NIR and Reduction Dual-Sensitive Polymeric Prodrug Nanoparticles for Bioimaging and Combined Chemo-Phototherapy
Abstract
:1. Introduction
2. Materials
2.1. Materials
2.2. Measurement
3. Methods
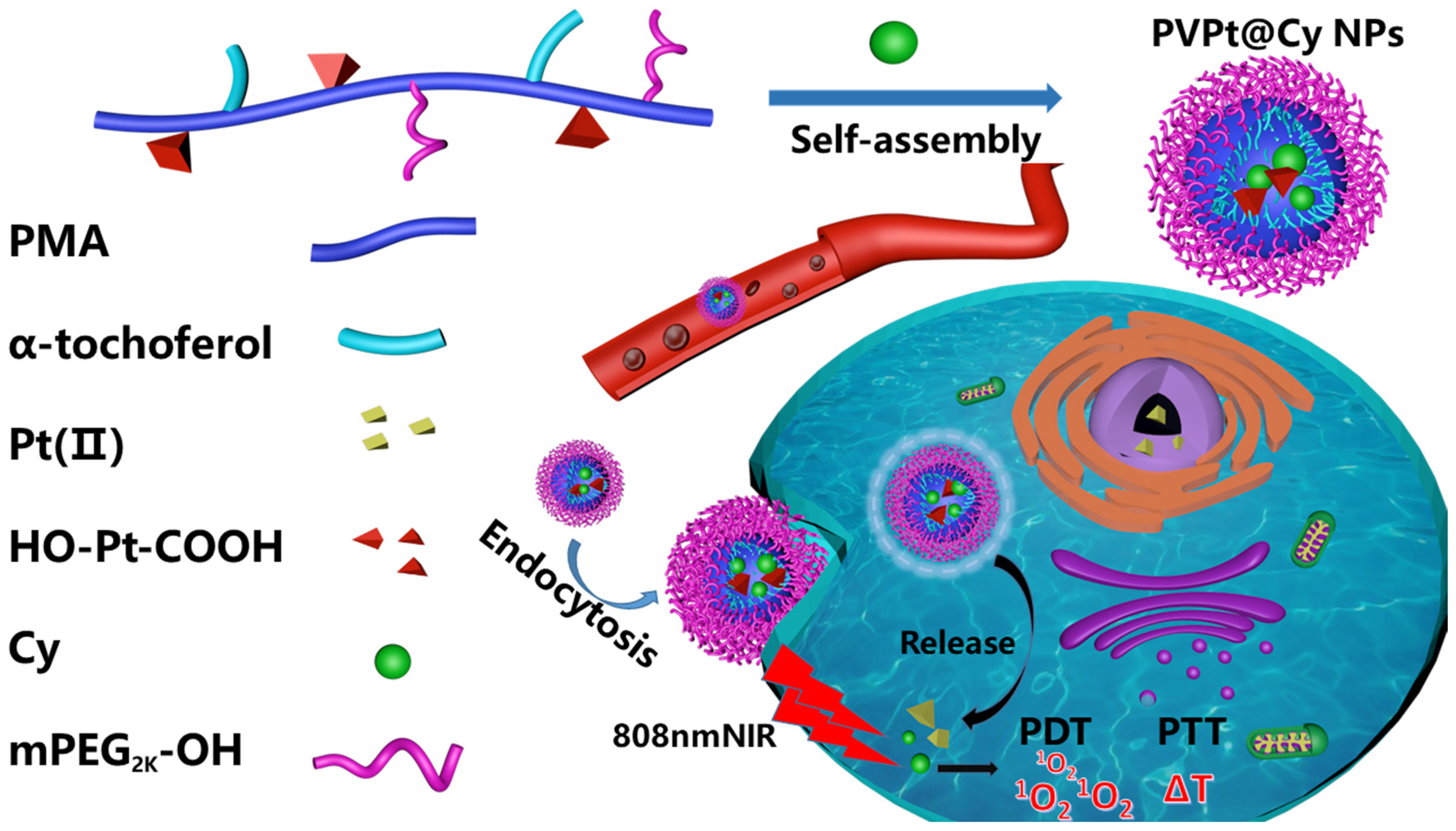
3.1. Synthesis and Formulation of the PVPt@Cy NPs
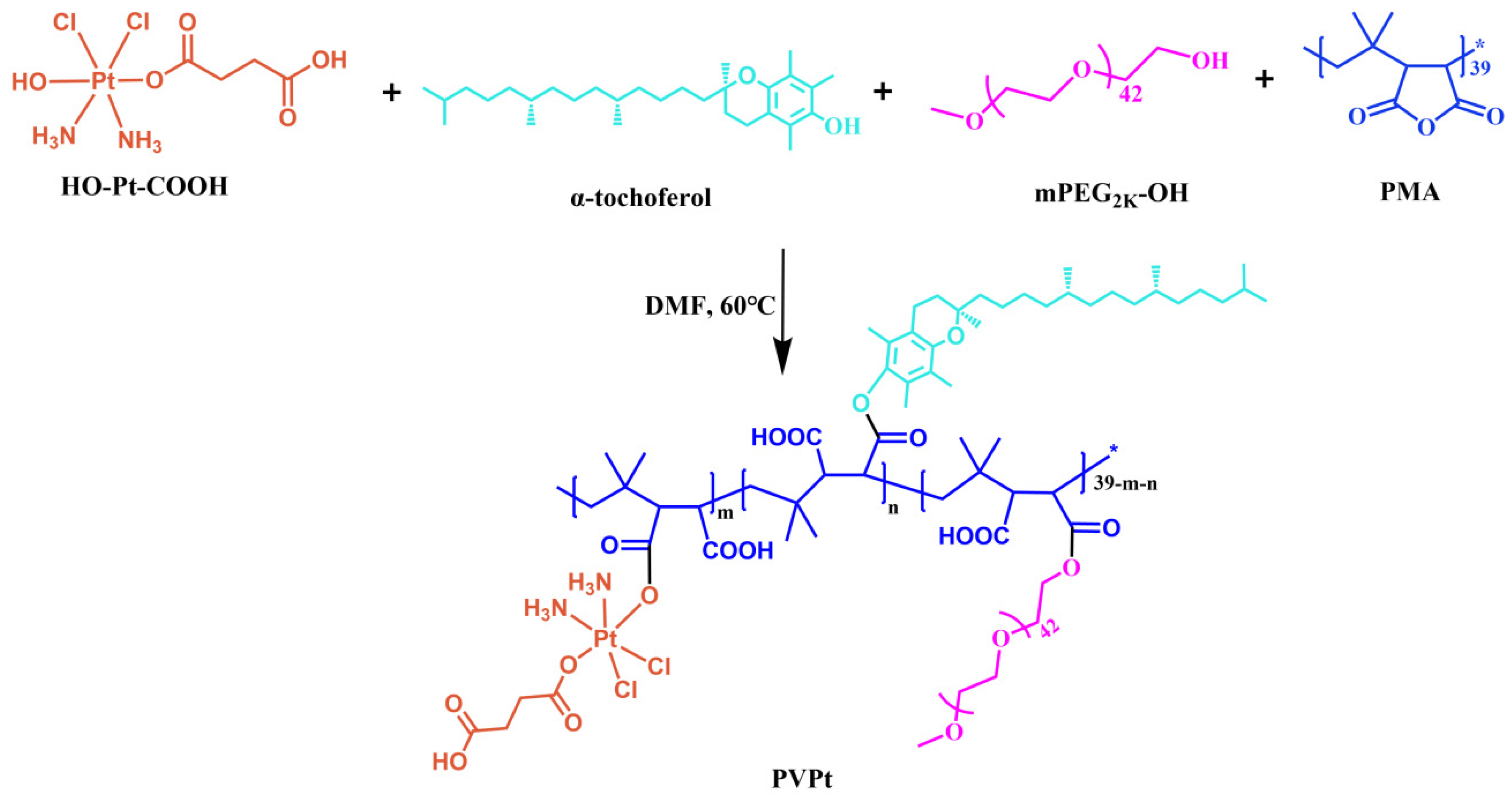
3.1.1. Synthesis of the Pt(IV)-Based Polymer Prodrug (PVPt)
3.1.2. Preparation of Cy-Loaded PVPt Nanoparticles (Denoted as PVPt@Cy NPs)
3.1.3. Drug Loading Capacity
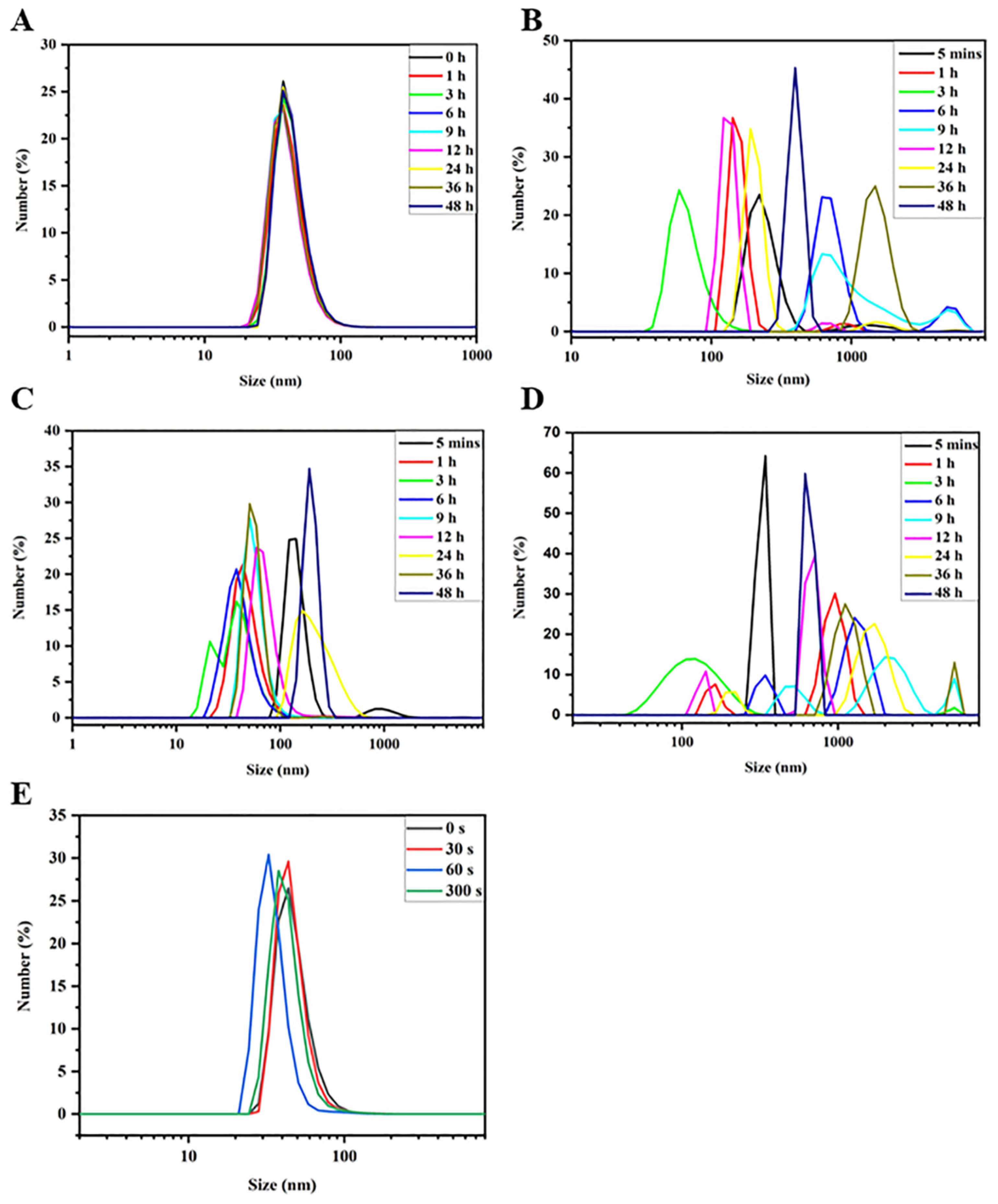
3.1.4. Stability and Responsiveness of PVPt NPs and PVPt@Cy NPs
3.1.5. In Vitro Photothermal and Photodynamic Performance of PVPt@Cy NPs
3.1.6. In Vitro Drug Release
3.2. Biological Activity
3.2.1. Cell Culture
3.2.2. Intracellular ROS Assay
3.2.3. In Vitro Intracellular Uptake and Intracellular Distribution
3.2.4. In Vitro Cytotoxicity
3.2.5. Animal Models
3.2.6. In Vivo Bioimaging
3.2.7. Statistical Analysis
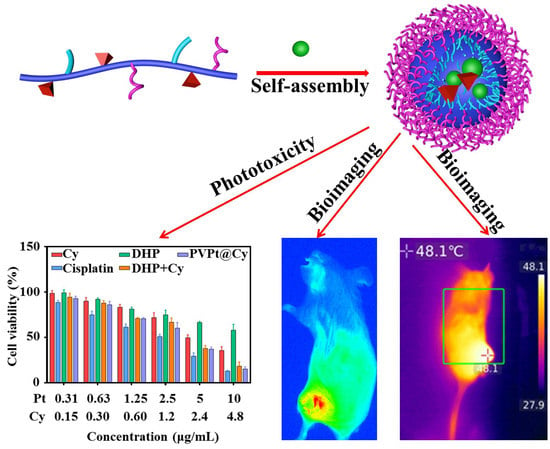
4. Results and Discussion
4.1. Preparation of Polymer Prodrug PVPt and Cy-Loaded PVPt Nanoparticles (PVPt@Cy NPs)
4.2. Stability Evaluation
4.3. Photothermal and Photodynamic Properties
4.4. In Vitro Pt Release
4.5. Intracellular ROS Detection and Cellular Uptake
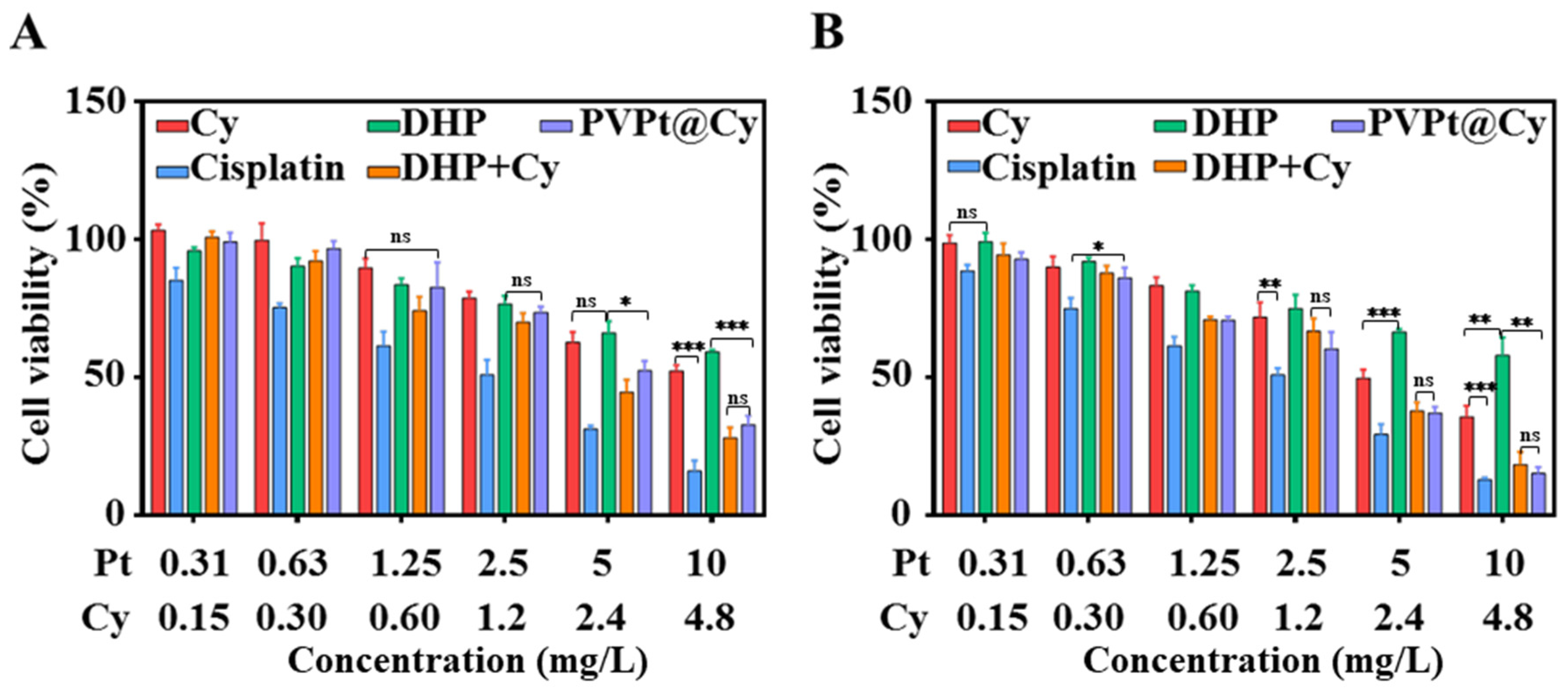
4.6. In Vitro Study of Intracellular Cytotoxicity
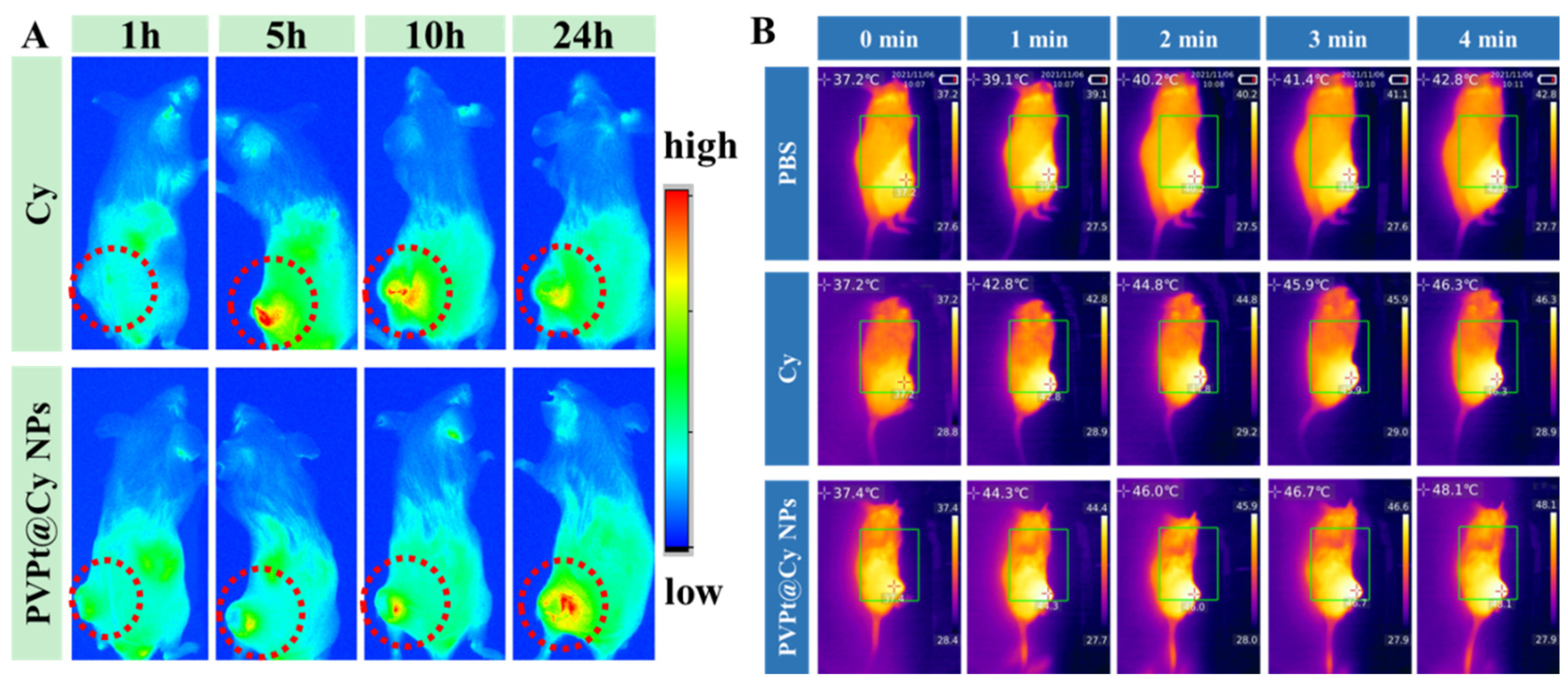
4.7. In Vivo Biodistribution and Imaging
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berger, N.A. Cancer chemotherapy: New strategies for success. J. Clin. Investig. 1986, 78, 1131–1135. [Google Scholar] [CrossRef]
- Lin, G.; Mi, P.; Chu, C.; Zhang, J.; Liu, G. Inorganic Nanocarriers Overcoming Multidrug Resistance for Cancer Theranostics. Adv. Sci. 2016, 3, 1600134. [Google Scholar] [CrossRef]
