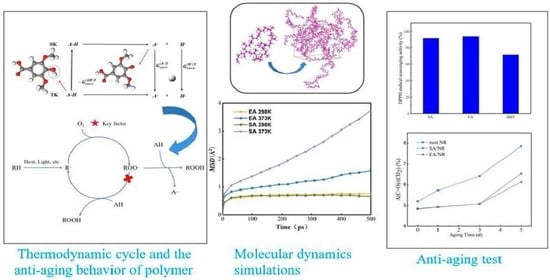
The Influence of Syringic Acid and Erucic Acid on the Antioxidant Properties of Natural Rubber: Experimental and Molecular Simulation Investigations
Abstract
1. Introduction
2. Molecular Simulations
2.1. Quantum Mechanics (QM) Simulation
2.2. Molecular Dynamics (MD) Simulation
3. Materials and Methods
3.1. Materials
3.2. Preparation of NR Composites
3.3. Measurements and Characterization
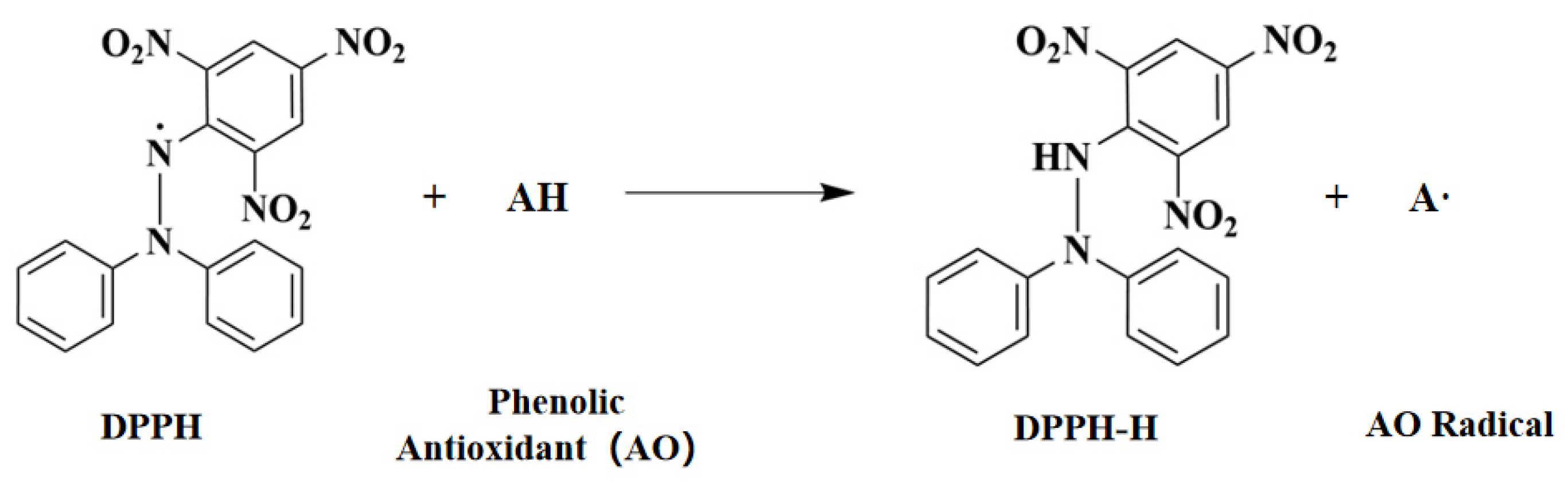
3.3.1. DPPH (2,2-Diphenyl-1-picrylhydrazyl) Radical Scavenging Test
3.3.2. Accelerated Thermal-Oxidative Aging Experiments
3.3.3. Mechanical Property Test
3.3.4. Fourier Transform Infrared Spectroscopy (FT-IR)
4. Results and Discussion
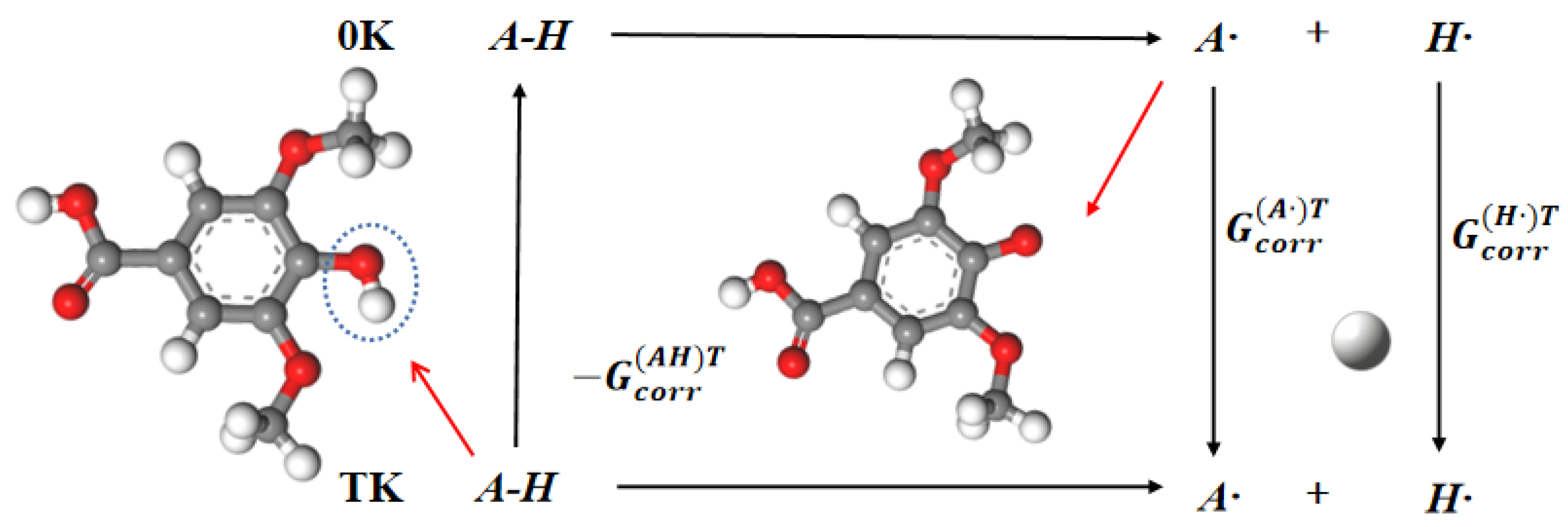
4.1. Hydrogen Dissociation Energy
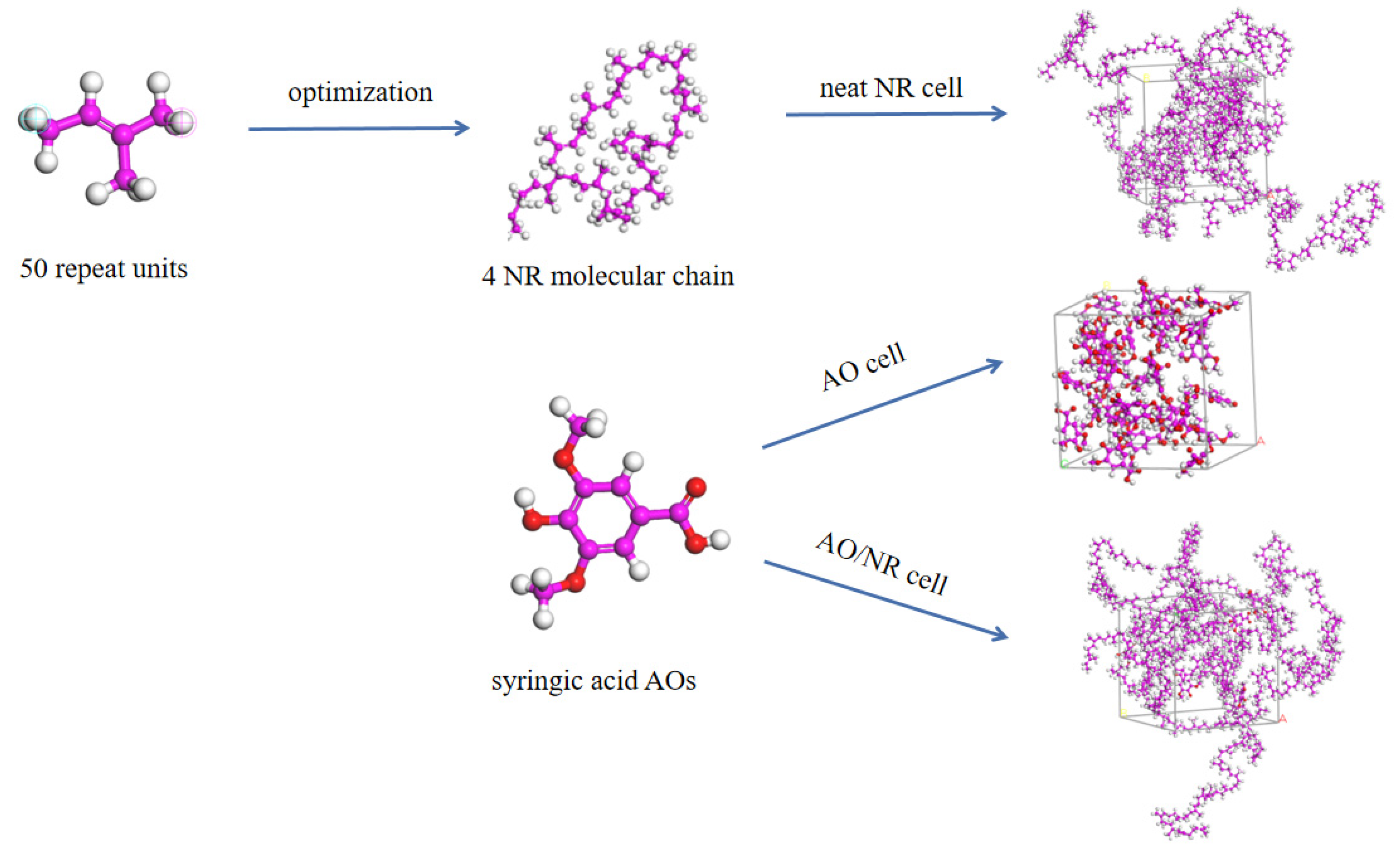
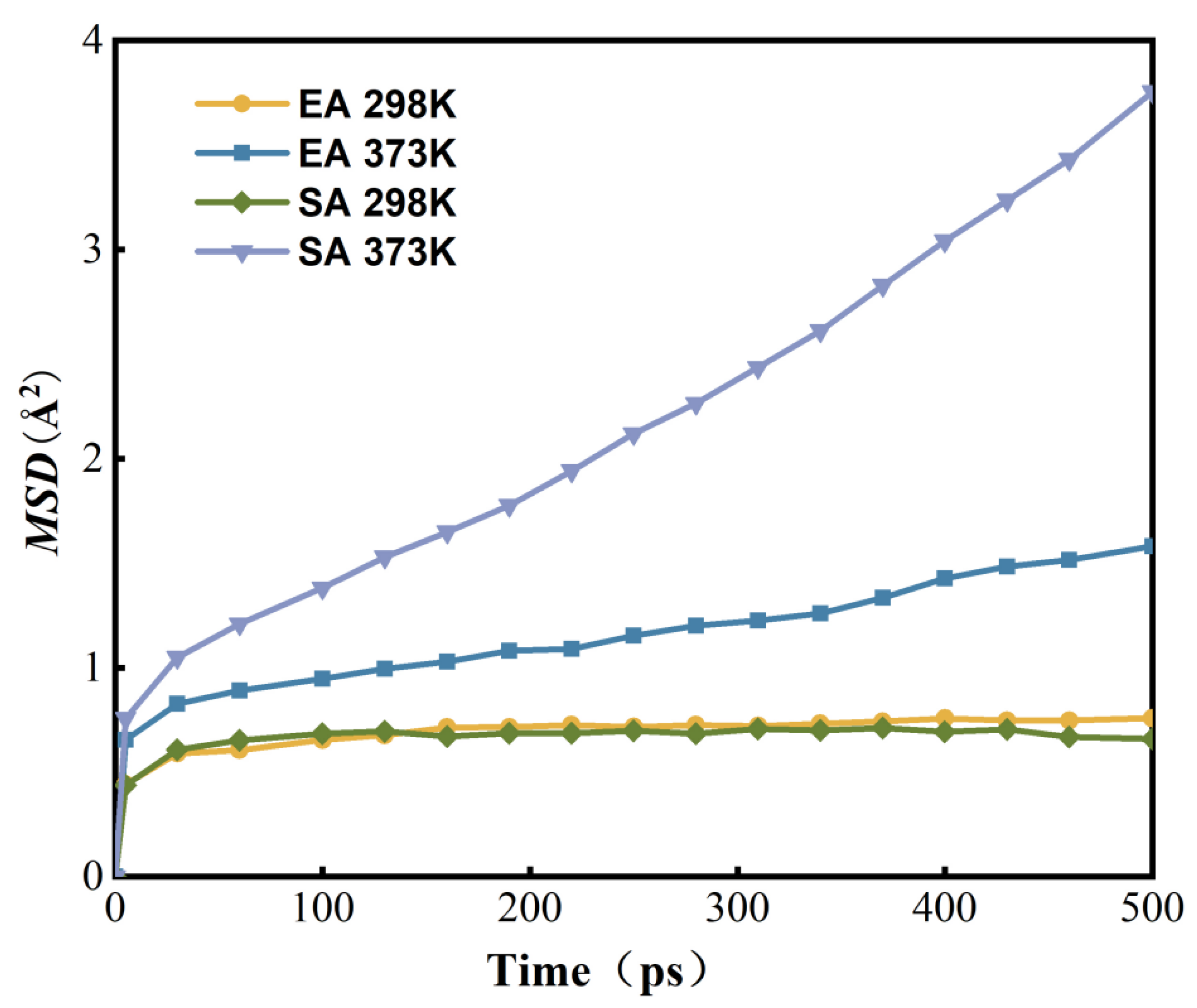
4.2. Dispersion and Migration of Antioxidants
4.3. Oxygen Permeability
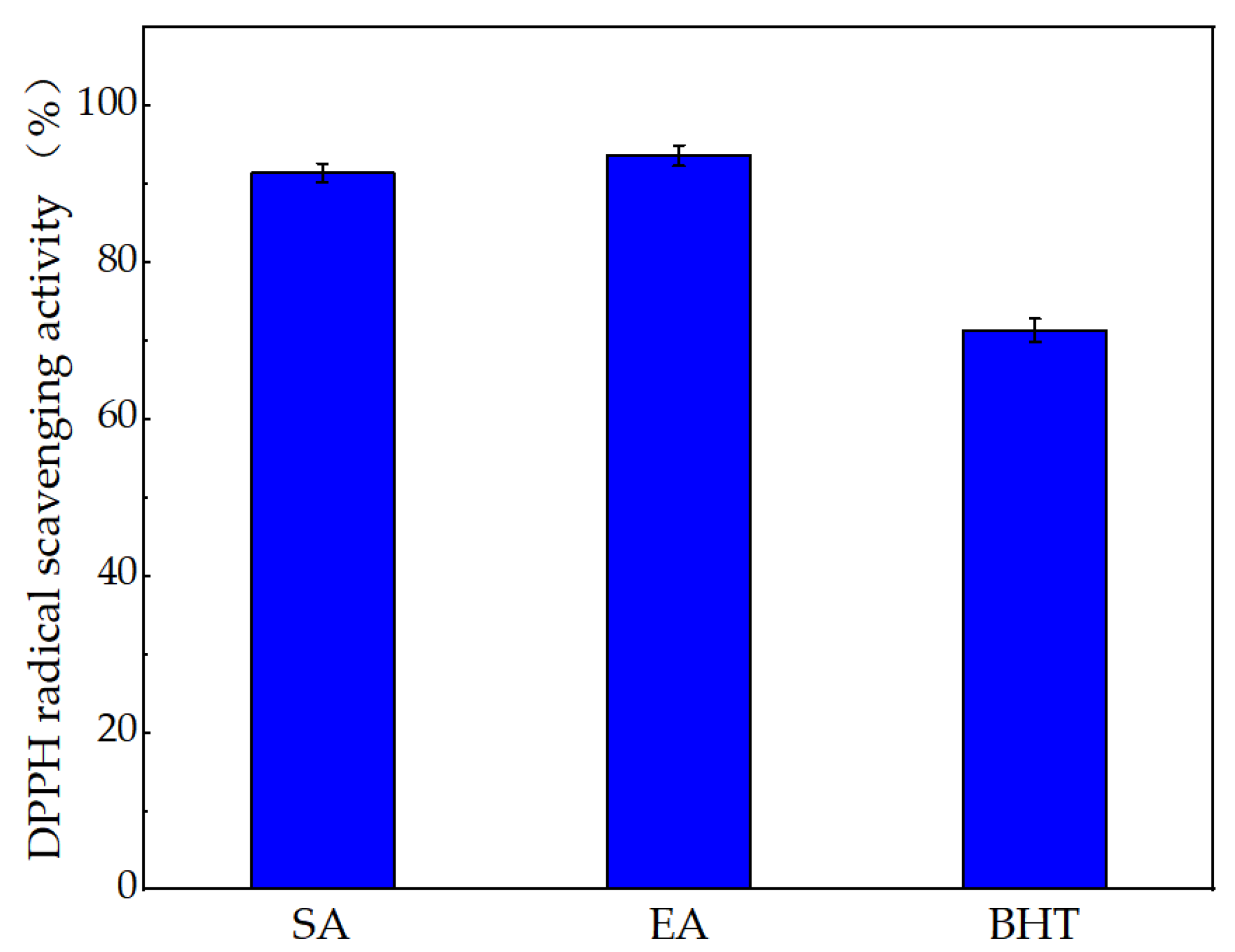
4.4. Analysis of Antioxidant Activity
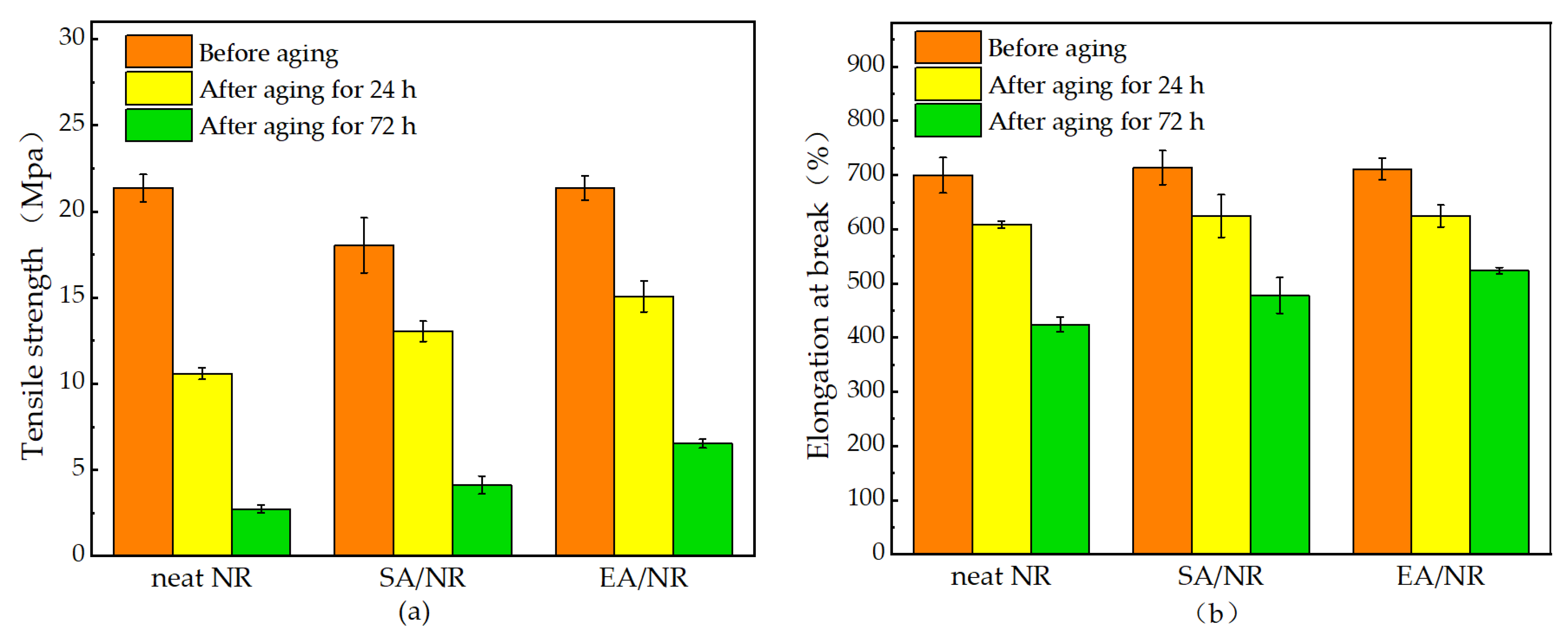
4.5. Analysis of Mechanical Properties of NR Composites
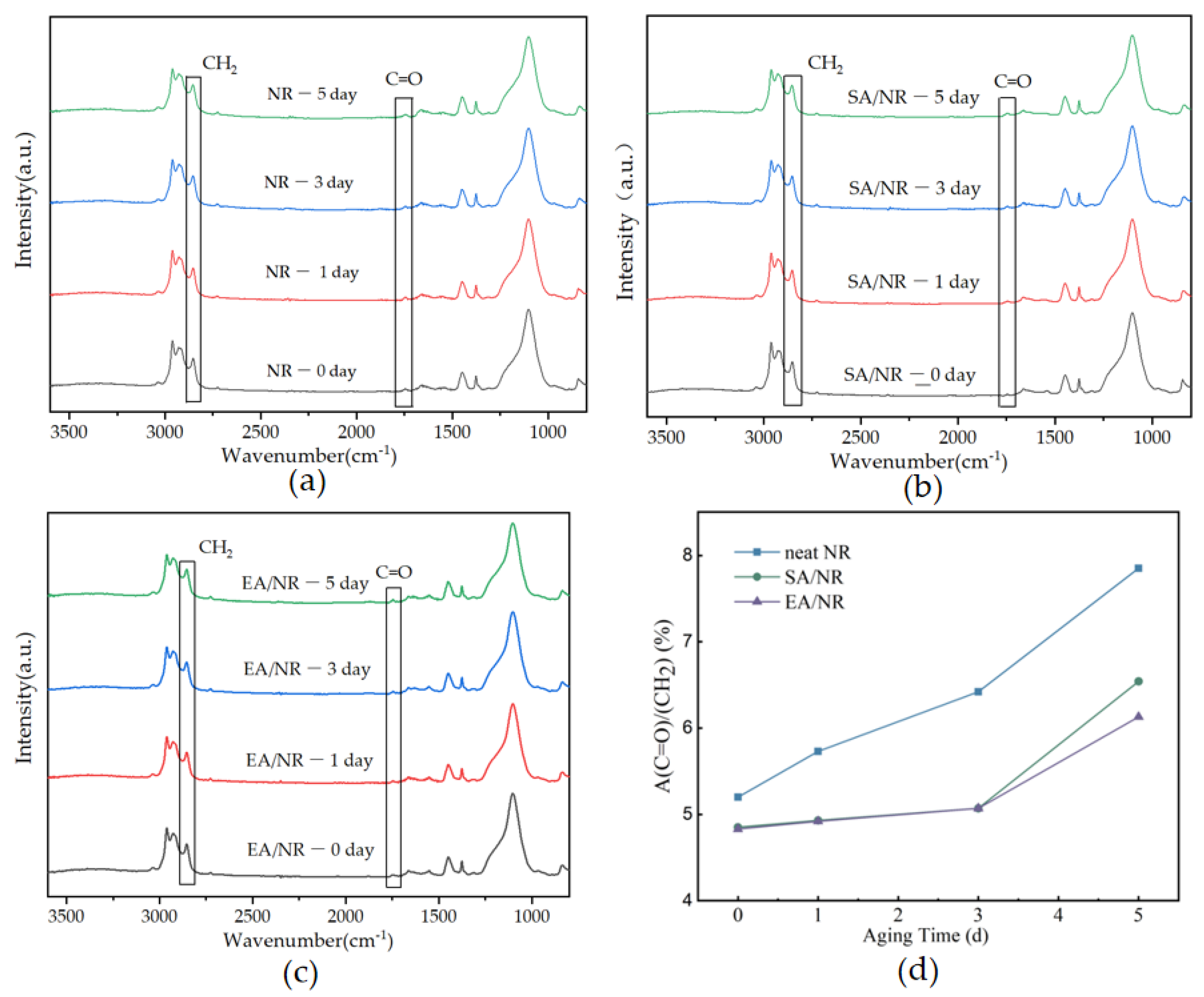
4.6. Microstructure Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong, B.; Dong, H.; Luo, Y.; Zhang, D.; Jia, Z.; Jia, D.; Liu, F. Simultaneous reduction and functionalization of graphene oxide via antioxidant for highly aging resistant and thermal conductive elastomer composites. Compos. Sci. Technol. 2017, 151, 156–163. [Google Scholar] [CrossRef]
