Can Supramolecular Polymers Become Another Material Choice for Polymer Flooding to Enhance Oil Recovery?
Abstract
:1. Introduction
2. Why Choosing Supramolecular Polymer as the New Material for Enhanced Oil Recovery?
2.1. Conventional Polymers Utilized for EOR
2.2. Limitations of Traditional Polymers in EOR
RG = 0.619{M × [ƞ])/1024}1/3
rH/RG > 5
2.3. Supramolecular Polymer: Infinite Network based on Non-Covalent Action
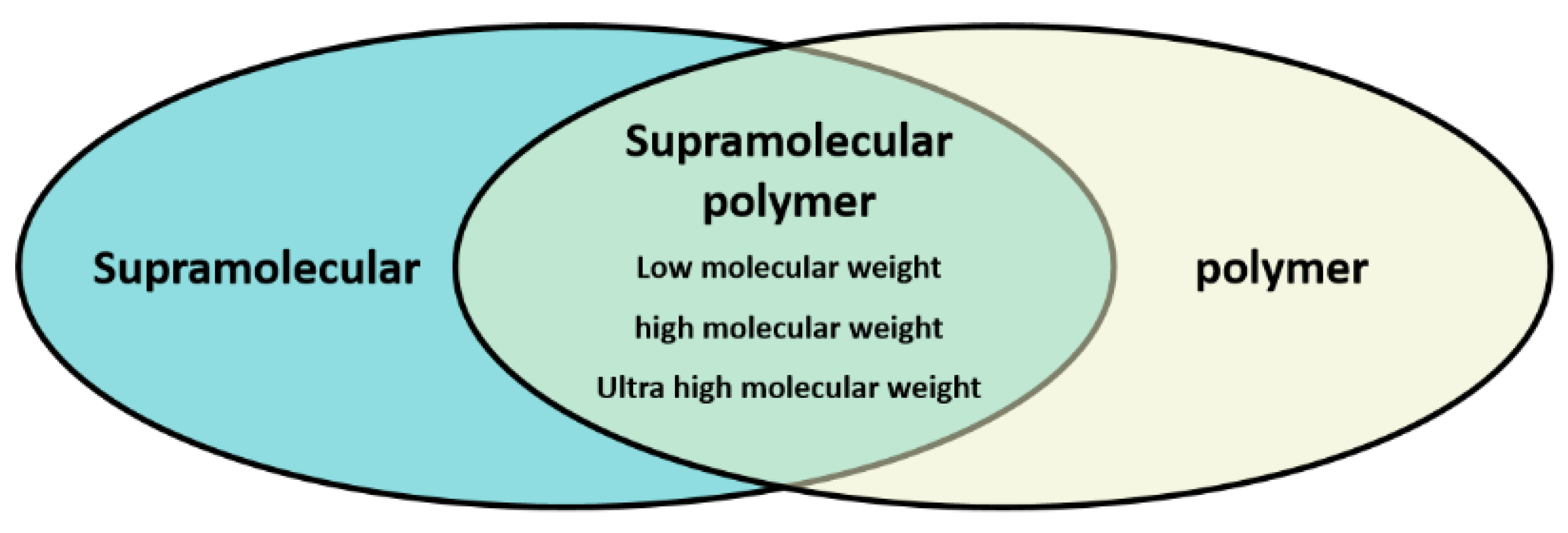
2.4. Differences between “Supramolecular Weight Polymers” and High Molecular Weight Polymers
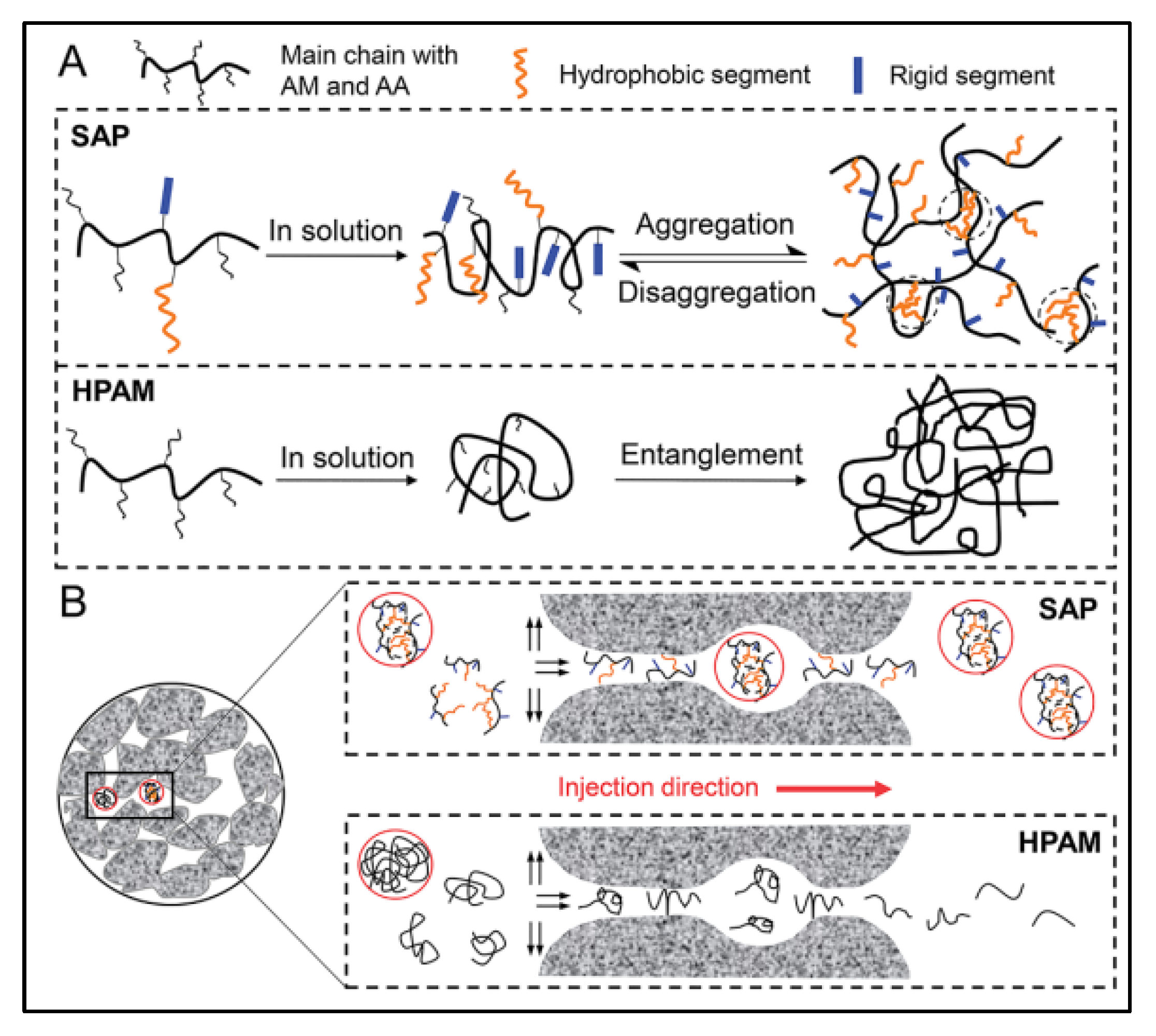
2.5. Major Superiority of Supramolecular Polymer Flooding in Enhanced Oil Recovery
3. How to Fabricate Supramolecular Polymers?
3.1. Synthesis of Supramolecular Polymers Suitable for EOR
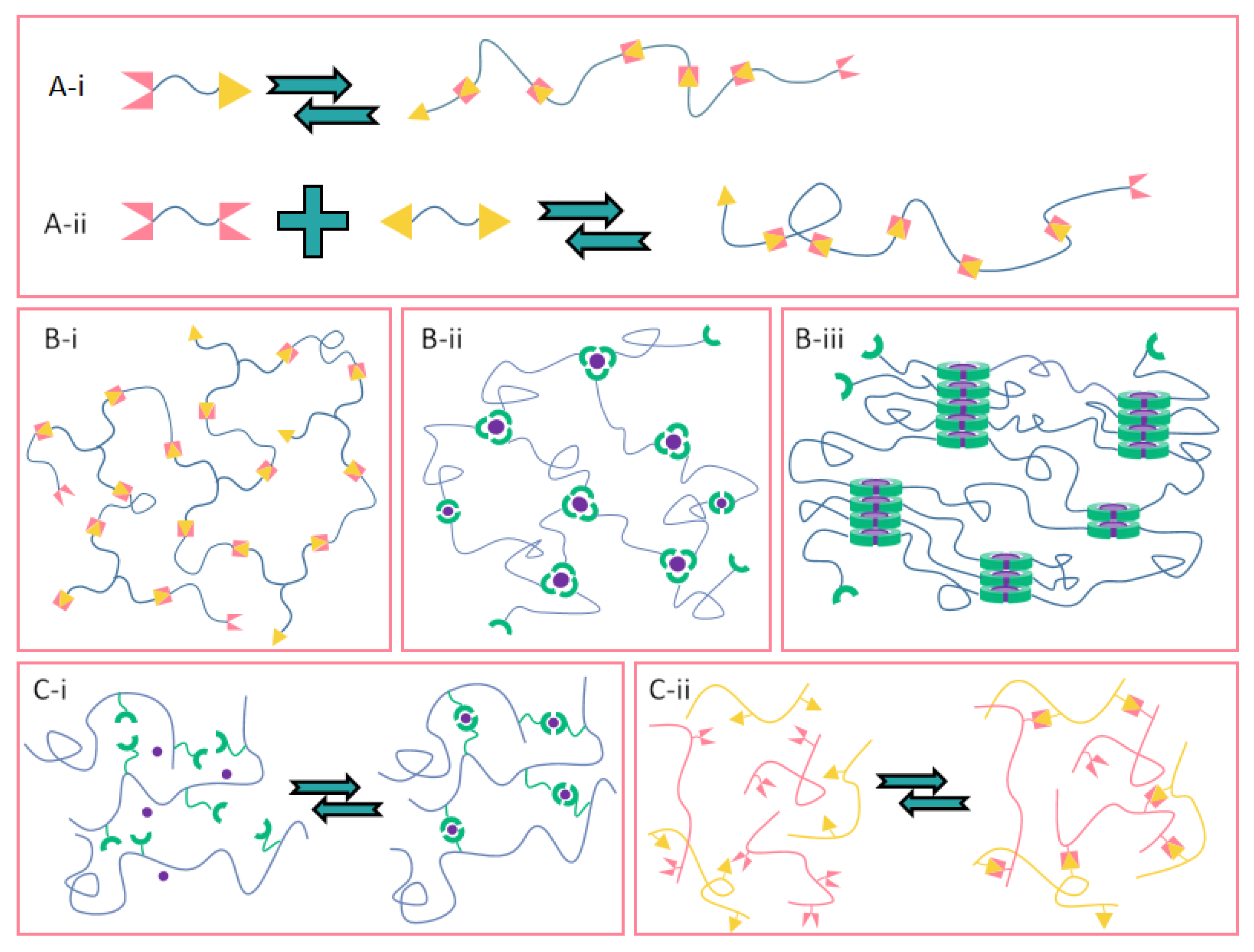
3.1.1. Types of Non-Covalent Interactions for the Construction of Supramolecular Polymers
3.1.2. Selection of Precursors for Supramolecular Polymers
3.1.3. Design Strategy of Supramolecular Polymer Network
3.2. Polymerization Techniques in Oil Field Chemistry
3.2.1. Free Radical Polymerization (FRP)
3.2.2. Reversible Deactivation Radical Polymerization (RDRP)
3.2.3. Post-Polymerization Modification
4. Characterization Methods of Supramolecular Polymers
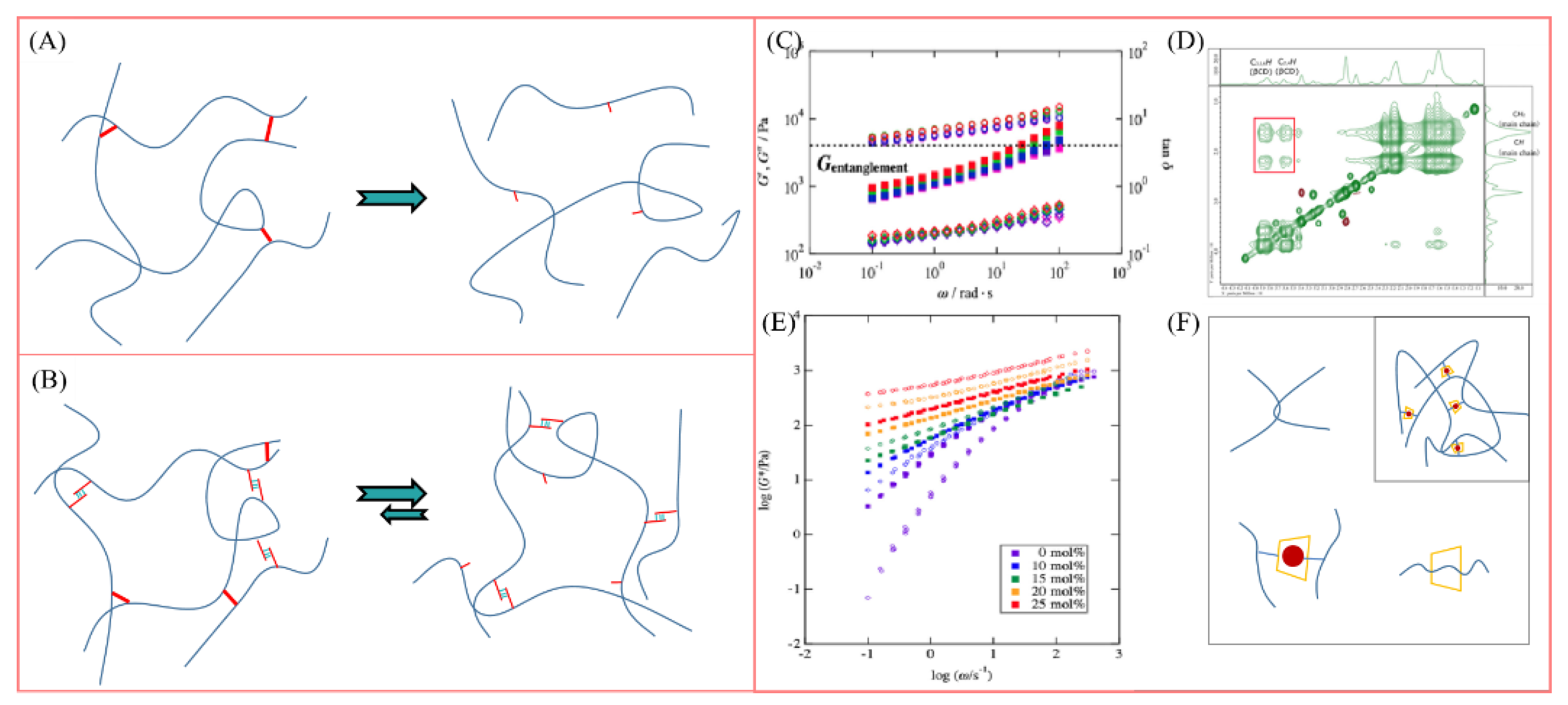
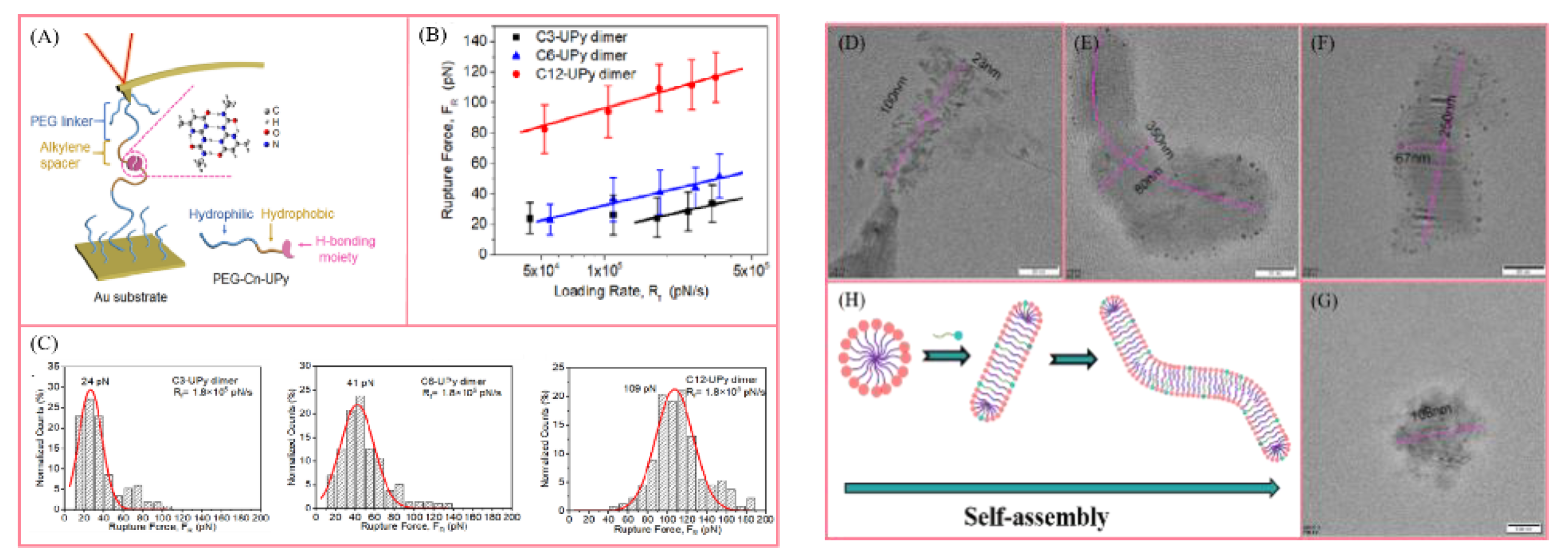
4.1. Rheological Characterization
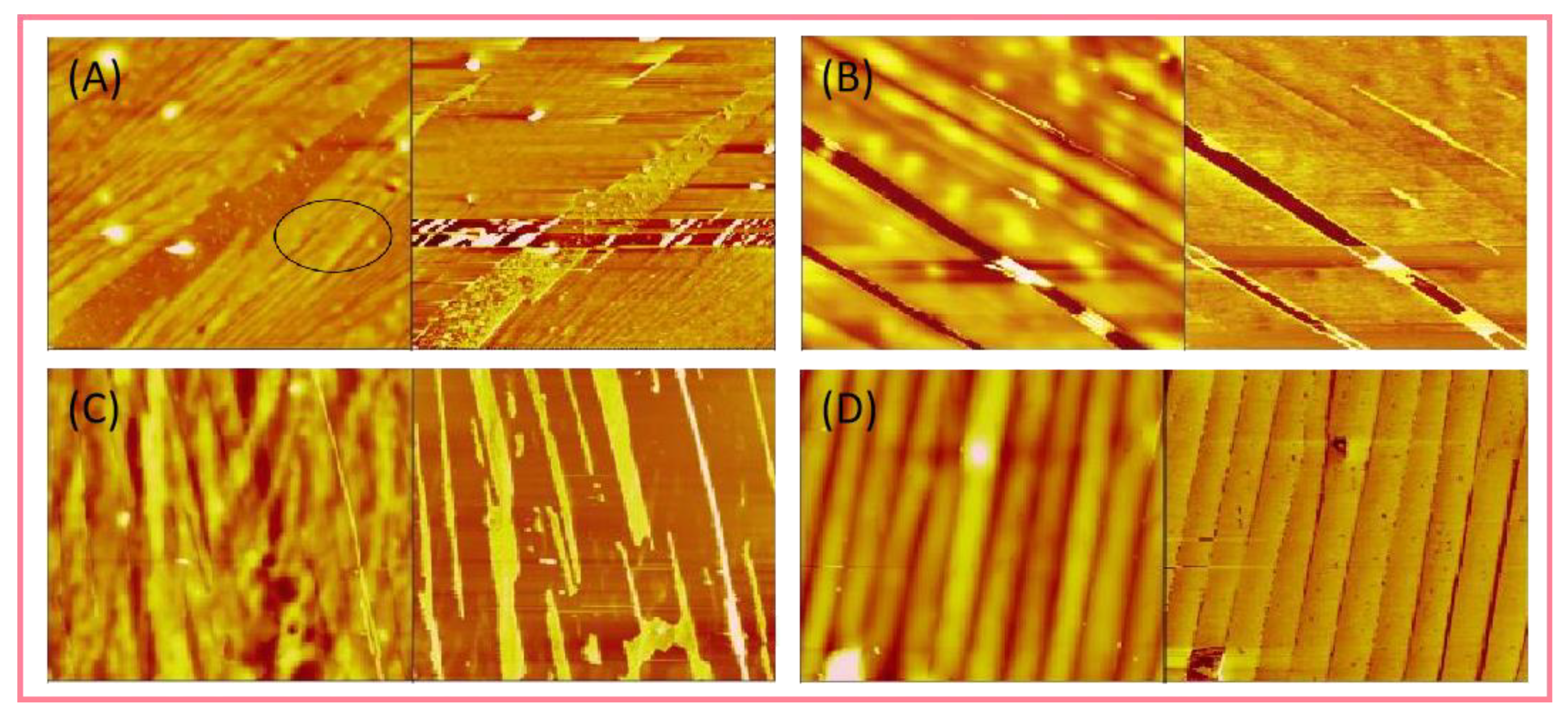
4.2. Structural Characterization of Supramolecular Polymers
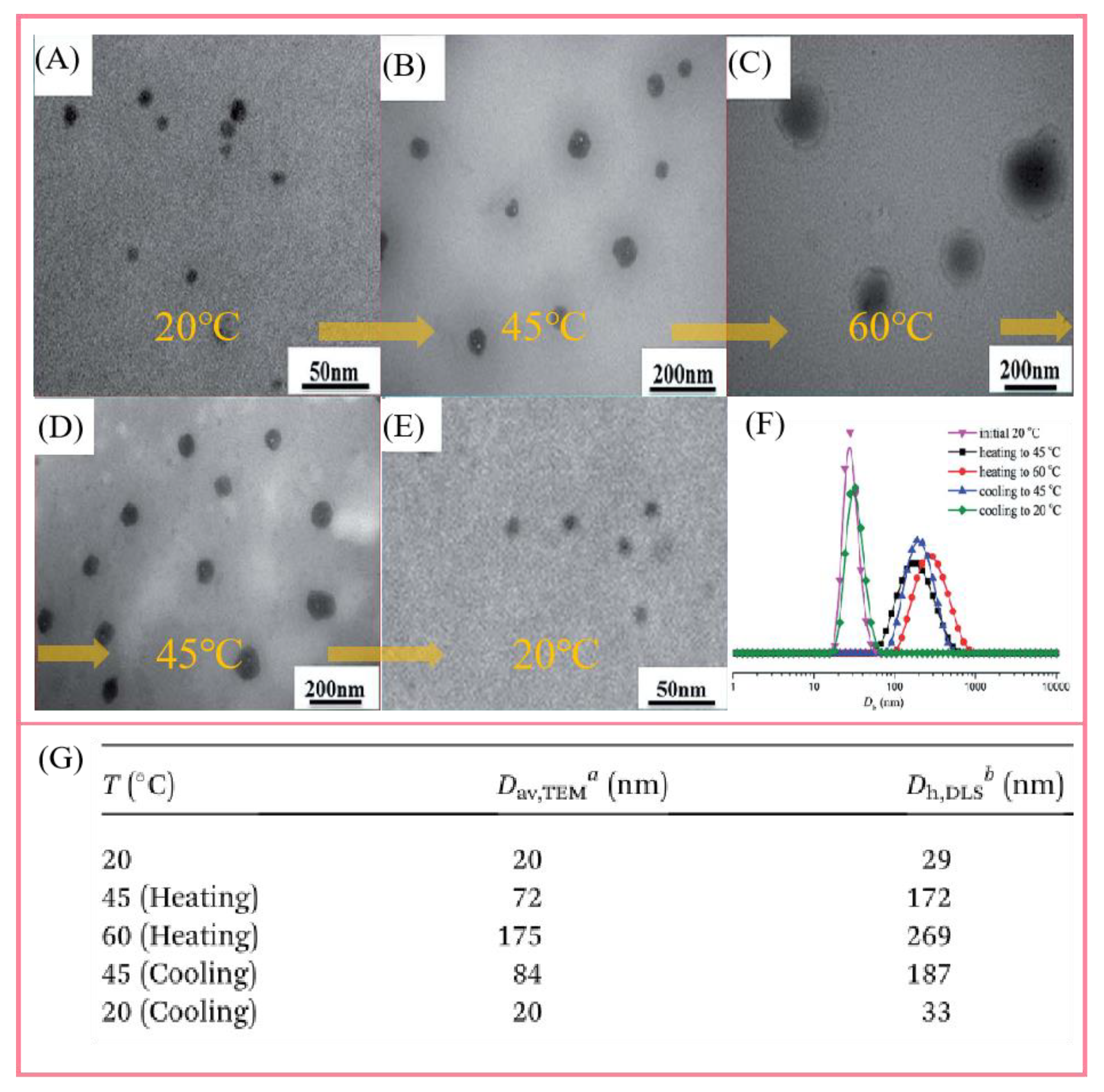
4.3. Study on the Mechanism of Supramolecular Self-Assembly
4.4. Summary
5. Application Progress of Supramolecular Polymers in EOR
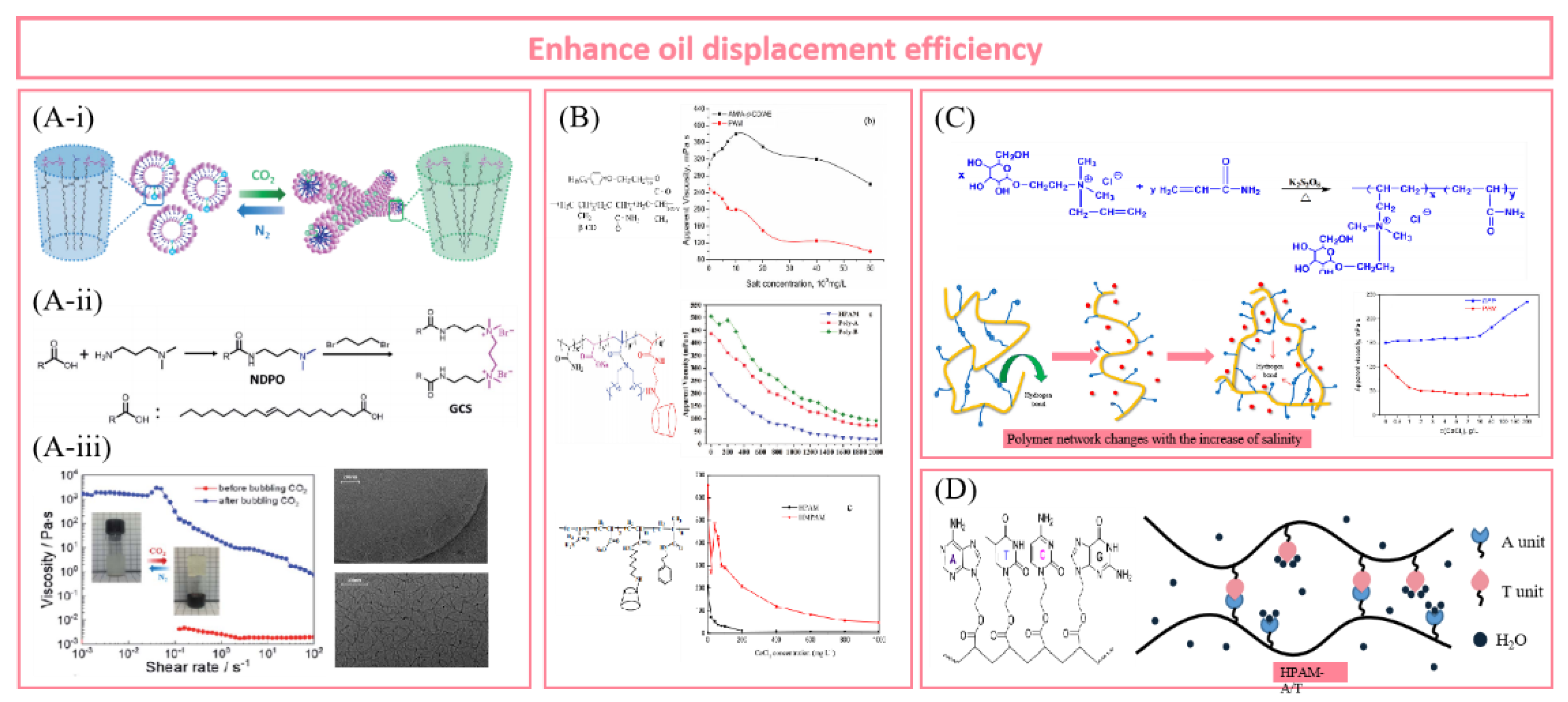
5.1. Enhance Oil Displacement Efficiency
5.2. Auxiliary Gas Drive
6. Challenges and Future Directions
6.1. Comprehensive Comparison between Conventional Polymer Materials and Supramolecular Polymers
6.2. Challenges Caused by Supramolecular Polymers in EOR
6.2.1. The Contradiction between Conventional Characterization Strategies and Supramolecular Polymers in Oilfield Chemistry
6.2.2. Precise Control of Structure and Performance
6.2.3. The Application of Supramolecular Polymer in Oil Field Is Limited
6.3. Future Directions
6.3.1. Combination of Covalent and Supramolecular Polymers
6.3.2. Development of Related Synthetic Processes and Characterization Techniques
6.3.3. Extending Complex Polymer Network
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| CEOR | chemical-enhanced oil recovery |
| WMM-100 | a cationic copper phthalide supramolecule |
| PAM | polyacrylamide |
| HPAM | partially hydrolyzed polyacrylamide |
| AMPS | 2-acrylamido-2-methyl-1-propane sulfonic acid |
| NVP | N-vinyl pyrrolidone |
| SAP | self-adaptive polymer |
| β-CD | β-cyclodextrin |
| PEG | polyethylene glycol |
| PAAS | sodium polyacrylate |
| SDS | sodium dodecyl sulfate |
| PPSA | a novel tetra-polymer |
| ANS | 1-anilinonaphthalene-8-sulfonic acid |
| PNIPAM– (2CD–2MPEG) | poly(N-isopropylacrylamide) with two hydrophilic methoxypolyethylene glycol and two β-CD |
| AM-co-A-β-cd-co-AE | poly(acrylamide-co-allyl-β-CD-co-methacrylic acid octylphenols poly(ethylene oxide) ester) |
| WAG | water-alternating-gas |
| LCST | lower critical solution temperature |
| IPN-PAASP | interpenetrating networks |
References
- Tavakkoli, O.; Kamyab, H.; Shariati, M.; Mustafa Mohamed, A.; Junin, R. Effect of nanoparticles on the performance of polymer/surfactant flooding for enhanced oil recovery: A review. Fuel 2022, 312, 122867. [Google Scholar] [CrossRef]
- Scott, A.J.; Romero-Zerón, L.; Penlidis, A. Evaluation of Polymeric Materials for Chemical Enhanced Oil Recovery. Processes 2020, 8, 361. [Google Scholar] [CrossRef] [Green Version]
- Gbadamosi, A.; Patil, S.; Kamal, M.S.; Adewunmi, A.A.; Yusuff, A.S.; Agi, A.; Oseh, J. Application of Polymers for Chemical Enhanced Oil Recovery: A Review. Polymers 2022, 14, 1433. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, J. New insights in the study of pyrene excimer fluorescence to characterize macromolecules and their supramolecular assemblies in solution. Langmuir 2012, 28, 6527–6538. [Google Scholar] [CrossRef]
- Atta, D.Y.; Negash, B.M.; Yekeen, N.; Habte, A.D. A state-of-the-art review on the application of natural surfactants in enhanced oil recovery. J. Mol. Liq. 2021, 321, 24. [Google Scholar] [CrossRef]
- Pogaku, R.; Mohd Fuat, N.H.; Sakar, S.; Cha, Z.W.; Musa, N.; Awang Tajudin, D.N.A.; Morris, L.O. Polymer flooding and its combinations with other chemical injection methods in enhanced oil recovery. Polym. Bull. 2017, 75, 1753–1774. [Google Scholar] [CrossRef]
- Lashari, N.; Ganat, T. Emerging applications of nanomaterials in chemical enhanced oil recovery: Progress and perspective. Chin. J. Chem. Eng. 2020, 28, 1995–2009. [Google Scholar] [CrossRef]
- Tackie-Otoo, B.N.; Ayoub Mohammed, M.A.; Yekeen, N.; Negash, B.M. Alternative chemical agents for alkalis, surfactants and polymers for enhanced oil recovery: Research trend and prospects. J. Pet. Sci. Eng. 2020, 187, 106828. [Google Scholar] [CrossRef]
- Rellegadla, S.; Prajapat, G.; Agrawal, A. Polymers for enhanced oil recovery: Fundamentals and selection criteria. Appl. Microbiol. Biotechnol. 2017, 101, 4387–4402. [Google Scholar] [CrossRef]
- Wei, B.; Romero-Zeron, L.; Rodrigue, D. Oil displacement mechanisms of viscoelastic polymers in enhanced oil recovery (EOR): A review. J. Pet. Explor. Prod. Technol. 2014, 4, 113–121. [Google Scholar] [CrossRef]
