ZIF-67 In Situ Grown on Attapulgite: A Flame Retardant Synergist for Ethylene Vinyl Acetate/Magnesium Hydroxide Composites
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Pretreatment of Attapulgite
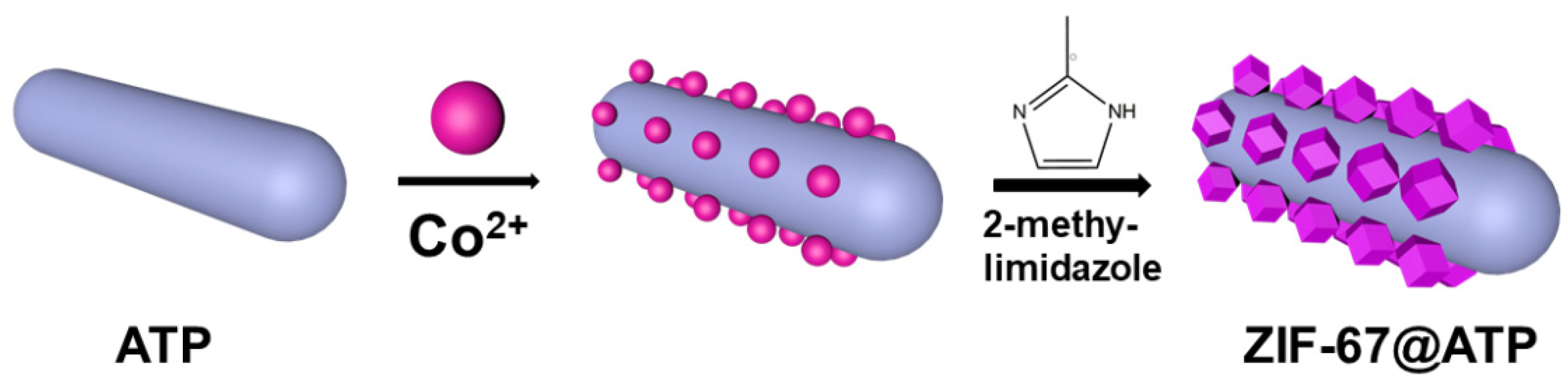
2.3. Preparation of ZIF-67@ATP
2.4. Fabrication of EVA/MH/ZIF-67@ATP Composites
2.5. Characterization
3. Results and Discussion
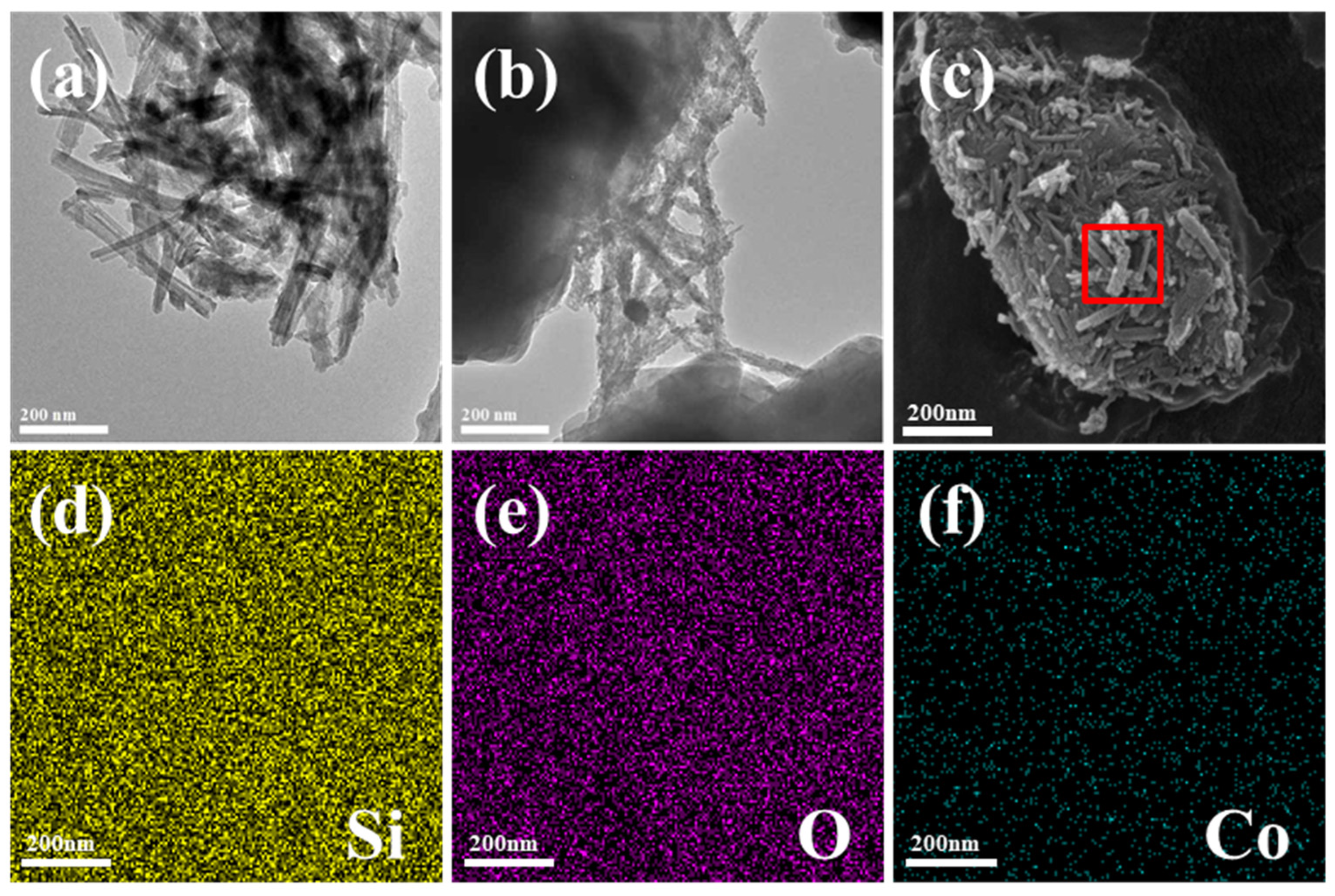
3.1. Structure and Morphology of ZIF-67@ATP Composites
0.8855x + 0.6124y = 5.53
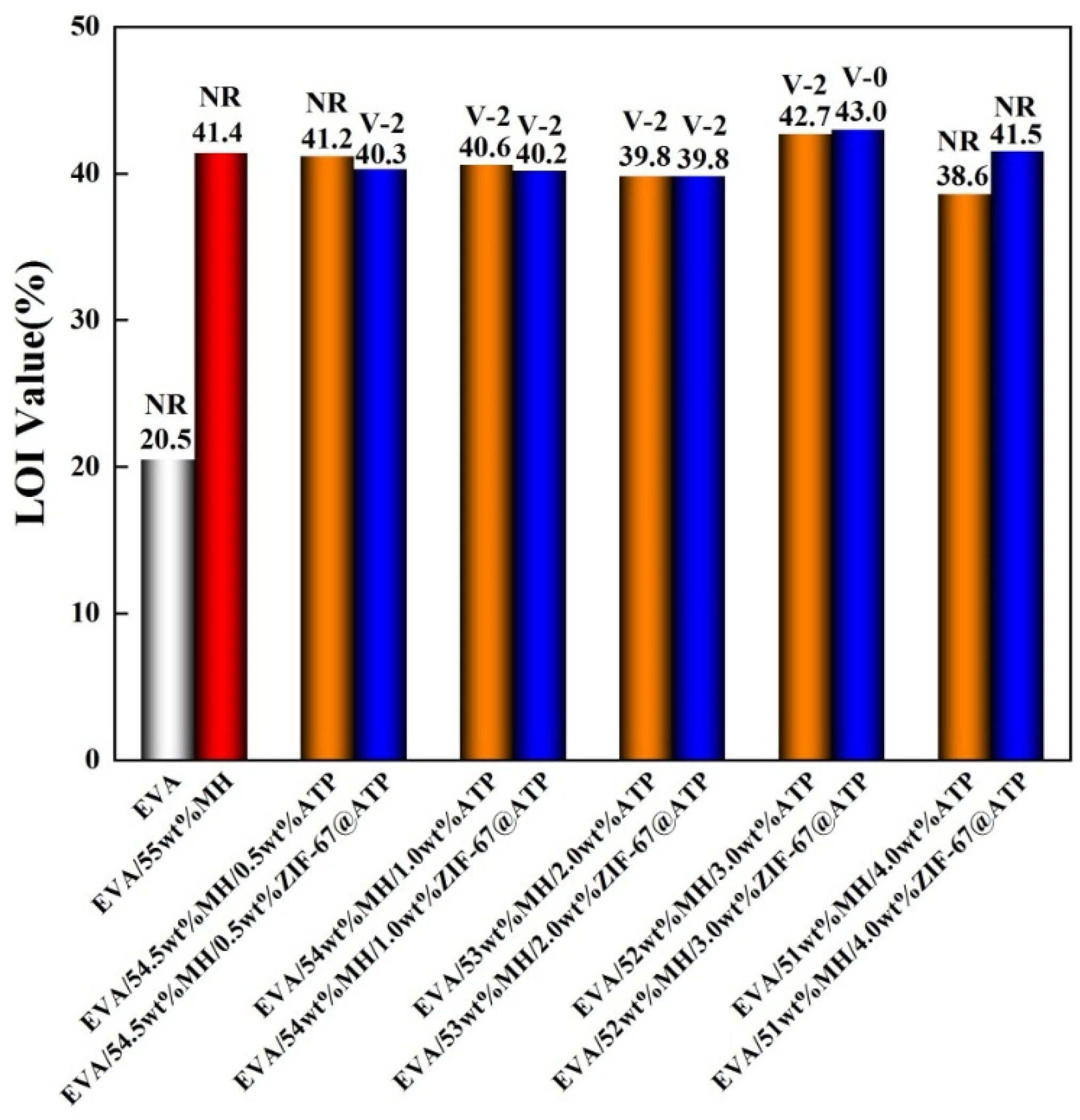
3.2. Characterization of Flame Retardancy of EVA/MH/ZIF-67@ATP Composites
3.3. Char Layer Analysis of EVA/MH/ZIF-67@ATP Composites
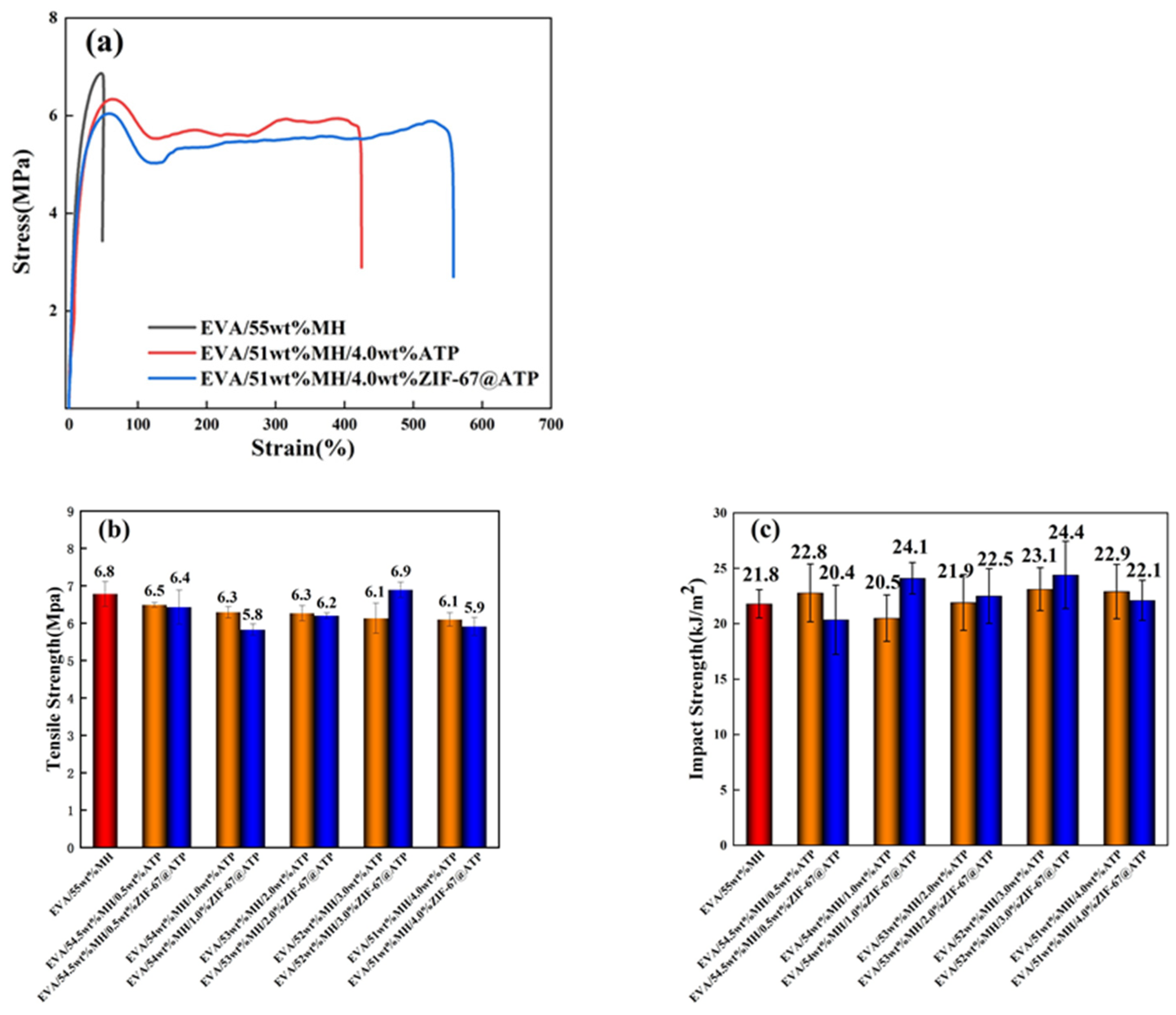
3.4. Mechanical Properties of EVA/MH/ZIF-67@ATP Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Wu, H.; Song, Q.; Zhang, J.; Li, W.; Qu, H. Core/shell structure magnesium hydroxide@polyphosphate metal salt: Preparation and its performance on the flame retardancy for ethylene vinyl acetate copolymer. J. Therm. Anal. Calorim. 2019, 141, 1341–1350. [Google Scholar] [CrossRef]
- Bidsorkhi, H.C.; Adelnia, H.; Heidar Pour, R.; Soheilmoghaddam, M. Preparation and characterization of ethylene-vinyl acetate/halloysite nanotube nanocomposites. J. Mater. Sci. 2015, 50, 3237–3245. [Google Scholar] [CrossRef]
- Albite-Ortega, J.; Sánchez-Valdes, S.; Ramirez-Vargas, E.; Nuñez-Figueredo, Y.; de Valle, L.R.; Martínez-Colunga, J.G.; Graciano-Verdugo, A.Z.; Sanchez-Martínez, Z.V.; Espinoza-Martínez, A.B.; Rodriguez-Gonzalez, J.A.; et al. Influence of keratin and DNA coating on fire retardant magnesium hydroxide dispersion and flammability characteristics of PE/EVA blends. Polym. Degrad. Stab. 2019, 165, 1–11. [Google Scholar] [CrossRef]
- Zhou, K.; Tang, G.; Jiang, S.; Gui, Z.; Hu, Y. Combination effect of MoS2 with aluminum hypophosphite in flame retardant ethylene-vinyl acetate composites. RSC Adv. 2016, 6, 37672–37680. [Google Scholar] [CrossRef]
