100719. [Google Scholar] [CrossRef]
- Imgharn, A.; Aarab, N.; Hsini, A.; Naciri, Y.; Elhoudi, M.; Haki, M.A.; Laabd, M.; Lakhmiri, R.; Albourine, A. Application of calcium alginate-PANI@sawdust wood hydrogel bio-beads for the removal of orange G dye from aqueous solution. Environ. Sci. Pollut. Res. 2022, 29, 60259–60268. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Saravanan, A.; Manohar, P.; Luong, J.; Gedanken, A. Photocatalytic degradation of organic dyes and antimicrobial activities by polyaniline–nitrogen-doped carbon dot nanocomposite. Nanomaterials 2021, 11, 1128. [Google Scholar] [CrossRef]
- Yuan, X.; Kobylanski, M.P.; Cui, Z.; Li, J.; Beaunier, P.; Dragoe, D.; Colbeau-Justin, C.; Zaleska-Medynska, A.; Remita, H. Highly active composite TiO2-polypyrrole nanostructures for water and air depollution under visible light irradiation. J. Environ. Chem. Eng. 2020, 8, 104178. [Google Scholar] [CrossRef]
- Demir, M.; Taymaz, B.H.; Sarıbel, M.; Kamış, H. Photocatalytic Degradation of Organic Dyes with Magnetically Separable PANI/Fe3O4 Composite under Both UV and Visible-light Irradiation. ChemistrySelect 2022, 7, e202103787. [Google Scholar] [CrossRef]
- Riyat, R.I.; Salam, A.; Molla, T.H.; Islam, S.; Bashar, A.; Chandra, D.; Ahsan, S.; Roy, D. Magnetically recyclable core–shell structured Co0.5Zn0.5Fe2O4@polyaniline nanocomposite: High stability and rapid photocatalytic degradation of commercial azo dyes and industrial effluents. React. Kinet. Mech. Catal. 2022, 135, 1077–1098. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Y.; Xue, Q.; Wen, Y.; Hong, X.; Ullah, K. TiO2/Cu-MOF/PPy composite as a novel photocatalyst for decomposition of organic dyes. J. Mater. Sci. Mater. Electron. 2021, 32, 4097–4109. [Google Scholar] [CrossRef]
- Mittal, H.; Khanuja, M. Hydrothermal in-situ synthesis of MoSe2-polypyrrole nanocomposite for efficient photocatalytic degradation of dyes under dark and visible light irradiation. Sep. Purif. Technol. 2020, 254, 117508. [Google Scholar] [CrossRef]
- Taymaz, B.H.; Kamiş, H.; Yoldaş, O. Photocatalytic degradation of malachite green dye using zero valent iron doped polypyrrole. Environ. Eng. Res. 2021, 27, 200638. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Z.; Wang, X.; Yang, G.; Liu, Y. Adsorption-photocatalysis performance of polyaniline/dicarboxyl acid cellulose@graphene oxide for dye removal. Int. J. Biol. Macromol. 2021, 182, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Mansor, E.S.; Geioushy, R.A.; Fouad, O.A. PANI/BiOCl nanocomposite induced efficient visible-light photocatalytic activity. J. Mater. Sci. Mater. Electron. 2021, 32, 1992–2000. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, D.; Khare, N. Hierarchical PANI/CdS nanoarchitecture system for visible light included photocatalytic dye degradation and photoelectrochemical water splitting. Polymer 2021, 231, 124117. [Google Scholar] [CrossRef]
- Qutub, N.; Singh, P.; Sabir, S.; Umar, K.; Sagadevan, S.; Oh, W.-C. Synthesis of polyaniline supported CdS/CdS-ZnS/CdS-TiO2 nanocomposite for efficient photocatalytic applications. Nanomaterials 2022, 12, 1355. [Google Scholar] [CrossRef]