- Yan, Y.Q.; Wang, L.H.; Sang, G.Q.; Han, X. Influence of Polymer Viscoelasticity on Microscopic Remaining Oil Production. Polymers 2022, 14, 19. [Google Scholar] [CrossRef]
- Clarke, A.; Howe, A.M.; Mitchell, J.; Staniland, J.; Hawkes, L.A. How Viscoelastic-Polymer Flooding Enhances Displacement Efficiency. SPE J. 2016, 21, 675–687. [Google Scholar] [CrossRef]
- Sandengen, K.; Melhuus, K.; Kristoffersen, A. Polymer “viscoelastic effect”; does it reduce residual oil saturation. J. Pet. Sci. Eng. 2017, 153, 355–363. [Google Scholar] [CrossRef]
- Evani, S.; Rose, G.D. Hydrophobe Association Polymers. Abstr. Pap. Am. Chem. Soc. 1987, 194, 100. [Google Scholar]
- Zhu, Z.; Kou, H.Q. The effect of NaCl on the solution properties of a betaine-type amphiphilic polymer and its performance enhancement mechanism. Colloid Polym. Sci. 2022, 300, 69–81. [Google Scholar] [CrossRef]
- Yu, B.; Kang, W.L.; Sarsenbekuly, B.; Yang, R.M.; Fan, H.M.; Yin, X. Thickening behavior and synergistic mechanism of mixed system of two hydrophobically associating polymers. J. Dispers. Sci. Technol. 2017, 38, 1196–1203. [Google Scholar] [CrossRef]
- Al-Hajri, S.; Negash, B.M.; Rahman, M.M.; Haroun, M.; Al-Shami, T.M. Perspective Review of Polymers as Additives in Water-Based Fracturing Fluids. ACS Omega 2022, 7, 7431–7443. [Google Scholar] [CrossRef]
- Balaga, D.K.; Kulkarni, S.D. A review of synthetic polymers as filtration control additives for water-based drilling fluids for high-temperature applications. J. Pet. Sci. Eng. 2022, 215, 10. [Google Scholar] [CrossRef]
- Caili, D.; Qing, Y.; Fulin, Z. In-depth Profile Control Technologies in China-A Review of the State of the Art. Pet. Sci. Technol. 2010, 28, 1307–1315. [Google Scholar] [CrossRef]
- Zhang, H.L.; Yu, H.L.; Sun, C.L.; Tong, Y.B.; Deng, J.J.; Wu, L.M.; Sun, L.Q.; Guo, S.J.; Liu, H.S. Evaluation of new hydrophobic association inorganic composite material as coagulant for oilfield wastewater treatment. Sep. Purif. Technol. 2021, 275, 7. [Google Scholar] [CrossRef]
- Kamal, M.S.; Sultan, A.S.; Al-Mubaiyedh, U.A.; Hussein, I.A. Review on Polymer Flooding: Rheology, Adsorption, Stability, and Field Applications of Various Polymer Systems. Polym. Rev. 2015, 55, 491–530. [Google Scholar] [CrossRef]
- Wang, D.; Tong, G.; Dong, R.; Zhou, Y.; Shen, J.; Zhu, X. Self-assembly of supramolecularly engineered polymers and their biomedical applications. Chem. Commun. 2014, 50, 11994–12017. [Google Scholar] [CrossRef]
- Yang, L.; Tan, X.; Wang, Z.; Zhang, X. Supramolecular Polymers: Historical Development, Preparation, Characterization, and Functions. Chem. Rev. 2015, 115, 7196–7239. [Google Scholar] [CrossRef]
- Jangizehi, A.; Ahmadi, M.; Seiffert, S. Dynamics of supramolecular associative polymer networks at the interplay of chain entanglement, transient chain association, and chain-sticker clustering. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 1209–1223. [Google Scholar] [CrossRef]
- Katashima, T. Molecular Understanding of Viscoelasticity in Transient Polymer Networks Based on Multiple Methods. Nihon Reoroji Gakkaishi 2022, 50, 51–56. [Google Scholar] [CrossRef]
- Hendrikse, S.I.S.; Wijnands, S.P.W.; Lafleur, R.P.M.; Pouderoijen, M.J.; Janssen, H.M.; Dankers, P.Y.W.; Meijer, E.W. Controlling and tuning the dynamic nature of supramolecular polymers in aqueous solutions. Chem. Commun. 2017, 53, 2279–2282. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Yuan, Q.; Hollett, G.; Zhao, W.; Kang, Y.; Wu, J. Cyclodextrin-based host–guest supramolecular hydrogel and its application in biomedical fields. Polym. Chem. 2018, 9, 3436–3449. [Google Scholar] [CrossRef]
- Zhou, J.; Li, J.; Du, X.; Xu, B. Supramolecular biofunctional materials. Biomaterials 2017, 129, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.M.; Islam, T.; Shah, S.S.; Awal, A.; Aziz, M.A.; Ahammad, A.J.S. Recent Advances in Carbon and Metal Based Supramolecular Technology for Supercapacitor Applications. Chem. Rec. 2022, 22, e202200041. [Google Scholar] [CrossRef]
- Tang, W.; Zou, C.; Da, C.; Cao, Y.; Peng, H. A review on the recent development of cyclodextrin-based materials used in oilfield applications. Carbohydr. Polym. 2020, 240, 116321. [Google Scholar] [CrossRef]
- Kang, W.; Mushi, S.J.; Yang, H.; Wang, P.; Hou, X. Development of smart viscoelastic surfactants and its applications in fracturing fluid: A review. J. Pet. Sci. Eng. 2020, 190, 107107. [Google Scholar] [CrossRef]
- Li, Z.; Kang, W.; Yang, H.; Zhou, B.; Jiang, H.; Liu, D.; Jia, H.; Wang, J. Advances of supramolecular interaction systems for improved oil recovery (IOR). Adv. Colloid Interface Sci. 2022, 301, 102617. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.L.; Li, Z.Q.; Zhang, Q.L.; Wang, G.; Xu, Y.Z. Multi-scale rheological perspective to polymer solutions and gels in EOR. J. Cent. South Univ. Technol. 2007, 14, 232–237. [Google Scholar] [CrossRef]
- Hu, X.; Ke, Y.; Zhao, Y.; Yu, C.; Lu, S.; Peng, F. Preparation and properties of nanocomposites of beta-cyclodextrin-functionalized polyacrylamide and its application for enhancing oil recovery. RSC Adv. 2018, 8, 30491–30501. [Google Scholar] [CrossRef] [Green Version]
- Zou, C.; Tang, Q.; Tan, N.; Xiao, P.; Hu, X. Cyclodextrin and methacrylic acid octyl phenols poly(ethylene oxide) ester-induced synergistic effect in a novel poly(AM-co-A-β-CD-co-AE) polymer. Starch—Stärke 2012, 64, 281–289. [Google Scholar] [CrossRef]