- Matchawet, S.; Kaesaman, A. Electrical and Mechanical Properties of Conductive Carbon Black Filled Epoxidized Natural Rubber. Adv. Mater. Res. 2014, 884, 255–258. [Google Scholar] [CrossRef]
- Seentrakoon, B.; Junhasavasdikul, B.; Chavasiri, W. Enhanced UV-protection and antibacterial properties of natural rubber/rutile-TiO2 nanocomposites. Polym. Degrad. 2013, 98, 566–578. [Google Scholar] [CrossRef]
- Palosuo, T.; Makinen-Kiljunen, H.; Alenius, H.; Reunala, E.; Yip, K.; Turjanmaa, K. Measurement of natural rubber latex allergen levels in medical gloves by allergen-specific IgE-ELISA inhibition, RAST inhibition, and skin prick test. Allergy 2010, 53, 59–67. [Google Scholar] [CrossRef]
- Barrera, C.S.; Cornis, K. Novel Mineral and Organic Materials from AgroIndustrial Residues as Fillers for Natural Rubber. Polym. Environ. 2015, 23, 437–448. [Google Scholar] [CrossRef]
- Deng, P.; Jiao, Q.; Ren, H. Synthesis of nitrogen-doped porous hollow carbon nanospheres with a high nitrogen content: A sustainable synthetic strategy using energetic precursors. Sci. Total Environ. 2020, 714, 136725. [Google Scholar] [CrossRef] [PubMed]
- Mente, P.; Motaung, T.E.; Hlangothi, S.P. Natural rubber and reclaimed rubber composites—A systematic review. Polym. Sci. 2016, 2, 1. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Dayang Habibah, A.I.H.; Devaraj, V.; Kamarularifin, H.; Suhawati, I. Cure Characteristics and Ageing Resistance of Recovered Waste Pre-Vulcanized Nitrile/Epoxidized Natural Rubber Latex Blends in Nitrile Butadiene Rubber Compounds. Adv. Mater. Res. 2015, 1119, 347–351. [Google Scholar] [CrossRef]
