Characterisation of Films Based on Exopolysaccharides from Alteromonas Strains Isolated from French Polynesia Marine Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Exopolysaccharide Production
2.2. Preparation and Rheological Characterisation of Filmogenic Solutions
2.3. Films’ Preparation
2.4. Optical Characterisation
2.5. Morphological Characterisation
2.6. Mechanical Properties
2.7. Water Vapour Permeability
2.8. Gas Permeability
3. Results
3.1. Rheology of Filmogenic Solutions
3.2. Film Appearance, Morphology, and Optical Characterisation
3.3. Mechanical Properties
| Film Composition | Mechanical Properties | Plasticiser (wt. wt.polymer−1% or g L−1) | RH (%) | WVP (10−11 mol m−1 s−1 Pa−1) | Driving Force (ΔRH%) | Permeability (10−16 mol m−1 s−1 Pa−1) | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Ꞇ (MPa) | ε (%) | εm (MPa) | O2 | CO2 | ||||||
| EPS A | 4.55 ± 0.36 | 47.0 ± 1.1 | 10 ± 0 | Gly (60% or 9 g L−1) | 53 | 5.8 ± 0.7 | 75.3–34.7 | 43.1 ± 10.1 | 62.6 ± 11.0 | This study |
| EPS B | 11.7 ± 1.1 | 37.7 ± 0.5 | 93 ± 12 | Gly (30% or 4.5 g L−1) | 2.7 ± 0.1 | 7.5 ± 0.4 | 24.0 ± 1.2 | This study | ||
| EPS C | 10.8 ± 1.1 | 35.6 ± 5.5 | 65 ± 5 | 6.1 ± 1.0 | 30.0 ± 1.5 | 28.5 ± 1.4 | This study | |||
| EPS D | 23.6 ± 2.8 | 5.58 ± 0.83 | 1100 ± 110 | 3.7 ± 0.4 | n.d. | n.d. | This study | |||
| EPS E | 21.1 ± 0.1 | 4.40 ± 0.42 | 597 ± 62 | 2.9 ± 0.0 | n.d. | n.d. | This study | |||
| EPS F | 16.6 ± 0.4 | 2.80 ± 0.46 | 885 ± 125 | 5.8 ± 0.2 | n.d. | n.d. | This study | |||
| Enterobacter A47 EPS | 3.8–15.5 | 5.4–22.1 | 14.5–457.8 | Gly (30%) | 45 | 1.7–2.3 | 80.9–53.4 | n.a. | n.a. | [26] |
| 3.1 | 54.9 | 2.8 | Citric acid (50%) | 44.3 | 1.0 | 76.9–22.5 | 0.7 | 42.7 | [2] | |
| Pseudomonas oleovorans EPS | 51 | 9.5 | 1738 | None | 44.3 | 1.1 | 64.8–22.0 | n.a. | 2.0 | [1] |
| 5.4 | 92.0–64.8 | |||||||||
| Kefir microflora EPS | 40.9 | 2.70 | n.a. | None | 75 | 0.32 | 75–0 | n.a. | n.a. | [19] |
| 15.2 | 116.7 | n.a. | Gly (25%) | 0.23 | ||||||
| Chitosan | 31.1 | 10.6 | n.a. | None | n.a. | n.a. | - | n.a. | n.a. | [20] |
| 41.6 | 24.7 | 1193 | Gly (15%) | n.a. | n.a. | - | n.a. | n.a. | [16] | |
| Chitosan/dextran-like EPS | 43.3 | 20.7 | n.a. | 1,3-propanediol (50%) | n.a. | n.a. | - | n.a. | n.a. | [20] |
| Chitosan/ Alteromonas sp. EPS | 39.5–42.7 | 16.6–23.7 | 1008–1186 | Gly (15%) | n.a. | n.a. | - | n.a. | n.a. | [16] |
| Gellan | 30 | 34 | n.a. | Gly (4%) | 54 | 2.0 | 54–0 | n.a. | n.a. | [30] |
| Alginate crosslinked with calcium | 64.7 | 2.8 | n.a. | Gly (40%) | 56 | n.a. | - | n.a. | n.a. | [31] |
| 24.1 | 7.6 | n.a. | 98 | 2.6 | 100–0 | n.a. | n.a. | |||
| 65.9 | 2.5 | n.a. | Sorbitol (40%) | 56 | n.a. | - | n.a. | n.a. | ||
| 18.4 | 6.6 | n.a. | 98 | 1.7 | 100–0 | n.a. | n.a. | |||
| Pectin/alginate/xanthan | 29.7 | 19.0 | n.a. | Gly (18 g L−1) | 50 | 1.01 | 100–0 | n.a. | n.a. | [25] |
| Hyaluronic acid (HA) | 70.7 | 5.6 | n.a. | none | 58 | n.a. | - | n.a. | n.a. | [32] |
| HA/CMC | 68.8–75.1 | 7.9–13.6 | n.a. | none | 58 | n.a. | - | n.a. | n.a. | |
| Cassava starch/CMC | 13.3 | 65.7 | n.a. | Gly (15 g L−1) | 55 | 0.92 | 100–0 | n.a. | n.a. | [6] |
3.4. Water Vapour Permeability
