Fully Automated Multi-Step Synthesis of Block Copolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.2. Synthesis of the Polymers
2.2.1. Manual Synthesis of P3c
2.2.2. Automated Synthesis of P4c
2.2.3. General Procedure of Manual Dialysis
2.2.4. Automated Dialysis
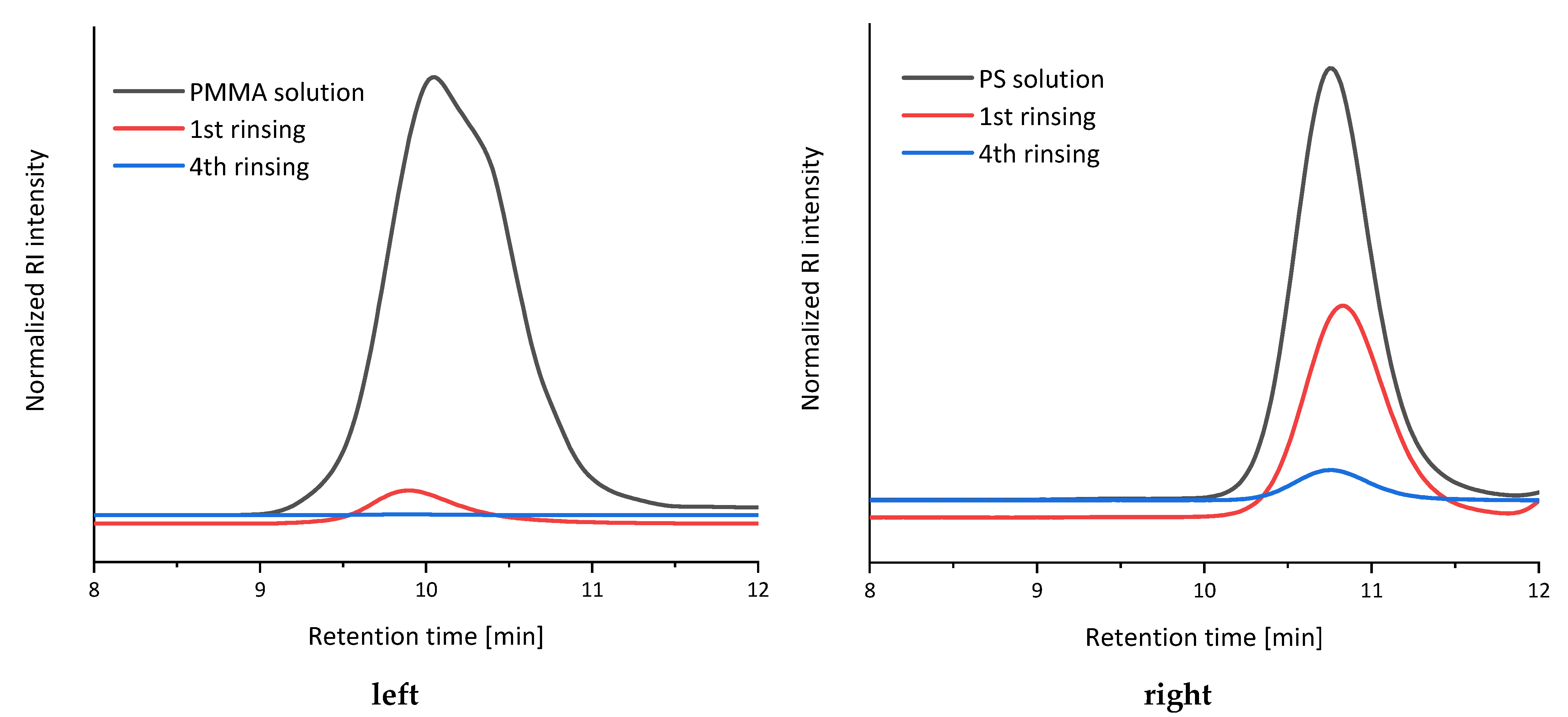
2.2.5. Reusability Test of Dialysis Tubing
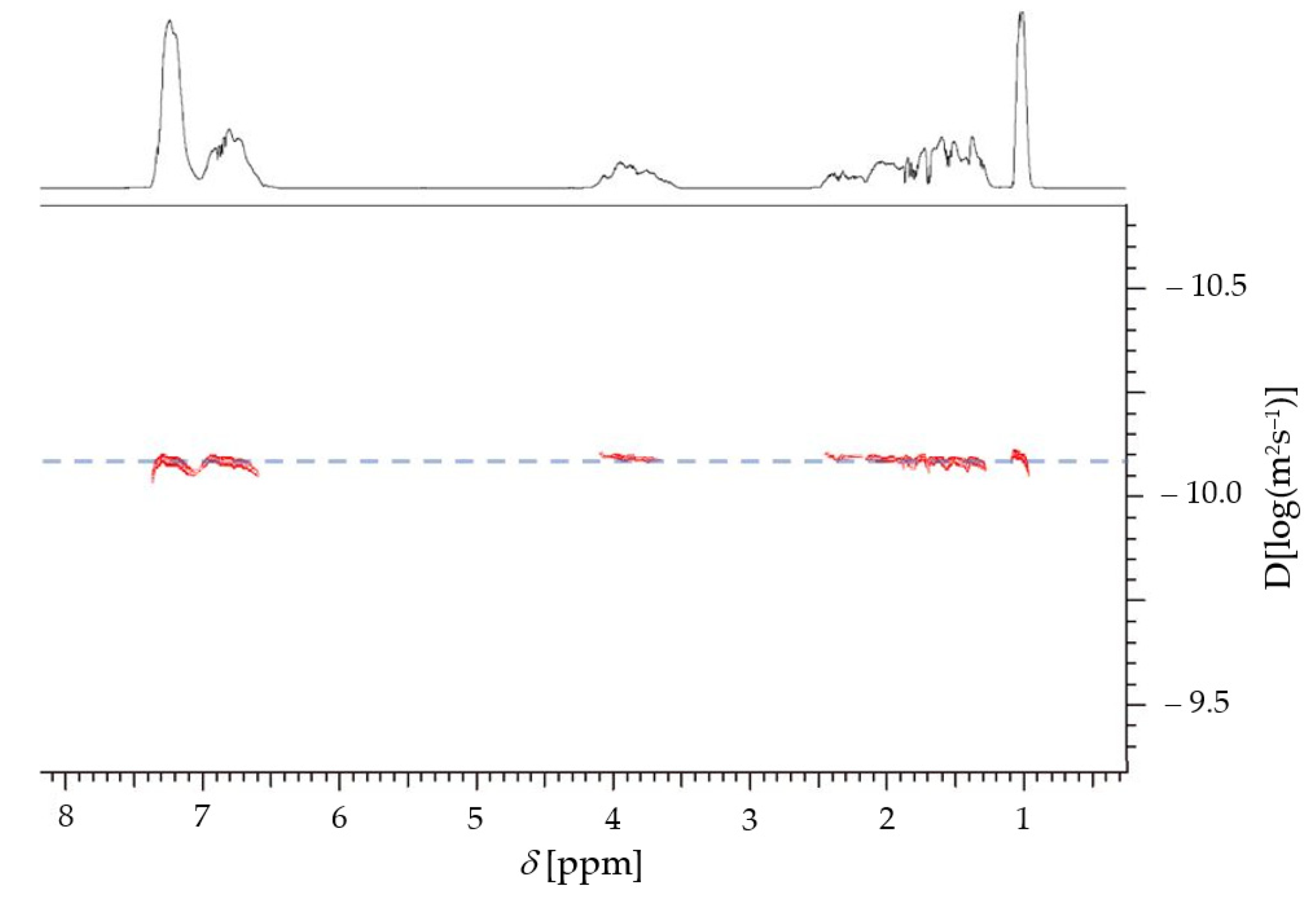
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riess, G.; Hurtrez, G.; Bahadur, P. Block Copolymers, Encyclopedia Polymer Science and Engineering; Wiley: New York, NY, USA, 1986; Volume 2, pp. 324–434. [Google Scholar]
- Riess, G. Micellization of block copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef] [Green Version]
- Burguière, C.; Pascual, S.; Coutin, B.; Polton, A.; Tardi, M.; Charleux, B.; Matyjaszewski, K.; Vairon, J.-P. Amphiphilic block copolymers prepared via controlled radical polymerization as surfactants for emulsion polymerization. Macromol. Symp. 2000, 150, 39–44. [Google Scholar] [CrossRef]
- Mu, B.; Cui, J.; Yang, B.; Cui, J.; Guo, J.; Tian, L. One-Pot Synthesis and Tribological Properties of Oil-Containing Self-Lubricating Polyurethane Materials. Macromol. Mater. Eng. 2020, 306, 2000509. [Google Scholar] [CrossRef]
- Auschra, C.; Eckstein, E.; Knischka, R.; Pirrung, F.; Harbers, P. Controlled polymers for pigment dispersants. Paint. Coat. Ind. 2005, 21, 98–108. [Google Scholar]
- Kwon, G.S.; Kataoka, K. Block copolymer micelles as long-circulating drug vehicles. Adv. Drug Deliv. Rev. 1995, 16, 295–309. [Google Scholar] [CrossRef]
- Ramesh, K.; Mishra, A.K.; Kim, J.K.; Jeong, Y.T.; Gal, Y.-S.; Lim, K.T. Preparation of Doxorubicin-Loaded Amphiphilic Poly(D,L-Lactide-Co-Glycolide)-b-Poly(N-Acryloylmorpholine) AB2 Miktoarm Star Block Copolymers for Anticancer Drug Delivery. Materials 2020, 13, 3713. [Google Scholar] [CrossRef]
- Kwon, G.S.; Okano, T. Polymeric micelles as new drug carriers. Adv. Drug Deliv. Rev. 1996, 21, 107–116. [Google Scholar] [CrossRef]
