Incorporation of Nanocatalysts for the Production of Bio-Oil from Staphylea holocarpa Wood
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Sources
2.2. Solvent Extractive
2.3. The Physicochemical Properties of the Catalyst–Wood Mixture
3. Results and Discussion
3.1. Extractive of S. holocarpa
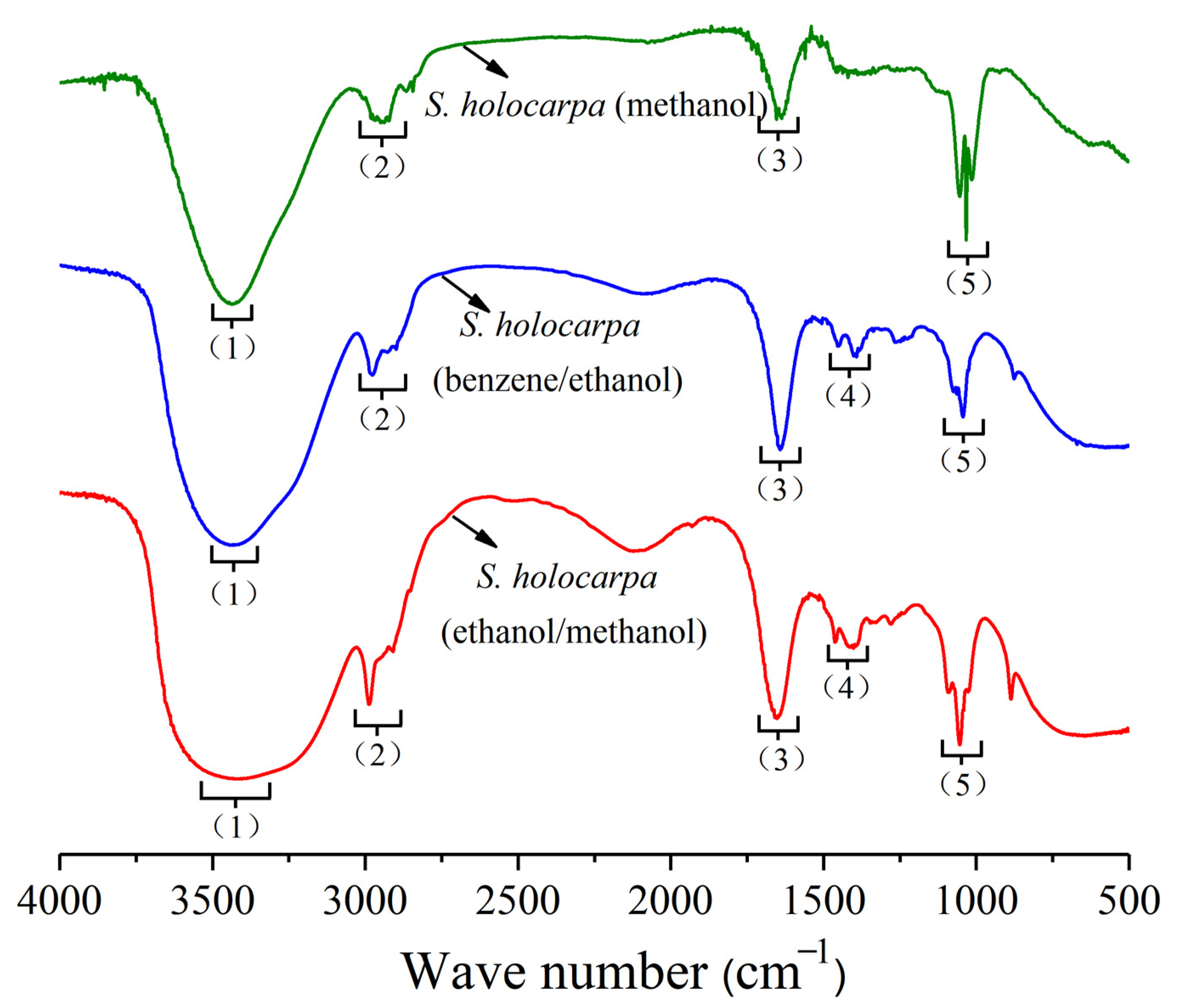
3.1.1. Analysis of FTIR
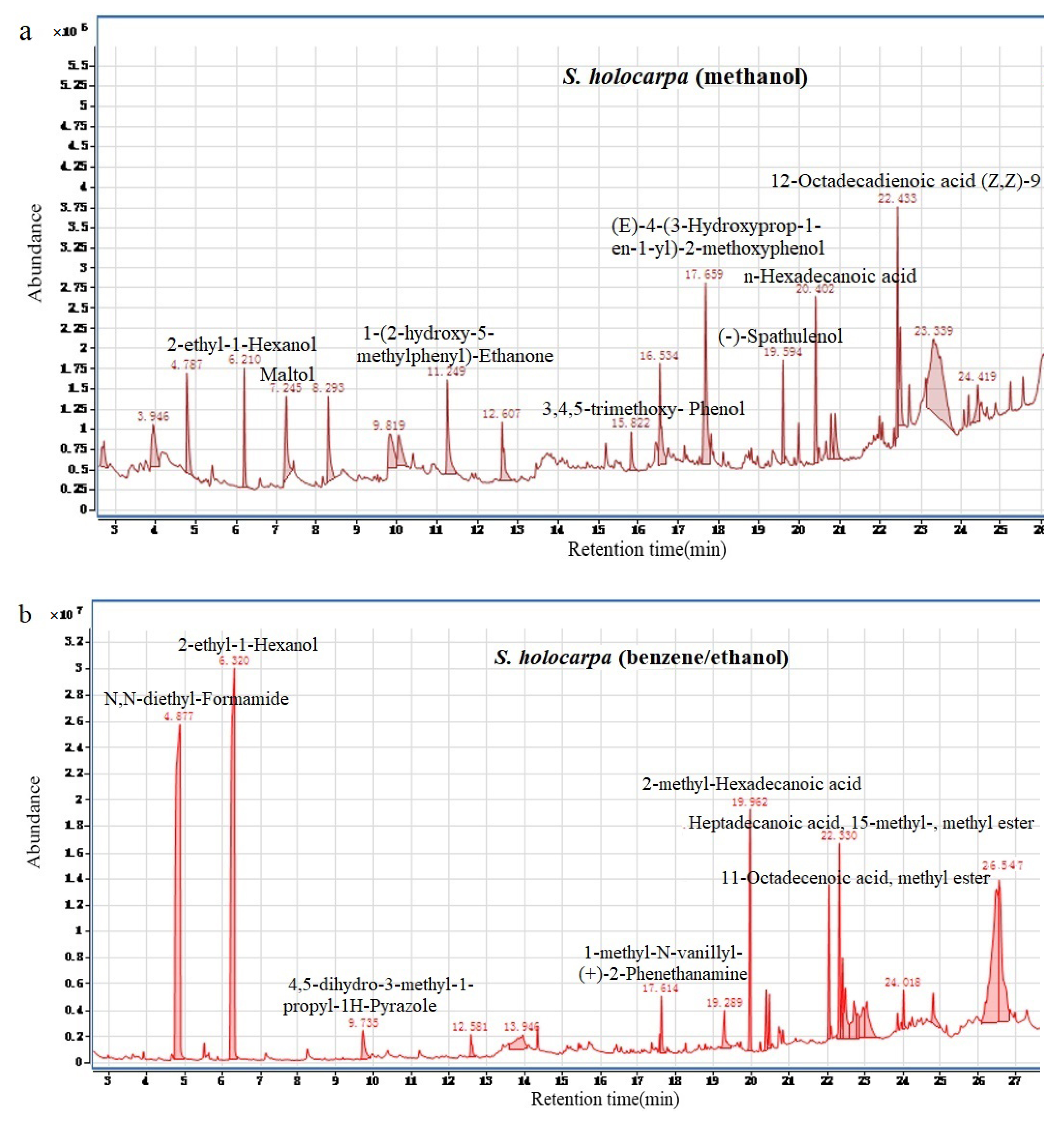
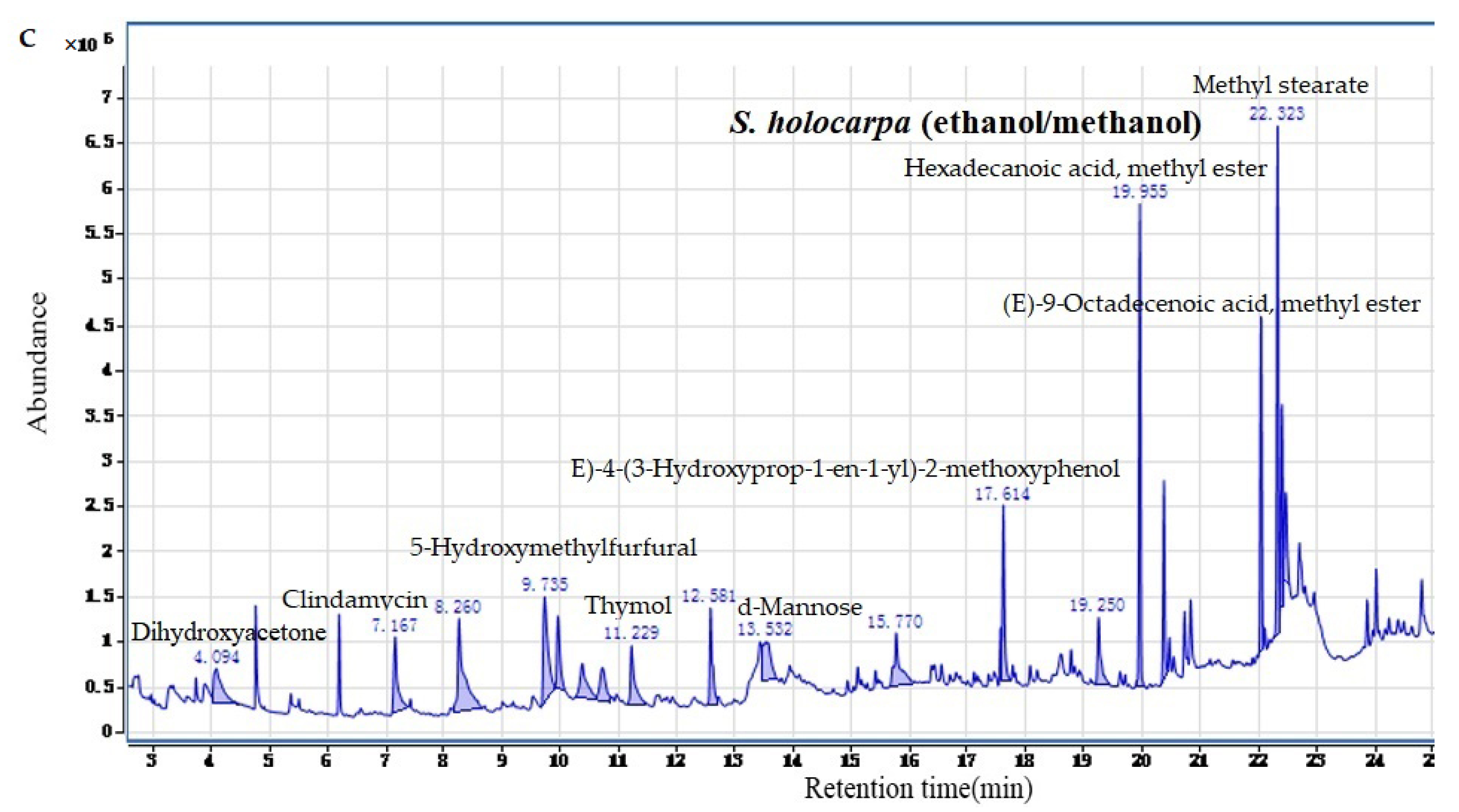
3.1.2. Analysis of GC–MS
3.1.3. LC–QTOF–MS Analysis
3.2. Incorporation of Nanocatalyst with S. holocarpa Wood
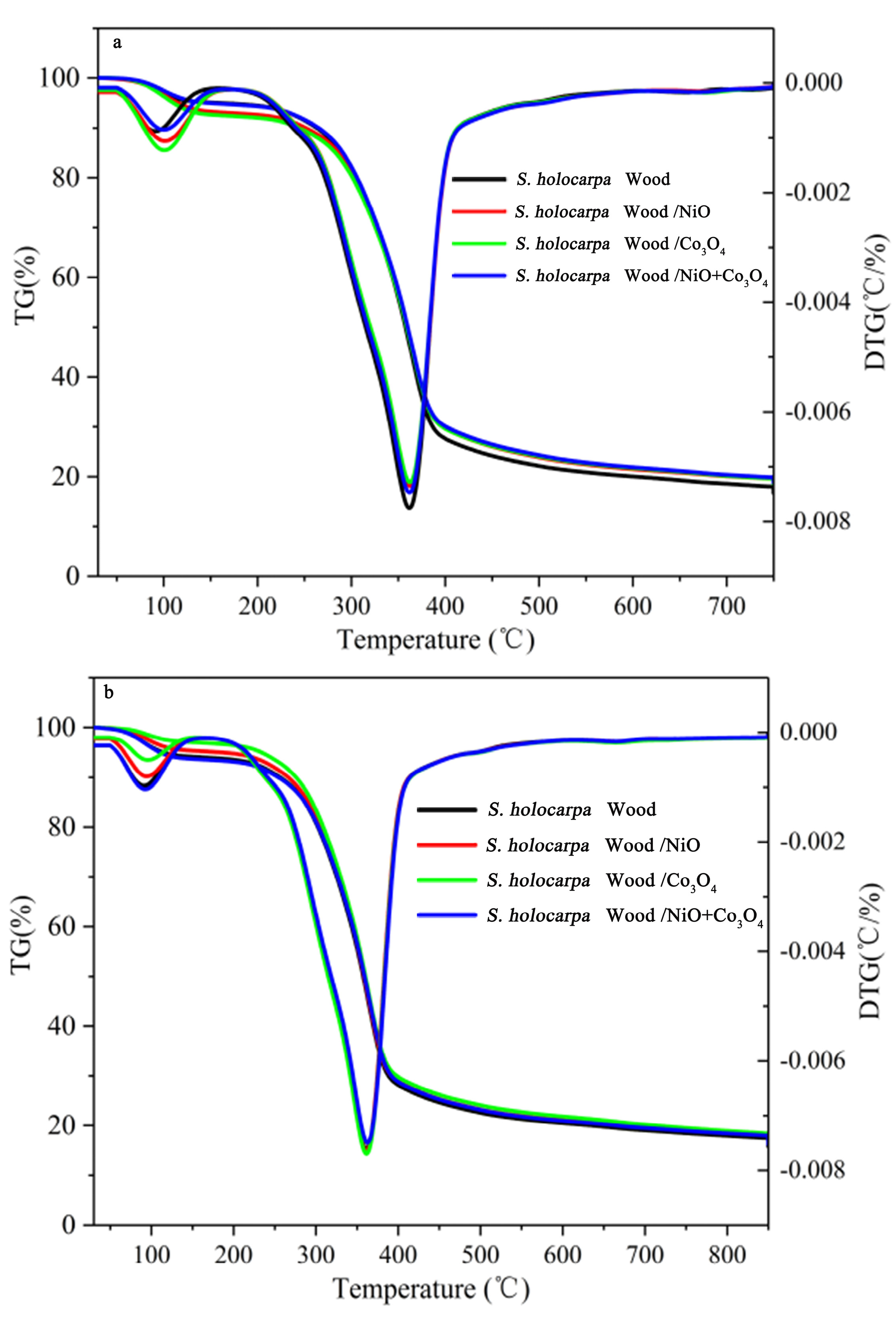
3.2.1. Analysis of TGA
3.2.2. TG−FTIR Analysis
3.2.3. Analysis of Py/GC–MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bildirici, M.E. Economic growth and biomass energy. Biomass Bioenergy 2013, 50, 19–24. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, W. Status quo and prospects of biomass energy. Trans. CSAE 2005, 21, 12–15. [Google Scholar]
- Wang, J.C.; Dai, L.; Tian, Y.S.; Qin, S.J. Analysis of the development status and trends of biomass energy industry in China. Trans. CSAE 2007, 23, 276–282. [Google Scholar]
- Lang, A.; Kopetz, H.; Parker, A. Biomass energy holds big promise. Nature 2012, 488, 590–591. [Google Scholar] [CrossRef]
- Van Dael, M.; Van Passel, S.; Pelkmans, L.; Guisson, R.; Reumermann, P.; Luzardo, N.M.; Witters, N.; Broeze, J. A techno-economic evaluation of a biomass energy conversion park. Appl. Energy 2013, 104, 611–622. [Google Scholar] [CrossRef]
- Moiseyev, A.; Solberg, B.; Kallio, A.M.I. Wood biomass use for energy in Europe under different assumptions of coal, gas and CO2 emission prices and market conditions. J. Forest Econ. 2013, 19, 432–449. [Google Scholar] [CrossRef]
- Kopetz, H. Build a biomass energy market. Nature 2013, 494, 29–31. [Google Scholar] [CrossRef]
- Berman, A.I. Biomass: Renewable energy. Science 1990, 249, 1228. [Google Scholar] [CrossRef] [PubMed]
- Field, C.B.; Campbell, J.E.; Lobell, D.B. Biomass energy: The scale of the potential resource. Trends Ecol. Evol. 2008, 23, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Shuvo, I.I.; Rahman, M.; Vahora, T.; Morrison, J.; DuCharme, S.; Choo-Smith, L.P. Producing light-weight bast fibers from canola biomass for technical textiles. Text. Res. J. 2020, 90, 1311–1325. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Sheng, Y.Q.; Huang, Q.G.; Yang, Z.L.; Shi, Y.; Guo, X.Q.; Ge, S.B. Influence of typical pretreatment on cotton stalk conversion activity and bio-oil property during low temperature (180–220 °C) hydrothermal process. Fuel 2022, 328, 125250. [Google Scholar] [CrossRef]
- O′ Callaghan, K. Technologies for the utilisation of biogenic waste in the bioeconomy. Food Chem. 2016, 198, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, Y.; Wang, D. Policy Measures for Biomass Energy Worldwide and Their Effect Analysis. World For. Res. 2014, 27, 89–92. [Google Scholar]