- Zimmermann, S.; Dziadziuszko, R.; Peters, S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat. Rev. 2014, 40, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Du, Y.; Guo, W.; Su, Y.; Meng, Y.; Shan, Z.; Feng, Y.; Meng, S. Methotrexate coated AZA-BODIPY nanoparticles for chemotherapy, photothermal and photodynamic synergistic therapy. Dye. Pigment. 2020, 179, 108351. [Google Scholar] [CrossRef]
- Chen, M.-M.; Hao, H.-L.; Zhao, W.; Zhao, X.; Chen, H.-Y.; Xu, J.-J. A plasmon-enhanced theranostic nanoplatform for synergistic chemo-phototherapy of hypoxic tumors in the NIR-II window. Chem. Sci. 2021, 12, 10848–10854. [Google Scholar] [CrossRef]
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Yue, C.; Ma, Y.; Gong, P.; Zhao, P.; Zheng, C.; Sheng, Z.; Zhang, P.; Wang, Z.; Cai, L. Single-Step Assembly of DOX/ICG Loaded Lipid–Polymer Nanoparticles for Highly Effective Chemo-photothermal Combination Therapy. ACS Nano 2013, 7, 2056–2067. [Google Scholar] [CrossRef]
- Lv, K.; Lin, H.; Qu, F. Biodegradable hollow Co3S4@N-doped carbon as enhanced PTT/PDT agent for multimodal MR/thermal imaging and synergistic antitumor therapy. Chem. Eng. J. 2020, 392, 124555. [Google Scholar] [CrossRef]
- Lv, R.; Yang, P.; He, F.; Gai, S.; Yang, G.; Dai, Y.; Hou, Z.; Lin, J. An imaging-guided platform for synergistic photodynam-ic/photothermal/chemo-therapy with pH/temperature-responsive drug release. Biomaterials 2015, 63, 115–127. [Google Scholar] [CrossRef]