- Lieske, A.; Jaeger, W. Synthesis and characterization of block copolymers containing cationic blocks. Macromol. Chem. Phys. 1998, 199, 255–260. [Google Scholar] [CrossRef]
- Szwarc, M.; Levy, M.; Milkovich, R. Polymerization Initiated by electron transfer to Monomer. A New Method of Formation of Block Polymers. J. Am. Chem. Soc. 1956, 78, 2656–2657. [Google Scholar] [CrossRef]
- Paraskeva, S.; Hadjichristidis, N. Synthesis of an exact graft copolymer of isoprene and styrene with two branches. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 931–935. [Google Scholar] [CrossRef]
- Natalello, A.; Morsbach, J.; Friedel, A.; Alkan, A.; Tonhauser, C.; Müller, A.H.E.; Frey, H. Living Anionic Polymerization in Continuous Flow: Facilitated Synthesis of High-Molecular Weight Poly(2-vinylpyridine) and Polystyrene. Org. Process. Res. Dev. 2014, 18, 1408–1412. [Google Scholar] [CrossRef]
- Tamasi, M.; Kosuri, S.; DiStefano, J.; Chapman, R.; Gormley, A.J. Automation of Controlled/Living Radical Polymerization. Adv. Intell. Syst. 2020, 2, 1900126. [Google Scholar] [CrossRef]
- Zhang, W.; D’Agosto, F.; Dugas, P.-Y.; Rieger, J.; Charleux, B. RAFT-mediated one-pot aqueous emulsion polymerization of methyl methacrylate in presence of poly(methacrylic acid-co-poly(ethylene oxide) methacrylate) trithiocarbonate macromolecular chain transfer agent. Polymer 2013, 54, 2011–2019. [Google Scholar]
- Parkinson, S.; Knox, S.T.; Bourne, R.A.; Warren, N.J. Rapid production of block copolymer nano-objects via continuous-flow ultrafast RAFT dispersion polymerisation. Polym. Chem. 2020, 11, 3465–3474. [Google Scholar] [CrossRef]
- Baeten, E.; Haven, J.J.; Junkers, T. RAFT multiblock reactor telescoping: From monomers to tetrablock copolymers in a continuous multistage reactor cascade. Polym. Chem. 2017, 8, 3815–3824. [Google Scholar] [CrossRef]
- Oliver, S.; Zhao, L.; Gormley, A.J.; Chapman, R.; Boyer, C. Living in the fast lane—High throughput controlled/living radical polymerization. Macromolecules 2019, 52, 3–23. [Google Scholar] [CrossRef] [Green Version]