- Li, X.; Shu, Z.; Luo, P.; Ye, Z. Associating Polymer Networks Based on Cyclodextrin Inclusion Compounds for Heavy Oil Recovery. J. Chem. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pu, W.; Du, D.; Liu, R.; Li, K.; Huang, T. Synthesis and evaluation of β-cyclodextrin-functionalized hydrophobically associating polyacrylamide. RSC Adv. 2016, 6, 96006–96014. [Google Scholar] [CrossRef]
- Zhu, Z.; Kou, H.Q.; Zhang, Z.; Wang, Y.X.; Wan, H.Q. Performance and mechanisms of enhanced oil recovery via amphiphilic polymer flooding in high salinity reservoir. Pet. Sci. Technol. 2022, 40, 1–11. [Google Scholar] [CrossRef]
- Machale, J.; Majumder, S.K.; Ghosh, P.; Sen, T.K. Role of chemical additives and their rheological properties in enhanced oil recovery. Rev. Chem. Eng. 2020, 36, 789–830. [Google Scholar] [CrossRef]
- Data, R.J.M.; Mattea, F.; Strumia, M.C.; Milanesio, J.M. Effect of including a hydrophobic comonomer on the rheology of an acrylamide-acrylic acid based copolymer. J. Appl. Polym. Sci. 2020, 137, 12. [Google Scholar] [CrossRef]
- Afolabi, R.O.; Oluyemi, G.F.; Officer, S.; Ugwu, J.O. Hydrophobically associating polymers for enhanced oil recovery—Part A: A review on the effects of some key reservoir conditions. J. Pet. Sci. Eng. 2019, 180, 681–698. [Google Scholar] [CrossRef]
- Chen, F.Z.; Gu, J.W.; Jiang, H.Q.; Yao, X.; Li, Y. Laboratory evaluation and numerical simulation of the alkali-surfactant-polymer synergistic mechanism in chemical flooding. RSC Adv. 2018, 8, 26476–26487. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Zhao, L.; He, Z.; Leng, Q.; Li, L.; Jia, Z. Performance Test of Supramolecular Oil Displacement Agent WMM-100. J. Southwest. Pet. Inst. 2005, 27, 54. [Google Scholar]
- Zou, C.J.; Wu, H.M.; Ma, L.; Lei, Y. Preparation and Application of a Series of Novel Anionic Acrylamide Polymers with Cyclodextrin Sides. J. Appl. Polym. Sci. 2011, 119, 953–961. [Google Scholar] [CrossRef]
- De Aguiar, K.; Palermo, L.C.M.; Mansur, C.R.E. Polymer viscosifier systems with potential application for enhanced oil recovery: A review. Oil Gas Sci. Technol. 2021, 76, 21. [Google Scholar] [CrossRef]
- Wever, D.A.Z.; Picchioni, F.; Broekhuis, A.A. Polymers for enhanced oil recovery: A paradigm for structure-property relationship in aqueous solution. Prog. Polym. Sci. 2011, 36, 1558–1628. [Google Scholar] [CrossRef]
- Rabiee, A.; Ershad-Langroudi, A.; Jamshidi, H. Polyacrylamide-based polyampholytes and their applications. Rev. Chem. Eng. 2014, 30, 501–519. [Google Scholar] [CrossRef]
- Li, X.; Wu, T. Rheological and mechanical properties of dynamic covalent polymers based on imine bond. J. Appl. Polym. Sci. 2021, 138, 50953. [Google Scholar] [CrossRef]
- Chen, C.C.; Sun, L.H.; Liu, X.G. Effect of degree of hydrolysis of polyacrylamide on the micromorphology of its solution. J. Appl. Polym. Sci. 2022, 139, 11. [Google Scholar] [CrossRef]
- Lai, N.J.; Wu, T.; Ye, Z.B.; Zhang, Y.; Zhou, N.; Zeng, F.H. Hybrid Hyperbranched Polymer Based on Modified Nano-SiO2 for Enhanced Oil Recovery. Chem. Lett. 2016, 45, 1189–1191. [Google Scholar] [CrossRef]
- Akhmetzhan, A.; Myrzakhmetova, N.; Amangeldi, N.; Kuanyshova, Z.; Akimbayeva, N.; Dosmaganbetova, S.; Toktarbay, Z.; Longinos, S.N. A Short Review on the N,N-Dimethylacrylamide-Based Hydrogels. Gels 2021, 7, 12. [Google Scholar] [CrossRef]
- Shao, Y.; Mao, J.C.; Yang, B.; Zhao, J.Z.; Yang, X.J. High Performance Hydrophobic Associated Polymer for Fracturing Fluids with Low-Dosage. Pet. Chem. 2020, 60, 219–225. [Google Scholar] [CrossRef]
- Ji, Y.F.; Cao, X.L.; Zhu, Y.W.; Xu, H.; Sun, X.Z.; Li, H.T. Synthesis of super high molecular weight copolymer of AM/NaA/AMPS by oxidation-reduction and controlled radical polymerization. Pet. Sci. 2020, 17, 242–254. [Google Scholar] [CrossRef] [Green Version]
- Mao, J.C.; Xue, J.X.; Zhang, H.; Yang, X.J.; Lin, C.; Wang, Q.H.; Li, C.; Liao, Z.J. Investigation of a hydrophobically associating polymer’s temperature and salt resistance for fracturing fluid thickener. Colloid Polym. Sci. 2022, 300, 569–582. [Google Scholar] [CrossRef]
- Nsengiyumva, E.M.; Alexandridis, P. Xanthan gum in aqueous solutions: Fundamentals and applications. Int. J. Biol. Macromol. 2022, 216, 583–604. [Google Scholar] [CrossRef]
- Petri, D.F.S. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 132, 13. [Google Scholar] [CrossRef] [Green Version]
- Pu, W.F.; Shen, C.; Wei, B.; Yang, Y.; Li, Y.B. A comprehensive review of polysaccharide biopolymers for enhanced oil recovery (EOR) from flask to field. J. Ind. Eng. Chem. 2018, 61, 1–11. [Google Scholar] [CrossRef]
- Sofia, G.B.; Djamel, A. A Rheological Study of Xanthan Polymer for Enhanced Oil Recovery. J. Macromol. Sci. Part B-Phys. 2016, 55, 793–809. [Google Scholar] [CrossRef]
- Khalid, N.; Zahoor, T.; Pasha, I.; Shahid, M. Rheological Characterization and Microstructural Depiction of Xanthan Gum and Its Hydrolysates. Pak. J. Agric. Sci. 2020, 57, 561–571. [Google Scholar] [CrossRef]
- Ge, M.-R.; Miao, S.-J.; Liu, J.-F.; Gang, H.-Z.; Yang, S.-Z.; Mu, B.-Z. Laboratory studies on a novel salt-tolerant and alkali-free flooding system composed of a biopolymer and a bio-based surfactant for oil recovery. J. Pet. Sci. Eng. 2021, 196, 107736. [Google Scholar] [CrossRef]