- Tuampoemsab, S. Influence of Amino Acids on Anti-Oxidative Properties of Green Natural Rubber and Natural Rubber Compound. Adv. Mater. Res. 2013, 747, 664–667. [Google Scholar] [CrossRef]
- He, S.J.; Wang, J.Q.; Hu, J.B.; Hu, J.B.; Zhou, H.F.; Hien, N.Y.; Luo, C.M.; Lin, J. Silicone rubber composites incorporating graphitic carbon nitride and modified by vinyl tri-methoxysilane. Polym. Test. 2019, 79, 106005. [Google Scholar] [CrossRef]
- Tang, M.Z.; Xing, W.; Wu, J.R.; Huang, G.S.; Xiang, K.W.; Guo, L.L.; Lia, G.X. Graphene as a prominent antioxidant for diolefin elastomers. J. Mater. Chem. A 2015, 3, 5942–5948. [Google Scholar] [CrossRef]
- Wu, W.J.; Zeng, X.R.; Li, H.Q.; Lai, X.J.; Li, F.; Guo, J.H. Synthesis and Characterization of A Novel Macromolecular Hindered Phenol Antioxidant and Its Thermo-Oxidative Aging Resistance for Natural Rubber. J. Macromol. Sci. B 2014, 53, 1244–1257. [Google Scholar] [CrossRef]
- Mizobuchi, M.; Ishidoh, K.; Kamemura, N. A comparison of cell death mechanisms of antioxidants, butylated hydroxyanisole and butylated hydroxytoluene. Compos. Sci. Technol. 2022, 45, 1899–1906. [Google Scholar] [CrossRef]
- Yehye, W.A.; Rahman, N.A.; Saad, O.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M.; Matlob, A.A. Rational Design and Synthesis of New, High Efficiency, Multipotent Schiff Base-1,2,4-triazole Antioxidants Bearing Butylated Hydroxytoluene Moieties. Molecules 2016, 21, 847. [Google Scholar] [CrossRef]
- Ning, N.Y.; Ma, Q.; Zhang, Y.Q.; Zhang, L.Q.; Wu, H.G.; Tian, M. Enhanced thermo-oxidative aging resistance of EPDM at high temperature by using synergistic antioxidants. Polym. Degrad. Stabil. 2014, 102, 1–8. [Google Scholar] [CrossRef]