- Rosales-Guzmán, M.; Pérez-Camacho, O.; Guerrero-Sánchez, C.; Harrisson, S.; Torres-Lubián, R.; Vitz, J.; Schubert, U.S.; Saldívar-Guerra, E. Semiautomated Parallel RAFT Copolymerization of Isoprene with Glycidyl Methacrylate. ACS Comb. Sci. 2019, 21, 771–781. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living Free-Radical Polymerization by Reversible Addition−Fragmentation Chain Transfer: The RAFT Process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Brocchini, S.; James, K.; Tangpasuthadol, V.; Kohn, J. A Combinatorial Approach for Polymer Design. J. Am. Chem. Soc. 1997, 119, 4553–4554. [Google Scholar] [CrossRef]
- Nasrullah, M.J.; Bahr, J.A.; Gallagher-Lein, C.; Webster, D.C.; Roesler, R.R.; Schmitt, P. Automated parallel polyurethane dispersion synthesis and characterization. J. Coat. Technol. Res. 2009, 6, 1–10. [Google Scholar] [CrossRef]
- Thomas, M.; Lu, J.J.; Zhang, C.; Chen, J.; Klibanov, A.M. Identification of novel superior polycationic vectors for gene delivery by high-throughput synthesis and screening of a combinatorial library. Pharm. Res. 2007, 24, 1564–1571. [Google Scholar] [PubMed]
- Szwarc, M. ‘Living’ Polymers. Nature 1956, 178, 1168–1169. [Google Scholar]
- Wang, J.-S.; Matyjaszewski, K. Controlled/”living” radical polymerization. atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar]
- Hawker, C.J.; Barclay, G.G.; Dao, J. Radical Crossover in Nitroxide Mediated “Living” Free Radical Polymerizations. J. Am. Chem. Soc. 1996, 118, 11467–11471. [Google Scholar] [CrossRef]
- Rojas, R.; Harris, N.; Piotrowska, K.; Kohn, J. Evaluation of Automated Synthesis for Chain and Step-Growth Polymerizations: Can Robots Replace the Chemists? J. Polym. Sci. Part A Polym. Chem. 2009, 47, 49–58. [Google Scholar]
- Haven, J.J.; Guerrero-Sanchez, C.; Keddie, D.J.; Moad, G.; Thang, S.H.; Schubert, U.S. One pot synthesis of higher order quasi-block copolymer libraries via sequential RAFT polymerization in an automated synthesizer. Polym. Chem. 2014, 5, 5236–5246. [Google Scholar] [CrossRef]