- Sowunmi, A.; Efeovbokhan, V.E.; Orodu, O.D.; Olabode, O.; Oputa, A.; Ameur, H. Comparative Study of Biopolymer Flooding: A Core Flooding and Numerical Reservoir Simulator Validation Analysis. Model. Simul. Eng. 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Li, D.; Liu, Z.; Zhang, G.; Zheng, Z.; Jia, J.; Gao, X.; Zan, X. Comparison and revelation of tight oil accumulation conditions, distribution characteristics and development status between China and U.S. Nat. Gas Geosci. 2017, 28, 1126–1138. [Google Scholar]
- Hu, W.; Bao, J.; Hu, B. Trend and progress in global oil and gas exploration. Pet. Explor. Dev. 2013, 40, 409–413. [Google Scholar] [CrossRef]
- Kianinejad, A.; Saidian, M.; Mavaddat, M.; Ghazanfari, M.H.; Kharrat, R.; Rashtchian, D. Worm-Like Micelles: A New Approach for Heavy Oil Recovery from Fractured Systems. Can. J. Chem. Eng. 2015, 93, 951–958. [Google Scholar] [CrossRef]
- Cheng, J.C.; Wang, D.M.; Wu, J.Z.; Li, Q.; Li, J.L.; Hu, Z.H. Molecular weight optimization of polymer for oil displacement. J. Pet. 2000, 1, 102–106. [Google Scholar]
- Al-Shakry, B.; Skauge, T.; Shiran, B.S.; Skauge, A. Polymer Injectivity: Investigation of Mechanical Degradation of Enhanced Oil Recovery Polymers Using In-Situ Rheology. Energies 2019, 12, 25. [Google Scholar] [CrossRef] [Green Version]
- Azad, M.S.; Trivedi, J.J. Does Polymer’s Viscoelasticity Influence Heavy-Oil Sweep Efficiency and Injectivity at 1 ft/D? SPE Reserv. Eval. Eng. 2020, 23, 446–462. [Google Scholar] [CrossRef]
- Gulikkrzywicki, T.; Fouquey, C.; Lehn, J.M. Electron-Microscopic Study of Supramolecular Liquid-Crystalline Polymers Formed by Molecular-Recognition-Directed Self-Assembly from Complementary Chiral Components. Proc. Natl. Acad. Sci. USA 1993, 90, 163–167. [Google Scholar] [CrossRef] [Green Version]
- Eelkema, R.; Pich, A. Pros and Cons: Supramolecular or Macromolecular: What Is Best for Functional Hydrogels with Advanced Properties? Adv. Mater. 2020, 32, e1906012. [Google Scholar] [CrossRef]
- Herbst, F.; Dohler, D.; Michael, P.; Binder, W.H. Self-healing polymers via supramolecular forces. Macromol. Rapid Commun. 2013, 34, 203–220. [Google Scholar] [CrossRef]
- Juang, G.C.; Wang, K.; He, Y.B.; Dong, T.F.; Luo, X.W.; Zao, L.; Wu, Y.C. A new drilling fluid technology based on supramolecular chemistry. J. China Univ. Pet. 2020, 44, 111–120. [Google Scholar]
- Pu, W.F.; Liu, R.; Li, B.; Jin, F.Y.; Peng, Q.; Sun, L.; Du, D.J.; Yao, F.S. Amphoteric hyperbranched polymers with multistimuli-responsive behavior in the application of polymer flooding. RSC Adv. 2015, 5, 88002–88013. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Li, B.; Han, P. Enhancing oil recovery from low-permeability reservoirs with a self-adaptive polymer: A proof-of-concept study. Fuel 2019, 251, 136–146. [Google Scholar] [CrossRef]
- Hutchins, K.M. Functional materials based on molecules with hydrogen-bonding ability: Applications to drug co-crystals and polymer complexes. R. Soc. Open Sci. 2018, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.L.; Hu, B.L.; Li, R.W.; Zhang, Q.C. Hydrogen Bonding in Self-Healing Elastomers. ACS Omega 2021, 6, 9319–9333. [Google Scholar] [CrossRef]
- Fan, Z.W.; Zou, Y.B.; Liu, C.L.; Xiang, S.C.; Zhang, Z.J. Hydrogen-Bonded Organic Frameworks: Functionalized Construction Strategy by Nitrogen-Containing Functional Group. Chem.-Eur. J. 2022, 28, 24. [Google Scholar] [CrossRef]
- Karas, L.J.; Wu, C.H.; Das, R.; Wu, J.I.C. Hydrogen bond design principles. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2020, 10, 15. [Google Scholar] [CrossRef]
- Zhou, F.F.; Wang, J.S.; Zhang, Y.P.; Wang, Q.H.; Guo, C.W.; Wang, F.K.; Zheng, X.; Zhang, H.J. Theoretical studies on the bond strength and electron density characteristics in multiple hydrogen bonded arrays. J. Mol. Graph. 2019, 93, 6. [Google Scholar] [CrossRef]
- Chen, J.; Wu, M.; Gong, L.; Zhang, J.; Yan, B.; Liu, J.; Zhang, H.; Thundat, T.; Zeng, H. Mechanistic Understanding and Nanomechanics of Multiple Hydrogen-Bonding Interactions in Aqueous Environment. J. Phys. Chem. C 2019, 123, 4540–4548. [Google Scholar] [CrossRef]
- Leenders, C.M.; Baker, M.B.; Pijpers, I.A.; Lafleur, R.P.; Albertazzi, L.; Palmans, A.R.; Meijer, E.W. Supramolecular polymerisation in water; elucidating the role of hydrophobic and hydrogen-bond interactions. Soft Matter 2016, 12, 2887–2893. [Google Scholar] [CrossRef] [Green Version]
- Leenders, C.M.; Albertazzi, L.; Mes, T.; Koenigs, M.M.; Palmans, A.R.; Meijer, E.W. Supramolecular polymerization in water harnessing both hydrophobic effects and hydrogen bond formation. Chem. Commun. 2013, 49, 1963–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chirila, T.V.; Lee, H.H.; Oddon, M.; Nieuwenhuizen, M.M.L.; Blakey, I.; Nicholson, T.M. Hydrogen-bonded supramolecular polymers as self-healing hydrogels: Effect of a bulky adamantyl substituent in the ureido-pyrimidinone monomer. J. Appl. Polym. Sci. 2014, 131, 39932. [Google Scholar] [CrossRef]
- Miyake, H. Supramolecular Chirality in Dynamic Coordination Chemistry. Symmetry 2014, 6, 880–895. [Google Scholar] [CrossRef] [Green Version]
- Bentz, K.C.; Cohen, S.M. Supramolecular Metallopolymers: From Linear Materials to Infinite Networks. Angew. Chem.-Int. Ed. 2018, 57, 14992–15001. [Google Scholar] [CrossRef] [PubMed]
- Buaksuntear, K.; Limarun, P.; Suethao, S.; Smitthipong, W. Non-Covalent Interaction on the Self-Healing of Mechanical Properties in Supramolecular Polymers. Int. J. Mol. Sci. 2022, 23, 30. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.P.; Guo, X.L.; Liu, G.Q.; Fan, A.P.; Wang, Z.; Zhao, Y.J. When self-assembly meets topology: An enhanced micelle stability. Chem. Commun. 2017, 53, 3822–3825. [Google Scholar] [CrossRef]