- Kumar, A.; Mittal, H.; Nagar, R.; Khanuja, M. The synergistic effect of acid-etched g-C3N4 nanosheets and polyaniline nanofibers for the adsorption and photocatalytic degradation of textile dyes: A study of charge transfer mechanism and intermediate products. Mater. Adv. 2022, 3, 5325–5336. [Google Scholar] [CrossRef]
- Ardani, M.R.; Pang, A.L.; Pal, U.; Zheng, R.; Arsad, A.; Hamzah, A.A.; Ahmadipour, M. Ultrasonic-assisted polyaniline-multiwall carbon nanotube photocatalyst for efficient photodegradation of organic pollutants. J. Water Process Eng. 2022, 46, 102557. [Google Scholar] [CrossRef]
- Bhaumik, M.; Maity, A.; Brink, H.G. Metallic nickel nanoparticles supported polyaniline nanotubes as heterogeneous Fenton-like catalyst for the degradation of brilliant green dye in aqueous solution. J. Colloid Interface Sci. 2021, 611, 408–420. [Google Scholar] [CrossRef]
- Fenniche, F.; Henni, A.; Khane, Y.; Aouf, D.; Harfouche, N.; Bensalem, S.; Zerrouki, D.; Belkhalfa, H. Electrochemical synthesis of reduced graphene oxide–wrapped polyaniline nanorods for improved photocatalytic and antibacterial activities. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1011–1025. [Google Scholar] [CrossRef]
- Kumar, H.; Luthra, M.; Punia, M.; Singh, D. Ag2O@PANI nanocomposites for advanced functional applications: A sustainable experimental and theoretical approach. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128464. [Google Scholar] [CrossRef]
- Sayed, M.A.; Ahmed, M.; El-Shahat, M.; El-Sewify, I.M. Mesoporous polyaniline/SnO2 nanospheres for enhanced photocatalytic degradation of bio-staining fluorescent dye from an aqueous environment. Inorg. Chem. Commun. 2022, 139, 109326. [Google Scholar] [CrossRef]
- Rahman, K.H.; Kar, A.K. Effect of band gap variation and sensitization process of polyaniline (PANI)-TiO2 p-n heterojunction photocatalysts on the enhancement of photocatalytic degradation of toxic methylene blue with UV irradiation. J. Environ. Chem. Eng. 2020, 8, 104181. [Google Scholar] [CrossRef]
- Aminuddin, N.; Nawi, M.; Bahrudin, N.; Jawad, A. Iron ion assisted photocatalytic-adsorptive removal of acid orange 52 by immobilized TiO2/polyaniline bilayer photocatalyst. Appl. Surf. Sci. Adv. 2021, 6, 100180. [Google Scholar] [CrossRef]
- Aminuddin, N.; Nawi, M.; Bahrudin, N. Enhancing the optical properties of immobilized TiO2/polyaniline bilayer photocatalyst for methyl orange decolorization. React. Funct. Polym. 2022, 174, 105248. [Google Scholar] [CrossRef]
- Naciri, Y.; Hsini, A.; Bouziani, A.; Tanji, K.; El Ibrahimi, B.; Ghazzal, M.; Bakiz, B.; Albourine, A.; Benlhachemi, A.; Navío, J.; et al. Z-scheme WO3/PANI heterojunctions with enhanced photocatalytic activity under visible light: A depth experimental and DFT studies. Chemosphere 2021, 292, 133468. [Google Scholar] [CrossRef]
- Hamdy, M.S.; Abd-Rabboh, H.S.; Benaissa, M.; Al-Metwaly, M.G.; Galal, A.; Ahmed, M. Fabrication of novel polyaniline/ZnO heterojunction for exceptional photocatalytic hydrogen production and degradation of fluorescein dye through direct Z-scheme mechanism. Opt. Mater. 2021, 117, 111198. [Google Scholar] [CrossRef]