- De Lucia, M.; Panzella, L.; Pezzella, A.; Napolitano, A.; d’Ischia, M. Oxidative chemistry of the natural antioxidant hydroxytyrosol: Hydrogen peroxide-dependent hydroxylation and hydroxyquinone/o-quinone coupling pathways. Tetrahedron 2006, 62, 1273–1278. [Google Scholar] [CrossRef]
- Liu, R.Z.; Mabury, S.A. Printing ink related chemicals, including synthetic phenolic antioxidants, organophosphite antioxidants, and photoinitiators, in printing paper products and implications for human exposure. Environ. Int. 2021, 149, 106412. [Google Scholar] [CrossRef]
- Lu, L.; Luo, K.; Yang, W.; Zhang, S.; Wang, W.; Xu, H.; Wu, S. Insight into the anti-aging mechanisms of natural phenolic antioxidants in natural rubber composites using a screening strategy based on molecular simulation. RSC Adv. 2020, 10, 21318–21327. [Google Scholar] [CrossRef]
- Lim, S.H.; Choi, C.I. Potentials of Raspberry Ketone as a Natural Antioxidant. Antioxidants 2021, 10, 482. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Antioxidant Behavior in Bulk Oil: Limitations of Polar Paradox Theory. J. Agric. Food Chem. 2012, 60, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Guitard, R.; Paul, J.F.; Nardello-Rataj, V.; Aubry, J.M. Myricetin, rosmarinic and carnosic acids as superior natural antioxidant alternatives to alpha-tocopherol for the preservation of omega-3 oils. Food Chem. 2016, 213, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, M.; Lecomtem, J.; Villeneuve, P. The use and effectiveness of antioxidants in lipids preservation. Handb. Antioxid. Food Preserv. 2015, 1, 349–372. [Google Scholar] [CrossRef]