- Zhou, Y.Z.; Yin, D.Y.; Chen, W.L.; Liu, B.; Zhang, X.R. A comprehensive review of emulsion and its field application for enhanced oil recovery. Energy Sci. Eng. 2019, 7, 1046–1058. [Google Scholar] [CrossRef] [Green Version]
- Olajire, A.A. Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: Prospects and challenges. Energy 2014, 77, 963–982. [Google Scholar] [CrossRef]
- Rossow, T.; Seiffert, S. Supramolecular Polymer Networks: Preparation, Properties, and Potential. In Supramolecular Polymer Networks and Gels; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–46. [Google Scholar]
- Zhang, X.F.; Shan, X.H.; Wang, F.; Yang, H.B.; Zhou, B.B.; Jiang, H.Z.; Kang, W.L.; Sarsenbekuly, B. Effect of aging time on the viscoelastic properties of amphiphilic polymer-polyacid supramolecular solution. Phys. Fluids 2020, 32, 8. [Google Scholar] [CrossRef]
- Liu, H.; Lin, S.; Feng, Y.; Theato, P. CO2-Responsive polymer materials. Polym. Chem. 2017, 8, 12–23. [Google Scholar] [CrossRef]
- Darabi, A.; Jessop, P.G.; Cunningham, M.F. CO2-responsive polymeric materials: Synthesis, self-assembly, and functional applications. Chem. Soc. Rev. 2016, 45, 4391–4436. [Google Scholar] [CrossRef]
- Sayed, M.; Pal, H. An overview from simple host-guest systems to progressively complex supramolecular assemblies. Phys. Chem. Chem. Phys. 2021, 23, 26085–26107. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, Z.Q.; Wang, Y.M.; Li, L.; Guo, X.H.; Pham, D.T.; Lincoln, S.F.; Prud’homme, R.K. Supramolecular polymer assembly in aqueous solution arising from cyclodextrin host-guest complexation. Beilstein J. Org. Chem. 2016, 12, 50–72. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Gou, S.; Wu, Q.; Zhou, L.; Zhang, H.; Fei, Y. Modified acrylamide copolymers based on β-cyclodextrin and twin-tail structures for enhanced oil recovery through host–guest interactions. New J. Chem. 2019, 43, 5363–5373. [Google Scholar] [CrossRef]
- Raffa, P.; Broekhuis, A.A.; Picchioni, F. Polymeric surfactants for enhanced oil recovery: A review. J. Pet. Sci. Eng. 2016, 145, 723–733. [Google Scholar] [CrossRef]
- Martín, V.I.; López-Cornejo, P.; López-López, M.; Blanco-Arévalo, D.; Moreno-Vargas, A.J.; Angulo, M.; Laschewsky, A.; Moyá, M.L. Influence of the surfactant degree of oligomerization on the formation of cyclodextrin: Surfactant inclusion complexes. Arab. J. Chem. 2020, 13, 2318–2330. [Google Scholar] [CrossRef]
- Huo, Y.F.; He, Z.F.; Wang, C.; Zhang, L.; Xuan, Q.Y.; Wei, S.Y.; Wang, Y.H.; Pan, D.; Dong, B.B.; Wei, R.B.; et al. The recent progress of synergistic supramolecular polymers: Preparation, properties and applications. Chem. Commun. 2021, 57, 1413–1429. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Yan, D.Y. Supramolecular self-assembly of amphiphilic hyperbranched polymers at all scales and dimensions: Progress, characteristics and perspectives. Chem. Commun. 2009, 10, 1172–1188. [Google Scholar] [CrossRef]
- McCune, J.A.; Mommer, S.; Parkins, C.C.; Scherman, O.A. Design Principles for Aqueous Interactive Materials: Lessons from Small Molecules and Stimuli-Responsive Systems. Adv. Mater. 2020, 32, 14. [Google Scholar] [CrossRef]
- Guo, X.; Jia, X.X.; Du, J.J.; Xiao, L.Q.; Li, F.F.; Liao, L.Q.; Liu, L.J. Host-guest chemistry of cyclodextrin carbamates and cellulose derivatives in aqueous solution. Carbohydr. Polym. 2013, 98, 982–987. [Google Scholar] [CrossRef]
- Liu, L.Z.; Lyu, D.; Xiang, M.Y.; Men, Y.F. Side chain packing states of chitosan-based supramolecular derivatives containing long alkyl side chains. Polym. Cryst. 2020, 3, 6. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, Y.; Ulrich, S.; Barboiu, M.; Ramstrom, O. Dynamic Covalent Polymers for Biomedical Applications. Mater. Chem. Front. 2020, 4, 489–506. [Google Scholar] [CrossRef]
- Vlassopoulos, D.; Pitsikalis, M.; Hadjichristidis, N. Linear dynamics of end-functionalized polymer melts: Linear chains, stars, and blends. Macromolecules 2000, 33, 9740–9746. [Google Scholar] [CrossRef]
- Clemons, T.D.; Stupp, S.I. Design of materials with supramolecular polymers. Prog. Polym. Sci. 2020, 111, 101310. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Dai, Z.; Jiang, F.; Tian, J.; Zhang, W. A super-stretchable, self-healing and injectable supramolecular hydrogel constructed by a host-guest crosslinker. Biomater. Sci. 2020, 8, 3359–3369. [Google Scholar] [CrossRef]
- Romero-Zeron, L.; Espinosa, C. Advantageous supramolecular system through self-association of xanthan gum/cationic surfactant via beta-cyclodextrin host-guest complexations for Enhanced Oil Recovery Applications. J. Pet. Sci. Eng. 2020, 185, 13. [Google Scholar] [CrossRef]
- Wei, B.; Romero-Zerón, L.; Rodrigue, D. Evaluation of Two New Self-assembly Polymeric Systems for Enhanced Heavy Oil Recovery. Ind. Eng. Chem. Res. 2014, 53, 16600–16611. [Google Scholar] [CrossRef]
- Juang, G.C.; Wang, K.; Xuan, Y.; He, Y.B.; Song, R.R. Xanthan gum—β Application of cyclodextrin self-assembly system in water-based cobalt drilling fluid without soil phase. Pet. Sci. Bull. 2016, 1, 279–285. [Google Scholar]
- Kunyuan, Q.I.U. Progress of Free Radical Polymerization in Recent Years. Polym. Bull. 2008, 7, 15–28. [Google Scholar]
- Szkurhan, A.R.; Georges, M.K. Aqueous stable free radical polymerization processes. Chin. J. Polym. Sci. 2004, 22, 309–312. [Google Scholar]