- Sanchez-Sanchez, A.; Izquierdo, M.T.; Mathieu, S.; Medjahdi, G.; Fierro, V.; Celzard, A. Activated carbon xerogels derived from phenolic oil: Basic catalysis synthesis and electrochemical performances. Fuel Process. Techol. 2020, 205, 106427. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; da Silva, E.L.; Cuna, A.; Malfatti, C.F.; Marcuzzo, J.S.; Baldan, M.R.; Celzard, A.; Fierro, V.; Silva, G.F.B.L.E. Sustainable Carbon Material from Kraft Black Liquor as Nickel-Based Electrocatalyst Support for Ethanol Electro-Oxidation. Waste Biomass Valoriz. 2021, 12, 2507–2519. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Munhoz, M.G.C.; Fonseca, B.C.D.; Boss, A.F.N.; de Almeida-Mattos, P.; Braghiroli, F.L.; Bouafif, H.; Koubaa, A.; Silva, G.F.B.; Baldan, M.R. Xerogel-like Materials from Sustainable Sources: Properties and Electrochemical Performances. Energies 2021, 14, 7977. [Google Scholar] [CrossRef]
- Fonseca, B.C.S.; Araújo, L.S.; Pinheiro, B.S.; Santos, A.S.; Amaral-Labat, G.; Matsushima, J.T.; Baldan, M.R. Bio-based Carbon Electrochemically Decorated with Cu Nanoparticles: Green Synthesis and Electrochemical Performance. Mater. Res. 2022, 25, e20220143. [Google Scholar] [CrossRef]
- Nikolic, S.; Mojovic, L.; Rakin, M.; Pejin, D.; Pejin, J. Ultrasound-assisted production of bioethanol by simultaneous saccharification and fermentation of corn meal. Food Chem. 2010, 122, 216–222. [Google Scholar] [CrossRef]
- Mehrabadi, A.; Farid, M.M.; Craggs, R. Potential of five different isolated colonial algal species for wastewater treatment and biomass energy production. Algal. Res. 2017, 21, 1–8. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Xie, G. Forest Biomass Energy Resources in China: Quantity and Distribution. Forests 2015, 6, 3970–3984. [Google Scholar] [CrossRef]
- Yan, J.D. Biomass to Energy in China: Development Status and Strategic Consideration. Chem. Ind. For. Prod. 2014, 34, 151–158. [Google Scholar]
- Ge, S.B.; Liang, Y.Y.; Zhou, C.X.; Sheng, Y.Q.; Zhang, M.L.; Cai, L.P.; Zhou, Y.H.; Huang, Z.H.; Manzo, M.; Wu, C.Y.; et al. The potential of Pinus armandii Franch for high-grade resource utilization. Biomass Bioenergy 2022, 158, 106345. [Google Scholar] [CrossRef]
- Saffar, T.; Bouafif, H.; Braghiroli, F.L.; Magdouli, S.; Langlois, A.; Koubaa, A. Production of Bio-based Polyol from Oxypropylated Pyrolytic Lignin for Rigid Polyurethane Foam Application. Waste Biomass Valorization 2020, 11, 6411–6427. [Google Scholar] [CrossRef]
- Pinheiro Pires, A.P.; Arauzo, J.; Fonts, I.; Domine, M.E.; Fernández Arroyo, A.; Garcia-Perez, M.E.; Montoya, J.; Chejne, F.; Pfromm, P.; Garcia-Perez, M. Challenges and Opportunities for Bio-oil Refining: A Review. Energy Fuels 2019, 33, 4683–4720. [Google Scholar] [CrossRef]
- Feng, S.; Cheng, S.; Yuan, Z.; Leitch, M.; Xu, C. Valorization of bark for chemicals and materials: A review. Sustain. Energy Rev. 2013, 26, 560–578. [Google Scholar] [CrossRef]
- Ge, S.B.; Shi, Y.; Xia, C.L.; Huang, Z.H.; Manzo, M.; Cai, L.P.; Ma, H.Z.; Zhang, S.; Jiang, J.C.; Sonne, C.; et al. Progress in pyrolysis conversion of waste into value-added liquid pyro-oil, with focus on heating source and machine learning analysis. Energy Convers. Manag. 2021, 245, 114638. [Google Scholar] [CrossRef]