- Li, C.Y.; Strachan, A. Molecular Scale Simulations on Thermoset Polymers: A Review. J. Polym. Sci. Pol. Phys. 2015, 53, 103–122. [Google Scholar] [CrossRef]
- Perilla, J.R.; Goh, B.C.; Cassidy, C.K.; Liu, B.; Bernardi, R.C.; Rudack, T.; Yu, H.; Wu, Z.; Schulten, K. Molecular dynamics simulations of large macromolecular complexes. Curr. Opin. Struct. Biol. 2015, 31, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.L.; Wang, R.G.; Zhao, S.H.; Chen, Y.Y.; Su, H.F.; Zhang, L.Q.; Chan, T.W.; Wu, S.Z. Experimental study and molecular dynamics simulation of dynamic properties and interfacial bonding characteristics of graphene/solution-polymerized styrene-butadiene rubber composites. RSC Adv. 2016, 6, 58077–58087. [Google Scholar] [CrossRef]
- Luo, K.Q.; You, G.H.; Zhao, X.Y.; Lu, L.; Wang, W.C.; Wu, S.Z. Synergistic effects of antioxidant and silica on enhancing thermo-oxidative resistance of natural rubber: Insights from experiments and molecular simulations. Mater. Des. 2019, 5, 181. [Google Scholar] [CrossRef]
- Yu, S.S.; Wang, Y.S.; Maa, Y.J.; Wang, L.M.; Zhu, J.; Liu, S.G. Structure, thermal stability, antioxidant activity and DFT studies of trisphenols and related phenols. Inorg. Chim. Acta 2017, 468, 159–170. [Google Scholar] [CrossRef]