- Chatterjee, S.; Guidi, M.; Seeberger, P.H.; Gilmore, K. Automated radial synthesis of organic molecules. Nature 2020, 579, 379–384. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Fijten, M.W.M.; Abeln, C.H.; Schubert, U.S. High-Throughput Investigation of Polymerization Kinetics by Online Monitoring of GPC and GC. Macromol. Rapid Commun. 2004, 25, 237–242. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Schubert, U.S. High-throughput synthesis equipment applied to polymer research. Rev. Sci. Instrum. 2005, 76, 062202. [Google Scholar] [CrossRef] [Green Version]
- Hoogenboom, R.; Fijten, M.W.M.; Meier, M.A.R.; Schubert, U.S. Living Cationic Polymerizations Utilizing an Automated Synthesizer: High-Throughput Synthesis of Polyoxazolines. Macromol. Rapid Commun. 2003, 24, 92–97. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Schubert, U.S. The fast and the curious: High-throughput experimentation in synthetic polymer chemistry. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2425–2434. [Google Scholar] [CrossRef]
- Zhang, H.; Marin, V.; Fijten, M.W.M.; Schubert, U.S. High-throughput experimentation in atom transfer radical polymerization: A general approach toward a directed design and understanding of optimal catalytic systems. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 1876–1885. [Google Scholar] [CrossRef]
- Zhang, H.; Fijten, M.W.M.; Hoogenboom, R.; Reinierkens, R.; Schubert, U.S. Application of a Parallel Synthetic Approach in Atom-Transfer Radical Polymerization: Set-Up and Feasibility Demonstration. Macromol. Rapid Commun. 2003, 24, 81–86. [Google Scholar] [CrossRef]
- Schuett, T.; Kimmig, J.; Zechel, S.; Schubert, U.S. Automated polymer purification using dialysis. Polymers 2020, 12, 2095. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, K.; Buckinx, A.-L.; Zaquen, N.; Junkers, T. Micelle Purification in Continuous Flow via Inline Dialysis. Macromolecules 2021, 54, 3865–3872. [Google Scholar] [CrossRef]