- Wan, G.; Chen, B.; Li, L.; Wang, D.; Shi, S.; Zhang, T.; Wang, Y.; Zhang, L.; Wang, Y. Nanoscaled red blood cells facilitate breast cancer treatment by combining photothermal/photodynamic therapy and chemotherapy. Biomaterials 2018, 155, 25–40. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Chen, Z.; Zhang, F.; Wang, Q.; Guo, W.; Wang, K.; Lin, H.; Qu, F. Construct of MoSe2/Bi2Se3 nanohetero-structure: Multimodal CT/PT imaging-guided PTT/PDT/chemotherapy for cancer treating. Biomaterials 2019, 217, 119282. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.R.; Wang, C.; An, R.; Wang, S.; Li, Z.-Q.; Wang, Y.; Ihsan, A.; Ye, D.; Xia, X.-H.; Younis, M.A. Low Power Single Laser Activated Synergistic Cancer Phototherapy Using Photosensitizer Functionalized Dual Plasmonic Photothermal Nanoagents. ACS Nano 2019, 13, 2544–2557. [Google Scholar] [CrossRef]
- Li, S.; Deng, Q.; Li, X.; Huang, Y.; Li, X.; Liu, F.; Wang, H.; Qing, W.; Liu, Z.; Lee, C.-S. Bis-diketopyrrolopyrrole conjugated polymer nanoparticles as photothermic nanoagonist for specific and synergistic glioblastoma therapy. Biomaterials 2019, 216, 119252. [Google Scholar] [CrossRef]
- Yi, H.; Lu, W.; Liu, F.; Zhang, G.; Xie, F.; Liu, W.; Wang, L.; Zhou, W.; Cheng, Z. ROS-responsive liposomes with NIR light-triggered doxorubicin release for combinatorial therapy of breast cancer. J. Nanobiotechnology 2021, 19, 134. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, Y.; Xin, X.; Huo, P.; Zhang, Y.; Chen, H.; Feng, N.; Feng, Q.; Zhang, Z. Dual-modal imaging-guided theranostic nanocarriers based on 2-methoxyestradiol and indocyanine green. Int. J. Pharm. 2021, 592, 120098. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nakamura, M.; Miki, H.; Ozaki, S.; Abe, M.; Matsumoto, T.; Kori, T.; Ishimura, K. Photostable Iodinated Sili-ca/Porphyrin Hybrid Nanoparticles with Heavy-Atom Effect for Wide-Field Photodynamic/Photothermal Therapy Using Single Light Source. Adv. Funct. Mater. 2014, 24, 503–513. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Qian, G.; Ito, A. Synergistical chemotherapy and cancer immunotherapy using dual drug-delivering and immunopotentiating mesoporous silica. Appl. Mater. Today 2019, 16, 102–111. [Google Scholar] [CrossRef]