- Belabed, C.; Tab, A.; Moulai, F.; Černohorský, O.; Boudiaf, S.; Benrekaa, N.; Grym, J.; Trari, M. ZnO nanorods-PANI heterojunction dielectric, electrochemical properties, and photodegradation study of organic pollutant under solar light. Int. J. Hydrogen Energy 2021, 46, 20893–20904. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, H. Polyaniline Plastic Nanocomposite as Multi-Functional Nanomaterial. ChemistrySelect 2022, 7, 202201475. [Google Scholar] [CrossRef]
- Kumaresan, A.; Arun, A.; Kalpana, V.; Vinupritha, P.; Sundaravadivel, E. Polymer-supported NiWO4 nanocomposites for visible light degradation of toxic dyes. J. Mater. Sci. Mater. Electron. 2022, 33, 9660–9668. [Google Scholar] [CrossRef]
- Ding, W.P.; Li, J.D.; Chen, S.W.; Li, X.G.; Wang, Q.; He, A.Y.; Yin, J.Z. Attapulgite/g-C3N4-Pt/polyaniline composites: Preparation and visible light photocatalytic properties. Chin. J. Inorg. Chem. 2022, 38, 253–260. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Pan, J.; Wang, M.; Chen, A.; Chen, Y. Fabrication, characterization and photocatalytic degradation activity of PS/PANI/CeO2 tri-layer nanostructured hybrids. Bull. Mater. Sci. 2022, 45, 1–9. [Google Scholar] [CrossRef]
- Shashikala, B.S.; Al-Gunaid, M.Q.A.; Somesh, T.E.; Anasuya, S.J. Core–shell synergistic effect of (PANI-NaBiO2) incorporated polycarbonate films to photodegradation of MG dye and photovoltaic activity. Polym. Bull. 2022, 79, 7531–7554. [Google Scholar] [CrossRef]
- Palliyalil, S.; Chola, R.K.V.; Vigneshwaran, S.; Poovathumkuzhi, N.C.; Chelaveettil, B.M.; Meenakshi, S. Ternary system of TiO2 confined chitosan–polyaniline heterostructure photocatalyst for the degradation of anionic and cationic dyes. Environ. Technol. Innov. 2022, 28, 102586. [Google Scholar] [CrossRef]
- Liu, S.; Hu, J.; Wu, D.; Zeng, H.; Zhou, T.; Yang, M.; Feng, Q. Preparation of spunlaced viscose/PANI-ZnO/GO fiber membrane and its performance of photocatalytic decolorization. J. Ind. Text. 2022, 51, 7359S–7373S. [Google Scholar] [CrossRef]
- Jumat, N.A.; Khor, S.-H.; Basirun, W.J.; Juan, J.-C.; Phang, S.-W. Highly Visible Light Active Ternary Polyaniline-TiO2-Fe3O4 Nanotube/Nanorod for Photodegradation of Reactive Black 5 Dyes. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2168–2181. [Google Scholar] [CrossRef]
- Zare, N.; Kojoori, R.K.; Abdolmohammadi, S.; Sadegh-Samiei, S. Ultrasonic-assisted synthesis of highly effective visible light Fe3O4/ZnO/PANI nanocomposite: Thoroughly kinetics and thermodynamic investigations on the Congo red dye decomposition. J. Mol. Struct. 2021, 1250, 131903. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, X.; Waterhouse, G.I.; Zhang, Z.-M.; Yu, L.-M. Protonated graphitic carbon nitride/polypyrrole/reduced graphene oxide composites as efficient visible light driven photocatalysts for dye degradation and E. coli disinfection. J. Alloy. Compd. 2021, 873, 159750. [Google Scholar] [CrossRef]