- Ge, S.B.; Manigandan, S.; Mathimani, T.; Basha, S.; Xia, C.L.; Brindhadevi, K.; Unpaprom, Y.; Whangchai, K.; Pugazhendhi, A. An assessment of agricultural waste cellulosic biofuel for improved combustion and emission characteristics. Sci. Total Environ. 2022, 813, 152418. [Google Scholar] [CrossRef]
- Hemalatha, K.; Madhumitha, G.; Kajbafvala, A.; Anupama, N.; Sompalle, R.; Roopan, S.M. Function of Nanocatalyst in Chemistry of Organic Compounds Revolution: An Overview. J. Nanomater. 2013, 2013, 4. [Google Scholar] [CrossRef]
- Ge, S.B.; Brindhadevi, K.; Xia, C.L.; Khalifa, A.S.; Elfasakhany, A.; Unpaprom, Y.; Whangchai, K. Performance, combustion and emission characteristics of the CI engine fueled with Botryococcus braunii microalgae with addition of TiO2 nanoparticle. Fuel 2022, 317, 121898. [Google Scholar] [CrossRef]
- Singh, N.; Dhanya, B.S.; Verma, M.L. Nano-Immobilized Biocatalysts and Their Potential Biotechnological Applications in Bioenergy Production. Mater. Sci. Energy Technol. 2020, 3, 808–824. [Google Scholar] [CrossRef]
- Hosseini, S.A. Nanocatalysts for biodiesel production. Arab. J. Chem. 2022, 15, 104152. [Google Scholar] [CrossRef]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Jafarzadeh, H.; Karaman, C.; Gungor, A.; Karaman, O.; Show, P.L.; Sami, P.; Mehrizi, A.A. Hydrogen production via sodium borohydride hydrolysis catalyzed by cobalt ferrite anchored nitrogen-and sulfur co-doped graphene hybrid nanocatalyst: Artificial neural network modeling approach. Chem. Eng. Res. Des. 2022, 183, 557–566. [Google Scholar] [CrossRef]
- Kucek, K.T.; Cesar-Oliveira, M.A.F.; Wilhelm, H.M.; Ramos, L.P. Ethanolysis of refined soybean oil assisted by sodium and potassium hydroxides. J. Am. Oil Chem. Soc. 2007, 84, 385–392. [Google Scholar] [CrossRef]
- Yigezu, Z.D.; Muthukumar, K. Catalytic Cracking of Vegetable Oil with Metal Oxides for Biofuel Production. Energy Convers. Manag. 2014, 84, 326–333. [Google Scholar] [CrossRef]
- Arya, I.; Poona, A.; Dikshit, P.K.; Pandit, S.; Kumar, J.; Singh, H.N.; Jha, N.K.; Rudayni, H.A.; Chaudhary, A.A.; Kumar, S. Current Trends and Future Prospects of Nanotechnology in Biofuel Production. Catalysts 2021, 11, 1308. [Google Scholar] [CrossRef]
- Shannon, M.; Lafeuille, J.L.; Fregiere-Salmomon, A.; Lefevre, S.; Galvin-King, P.; Haughey, S.A.; Burns, D.T.; Shen, X.Q.; Kapil, A.; McGrath, T.F.; et al. The detection and determination of adulterants in turmeric using fourier-transform infrared (FTIR) spectroscopy coupled to chemometric analysis and micro-FTIR imaging. Food Control 2022, 139, 109093. [Google Scholar] [CrossRef]
- Lourencon, T.V.; Hansel, F.A.; da Silva, T.A.; Ramos, L.P.; de Muniz, G.I.B.; Magalhaes, W.L.E. Hardwood and softwood kraft lignins fractionation by simple sequential acid precipitation. Sep. Purif. Technol. 2015, 154, 82–88. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Z.; Li, H.; He, F. Low temperature pyrolysis characteristics of major components of biomass. J. Fuel Chem. Technol. 2009, 37, 427–432. [Google Scholar] [CrossRef]