- Sun, W.; Li, S.; Haupler, B.; Liu, J.; Jin, S.; Steffen, W.; Schubert, U.S.; Butt, H.J.; Liang, X.J.; Wu, S. An Amphiphilic Ruthenium Polymetallodrug for Combined Photodynamic Therapy and Photochemotherapy In Vivo. Adv. Mater. 2017, 29, 3702. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Sun, Y.; Wang, L.; Ding, L.; Zhu, W.H.; Di, W.; Duan, Y.R. Gold-caged copolymer nanoparticles as multimodal synergistic photodynamic/photothermal/chemotherapy platform against lethality androgen-resistant prostate cancer. Biomaterials 2019, 212, 73–86. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Zheng, Y.; Tan, C.-P.; Sun, J.-H.; Zhang, W.; Ji, L.-N.; Mao, Z.-W. Graphene Oxide Decorated with Ru(II)–Polyethylene Glycol Complex for Lysosome-Targeted Imaging and Photodynamic/Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 6761–6771. [Google Scholar] [CrossRef]
- Li, P.; Liu, L.; Lu, Q.; Yang, S.; Yang, L.; Cheng, Y.; Wang, Y.; Wang, S.; Song, Y.; Tan, F.; et al. Ultrasmall MoS2 Nanodots-Doped Biodegradable SiO2 Nanoparticles for Clearable FL/CT/MSOT Imaging-Guided PTT/PDT Combination Tumor Therapy. ACS Appl. Mater. Interfaces 2019, 11, 5771–5781. [Google Scholar] [CrossRef]
- Liu, B.; Li, C.; Chen, G.; Liu, B.; Deng, X.; Wei, Y.; Li, C.; Xing, B.; Ma, P.; Lin, J. Synthesis and Optimization of MoS2 @Fe3O4-ICG/Pt(IV) Nanoflowers for MR/IR/PA Bioimaging and Combined PTT/PDT/Chemotherapy Triggered by 808 nm Laser. Adv. Sci. 2017, 4, 1600540. [Google Scholar] [CrossRef]