- Bahadoran, A.; Baghbadorani, N.B.; De Lile, J.R.; Masudy-Panah, S.; Sadeghi, B.; Li, J.; Ramakrishna, S.; Liu, Q.; Janani, B.J.; Fakhri, A. Ag doped Sn3O4 nanostructure and immobilized on hyperbranched polypyrrole for visible light sensitized photocatalytic, antibacterial agent and microbial detection process. J. Photochem. Photobiol. B Biol. 2022, 228, 112393. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, R.; Ying, G.; Lu, Z.; Peng, C.; Shen, M.; Zhang, J. Absolute film separation of dyes/salts and emulsions with a superhigh water permeance through graded nanofluidic channels. Mater. Horizons 2022, 9, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-Y.; Gao, M.-H.; Hu, W.-W.; Luo, D.; Hu, S.-Z.; Huang, T.; Zhang, N.; Wang, Y. Largely enhanced adsorption performance and stability of MXene through in-situ depositing polypyrrole nanoparticles. Sep. Purif. Technol. 2022, 287, 120596. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Zhou, P.Z.; Zhang, S.; Yin, X.Y.; Zeng, X.J.; Pi, P.H.; Nong, Y.J.; Wen, X.F. Facile preparation of ultralong polypyrrole nanowires-coated membrane for switchable emulsions separation and dyes adsorption. J. Water Process Eng. 2022, 49, 102942. [Google Scholar] [CrossRef]
- Lai, X.; Wang, C.; Wang, L.; Xiao, C. A novel PPTA/PPy composite organic solvent nanofiltration (OSN) membrane prepared by chemical vapor deposition for organic dye wastewater treatment. J. Water Process Eng. 2022, 45, 102533. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, J.; Qiao, G.; Wang, J.; Li, H. Facile two-step synthesis of nanofiber polyaniline/graphene/cuprous oxide composite with enhanced photocatalytic performance. Appl. Nanosci. 2021, 11, 983–993. [Google Scholar] [CrossRef]
- Attia, N.F.; Shaltout, S.M.; Salem, I.A.; Zaki, A.B.; El-Sadek, M.H.; Salem, M.A. Sustainable and smart hybrid nanoporous adsorbent derived biomass as efficient adsorbent for cleaning of wastewater from Alizarin Red dye. Biomass Convers. Biorefinery 2022, 1–16. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Dai, Z.; Yu, Q.; Miao, Y.; Xu, R. Facile preparation of a polypyrrole modified Chinese yam peel-based adsorbent: Characterization, performance, and application in removal of Congo red dye. RSC Adv. 2022, 12, 9424–9434. [Google Scholar] [CrossRef]
- Huang, F.; Tian, X.; Wei, W.; Xu, X.; Li, J.; Guo, Y.; Zhou, Z. Wheat straw-core hydrogel spheres with polypyrrole nanotubes for the removal of organic dyes. J. Clean. Prod. 2022, 344, 131100. [Google Scholar] [CrossRef]
- Heybet, E.N.; Ugraskan, V.; Isik, B.; Yazici, O. Adsorption of methylene blue dye on sodium alginate/polypyrrole nanotube composites. Int. J. Biol. Macromol. 2021, 193, 88–99. [Google Scholar] [CrossRef]
- Maqbool, M.; Sadaf, S.; Bhatti, H.N.; Rehmat, S.; Kausar, A.; Alissa, S.A.; Iqbal, M. Sodium alginate and polypyrrole composites with algal dead biomass for the adsorption of Congo red dye: Kinetics, thermodynamics and desorption studies. Surfaces Interfaces 2021, 25, 101183. [Google Scholar] [CrossRef]
- Qi, F.-F.; Ma, T.-Y.; Liu, Y.; Fan, Y.-M.; Li, J.-Q.; Yu, Y.; Chu, L.-L. 3D superhydrophilic polypyrrole nanofiber mat for highly efficient adsorption of anionic azo dyes. Microchem. J. 2020, 159, 105389. [Google Scholar] [CrossRef]