- Yao, X.; Xu, K. Pyrolysis characteristics of corn cob and release rule of gas products. Trans. Chin. Soc. Agric. Eng. 2015, 31, 275–282. [Google Scholar]
- Li, Y.; Bi, H.; Zheng, D.; Zhao, Y.; Wang, Y.; Gu, H.; Chen, X.; Liu, Z. Medical molecules of waste chinese buckeye seed from pharmacy. Therm. Sci. 2020, 24, 1681–1687. [Google Scholar] [CrossRef]
- Seo, M.J.; Shin, K.C.; Oh, D.K. Production of 5,8-dihydroxy-9,12(Z,Z)-octadecadienoic acid from linoleic acid by whole recombinant Escherichia coli cells expressing diol synthase from Aspergillus nidulans. Appl. Microbiol. Biotechnol. 2014, 98, 7447–7456. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; Seo, M.J.; Shin, K.C.; Oh, D.K. Production of 8-hydroxy-9,12(Z,Z)-octadecadienoic acid from linoleic acid by recombinant cells expressing H1004A-C1006S variant of Aspergillus nidulans diol synthase. J. Mol. Catal. B Enzym. 2015, 115, 35–42. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Q.; Hu, J.; Han, X.; Li, W.; Zhao, L. Maltol, a Food Flavoring Agent, Attenuates Acute Alcohol-Induced Oxidative Damage in Mice. Nutrients 2015, 7, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ma, Y.; Wang, L.; Zhang, Y.; Zhou, J. Multispectroscopic studies on the interaction of maltol, a food additive, with bovine serum albumin. Food Chem. 2012, 133, 264–270. [Google Scholar] [CrossRef]
- Kandioller, W.; Hartinger, C.G.; Nazarov, A.A.; Bartel, C.; Skocic, M.; Jakupec, M.A.; Arion, V.B.; Keppler, B.K. Maltol-Derived Ruthenium-Cymene Complexes with Tumor Inhibiting Properties: The Impact of Ligand-Metal Bond Stability on Anticancer Activity In Vitro. Chem. A Eur. J. 2009, 15, 12283–12291. [Google Scholar] [CrossRef]
- Gasche, C.; Ahmad, T.; Tulassay, Z.; Baumgart, D.C.; Bokemeyer, B.; Buening, C.; Howaldt, S.; Stallmach, A.; Grp, A.S. Ferric Maltol Is Effective in Correcting Iron Deficiency Anemia in Patients with Inflammatory Bowel Disease: Results from a Phase-3 Clinical Trial Program. Inflamm. Bowel Dis. 2015, 21, 579–588. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-Inflammatory Property of n-Hexadecanoic Acid: Structural Evidence and Kinetic Assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef]
- Chellappan, S.R.; Kandhan, V.; Chandrasekaran, T.; Abubakker, A.; Appakan, S. Rhizogenesis in Cell Suspension Culture from Mango Ginger: A Source of Isosorbide and n-Hexadecanoic Acid. Adv. Bot. 2015, 2015, 1–7. [Google Scholar]
- Chatzivasiloglou, E.; Bernard, M.C.; Tsoukleris, D.S.; Falaras, P.; Kontos, A.I.; Belessi, V.; Arabatzis, I.M. 2-Ethyl-1-hexanol based screen-printed titania thin films for dye-sensitized solar cells. Sol. Energy 2005, 79, 236–240. [Google Scholar]
- Huth, H.; Wang, L.M.; Schick, C.; Richert, R. Comparing calorimetric and dielectric polarization modes in viscous 2-ethyl-1-hexanol. J. Chem. Phys. 2007, 126, 104503. [Google Scholar] [CrossRef] [PubMed]
- Ghaziaskar, H.S.; Eskandari, H.; Daneshfar, A. Solubility of 2-ethyl-1-hexanol, 2-ethylhexanoic acid, and their mixtures in supercritical carbon dioxide. J. Chem. Eng. Data 2003, 48, 236–240. [Google Scholar] [CrossRef]