- Shen, L.; Shao, C.; Li, R.; Xu, Y.; Li, J.; Lin, H. Preparation and characterization of ethylene–vinyl acetate copolymer (EVA)–magnesium hydroxide (MH)–hexaphenoxycyclotriphosphazene (HPCTP) composite flame-retardant materials. Polym. Bull. 2018, 76, 2399–2410. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Li, Y.; Wang, J.; Liu, X.; Song, T.; Yang, X.; Hao, J. Spray-Drying-Assisted Layer-by-Layer Assembly of Alginate, 3-Aminopropyltriethoxysilane, and Magnesium Hydroxide Flame Retardant and Its Catalytic Graphitization in Ethylene-Vinyl Acetate Resin. ACS Appl. Mater. Interfaces 2018, 10, 10490–10500. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.A.; Iqbal, M.A.; Fedel, M. Fire Retardancy of Aluminum Hydroxide Reinforced Flame Retardant Modified Epoxy Resin Composite. Russ. J. Appl. Chem. 2018, 91, 680–686. [Google Scholar] [CrossRef]
- Lyu, B.; Luo, K.; Gao, D.; Wang, Y.; Ma, J. Modified layered double hydroxide/zanthoxylum bungeanum seed oil composites to improve the flame retardant of leather. Polym. Degrad. Stab. 2021, 183, 109430–109464. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, C.; Xu, S. Mechanical, thermal and flame retardant properties of magnesium hydroxide filled poly(vinyl chloride) composites: The effect of filler shape. Compos. Part A Appl. Sci. Manuf. 2018, 113, 1–11. [Google Scholar] [CrossRef]
- Ai, L.; Chen, S.; Zeng, J.; Yang, L.; Liu, P. Synergistic Flame Retardant Effect of an Intumescent Flame Retardant Containing Boron and Magnesium Hydroxide. ACS Omega 2019, 4, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Shen, M.; Xue, Y.; Zheng, Y.; Zhang, K.; Han, Y.; Yang, L. Controllable mechanical properties of epoxy composites by incorporating self-assembled carbon nanotube–montmorillonite. Compos. Part B Eng. 2019, 164, 368–376. [Google Scholar] [CrossRef]