- Yu, Y.; Su, J.; Liu, J.; Li, W. Magnetic poly(glycidyl methacrylate) microspheres with grafted polypyrrole chains for the high-capacity adsorption of Congo red dye from aqueous solutions. Coatings 2022, 12, 168. [Google Scholar] [CrossRef]
- Li, Y.; Yan, S.; Jia, X.; Wu, J.; Yang, J.; Zhao, C.; Wang, S.; Song, H.; Yang, X. Uncovering the origin of full-spectrum visible-light-responsive polypyrrole supramolecular photocatalysts. Appl. Catal. B: Environ. 2021, 287, 119926. [Google Scholar] [CrossRef]
- Taymaz, B.H.; Taş, R.; Kamış, H.; Can, M. Photocatalytic activity of polyaniline and neutral polyaniline for degradation of methylene blue and malachite green dyes under UV Light. Polym. Bull. 2021, 78, 2849–2865. [Google Scholar] [CrossRef]
- Hussain, D.; Siddiqui, M.F.; Shirazi, Z.; Alam Khan, T. Evaluation of adsorptive and photocatalytic degradation properties of FeWO4/polypyrrole nanocomposite for rose bengal and alizarin red S from liquid phase: Modeling of adsorption isotherms and kinetics data. Environ. Prog. Sustain. Energy 2022, 41, 13822. [Google Scholar] [CrossRef]
- Pang, A.L.; Arsad, A.; Ahmadipour, M.; Hamzah, A.A.; Zaini, M.A.A.; Mohsin, R. High efficient degradation of organic dyes by polypyrrole-multiwall carbon nanotubes nanocomposites. Polym. Adv. Technol. 2022, 33, 1402–1411. [Google Scholar] [CrossRef]
- Punnakkal, V.S.; Jos, B.; Anila, E.I. Polypyrrole-silver nanocomposite for enhanced photocatalytic degradation of methylene blue under sunlight irradiation. Mater. Lett. 2021, 298, 130014. [Google Scholar] [CrossRef]
- Koysuren, H.N.; Koysuren, O. Improving UV light photocatalytic activity of WO3 by doping with boron and compounding with polypyrrole. Biointerface Res. Appl. Chem. 2022, 13, 86. [Google Scholar] [CrossRef]
- Capilli, G.; Sartori, D.R.; Gonzalez, M.C.; Laurenti, E.; Minero, C.; Calza, P. Non-purified commercial multiwalled carbon nanotubes supported on electrospun polyacrylonitrile@polypyrrole nanofibers as photocatalysts for water decontamination. RSC Adv. 2021, 11, 9911–9920. [Google Scholar] [CrossRef]
- Yu, H.; He, Y.; Li, H.; Li, Z.; Ren, B.; Chen, G.; Hu, X.; Tang, T.; Cheng, Y.; Ou, J.Z. Core-shell PPy@TiO2 enable GO membranes with controllable and stable dye desalination properties. Desalination 2022, 526, 115523. [Google Scholar] [CrossRef]
- Elkady, M.; Hassan, H. Photocatalytic Degradation of malachite green dye from aqueous solution using environmentally compatible Ag/ZnO polymeric nanofibers. Polymers 2021, 13, 2033. [Google Scholar] [CrossRef] [PubMed]
- Habtamu, F.; Berhanu, S.; Mender, T. Polyaniline supported Ag-doped ZnO nanocomposite: Synthesis, characterization, and kinetics study for photocatalytic degradation of malachite green. J. Chem. 2021, 2021, 2451836. [Google Scholar] [CrossRef]
- Biju, R.; Ravikumar, R.; Thomas, C.; Indulal, C.R. Enhanced photocatalytic degradation of Metanil Yellow dye using polypyrrole-based copper oxide–zinc oxide nanocomposites under visible light. J. Nanoparticle Res. 2022, 24, 1–16. [Google Scholar] [CrossRef]
- Mohamed, H.G.; Aboud, A.A.; El-Salam, H.A. Synthesis and characterization of chitosan/polyacrylamide hydrogel grafted poly(N-methylaniline) for methyl red removal. Int. J. Biol. Macromol. 2021, 187, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, J. Polymers of phenylenediamines. Prog. Polym. Sci. 2015, 41, 1–31. [Google Scholar] [CrossRef]