- Wei, D.; Wang, M.; Jiang, B.; Shi, J.; Hao, J. Role of dihydroxyacetone kinases I and II in the dha regulon of Klebsiella pneumoniae. J. Biotechnol. 2014, 177, 13–19. [Google Scholar] [CrossRef]
- Wang, L.; Chauliac, D.; Rhee, M.S.; Panneerselvam, A.; Ingram, L.O.; Shanmugam, K.T. Fermentation of dihydroxyacetone by engineered Escherichia coli and Klebsiella variicola to products. Proc. Natl. Acad. Sci. USA 2018, 115, 4381–4386. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.C.; Kochevar, I.E. Factors influencing sunless tanning with dihydroxyacetone. Br. J. Dermatol. 2003, 149, 332–340. [Google Scholar] [CrossRef]
- El-Ansary, A.; Ben Bacha, A.; Bjorklund, G.; Al-Orf, N.; Bhat, R.S.; Moubayed, N.; Abed, K. Probiotic treatment reduces the autistic-like excitation/inhibition imbalance in juvenile hamsters induced by orally administered propionic acid and clindamycin. Metab. Brain Dis. 2018, 33, 1155–1164. [Google Scholar] [CrossRef]
- Khamash, D.F.; Voskertchian, A.; Tamma, P.D.; Akinboyo, I.C.; Carroll, K.C.; Milstone, A.M. Increasing Clindamycin and Trimethoprim-Sulfamethoxazole Resistance in Pediatric Staphylococcus aureus Infections. J. Pediatr. Infect. Dis. Soc. 2019, 8, 351–353. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Asadbegi, M.; Yaghmaei, P.; Salehi, I.; Komaki, A.; Ebrahim-Habibi, A. Investigation of thymol effect on learning and memory impairment induced by intrahippocampal injection of amyloid beta peptide in high fat diet- fed rats. Metab. Brain Dis. 2017, 32, 827–839. [Google Scholar] [CrossRef]
- Hu, X.; Shi, Y.; Zhang, P.; Miao, M.; Zhang, T.; Jiang, B. d-Mannose: Properties, Production, and Applications: An Overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 773–785. [Google Scholar] [CrossRef]
- Duan, W.; Ren, J.; Li, Y.; Liu, T.; Song, X.; Chen, Z.; Huang, Z.; Hou, X.; Li, Y. Conservation and Expression Patterns Divergence of Ascorbic Acid D-mannose/L-galactose Pathway Genes in Brassica rapa. J. Front Plant Sci. 2016, 7, 778. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Luo, W.; Liu, L.; Pang, X.; Zhu, H.; Liu, A.; Lu, J.; Ma, D.L.; Leung, C.H.; Wang, Y.; et al. Isocryptotanshinone, a STAT3 inhibitor, induces apoptosis and pro-death autophagy in A549 lung cancer cells. J. Drug Target. 2016, 24, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lee, J.; Hernandez, M.A.S.; Mazitschek, R.; Ozcan, U. Treatment of Obesity with Celastrol. Cell 2015, 161, 999–1011. [Google Scholar] [CrossRef]
- Lee, H.W.; Jang, K.S.B.; Choi, H.J.; Jo, A.; Cheong, J.H.; Chun, K.H. Celastrol inhibits gastric cancer growth by induction of apoptosis and autophagy. J. BMB Rep. 2014, 47, 697–702. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Yang, X.; Wu, J. Synergistic Effect of Celastrol on Palmitic Acid induced Cardiomyocyte Apoptosis. J. Hubei Univ. Sci. Technol. 2018, 47, 93–97. [Google Scholar]