- Haidasz, E.A.; Shah, R.; Pratt, D.A. The Catalytic Mechanism of Diarylamine Radical-Trapping Antioxidants. J. Am. Chem. Soc. 2014, 136, 16643–16650. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Zhao, X.Y.; Yue, D.M.; Zhang, L.Q.; Wu, S.Z. A combined experiment and molecular dynamics simulation study of hydrogen bonds and free volume in nitrile-butadiene rubber/hindered phenol damping mixtures. J. Mater. Chem. 2012, 22, 12339–12348. [Google Scholar] [CrossRef]
- Allen, T.E.H.; Goodman, J.M.; Grayson, M.N.; Gutsell, S.; Russell, P.J. Computational approaches for predicting Molecular Initiating Events. Toxicol. Lett. 2018, 295, S99–S100. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of Antioxidants in the Oxidation of Foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Zou, Y.K.; Sun, Y.K.; He, J.W.; Tang, Z.H.; Zhu, L.X.; Luo, Y.F.; Liu, F. Enhancing mechanical properties of styrene-butadiene rubber/silica nanocomposites by in situ interfacial modification with a novel rare-earth complex. Compos. Part A-Appl. Sci. Manuf. 2016, 87, 297–309. [Google Scholar] [CrossRef]
- Ho, J. Are thermodynamic cycles necessary for continuum solvent calculation of pKas and reduction potentials. Phys. Chem. Chem. Phys. 2015, 17, 2859–2868. [Google Scholar] [CrossRef]
- Wang, X.; Song, M.; Liu, S.; Wu, S.; Thu, A.M. Analysis of phthalate plasticizer migration from PVDC packaging materials to food simulants using molecular dynamics simulations and artificial neural network. Food Chem. 2020, 317, 126465–126474. [Google Scholar] [CrossRef]