- Zhang, L.Y.; Juang, G.C.; An, Y.X. Development and evaluation of ZJA, a supramolecular viscosity increasing and cutting agent for drilling fluid. Mod. Chem. Ind. 2016, 36, 131–134. [Google Scholar]
- Jiang, F.; Pu, W.F.; Li, Y.B.; Du, D.J. A double-tailed acrylamide hydrophobically associating polymer: Synthesis, characterization, and solution properties. J. Appl. Polym. Sci. 2015, 132, 10. [Google Scholar] [CrossRef]
- Liao, Y.; Zheng, H.L.; Dai, L.; Li, F.T.; Zhu, G.C.; Guan, Q.Q.; Sun, Y.J.; Tang, X.M. Hydrophobically Modified Polyacrylamide Synthesis and Application in Water Treatment. Asian J. Chem. 2014, 26, 5923–5927. [Google Scholar] [CrossRef]
- Szwarc, M. ‘Living’ Polymers. Nature 1956, 178, 1168–1169. [Google Scholar] [CrossRef]
- McLeary, J.B.; Klumperman, B. RAFT mediated polymerisation in heterogeneous media. Soft Matter 2006, 2, 45–53. [Google Scholar] [CrossRef]
- Zetterlund, P.B.; Kagawa, Y.; Okubo, M. Controlled/living radical polymerization in dispersed systems. Chem. Rev. 2008, 108, 3747–3794. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, H.C.; Antonietti, M.; Schmidt, B. Free radical and RAFT polymerization of vinyl esters in metal-organic-frameworks. Polym. Chem. 2017, 8, 6204–6208. [Google Scholar] [CrossRef] [Green Version]
- Petton, L.; Ciolino, A.E.; Stamenovic, M.M.; Espeel, P.; Du Prez, F.E. From NMP to RAFT and Thiol-Ene Chemistry by In Situ Functionalization of Nitroxide Chain Ends. Macromol. Rapid Commun. 2012, 33, 1310–1315. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Choi, B.; Wei, X.; Feng, A.; Thang, S.H. Synthesis, self-assembly, and base-pairing of nucleobase end-functionalized block copolymers in aqueous solution. Polym. Chem. 2018, 9, 5086–5094. [Google Scholar] [CrossRef]
- Bernard, J.; Lortie, F.; Fenet, B. Design of Heterocomplementary H-Bonding RAFT Agents—Towards the Generation of Supramolecular Star Polymers. Macromol. Rapid Commun. 2009, 30, 83–88. [Google Scholar] [CrossRef]
- Chen, S.B.; Bertrand, A.; Chang, X.J.; Alcouffe, P.; Ladaviere, C.; Gerard, J.F.; Lortie, F.; Bernard, J. Heterocomplementary H-Bonding RAFT Agents as Tools for the Preparation of Supramolecular Miktoarm Star Copolymers. Macromolecules 2010, 43, 5981–5988. [Google Scholar] [CrossRef]
- Moad, G. RAFT polymerization to form stimuli-responsive polymers. Polym. Chem. 2017, 8, 177–219. [Google Scholar] [CrossRef]
- Bai, Y.R.; Shang, X.S.; Wang, Z.B.; Zhao, X.T. Experimental Study on Hydrophobically Associating Hydroxyethyl Cellulose Flooding System for Enhanced Oil Recovery. Energy Fuels 2018, 32, 6713–6725. [Google Scholar] [CrossRef]
- Zuo, F.; Luo, C.; Ding, X.; Zheng, Z.; Cheng, X.; Peng, Y. Redox-responsive inclusion complexation between beta-cyclodextrin and ferrocene-functionalized poly(N-isopropylacrylamide) and its effect on the solution properties of this polymer. Supramol. Chem. 2008, 20, 559–564. [Google Scholar] [CrossRef]
- Jiang, Z.P.; Shangguan, Y.G.; Zheng, Q. Rheological Behavior of Ferrocene-modified Polyacrylamide in Aqueous Solutions. Acta Polym. Sin. 2015, 1, 120–126. [Google Scholar]
- Liu, Y.; Wang, Z.; Zhang, X. Characterization of supramolecular polymers. Chem. Soc. Rev. 2012, 41, 5922–5932. [Google Scholar] [CrossRef]
- Hartlieb, M.; Mansfield, E.D.H.; Perrier, S. A guide to supramolecular polymerizations. Polym. Chem. 2020, 11, 1083–1110. [Google Scholar] [CrossRef]
- Kashiwagi, Y.; Katashima, T.; Nakahata, M.; Takashima, Y.; Harada, A.; Inoue, T. Linear viscoelastic studies on a transient network formed by host-guest interaction. J. Polym. Sci. Part B—Polym. Phys. 2018, 56, 1109–1117. [Google Scholar] [CrossRef]
- Kubota, R.; Tanaka, W.; Hamachi, I. Microscopic Imaging Techniques for Molecular Assemblies: Electron, Atomic Force, and Confocal Microscopies. Chem. Rev. 2021, 121, 14281–14347. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, W.; Yang, M. Synthesis and solution properties of an associative polymer with excellent salt-thickening. J. Appl. Polym. Sci. 2012, 125, 4049–4059. [Google Scholar] [CrossRef]
- Zhong, C.R.; Zhang, H.; Feng, L.M. Solution behavior and associating structures of a salt-tolerant tetra-polymer containing an allyl-capped macromonomer. J. Polym. Res. 2014, 21, 9. [Google Scholar] [CrossRef]
- Roy, A.; Comesse, S.; Grisel, M.; Hucher, N.; Souguir, Z.; Renou, F. Hydrophobically modified xanthan: An amphiphilic but not associative polymer. Biomacromolecules 2014, 15, 1160–1170. [Google Scholar] [CrossRef]
- Kubota, R.; Nakamura, K.; Torigoe, S.; Hamachi, I. The Power of Confocal Laser Scanning Microscopy in Supramolecular Chemistry: In situ Real-time Imaging of Stimuli-Responsive Multicomponent Supramolecular Hydrogels. ChemistryOpen 2020, 9, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Stepanek, P. Dynamic Light Scattering in Polymer Systems at Extremely Low Temperatures: Experimental Setup and Typical Results. Chem. Listy 2018, 112, 546–549. [Google Scholar]
- Pinault, T.; Andrioletti, B.; Bouteiller, L. Chain stopper engineering for hydrogen bonded supramolecular polymers. Beilstein J. Org. Chem. 2010, 6, 869–875. [Google Scholar] [CrossRef] [Green Version]
- Lortie, F.; Boileau, S.B.; Bouteiller, L.; Chassenieux, C.; Laupretre, F. Chain stopper-assisted characterization of supramolecular polymers. Macromolecules 2005, 38, 5283–5287. [Google Scholar] [CrossRef]