- Zhang, C.; Chung, J.W.; Priestley, R.D. Dialysis Nanoprecipitation of Polystyrene Nanoparticles. Macromol. Rapid Commun. 2012, 33, 1798–1803. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, B.E.; De Visser, J.F.; Portale, G.; Hermida-Merino, D.; Friedrich, H.; Bomans, P.H.H.; Bras, W.; Monaghan, O.R.; Holder, S.J.; Sommerdijk, N.A.J.M. The evolution of bicontinuous polymeric nanospheres in aqueous solution. Soft Matter 2016, 12, 4113–4122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuett, T.; Geitner, R.; Zechel, S.; Schubert, U.S. Dialysis Diffusion Kinetics in Polymer Purification. Macromolecules 2021, 54, 9410–9417. [Google Scholar] [CrossRef]
- Enke, M.; Bose, R.K.; Bode, S.; Vitz, J.; Schacher, F.H.; Garcia, S.J.; van der Zwaag, S.; Hager, M.D.; Schubert, U.S. A Metal Salt Dependent Self-Healing Response in Supramolecular Block Copolymers. Macromolecules 2016, 49, 8418–8429. [Google Scholar] [CrossRef]


| Number | Polymer | Synthesis Approach | Mn (g mol−1) | Mw (g mol−1) | Đ | Yield | |
|---|---|---|---|---|---|---|---|
| [g] | [%] | ||||||
| P1a | PS | manual | 3800 | 4900 | 1.29 | 1.00 | 20 |
| P1b | PS-b-PBA-b-PS | manual | 8100 | 10,500 | 1.30 | 0.31 | 17 |
| P2a | PS | automated | 4100 | 5100 | 1.25 | 0.78 | 16 |
| P2b | PS-b-PBA-b-PS | automated | 12,700 | 18,700 | 1.48 | 0.22 | 8 |
| P3a | PS | manual | 5900 | 7500 | 1.28 | 3.00 | 30 |
| P3b | PS-b-PBA-b-PS | manual | 17,800 | 21,500 | 1.20 | 7.33 | 60 |
| P3c | PS-b-PBA-b-PS-b-PBA-b-PS | manual | 20,800 | 26,000 | 1.25 | 6.89 | 50 |
| P4a | PS | automated | 5200 | 6700 | 1.28 | 3.00 | 30 |
| P4b | PS-b-PBA-b-PS | automated | 13,700 | 17,100 | 1.25 | 7.00 | 51 |
| P4c | PS-b-PBA-b-PS-b-PBA-b-PS | automated | 22,200 | 26,800 | 1.21 | 11.40 | 76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuett, T.; Kimmig, J.; Zechel, S.; Schubert, U.S. Fully Automated Multi-Step Synthesis of Block Copolymers. Polymers 2022, 14, 292. https://doi.org/10.3390/polym14020292
Schuett T, Kimmig J, Zechel S, Schubert US. Fully Automated Multi-Step Synthesis of Block Copolymers. Polymers. 2022; 14(2):292. https://doi.org/10.3390/polym14020292
Chicago/Turabian StyleSchuett, Timo, Julian Kimmig, Stefan Zechel, and Ulrich S. Schubert. 2022. "Fully Automated Multi-Step Synthesis of Block Copolymers" Polymers 14, no. 2: 292. https://doi.org/10.3390/polym14020292