- Mejía, A.; Devaraj, S.; Guzmán, J.; Lopez del Amo, J.M.; García, N.; Rojo, T.; Armand, M.; Tiemblo, P. Scalable plasticized polymer electrolytes reinforced with surface-modified sepiolite fillers—A feasibility study in lithium metal polymer batteries. J. Power Sources 2016, 306, 772–778. [Google Scholar] [CrossRef]
- Dhanumalayan, E.; Kaleemulla, S. Enhanced structure, dielectric, and thermal properties of attapulgite clay and hexagonal boron nitride admixture loaded polymer blends. J. Mater. Sci. Mater. Electron. 2020, 31, 17828–17842. [Google Scholar] [CrossRef]
- Li, P.; Zhang, J.; Wang, A. A NovelN-Succinylchitosan-graft-Polyacrylamide/Attapulgite Composite Hydrogel Prepared through Inverse Suspension Polymerization. Macromol. Mater. Eng. 2007, 292, 962–969. [Google Scholar] [CrossRef]
- Yang, C.; Liu, P. Core-shell attapulgite@polypyrrole composite with well-defined corn cob-like morphology via self-assembling and in situ oxidative polymerization. Synth. Met. 2009, 159, 2056–2062. [Google Scholar] [CrossRef]
- Xie, L.; Liu, M.; Ni, B.; Zhang, X.; Wang, Y. Slow-release nitrogen and boron fertilizer from a functional superabsorbent formulation based on wheat straw and attapulgite. Chem. Eng. J. 2011, 167, 342–348. [Google Scholar] [CrossRef]
- Ti, Y.; Wen, Q.; Chen, D. Characterization of the hydrogen bond in polyurethane/attapulgite nanocomposites. J. Appl. Polym. Sci. 2016, 133, 43069–43075. [Google Scholar] [CrossRef]
- Yang, J.; Tang, Y.; Xu, J.; Chen, B.; Tang, H.; Li, C. Durable superhydrophobic/superoleophilic epoxy/attapulgite nanocomposite coatings for oil/water separation. Surf. Coat. Technol. 2015, 272, 285–290. [Google Scholar] [CrossRef]