- Yao, H.; Tian, W.; Liu, Y.; Bai, Y.; Liu, D.; Liu, T.; Qi, M.; Wang, M.; Liu, Y. Cyclodextrin-tunable reversible self-assembly of a thermoresponsive Y-shaped polymer. RSC Adv. 2015, 5, 34557–34565. [Google Scholar] [CrossRef]
- Vancso, G.J. Feeling the force of supramolecular bonds in polymers. Angew. Chem. Int. Ed. 2007, 46, 3794–3796. [Google Scholar] [CrossRef]
- Zhang, W.; Hou, J.; Li, N.; Zhang, W.K. Application of Atomic Force Microscopy (AFM)-based Single-molecule Force Spectroscopy (SMFS) in Polymer Characterization. Acta Polym. Sin. 2021, 52, 1523–1546. [Google Scholar] [CrossRef]
- Zou, S.; Schonherr, H.; Vancso, G.J. Force spectroscopy of quadruple H-bonded dimers by AFM: Dynamic bond rupture and molecular time-temperature superposition. J. Am. Chem. Soc. 2005, 127, 11230–11231. [Google Scholar] [CrossRef]
- Feng, C.; Sun, L.; Liu, W.; Chen, C.; Li, B.; Sun, D. Effects of surfactant on the molecules of different polarity of solubilization: Based on the study of micellar microscopic morphology mechanism. J. Pet. Sci. Eng. 2022, 208, 109563. [Google Scholar] [CrossRef]
- Friedrich, H.; Frederik, P.M.; de With, G.; Sommerdijk, N. Imaging of Self-Assembled Structures: Interpretation of TEM and Cryo-TEM Images. Angew. Chem.-Int. Ed. 2010, 49, 7850–7858. [Google Scholar] [CrossRef]
- Franken, L.E.; Boekema, E.J.; Stuart, M.C.A. Transmission Electron Microscopy as a Tool for the Characterization of Soft Materials: Application and Interpretation. Adv. Sci. 2017, 4, 1600476. [Google Scholar] [CrossRef] [Green Version]
- Danino, D. Cryo-TEM of soft molecular assemblies. Curr. Opin. Colloid Interface Sci. 2012, 17, 316–329. [Google Scholar] [CrossRef]
- Weissman, H.; Rybtchinski, B. Noncovalent self-assembly in aqueous medium: Mechanistic insights from time-resolved cryogenic electron microscopy. Curr. Opin. Colloid Interface Sci. 2012, 17, 330–342. [Google Scholar] [CrossRef]
- Chen, I.C.; Yegin, C.; Zhang, M.; Akbulut, M. Use of pH-Responsive Amphiphilic Systems as Displacement Fluids in Enhanced Oil Recovery. SPE J. 2014, 19, 1035–1046. [Google Scholar] [CrossRef]
- Xiong, C.; Wei, F.; Zhou, Q.; Peng, K.; Ye, Z.; Yang, H. A CO2-responsive smart fluid based on supramolecular assembly structures varying reversibly from vesicles to wormlike micelles. RSC Adv. 2020, 10, 25311–25318. [Google Scholar] [CrossRef]
- Zhao, T.; Peng, J.; Zhang, Y.; Chen, J.; Chen, Y.; Sun, W.; Li, S. Synthesis of ultra-high concentration of salt-resistant polyacrylamide. Polym. Adv. Technol. 2020, 31, 2980–2989. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Hua, Z.; Tang, C. Supramolecular nucleobase-functionalized polymers: Synthesis and potential biological applications. J. Mater. Chem. B 2020, 8, 1576–1588. [Google Scholar] [CrossRef]
- Hua, Z.; Pitto-Barry, A.; Kang, Y.; Kirby, N.; Wilks, T.R.; O’Reilly, R.K. Micellar nanoparticles with tuneable morphologies through interactions between nucleobase-containing synthetic polymers in aqueous solution. Polym. Chem. 2016, 7, 4254–4262. [Google Scholar] [CrossRef] [Green Version]
- Obert, E.; Bellot, M.; Bouteiller, L.; Andrioletti, F.; Lehen-Ferrenbach, C.; Boue, F. Both water- and organo-soluble supramolecular polymer stabilized by hydrogen-bonding and hydrophobic interactions. J. Am. Chem. Soc. 2007, 129, 15601–15605. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.Q.; Sun, L.M.; Wang, L.S.; Tian, Z.Q.; Tang, H.T.; Wang, J. Phenolic Resin Based Supramolecular Aggregates-Novel In-Depth Profile Modifier for Oil Reservoir. Adv. Mater. Res. 2012, 554–556, 31–322. [Google Scholar] [CrossRef]
- Bai, Y.; He, S.; Lian, Y.; Yu, T.; Dai, C.; Zhao, J.; Zhang, H. Self-Lubricating Supramolecular Hydrogel for In-Depth Profile Control in Fractured Reservoirs. ACS Omega 2020, 5, 7244–7253. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.-f.; Du, D.-j.; Fan, H.-c.; Chen, B.-w.; Yuan, C.-D.; Varfolomeev, M.A. CO2-responsive preformed gel particles with interpenetrating networks for controlling CO2 breakthrough in tight reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126065. [Google Scholar] [CrossRef]
- Luo, X.; Zheng, P.; Gao, K.; Wei, B.; Feng, Y. Thermo- and CO2-triggered viscosifying of aqueous copolymer solutions for gas channeling control during water-alternating-CO2 flooding. Fuel 2021, 291, 120171. [Google Scholar] [CrossRef]
- Chen, L.; Liu, R.; Hao, X.; Yan, Q. CO2 -Cross-Linked Frustrated Lewis Networks as Gas-Regulated Dynamic Covalent Materials. Angew. Chem. Int. Ed. Engl. 2019, 58, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Nudelman, F.; Matthes, R.R.; Shaver, M.P. Frustrated Lewis Pair Polymers as Responsive Self-Healing Gels. J. Am. Chem. Soc. 2017, 139, 14232–14236. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Wang, Y.; Yan, Q. CO2 -Strengthened Double-Cross-Linked Polymer Gels from Frustrated Lewis Pair Networks. Macromol. Rapid Commun. 2021, 42, e2000699. [Google Scholar] [CrossRef]
- Zhu, M.S.Q.; Liu, J.; Gan, L.H.; Long, M.N. Research progress in bio-based self-healing materials. Eur. Polym. J. 2020, 129, 19. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Yamaguchi, H. Cyclodextrin-based supramolecular polymers. Chem. Soc. Rev. 2009, 38, 875–882. [Google Scholar] [CrossRef]
- Aeridou, E.; Diaz, D.D.; Aleman, C.; Perez-Madrigal, M.M. Advanced Functional Hydrogel Biomaterials Based on Dynamic B-O Bonds and Polysaccharide Building Blocks. Biomacromolecules 2020, 21, 3984–3996. [Google Scholar] [CrossRef]