- Ma, G.; Zhao, S.; Wang, Y.; Wang, Z.; Wang, J. Conjugated polyaniline derivative membranes enable ultrafast nanofiltration and organic-solvent nanofiltration. J. Membr. Sci. 2022, 645, 120241. [Google Scholar] [CrossRef]
- Trchová, M.; Konyushenko, E.N.; Stejskal, J.; Kovářová, J.; Ćirić-Marjanović, G. The conversion of polyaniline nanotubes to nitrogen-containing carbon nanotubes and their comparison with multi-walled carbon nanotubes. Polym. Degrad. Stab. 2009, 94, 929–938. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, W.; Liu, X.; Tang, T.; Xiong, J. Fabrication of Titanium Dioxide/Carbon Fiber (TiO2/CF) Composites for Removal of Methylene Blue (MB) from Aqueous Solution with Enhanced Photocatalytic Activity. J. Chem. 2021, 2021, 9986158. [Google Scholar] [CrossRef]
- Munusamy, S.; Sivaranjan, K.; Sabhapathy, P.; Narayanan, V.; Mohammad, F.; Sagadevan, S. Enhanced electrochemical and photocatalytic activity of g-C3N4-PANI-PPy nanohybrids. Synth. Met. 2020, 272, 116669. [Google Scholar] [CrossRef]
- Mahmud, H.N.M.E.; Kamal, S.J.; Mohamad, N.; Sharma, A.K.; Saharan, P.; Santos, J.H.; Zakaria, S.N.A. Nanoconducting polymer: An effective adsorbent for dyes. Chem. Pap. 2021, 75, 5173–5185. [Google Scholar] [CrossRef]
- Stejskal, J.; Kohl, M.; Trchová, M.; Kolská, Z.; Pekárek, M.; Křivka, I.; Prokeš, J. Conversion of conducting polypyrrole nanostructures to nitrogen containing carbons and its impact on the adsorption of organic dye. Mater. Adv. 2021, 2, 706–717. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M.; Lapčák, L.; Kolská, Z.; Kohl, M.; Pekárek, M.; Prokeš, J. Comparison of carbonized and activated polypyrrole globules, nanofibers, and nanotubes as conducting nanomaterials and adsorbents of organic dye. Carbon Trends 2021, 4, 100068. [Google Scholar] [CrossRef]
- Stejskal, J. Conducting polymers are not just conducting: A perspective for emerging technology. Polym. Int. 2020, 69, 662–664. [Google Scholar] [CrossRef]



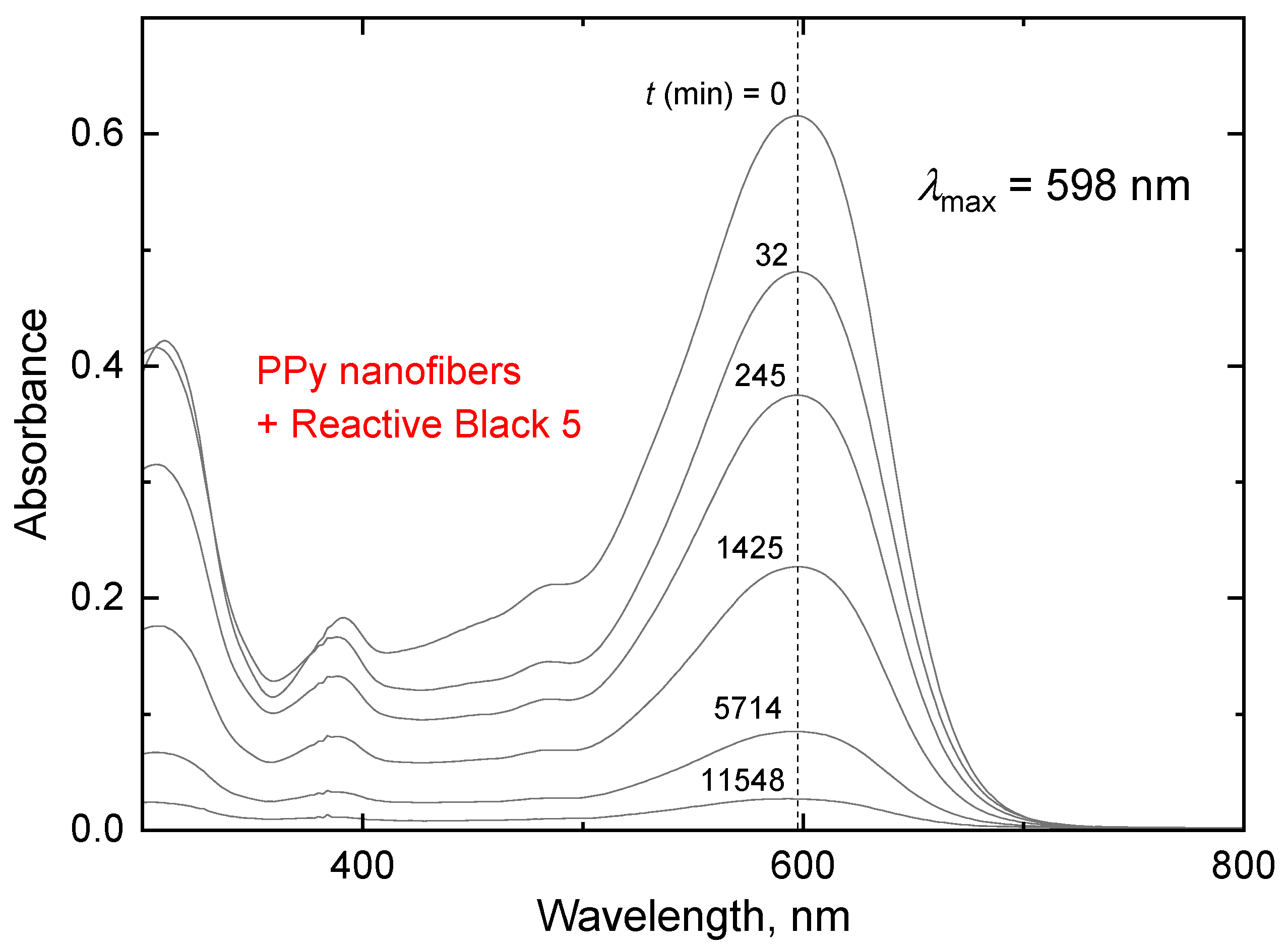

| Dye | PANI Composite with | Reference | |
|---|---|---|---|
| BINARY COMPOSITES | |||
| Acid Blue 74 | − | Tea saponin | [41] |
| Acid Red 18 | − | CoFe2O4 | [28] |
| Brilliant green | + | Reduced graphene oxide | [69] |
| Brilliant green | + | SiO2 | [55] |
| Congo red | − | Activated carbon | [66] |
| BaTiO3 | [72] | ||
| Graphene | [68] | ||
| Kevlar fibers | [73] | ||
| Polyimide | [74] | ||
| SiO2 | [55] | ||
| Tea saponin | [41] | ||
| TiO2 | [58] | ||
| ZnO | [60] | ||
| Crystal violet | + | SiO2 | [55] |
| Direct Blue 14 | − | Activated carbon | [64] |
| Polystyrene | [64] | ||
| Direct Blue 15 | − | SnO2 | [56] |
| Eosin Y | − | FeS | [21] |
| Methyl orange | − | Activated carbon | [7] |
| Bacterial cellulose | [75] | ||
| Cellulose | [24] | ||
| CuO | [71] | ||
| Fe3O4 | [50] | ||
| Manganese ferrite | [51] | ||
| Multiwall carbon nanotubes | [8] | ||
| PEO | [76] | ||
| Polyurethane foam | [77] | ||
| TiO2 | [22] | ||
| Methyl red | − | MgFe2O4 | [52] |