- Abraham, C.S.; Muthu, S.; Prasana, J.C.; Rizwana, B.F.; Armakovic, S.; Armakovic, S.J. Vibrational and electronic absorption spectroscopic profiling, natural hybrid orbital, charge transfer, electron localization function and molecular docking analysis on 3-amino-3-(2-nitrophenyl) propionic acid. J. Mol. Struct. 2018, 1171, 733–746. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N.; Yousif, E. Chemically modified biolubricant basestocks from epoxidized oleic acid: Improved low temperature properties and oxidative stability. J. Saudi Chem. Soc. 2011, 15, 195–201. [Google Scholar] [CrossRef]
- Hu, C.; You, G.; Liu, J.; Du, S.; Zhao, X.; Wu, S. Study on the mechanisms of the lubricating oil antioxidants: Experimental and molecular simulation. J. Mol. Liq. 2021, 324, 115099. [Google Scholar] [CrossRef]
- Barton, A.M. CRC Handbook of Solubility Parameters and Other Cohesion Parameters; CRC Press: Boca Raton, FL, USA, 1983. [Google Scholar]
- Golzar, K.; Amjad-Iranagh, S.; Amani, M.; Modarress, H. Molecular simulation study of penetrant gas transport properties into the pure and nanosized silica particles filled polysulfone membranes. J. Membr. Sci. 2014, 451, 117–134. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, L.; Zhao, X.Y.; He, J.W.; Wang, A.; Chan, T.W.; Wu, S.Z. Effects of lanthanum complex on the thermo-oxidative aging of natural rubber. Polym. Degrad. Stabil. 2015, 120, 377–383. [Google Scholar] [CrossRef]


| Ingredient (phr) 1 | Samples | ||
|---|---|---|---|
| NR | SA/NR | EA/NR | |
| NR | 100 | 100 | 100 |
| SA | 0 | 1.2 | 0 |
| EA | 0 | 0 | 1.2 |
| Dissociation Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| ΔG298K (kJ·mol−1) | 98.02 | 78.97 | 375.05 | 411.06 | 342.20 |
| Samples | Solubility Parameter δ (J·cm−3)1/2 | ||