- Xu, J.F.; Zhang, X. Study on Supramolecular Polymers in China: An Overview and Outlook. Acta Polym. Sin. 2017, 1, 37–49. [Google Scholar] [CrossRef]
- Slouf, M.; Strachota, B.; Strachota, A.; Gajdosova, V.; Bertschova, V.; Nohava, J. Macro-, Micro- and Nanomechanical Characterization of Crosslinked Polymers with Very Broad Range of Mechanical Properties. Polymers 2020, 12, 26. [Google Scholar] [CrossRef]
- Xu, D.; Hawk, J.L.; Loveless, D.M.; Jeon, S.L.; Craig, S.L. Mechanism of Shear Thickening in Reversibly Cross-linked Supramolecular Polymer Networks. Macromolecules 2010, 43, 3556–3565. [Google Scholar] [CrossRef] [Green Version]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Bjorn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [Green Version]
- Li, A.C. Experimental Analysis on Water Quality of Produced Sewage in Polymer Flooding Oilfield. Fresenius Environ. Bull. 2021, 30, 349–357. [Google Scholar]
- Al-Sabahi, J.; Bora, T.; Claereboudt, M.; Al-Abri, M.; Dutta, J. Visible light photocatalytic degradation of HPAM polymer in oil produced water using supported zinc oxide enanorods. Chem. Eng. J. 2018, 351, 56–64. [Google Scholar] [CrossRef]
- Abbasi, A.; Nasef, M.M.; Yahya, W.Z.N. Copolymerization of vegetable oils and bio-based monomers with elemental sulfur: A new promising route for bio-based polymers. Sustain. Chem. Pharm. 2019, 13, 8. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, Y.J. Dependence of intrinsic viscosity and molecular size on molecular weight of partially hydrolyzed polyacrylamide. J. Appl. Polym. Sci. 2021, 138, 11. [Google Scholar] [CrossRef]
- Hinton, Z.R.; Shabbir, A.; Alvarez, N.J. Dynamics of Supramolecular Self-Healing Recovery in Extension. Macromolecules 2019, 52, 2231–2242. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, S.; Yadav, H.; Rellegadla, S.; Agrawal, A. Polymers for enhanced oil recovery: Fundamentals and selection criteria revisited. Appl. Microbiol. Biotechnol. 2021, 105, 8073–8090. [Google Scholar] [CrossRef]
- Ayirala, S.C.; Yousef, A.A. A State-of-the-Art Review to Develop Injection-Water-Chemistry Requirement Guidelines for IOR/EOR Projects. SPE Prod. Oper. 2015, 30, 26–42. [Google Scholar] [CrossRef]
- Bezrukov, A.; Galyametdinov, Y. Characterizing properties of polymers and colloids by their reaction-diffusion behavior in microfluidic channels. Colloid Surf. A Physicochem. Eng. Asp. 2021, 630, 10. [Google Scholar] [CrossRef]
- Feiner-Gracia, N.; Mares, A.G.; Buzhor, M.; Rodriguez-Trujillo, R.; Marti, J.S.; Amir, R.J.; Pujals, S.; Albertazzi, L. Real-Time Ratiometric Imaging of Micelles Assembly State in a Microfluidic Cancer-on-a-Chip. ACS Appl. Bio Mater. 2021, 4, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef] [Green Version]
- Draper, E.R.; Eden, E.G.B.; McDonald, T.O.; Adams, D.J. Spatially resolved multicomponent gels. Nat. Chem. 2015, 7, 849–853. [Google Scholar] [CrossRef]
- Shibaev, A.V.; Philippova, O.E. New Approaches to the Design of Double Polymer Networks: A Review. Polym. Sci. Ser. C 2022, 64, 26–39. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Zhao, J.; Yan, X.Z. Synergistic Covalent-and-Supramolecular Polymers. Acta Polym. Sin. 2022, 53, 691–706. [Google Scholar] [CrossRef]


| Conventional Polymer Materials | Supramolecular Polymer | |
|---|---|---|
| Source and cost of materials | Limited, petroleum-based monomer Low cost | Wide range of source [160,161,162] Medium cost |
| Characterization items | Molecular structure Rheological properties | Self-assembly research [163] Binding constant |
| Polymer network | Static, permanent network [164,165,166] Chemical covalent cross-link | Reversible, dynamic network Both physical non-covalent and chemical covalent cross-link |
| Environment compatibility | Poor [167,168] | Not yet clear |
| Stimulus responsiveness | Poor | pH, temperature, CO2, pressure, ion concentration response |
| Thickening ability | The higher the molecular The greater the viscosity | Good |
| Temperature and salt tolerance | Degradation under high temperature and high salinity | Insensitive to temperature and salinity |
| Shear resistance | Low viscosity retention | High viscosity retention |
| Injectability | Difficult to inject Cause blockage Viscosity loss | Viscosity controllable Easy to inject |
| Applicable reservoir | Medium high-permeability Reservoir (50–10,000 mD) T < 85 °C,K < 60,000 mg·L−1 | Applicable to many types of reservoirs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Zhang, Z.; Leng, K.; Li, B.; Feng, C.; Huo, X. Can Supramolecular Polymers Become Another Material Choice for Polymer Flooding to Enhance Oil Recovery? Polymers 2022, 14, 4405. https://doi.org/10.3390/polym14204405
Sun L, Zhang Z, Leng K, Li B, Feng C, Huo X. Can Supramolecular Polymers Become Another Material Choice for Polymer Flooding to Enhance Oil Recovery? Polymers. 2022; 14(20):4405. https://doi.org/10.3390/polym14204405
Chicago/Turabian StyleSun, Linghui, Zhirong Zhang, Kaiqi Leng, Bowen Li, Chun Feng, and Xu Huo. 2022. "Can Supramolecular Polymers Become Another Material Choice for Polymer Flooding to Enhance Oil Recovery?" Polymers 14, no. 20: 4405. https://doi.org/10.3390/polym14204405
APA StyleSun, L., Zhang, Z., Leng, K., Li, B., Feng, C., & Huo, X. (2022). Can Supramolecular Polymers Become Another Material Choice for Polymer Flooding to Enhance Oil Recovery? Polymers, 14(20), 4405. https://doi.org/10.3390/polym14204405