- Jeon, Y.J.; Kim, B.H.; Kim, S.; Oh, I.; Lee, S.; Shin, J.; Kim, T.Y. Rhododendrin ameliorates skin inflammation through inhibition of NF-κB, MAPK, and PI3K/Akt signaling. Eur. J. Pharmacol. 2013, 714, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Iwadate, T.; Kashiwakura, Y.; Masuoka, N.; Yamada, Y.; Nihei, K.I. Chemical synthesis and tyrosinase inhibitory activity of rhododendrol glycosides. J. Bioorganic Med. Chem. Lett. 2014, 24, 122–125. [Google Scholar] [CrossRef]
- Migas, P.; Krauze-Baranowska, M. The significance of arbutin and its derivatives in therapy and cosmetics. J. Phytochem Lett. 2015, 13, 35–40. [Google Scholar] [CrossRef]
- Jeon, J.S.; Kim, B.H.; Lee, S.H.; Kwon, H.J.; Bae, H.J.; Kim, S.K.; Park, J.A.; Shim, J.H.; Abd El-Aty, A.M.; Shin, H.C. Simultaneous determination of arbutin and its decomposed product hydroquinone in whitening creams using high-performance liquid chromatography with photodiode array detection: Effect of temperature and pH on decomposition. Int. J. Cosmet. Sci. 2015, 37, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Luo, W.; Zhao, W.; Lu, J.; Chen, X. Isocryptotanshinone Induced Apoptosis and Activated MAPK Signaling in Human Breast Cancer MCF-7 Cells. J. Breast Cancer 2015, 18, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Mathi, P.; Veeramachaneni, G.K.; Raj, K.K.; Talluri, V.R.; Bokka, V.R.; Botlagunta, M. In vitro and in silico characterization of angiogenic inhibitors from Sophora interrupta. J. Mol. Model. 2016, 22, 247. [Google Scholar] [CrossRef]
- Fu, X.; Zhong, Z.; Xiao, G.; Li, R. Comparative study on pyrolysis characteristics and dynamics of grass biomass. Trans. Chin. Soc. Agric. Eng. 2009, 25, 199–202. [Google Scholar]
- Kristanto, J.; Azis, M.M.; Purwono, S. Multi-distribution activation energy model on slow pyrolysis of cellulose and lignin in TGA/DSC. Heliyon 2021, 7, e07669. [Google Scholar] [CrossRef] [PubMed]
- Rami, J.M.; Patel, C.D.; Patel, C.M.; Patel, M.V. Thermogravimetric analysis (TGA) of some synthesized metal oxide nanoparticles. Mater. Today 2021, 43, 655–659. [Google Scholar] [CrossRef]
- Farrow, S.T.; Sun, C.G.; Liu, H.; Manquais, K.L.; Snape, C.E. Comparative study of the inherent combustion reactivity of sawdust chars produced by TGA and in the drop tube furnace. Fuel Process. Technol. 2020, 201, 106361. [Google Scholar] [CrossRef]
- Anca-Couce, A.; Tsekos, C.; Retschitzegger, S.; Zimbardi, F.; Funke, A.; Banks, S.; Kraia, T.; Marques, P.; Scharler, R.; de Jong, W.; et al. Biomass pyrolysis TGA assessment with an international round robin. Fuel 2020, 276, 118002. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Jing, X.; Gao, J.; Li, C.; Ma, Z. An approach for upgrading biomass and pyrolysis product quality using a combination of aqueous phase bio-oil washing and torrefaction pretreatment. Bioresour. Technol. 2017, 233, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Liu, Z.; Yan, D.; Peng, W.; Baghban, A. Application of LSSVM algorithm for estimating higher heating value of biomass based on ultimate analysis. Energy Sources Part A 2018, 40, 709–715. [Google Scholar] [CrossRef]