| Methylene blue | + | Boron cluster | [27] |
| Carbonized peanut shells | [65] | ||
| CMC | [78] | ||
| MnO2 | [34] | ||
| Poly(phenyl sulfone) | [79] | ||
| Reduced graphene oxide | [30] | ||
| ZnO | [60] | ||
| ZnO/SiO2 | [62] | ||
| ZrO2 | [63] | ||
| Cellulose | [24] | ||
| Mordant Blue 9 | − | CMC | [78] |
| Orange G | − | Opuntia ficus | [80] |
| Nut shells | [81] | ||
| Zeolite | [59] | ||
| Orange II | − | FeO(OH) | [49] |
| Reactive Black 5 | − | Manganese dioxide | [34] |
| Reactive Green 19 | − | Montmorillonite | [54] |
| 4Rhodamine 6G | + | Boron cluster | [27] |
| Rhodamine B | + | Carbonized tea waste | [67] |
| CMC | [78] | ||
| Sunset yellow | − | Reduced graphene oxide | [30] |
| TERNARY COMPOSITES | |||
| Allura Red | − | Bentonite/PEO | [40] |
| Congo red | − | CoS/graphite | [46] |
| Fly ash/Fe3O4 | [48] | ||
| L-cysteine/reduced graphene oxide | [70] | ||
| Crystal violet | + | Fe3O4/chitosan | [82] |
| Methyl orange | − | Cu/TiO2 | [22] |
| Fe3O4/chitosan | [82] | ||
| Fly ash/Fe3O4 | [48] | ||
| MnO2/NiO | [40] | ||
| Montmorillonite/PVAL | [43] | ||
| PAA/PAN | [83] | ||
| SiO2 | [55] | ||
| TiO2/PVDF | [84] | ||
| TiO2/PVDF | [85] | ||
| Methylene blue | + | MoO3/Fe3O4 | [53] |
| PAA/PAN | [83] | ||
| Orange G | − | Calcium alginate/sawdust | [86] |
| Reactive Black 5 | − | Montmorillonite/PVAL | [43] |
| Fe3O4/PVAL | [42] | ||
| Reactive Orange 5 | − | TiO2/zeolite | [33] |
| Rhodamine B | + | CuO/graphene | [47] |
| Safranin | + | Montmorillonite/PVAL | [43] |
| Dye | PANI Composite with | Reference | |
|---|---|---|---|
| BINARY COMPOSITES | |||
| Acid Blue 29 | − | CdS | [97] |
| Acid Orange 52 | − | TiO2 | [105] |
| Brilliant green | + | Ni | [100] |
| Congo red | − | Carbon nanodots | [87] |
| g-C3N4 | [98] | ||
| Reduced graphene oxide | [101] | ||
| ZrO2 | [9] | ||
| Crystal violet | + | Carbon nanodots | [87] |
| NiWO4 | [111] | ||
| Disperse Red 1 | − | Co0.5Zn0.5Fe2O4 | [90] |
| Fluorescein | − | ZnO | [108] |
| Levafix red | − | Fe3O4 | [89] |
| Malachite green | + | Fe3O4 | [89] |
| Methyl orange | − | Ag2O | [102] |
| g-C3N4 | [98] | ||
| MWCNT | [99] | ||
| TiO2 | [106] | ||
| Methylene blue | + | Carbon nanodots | [87] |
| MWCNT | [99] | ||
| Nb2O5 | [101] | ||
| NiWO4 | [111] | ||
| TiO2 | [104] | ||
| ZnO | [61] | ||
| Orange II | − | ZnO | [109] |
| Reactive Blue 220 | − | BiOCl | [95] |
| Reactive Orange 14 | − | Co0.5Zn0.5Fe2O4 | [90] |
| Rhodamine B | + | Carbon nanodots | [87] |
| CdS | [96] | ||
| MWCNT | [99] | ||
| SnO2 | [103] | ||
| WO3 | [107] | ||
| TERNARY COMPOSITES | |||
| Acid Blue 29 | − | TiO2/CdS | [97] |
| Allura red | − | TiO2/PVDF | [84] |
| Congo red | − | Zn/Fe3O4 | [118] |
| Methyl orange | − | Ag/AgMoO4 | [29] |
| g-C3N4/attapulgite | [112] | ||
| TiO2/chitosan | [115] | ||
| TiO2/PVDF | [84] | ||
| Methyl red | − | ZnO/Cu/Ni | [110] |
| Methylene blue | + | CeO2/polystyrene | [113] |
| TiO2/chitosan | [115] | ||
| ZnO/graphene oxide | [116] | ||
| Methylene green | + | NaBiO2/polycarbonate | [114] |
| Reactive Black 5 | − | TiO2/Fe3O4 | [117] |
| Reactive Brilliant Red K-2K | − | Graphene oxide/cellulose | [94] |