- Xu, J.; Gulzar, A.; Liu, Y.; Bi, H.; Gai, S.; Liu, B.; Yang, D.; He, F.; Yang, P. Integration of IR-808 Sensitized Upconversion Nanostructure and MoS2 Nanosheet for 808 nm NIR Light Triggered Phototherapy and Bioimaging. Small 2017, 13, 1841. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, M.; Ouyang, N.; Tang, Y.; Miao, P. Synergistic Chemo-thermal Therapy of Cancer by DNA-Templated Silver Nanoclusters and Polydopamine Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 21653–21660. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-Q.; Wang, Z.; Hao, S.; He, H.; Wan, Y.; Zhu, C.; Sun, L.-P.; Cheng, G.; Zheng, S.-Y. Mitochondria-Targeting Polydopamine Nanoparticles To Deliver Doxorubicin for Overcoming Drug Resistance. ACS Appl. Mater. Interfaces 2017, 9, 16793–16802. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Hu, H.; Wan, J.; Li, Y.; Wu, Y.; Tang, Y.; Xiao, C.; Xu, H.; Yang, X.; Li, Z. Hydroxyethyl starch stabilized polydopamine nanoparticles for cancer chemotherapy. Chem. Eng. J. 2018, 349, 129–145. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, M.; Zeng, Y.; Wu, L.; Wang, Q.; Han, X.; Liu, X.; Liu, J. Chlorin e6 Conjugated Poly (dopamine) Nanospheres as PDT/PTT Dual-Modal Therapeutic Agents for Enhanced Cancer Therapy. ACS Appl. Mater. Interfaces 2015, 7, 8176–8187. [Google Scholar] [CrossRef]
- Fu, X.; Huang, Y.; Zhao, H.; Zhang, E.; Shen, Q.; Di, Y.; Lv, F.; Liu, L.; Wang, S. Near-Infrared-Light Remote-Controlled Ac-tivation of Cancer Immunotherapy Using Photothermal Conjugated Polymer Nanoparticles. Adv. Mater. 2021, 33, e2102570. [Google Scholar] [CrossRef]
- Li, J.; Rao, J.; Pu, K. Recent progress on semiconducting polymer nanoparticles for molecular imaging and cancer photo-therapy. Biomaterials 2018, 155, 217–235. [Google Scholar] [CrossRef]