- Niu, X.; Huo, L.; Cai, C.; Guo, J.; Zhou, H. Rod-Like Attapulgite Modified by Bifunctional Acrylic Resin As Reinforcement for Epoxy Composites. Ind. Eng. Chem. Res. 2014, 53, 16359–16365. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Y.; Lyu, B.; Wang, P.; Ma, J. Nanocomposite based on poly(acrylic acid) / attapulgite towards flame retardant of cotton fabrics. Carbohydr. Polym. 2019, 206, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Wang, T.; Huang, Y.; Zhou, L.; Yang, Y.; Liao, F.; Wang, X. The flame-retardance polylactide nanocomposites with nano attapulgite coated by resorcinol bis(diphenyl phosphate). J. Vinyl Addit. Technol. 2016, 22, 506–513. [Google Scholar] [CrossRef]
- Nabipour, N.; Wang, X.; Song, L.; Hu, Y. Metal-organic frameworks for flame retardant polymers application: A critical review. Compos. Part A 2020, 139, 106113–106122. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, L.; Qiu, S.; Zhou, X.; Gui, Z.; Hu, Y. DOPO-Modified Two-Dimensional Co-Based Metal−Organic Framework: Preparation and Application for Enhancing Fire Safety of Poly(lactic acid). ACS Appl. Mater. Interfaces 2018, 10, 8274–8286. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Yu, B.; Qin, Y. Templated interfacial synthesis of metal-organic framework (MOF) nano- and micro-structures with precisely controlled shapes and sizes. Commun. Chem. 2021, 4, 1–10. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, D.; Zhang, J. The Application of ZIF-67 and Its Derivatives: Adsorption, Separation, Electrochemistry and Catalysts. J. Mater. Chem. A 2018, 6, 1887–1899. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, M.; Wei, C.; Zhu, J.; Wang, X.; Du, X. In situ growth of four MOF/LZH composites on layered zinc hydroxide and their photocatalytic performance in decomposition of organic dyes. Mendeleev Commun. 2020, 30, 282–284. [Google Scholar] [CrossRef]
- Hou, B.; Wu, J. Halloysite nanotubes (HNTs)@ZIF-67 composites-a new type of heterogeneous catalyst for the Knoevenagel condensation reaction. Dalton Trans. 2020, 49, 17621–17628. [Google Scholar] [CrossRef]