|---|---|---|---|
| Simulation | Experiment | Δδ = |δantioxidant − δNR| | |
| NR | 16.38 | 16.2–17.0 | - |
| SA | 14.77 | - | 1.61 |
| EA | 15.42 | - | 0.96 |
| System | D (10−6 cm2 s−1) | S (10−3 cm3 (STP) cm−3 kPa−1) | P (10−9 cm2 s−1 kPa−1) |
|---|---|---|---|
| NR | 3.7 | 1.70 | 6.29 |
| SA/NR | 1.8 | 2.99 | 5.38 |
| EA/NR | 1.9 | 0.67 | 1.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Wang, X.; Wang, X.; Zheng, W.; He, S.; Song, M.; Wang, H. The Influence of Syringic Acid and Erucic Acid on the Antioxidant Properties of Natural Rubber: Experimental and Molecular Simulation Investigations. Polymers 2022, 14, 4254. https://doi.org/10.3390/polym14204254
Chen S, Wang X, Wang X, Zheng W, He S, Song M, Wang H. The Influence of Syringic Acid and Erucic Acid on the Antioxidant Properties of Natural Rubber: Experimental and Molecular Simulation Investigations. Polymers. 2022; 14(20):4254. https://doi.org/10.3390/polym14204254
Chicago/Turabian StyleChen, Shihao, Xiujuan Wang, Xueting Wang, Wei Zheng, Shaojian He, Meng Song, and Hongzhen Wang. 2022. "The Influence of Syringic Acid and Erucic Acid on the Antioxidant Properties of Natural Rubber: Experimental and Molecular Simulation Investigations" Polymers 14, no. 20: 4254. https://doi.org/10.3390/polym14204254
APA StyleChen, S., Wang, X., Wang, X., Zheng, W., He, S., Song, M., & Wang, H. (2022). The Influence of Syringic Acid and Erucic Acid on the Antioxidant Properties of Natural Rubber: Experimental and Molecular Simulation Investigations. Polymers, 14(20), 4254. https://doi.org/10.3390/polym14204254