- Braga, C.B.; Perli, G.; Becher, T.B.; Ornelas, C. Biodegradable and pH-Responsive Acetalated Dextran (Ac-Dex) Nanoparticles for NIR Imaging and Controlled Delivery of a Platinum-Based Prodrug into Cancer Cells. Mol. Pharm. 2019, 16, 2083–2094. [Google Scholar] [CrossRef]
- Cao, J.; Chi, J.; Xia, J.; Zhang, Y.; Han, S.; Sun, Y. Iodinated Cyanine Dyes for Fast Near-Infrared-Guided Deep Tissue Synergistic Phototherapy. ACS Appl. Mater. Interfaces 2019, 11, 25720–25729. [Google Scholar] [CrossRef]
- Guo, M.; Huang, J.; Deng, Y.; Shen, H.; Ma, Y.; Zhang, M.; Zhu, A.; Li, Y.; Hui, H.; Wang, Y.; et al. pH-Responsive Cyanine-Grafted Graphene Oxide for Fluorescence Resonance Energy Transfer-Enhanced Photothermal Therapy. Adv. Funct. Mater. 2015, 25, 59–67. [Google Scholar] [CrossRef]
- Tan, X.; Wang, J.; Pang, X.; Liu, L.; Sun, Q.; You, Q.; Tan, F.; Li, N. Indocyanine Green-Loaded Silver Nanoparticle@Polyaniline Core/Shell Theranostic Nanocomposites for Photoacoustic/Near-Infrared Fluorescence Imaging-Guided and Single-Light-Triggered Photothermal and Photodynamic Therapy. ACS Appl Mater. Interfaces 2016, 8, 34991–35003. [Google Scholar] [CrossRef]
- Wang, C.; Huang, B.; Yang, G.; Ouyang, Y.; Tian, J.; Zhang, W. NIR-Triggered Multifunctional and Degradable Nano-platform Based on an ROS-Sensitive Block Copolymer for Imaging-Guided Chemo-Phototherapy. Biomacromolecules 2019, 20, 4218–4229. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Han, Y.; Sun, B.; Zhao, Z.; Opoku-Damoah, Y.; Cheng, H.; Zhang, H.; Zhou, J.; Ding, Y. Deep Tumor Penetrating Bioparticulates Inspired Burst Intracellular Drug Release for Precision Chemo-Phototherapy. Small 2018, 14, e1703110. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-S.; Su, C.-H.; Ho, W.