- Liu, D.; Zheng, H.; Yang, W.; Chen, Y. Efficient removal of Sr(II) from aqueous solution by melamine-trimesic acid modified attapulgite composite. J. Radioanal. Nucl. Chem. 2019, 321, 97–108. [Google Scholar] [CrossRef]
- Han, W.J.; Piao, S.H.; Choi, H.J. Synthesis and electrorheological characteristics of polyaniline@attapulgite nanoparticles via Pickering emulsion polymerization. Mater. Lett. 2017, 204, 42–44. [Google Scholar] [CrossRef]
- Duan, Y.; Yin, G.Z.; Wang, D.Y.; Costa, R.D. In Situ Ambient Preparation of Perovskite-Poly(Llactic acid) Phosphors for Highly Stable and Efficient Hybrid Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2021, 13, 21800–21809. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Ai, L.; Jiang, J. Mechanistic insight into the interaction and adsorption of Cr(VI) with zeolitic imidazolate framework-67 microcrystals from aqueous solution. Chem. Eng. J. 2015, 274, 238–246. [Google Scholar] [CrossRef]
- Jaberi, H.; Mosleh, S.; Dashtian, K. Development of Cigarette Carbonaceous Hydrochar/ZIF-67-Based Fluids for CO2 Capture from a Gas Stream in a Packed Column: Mass-Transfer Performance Evaluation. Energy Fuels 2020, 34, 7295–7306. [Google Scholar] [CrossRef]
- Yin, H.; Yan, X.; Gu, X. Evaluation of thermally-modified calcium-rich attapulgite as a low-cost substrate for rapid phosphorus removal in constructed wetlands. Water Res 2017, 115, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Che, R.; Liu, Q.; Su, S.; Li, Z.; Zhang, H.; Liu, J.; Liu, L.; Wang, J. Hierarchically structured layered-double-hydroxides derived by ZIF-67 for uranium recovery from simulated seawater. J Hazard Mater 2017, 338, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Hou, H.; Chen, J.; Li, H.; Wang, L. Adsorption of arsenic on iron modified attapulgite (Fe/ATP): Surface complexation model and DFT studies. Adsorption 2018, 24, 459–469. [Google Scholar] [CrossRef]