| Rhodamine B | + | BiOI/reduced graphene oxide | [10] |
| NaBiO2/polycarbonate | [114] | ||
| Dye | Composite with | Reference | |
|---|---|---|---|
| BINARY COMPOSITES | |||
| Acid Orange 7 | − | nanosilica | [23] |
| alizarine red | − | carbonized chicken feathers | [126] |
| chrysoidine | + | melamine | [16] |
| Congo red | − | Chinese yam peel | [127] |
| PPT | [124] | ||
| Eosin Y | − | PPT | [124] |
| wheat straw | [128] | ||
| fuchsin | − | bacterial cellulose | [125] |
| malachite green | + | steel mesh | [123] |
| methyl orange | − | MXene | [122] |
| methylene blue | + | g-C3N4 | [121] |
| MXene | [122] | ||
| sodium alginate | [129] | ||
| Reactive Black 5 | − | melamine | [15,16,17,18] |
| Rhodamine B | + | g-C3N4 | [121] |
| steel mesh | [123] | ||
| TERNARY COMPOSITES | |||
| Acid Orange 7 | − | PAN/PVP nanofibers | [131] |
| Acid Yellow 9 | − | PAN/PVP nanofibers | [131] |
| Congo red | − | CoO/graphene | [25] |
| PEGMA/Fe3O4 | [132] | ||
| sodium alginate/algae biomass | [130] | ||
| metanil yellow | − | PAN/PVP nanofibers | [131] |
| methyl orange | − | magnetite/chitosan | [26] |
| methylene blue | + | CoO/graphene | [25] |
| MoO3/Fe3O4 | [53] | ||
| Dye | Composite with | Reference | |
|---|---|---|---|
| BINARY COMPOSITES | |||
| alizarine red | − | FeWO4 | [135] |
| Congo red | − | MoSe2 | [92] |
| malachite green | + | Fe | [134] |
| methyl orange | − | AgMnO2 | [32] |
| TiO2 | [58] | ||
| methylene blue | + | Ag | [137] |
| + | MWCNT | [136] | |
| + | WO3 | [138] | |
| Rhodamine B | + | MoSe2 | [92] |
| + | MWCNT | [136] | |
| rose bengal | − | FeWO4 | [135] |
| TERNARY COMPOSITES | |||
| brilliant red | − | g-C3N4/reduced graphene oxide | [119] |
| Congo red | − | Ag/Sn3O4 | [120] |
| malachite green | + | Ag/ZnO | [142] |
| + | Ag/ZnO/cellulose acetate | [141] | |
| metanil yellow | − | CuO/ZnO | [143] |
| methyl orange | − | MWCNT/polyacrylonitrile | [139] |
| TiO2/Cu-MOF | [91] | ||
| methylene blue | + | TiO2/graphene oxide | [140] |
| Ag/Sn3O4 | [120] | ||
| TiO2/Cu-MOF | [91] | ||
| ZnO/activated carbon | [141] | ||
| ZnO/Fe3O4 | [19] | ||
| Rhodamine B | + | MWCNT/polyacrylonitrile | [139] |
| TiO2/Cu-MOF | [91] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stejskal, J. Recent Advances in the Removal of Organic Dyes from Aqueous Media with Conducting Polymers, Polyaniline and Polypyrrole, and Their Composites. Polymers 2022, 14, 4243. https://doi.org/10.3390/polym14194243
Stejskal J. Recent Advances in the Removal of Organic Dyes from Aqueous Media with Conducting Polymers, Polyaniline and Polypyrrole, and Their Composites. Polymers. 2022; 14(19):4243. https://doi.org/10.3390/polym14194243
Chicago/Turabian StyleStejskal, Jaroslav. 2022. "Recent Advances in the Removal of Organic Dyes from Aqueous Media with Conducting Polymers, Polyaniline and Polypyrrole, and Their Composites" Polymers 14, no. 19: 4243. https://doi.org/10.3390/polym14194243
APA StyleStejskal, J. (2022). Recent Advances in the Removal of Organic Dyes from Aqueous Media with Conducting Polymers, Polyaniline and Polypyrrole, and Their Composites. Polymers, 14(19), 4243. https://doi.org/10.3390/polym14194243