-Y.; Huang, C.-C.; Chang, J.-C.; Chien, Y.-H.; Hung, S.-T.; Liau, M.-C.; Ho, H.-Y. Tumor targeting and MR imaging with lipophilic cyanine-mediated near-infrared responsive porous Gd silicate nanoparticles. Biomaterials 2013, 34, 5677–5688. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Wu, Y.; Xu, X.; Xu, B.; Chen, Z.; Li, T. pH and Reduction Dual-Responsive Bi-Drugs Conjugated Dextran Assemblies for Combination Chemotherapy and In Vitro Evaluation. Polymers 2021, 13, 1515. [Google Scholar] [CrossRef] [PubMed]
- Elis, L.T.; Er, H.M.; Hambley, T.W. The Influence of the Axial Ligands of a Series of Platinum(1v) Anti-Cancer Complexes on their Reduction to Platinum(11) and React ion with DNA. Aust. J. Chem. 1995, 48, 793–806. [Google Scholar] [CrossRef]
- Guo, D.; Xu, S.; Huang, Y.; Jiang, H.; Yasen, W.; Wang, N.; Su, Y.; Qian, J.; Li, J.; Zhang, C.; et al. Platinum(IV) complex-based two-in-one polyprodrug for a combinatorial chemo-photodynamic therapy. Biomaterials 2018, 177, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, H.; Tang, J.; Li, Z.; Zhou, X.; Zhang, R.; Chen, L.; Mao, Y.; Li, C. Noninvasively Imaging Subcutaneous Tumor Xenograft by a Handheld Raman Detector, with the Assistance of an Optical Clearing Agent. ACS Appl. Mater. Interfaces 2017, 9, 17769–17776. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Mohammed, F.; Wang, Y.; Wang, J.; Lu, N.; Li, J.; Ge, Z. Hypoxia-responsive block copolymer polyprodrugs for complementary photodynamic-chemotherapy. J. Control. Release 2021, 339, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Hou, M.; Ma, X.; Bai, S.; Zhang, T.; Xue, P.; Zhang, X.; Liu, G.; Kang, Y.; Xu, Z. Starburst Diblock Polyprodrugs: Reduction-Responsive Unimolecular Micelles with High Drug