- Payra, S.; Challagulla, S.; Chakraborty, C.; Roy, S. A hydrogen evolution reaction induced unprecedentedly rapid electrocatalytic reduction of 4-nitrophenol over ZIF-67 compare to ZIF-8. J. Electroanal. Chem. 2019, 853, 113545–113569. [Google Scholar] [CrossRef]
- Jiao, C.; Zhao, L.; Chen, X. Preparation of modified hollow glass microspheres using Fe2O3 and its flame retardant properties in thermoplastic polyurethane. J. Therm. Anal. Calorim. 2016, 127, 2101–2112. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.; Li, X.; Yu, L.; Zhang, Z.; Wu, Z. Preparation of Cobalt Ferrite Nanoparticle-Decorated Boron Nitride Nanosheet Flame Retardant and Its Flame Retardancy in Epoxy Resin. Nano 2019, 14, 1950063–1950078. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Xing, W.; Hou, Y.; Zou, B.; Han, L.; Hu, W.; Hu, Y. The combustion and pyrolysis process of flame-retardant polystyrene/cobalt-based metal organic frameworks (MOF) nanocomposite. Combust. Flame 2021, 226, 108–116. [Google Scholar] [CrossRef]
- Shi, X.; Jiang, S.; Hu, Y.; Peng, X.; Yang, H.; Qian, X. Phosphorylated chitosan-cobalt complex: A novel green flame retardant for polylactic acid. Polym. Adv. Technol. 2018, 29, 860–866. [Google Scholar]
- Xu, B.; Wu, X.; Ma, W.; Qian, L.; Xin, F.; Qiu, Y. Synthesis and characterization of a novel organic-inorganic hybrid char-forming agent and its flame-retardant application in polypropylene composites. J. Anal. Appl. Pyrolysis 2018, 134, 231–242. [Google Scholar] [CrossRef]
- Yin, H.; Chen, H.; Chen, D. Morphology and mechanical properties of polyacrylonitrile/attapulgite nanocomposite. J. Mater. Sci. 2010, 45, 2372–2380. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.; Li, N.; Zhao, X.; Han, X.; Li, S.; Lu, W.; Wang, K.; Du, S. Enhanced interfacial strength of carbon fiber/PEEK composites using a facile approach via PEI&ZIF-67 synergistic modification. J. Mater. Res. Technol. 2019, 8, 6289–6300. [Google Scholar]