- Li, Y.Y.; Chen, J.; Yang, Y.; Li, C.; Peng, W. Molecular characteristics of volatile components from willow bark. J. King Saud Univ. Sci. 2020, 32, 1932–1936. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, J.; Zhang, Q. Effects of Torrefaction on the Pyrolysis Behavior and Bio-Oil Properties of Rice Husk by Using TG-FTIR and Py-GC/MS. Energy Fuels 2014, 28, 5857–5863. [Google Scholar] [CrossRef]
- Xu, G.; Wang, L.; Liu, J.; Wu, J. FTIR and XPS analysis of the changes in bamboo chemical structure decayed by white-rot and brown-rot fungi. Appl. Surf. Sci. 2013, 280, 799–805. [Google Scholar] [CrossRef]
- Wen, J.L.; Sun, S.L.; Yuan, T.Q.; Xu, F.; Sun, R.C. Understanding the chemical and structural transformations of lignin macromolecule during torrefaction. Appl. Energy 2014, 121, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Q.Y.; Liu, D.J.; Yang, S.H.; Hu, L.Y.; Gu, Y.H.; Shi, H.; Qiao, J.D. Electrocatalytic oxygen evolution of ultrafine nano-Co3O4 coupled with N-rich carbon composites. J. Fuel Chem. Technol. 2022, 50, 904–911. [Google Scholar] [CrossRef]
- Bharath, G.; Rambabu, K.; Hai, A.; Othman, L.; Ponpandian, N.; Banat, F.; Show, P.L. Hybrid Pd50-Ru50/MXene (Ti3C2Tx) nanocatalyst for effective hydrogenation of CO2 to methanol toward climate change control. Chem. Eng. J. 2021, 414, 128869. [Google Scholar] [CrossRef]
- Belles, L.; Moularas, C.; Smykała, S.; Deligiannakis, Y. Flame Spray Pyrolysis Co3O4/CoO as Highly-Efficient Nanocatalyst for Oxygen Reduction Reaction. Nanomaterials 2021, 11, 925. [Google Scholar] [CrossRef] [PubMed]
- Bharath, G.; Rambabu, K.; Hai, A.; Banat, F.; Taher, H.; Schmidt, J.E.; Show, P.L. Catalytic hydrodeoxygenation of biomass-derived pyrolysis oil over alloyed bimetallic Ni3Fe nanocatalyst for high-grade biofuel production. Energy Convers. Manag. 2020, 213, 112859. [Google Scholar] [CrossRef]
- Zeng, L.P.; Li, K.Z.; Huang, F.; Zhu, X.; Li, H.C. Effects of Co3O4 nanocatalyst morphology on CO oxidation: Synthesis process map and catalytic activity. Chin. J. Catal. 2016, 37, 908–922. [Google Scholar] [CrossRef]
- Bano, S.; Ganie, A.S.; Sultana, S.; Sabir, S.; Khan, M.Z. Fabrication and Optimization of Nanocatalyst for Biodiesel Production: An Overview. Front. Energy Res. 2020, 8, 579014. [Google Scholar] [CrossRef]
- Dawood, S.; Koyande, A.K.; Ahmad, M.; Mubashir, M.; Asif, S.; Klemeš, J.J.; Bokhari, A.; Saqib, S.; Lee, M.; Qyyum, M.A. Show PL. Synthesis of biodiesel from non-edible (Brachychiton populneus) oil in the presence of nickel oxide nanocatalyst: Parametric and optimisation studies. Chemosphere 2021, 278, 130469. [Google Scholar] [CrossRef]
- Zhou, L.; Cao, S.; Zhang, L.; Xiang, G.; Wang, J.; Zeng, X.; Chen, J. Facet effect of Co3O4 nanocatalysts on the catalytic decomposition of ammonium perchlorate. J. Hazard. Mater. 2020, 392, 122358. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Ross, J.R.H. Catalysis for conversion of biomass to fuels via pyrolysis and gasification: A review. Catal. Today 2011, 17, 1–13. [Google Scholar] [CrossRef]