Loading and Robust Micellar Stability for Programmed Delivery of Anticancer Drugs. Biomacromolecules 2019, 20, 1190–1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liang, G.; Zhang, W.; Xing, D.; Hu, X. Cascade-Promoted Photo-Chemotherapy against Resistant Cancers by Enzyme-Responsive Polyprodrug Nanoplatforms. Chem. Mater. 2018, 30, 3486–3498. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, D.R.; Domínguez-Ríos, R.; Juárez, J.; Valdés, M.; Hassan, N.; Quintero-Ramos, A.; del Toro-Arreola, A.; Barbosa, S.; Taboada, P.; Topete, A.; et al. Biodegradable photoresponsive nanoparticles for chemo-, photothermal- and photodynamic therapy of ovarian cancer. Mater. Sci. Eng. C 2020, 116, 111196. [Google Scholar] [CrossRef]
- Mao, J.; Li, Y.; Cai, Q.; Tang, Z.; Yang, Y.; Yuan, C.; Xu, Y.; Zeng, B.; Luo, W.; Kuo, S.; et al. Tumor microenvironment-activated self-charge-generable metallosupramolecular polymer nanocapsules for photoacoustic imaging-guided targeted synergistic photothermal-chemotherapy. Chem. Eng. J. 2021, 405, 126690. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wu, Y.; Xue, X.; Liu, S. NIR and Reduction Dual-Sensitive Polymeric Prodrug Nanoparticles for Bioimaging and Combined Chemo-Phototherapy. Polymers 2022, 14, 287. https://doi.org/10.3390/polym14020287
Li S, Wu Y, Xue X, Liu S. NIR and Reduction Dual-Sensitive Polymeric Prodrug Nanoparticles for Bioimaging and Combined Chemo-Phototherapy. Polymers. 2022; 14(2):287. https://doi.org/10.3390/polym14020287
Chicago/Turabian StyleLi, Shuying, Yanjuan Wu, Xiukun Xue, and Siyuan Liu. 2022. "NIR and Reduction Dual-Sensitive Polymeric Prodrug Nanoparticles for Bioimaging and Combined Chemo-Phototherapy" Polymers 14, no. 2: 287. https://doi.org/10.3390/polym14020287