- Wen, X.; Liu, Z.; Li, Z.; Zhang, J.; Wang, D.-Y.; Szymańska, K.; Chen, X.; Mijowska, E.; Tang, T. Constructing multifunctional nanofiller with reactive interface in PLA/CB-g-DOPO composites for simultaneously improving flame retardancy, electrical conductivity and mechanical properties. Compos. Sci. Technol. 2020, 188, 107988–107997. [Google Scholar] [CrossRef]




| Samples | EVA/g | MH/g | ATP/g | ZIF-67@ATP/g |
|---|---|---|---|---|
| EVA | 150 | / | / | / |
| EVA/55wt%MH | 67.5 | 82.5 | / | / |
| EVA/54.5wt%MH/0.5wt%ATP | 67.5 | 81.75 | 0.75 | / |
| EVA/54wt%MH/1.0wt%ATP | 67.5 | 81 | 1.5 | / |
| EVA/53wt%MH/2.0wt%ATP | 67.5 | 79.5 | 3 | / |
| EVA/52wt%MH/3.0wt%ATP | 67.5 | 78 | 4.5 | / |
| EVA/51wt%MH/4.0wt%ATP | 67.5 | 76.5 | 6 | / |
| EVA/54.5wt%MH/0.5wt%ZIF-67@ATP | 67.5 | 81.75 | / | 0.75 |
| EVA/54wt%MH/1.0wt%ZIF-67@ATP | 67.5 | 81 | / | 1.5 |
| EVA/53wt%MH/2.0wt%ZIF-67@ATP | 67.5 | 79.5 | / | 3 |
| EVA/52wt%MH/3.0wt%ZIF-67@ATP | 67.5 | 78 | / | 4.5 |
| EVA/51wt%MH/4.0wt%ZIF-67@ATP | 67.5 | 76.5 | / | 6 |
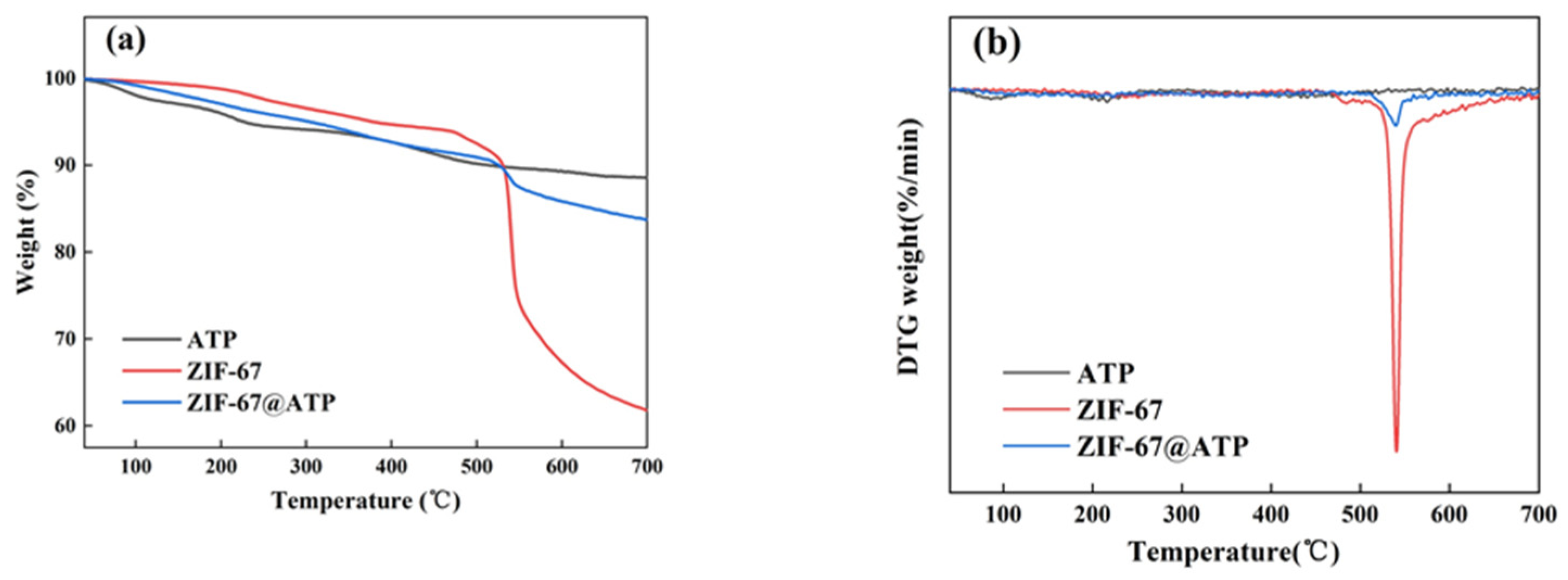
| Samples | T5 wt% (°C) | Tmax (°C) | M1 (mg) | R700 (%) | M2 (mg) |
|---|---|---|---|---|---|
| ATP | 227 | 199 | 6.25 | 88.55 | 5.53 |
| ZIF-67 | 371 | 535 | 6.01 | 61.24 | 3.68 |
| ZIF-67@ATP | 297 | 534 | 6.62 | 83.46 | 5.53 |
| Samples | TTI (s) | pHRR (kW/m2) | TpHRR (s) | THR (MJ/m2) | TSP (m2) | FIGRA (kW/(m2.s)) |
|---|---|---|---|---|---|---|
| EVA | 38 ± 3 | 1208 ± 34 | 153 ± 4 | 131 ± 1 | 12.8 ± 0.1 | 7.9 |
| EVA/MH | 51 ± 1 | 240 ± 3 | 130 ± 0 | 109 ± 2 | 10.0 ± 0.7 | 1.8 |
| EVA/52%MH/3%ATP | 54 ± 2 | 275 ± 36 | 120 ± 0 | 110 ± 2 | 12.3 ± 0.1 | 2.1 |
| EVA/52%MH/3%ZIF-67@ATP | 56 ± 5 | 268 ± 28 | 175 ± 0 | 106 ± 1 | 10.9 ± 1.9 | 1.4 |