- Zou, L.; Xu, Q. Synthesis of a Hierarchically Porous C/Co3O4 Nanostructure with Boron Doping for Oxygen Evolution Reaction. Chem. Asian J. 2020, 15, 490–493. [Google Scholar] [CrossRef] [PubMed]

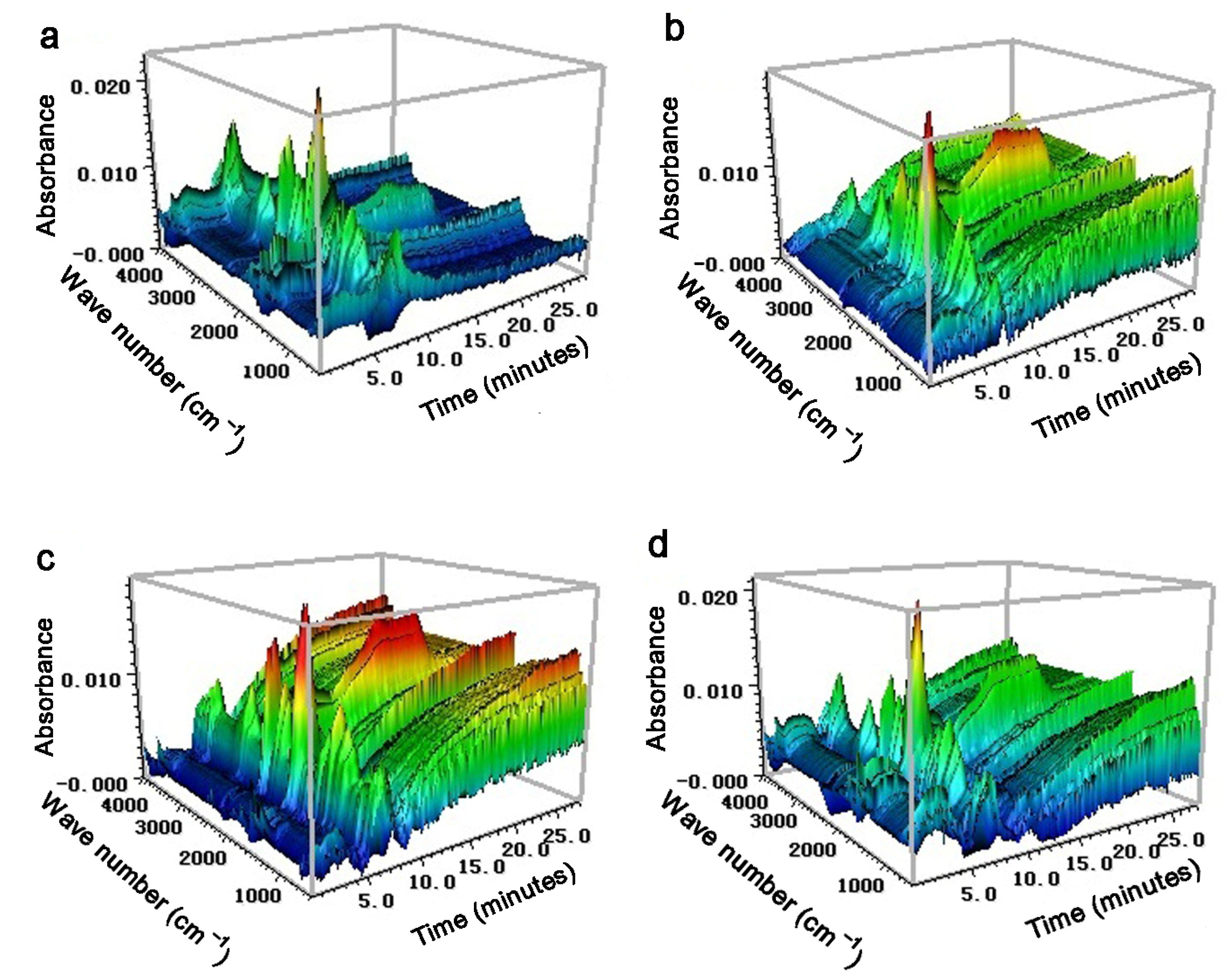
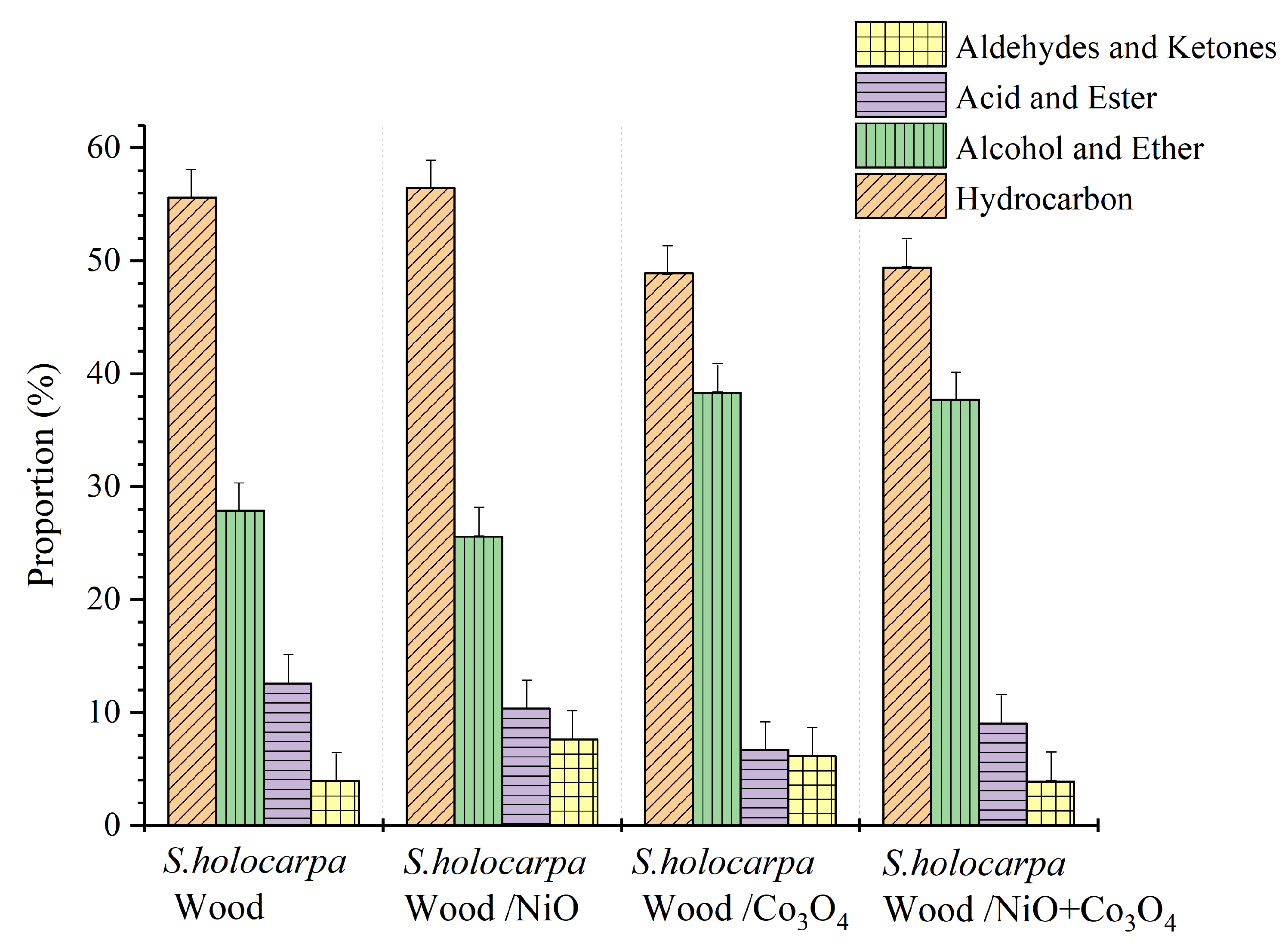
| (a) Label | Extractive Solvents |
| S. holocarpa (methanol) | Pure methanol |
| S. holocarpa (benzene/ethanol) | Mixture of benzene and ethanol in 1:1 ratio |
| S. holocarpa (ethanol/methanol) | Mixture of methanol and ethanol in 1:1 ratio |
| (b) Label | Type of Nanocatalyst Treatments |
| S. holocarpa Wood | S. holocarpa Wood (5 g) |
| S. holocarpa Wood/NiO | S. holocarpa Wood (5 g)+NiO (0.05 g) |
| S. holocarpa Wood/Co3O4 | S. holocarpa Wood (5 g)+Co3O4 (0.05 g) |
| S. holocarpa Wood/NiO+Co3O4 | S. holocarpa Wood (5 g)+NiO (0.025 g)+Co3O4 (0.025 g) |
| Frequency Range (cm−1) | Frequency (cm−1) | Functional Group | Classification of Compounds | ||
|---|---|---|---|---|---|
| Methanol Extract | Benzene/Ethanol Extract | Methanol/Ethanol Extract | |||
| (1) 3500–3000 | 3440 | 3444 | 3416 | O–H stretching | Alcohol, carboxylic acids |
| (2) 3000–2800 | 2970 | 2976 | 2978 | C–H stretching | Alkane |
| (3) 1680–1610 | 1641 | 1640 | 1644 | C=C stretching | Alkenes |
| (4) 1470–1340 | – | 1454, 1391 | 1449, 1398 | C–H bending | Alkanes |
| (5) 1200–1000 | 1053, 1034, 1016 | 1042 | 1081, 1044 | C–O stretching | Alcohol, ether, carboxylic acids |
| (6) 900–690 | – | 874 | 878 | C–H out of plane bending | Aromatic rings |
| No. | Rt (min) | Measured m/z | Identified Compound | Chemical Structure | Type of Compound | Molecular Formula | References |
|---|---|---|---|---|---|---|---|
| 1 | 12.30 | 296.14 | Isocryptotan– shinone | 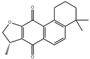 | Ketones | C19H20O3 | [62] |
| 2 | 12.50 | 550.31 | Celastrol | 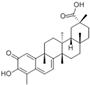 | Alcohols | C30H46O9 | [63,64,65] |
| 3 | 13.10 | 328.15 | Rhododendrin | 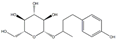 | Organic compounds | C16H24O7 | [66,67] |
| 4 | 13.10 | 272.09 | Arbutin | 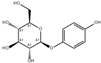 | Glycosides | C12H16O7 | [68,69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, G.; Yang, Y.; Chen, X.; Peng, W.; Li, H. Incorporation of Nanocatalysts for the Production of Bio-Oil from Staphylea holocarpa Wood. Polymers 2022, 14, 4385. https://doi.org/10.3390/polym14204385
Li Y, Li G, Yang Y, Chen X, Peng W, Li H. Incorporation of Nanocatalysts for the Production of Bio-Oil from Staphylea holocarpa Wood. Polymers. 2022; 14(20):4385. https://doi.org/10.3390/polym14204385
Chicago/Turabian StyleLi, Yiyang, Guanyan Li, Yafeng Yang, Xiangmeng Chen, Wanxi Peng, and Hanyin Li. 2022. "Incorporation of Nanocatalysts for the Production of Bio-Oil from Staphylea holocarpa Wood" Polymers 14, no. 20: 4385. https://doi.org/10.3390/polym14204385
APA StyleLi, Y., Li, G., Yang, Y., Chen, X., Peng, W., & Li, H. (2022). Incorporation of Nanocatalysts for the Production of Bio-Oil from Staphylea holocarpa Wood. Polymers, 14(20), 4385. https://doi.org/10.3390/polym14204385