| Samples | Tensile Strength (Mpa) | Impact Strength (kJ/m2) | Elongation at Break (%) |
|---|---|---|---|
| EVA/55wt%MH | 6.8 ± 0.3 | 21.8 ± 1.3 | 69.5 ± 23.8 |
| EVA/54.5wt%MH/0.5wt%ATP | 6.5 ± 0.1 | 22.8 ± 2.6 | 104.6 ± 30.5 |
| EVA/54wt%MH/1.0wt%ATP | 6.3 ± 0.2 | 20.5 ± 2.1 | 160.8 ± 39.7 |
| EVA/53wt%MH/2.0wt%ATP | 6.3 ± 0.2 | 21.9 ± 2.5 | 222.4 ± 51.5 |
| EVA/51wt%MH/4.0wt%ATP | 6.1 ± 0.2 | 22.9 ± 2.4 | 444.3 ± 27.7 |
| EVA/54.5wt%MH/0.5wt%ZIF-67@ATP | 6.4 ± 0.5 | 20.4 ± 3.1 | 161.4 ± 24.1 |
| EVA/54wt%MH/1.0wt%ZIF-67@ATP | 5.8 ± 0.2 | 24.1 ± 1.4 | 118.1 ± 12.9 |
| EVA/53wt%MH/2.0wt%ZIF-67@ATP | 6.2 ± 0.1 | 22.5 ± 2.5 | 309.7 ± 0.4 |
| EVA/51wt%MH/4.0wt%ZIF-67@ATP | 5.9 ± 0.2 | 22.1 ± 1.8 | 522.2 ± 51.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, D.-X.; Yang, Y.; Yin, G.-Z.; Vázquez-López, A.; Jiang, Y.; Wang, N.; Wang, D.-Y. ZIF-67 In Situ Grown on Attapulgite: A Flame Retardant Synergist for Ethylene Vinyl Acetate/Magnesium Hydroxide Composites. Polymers 2022, 14, 4408. https://doi.org/10.3390/polym14204408
Ma D-X, Yang Y, Yin G-Z, Vázquez-López A, Jiang Y, Wang N, Wang D-Y. ZIF-67 In Situ Grown on Attapulgite: A Flame Retardant Synergist for Ethylene Vinyl Acetate/Magnesium Hydroxide Composites. Polymers. 2022; 14(20):4408. https://doi.org/10.3390/polym14204408
Chicago/Turabian StyleMa, De-Xin, Yuan Yang, Guang-Zhong Yin, Antonio Vázquez-López, Yan Jiang, Na Wang, and De-Yi Wang. 2022. "ZIF-67 In Situ Grown on Attapulgite: A Flame Retardant Synergist for Ethylene Vinyl Acetate/Magnesium Hydroxide Composites" Polymers 14, no. 20: 4408. https://doi.org/10.3390/polym14204408
APA StyleMa, D.-X., Yang, Y., Yin, G.-Z., Vázquez-López, A., Jiang, Y., Wang, N., & Wang, D.-Y. (2022). ZIF-67 In Situ Grown on Attapulgite: A Flame Retardant Synergist for Ethylene Vinyl Acetate/Magnesium Hydroxide Composites. Polymers, 14(20), 4408. https://doi.org/10.3390/polym14204408