3.5. Gas Permeability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Alves, V.D.; Ferreira, A.R.; Costa, N.; Freitas, F.; Reis, M.A.M.; Coelhoso, I.M. Characterization of biodegradable films from the extracellular polysaccharide produced by Pseudomonas oleovorans grown on glycerol byproduct. Carbohydr. Polym. 2011, 83, 1582–1590. [Google Scholar] [CrossRef]
- Ferreira, A.R.V.; Torres, C.A.V.; Freitas, F.; Reis, M.A.M.; Alves, V.D.; Coelhoso, I.M. Biodegradable films produced from the bacterial polysaccharide FucoPol. Int. J. Biol. Macromol. 2014, 71, 111–116. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Li, C.; Xu, Y.; Luo, Y.; Liang, D.; Huang, C. Comprehensive Review of Polysaccharide-Based Materials in Edible Packaging: A Sustainable Approach. Foods 2021, 10, 1845. [Google Scholar] [CrossRef] [PubMed]
- Antosik, A.K.; Miądlicki, P.; Wilpiszewska, K.; Markowska-Szczupak, A.; Koren, Z.C.; Wróblewska, A. Polysaccharide films modified by compounds of natural origin and silver having potential medical applications. Cellulose 2021, 28, 7257–7271. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Jaisan, C.; Klunklin, W.; Phongthai, S.; Rawdkuen, S.; Tongdeesoontorn, W. Mechanical and Physicochemical Properties of Composite Biopolymer Films Based on Carboxymethyl Cellulose from Young Palmyra Palm Fruit Husk and Rice Flour. Polymers 2022, 14, 1872. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, Y.; Ji, T.; Sameen, D.E.; Ahmed, S.; Qin, W.; Dai, J.; Li, S.; Liu, Y. Cassava starch/carboxymethylcellulose edible films embedded with lactic acid bacteria to extend the shelf life of banana. Carbohydr. Polym. 2020, 248, 116805. [Google Scholar] [CrossRef] [PubMed]
- Laubach, J.; Joseph, M.; Brenza, T.; Gadhamshetty, V.; Sani, R.K. Exopolysaccharide and biopolymer-derived films as tools for transdermal drug delivery. J. Control. Release 2020, 329, 971–987. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, A.; Cardea, S. Microbial Exopolysaccharides as Drug Carriers. Polymers 2020, 12, 2142. [Google Scholar] [CrossRef]
- Costa, R.R.; Neto, A.I.; Calgeris, I.; Correia, C.R.; Pinho, A.C.M.; Fonseca, J.; Öner, E.T.; Mano, J.F. Adhesive nanostructured multilayer films using a bacterial exopolysaccharide for biomedical applications. J. Mater. Chem. B 2013, 1, 2367–2374. [Google Scholar] [CrossRef]
- Shen, S.; Chen, X.; Shen, Z.; Chen, H. Marine Polysaccharides for Wound Dressings Application: An Overview. Pharmaceutics 2021, 13, 1666. [Google Scholar] [CrossRef] [PubMed]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M. Exopolysaccharides from Marine and Marine Extremophilic Bacteria: Structures, Properties, Ecological Roles and Applications. Mar. Drugs 2018, 16, 69. [Google Scholar] [CrossRef]
- Ferreira, A.R.V.; Torres, C.A.V.; Freitas, F.; Sevrin, C.; Grandfils, C.; Reis, M.A.M.; Alves, V.D.; Coelhoso, I.M. Development and characterization of bilayer films of FucoPol and chitosan. Carbohydr. Polym. 2016, 147, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gruppuso, M.; Turco, G.; Marsich, E.; Porrelli, D. Polymeric wound dressings, an insight into polysaccharide-based electrospun membranes. Appl. Mater. Today 2021, 24, 101148. [Google Scholar] [CrossRef]
- Concórdio-Reis, P.; Alves, V.D.; Moppert, X.; Guézennec, J.; Freitas, F.; Reis, M.A.M. Characterization and Biotechnological Potential of Extracellular Polysaccharides Synthesized by Alteromonas Strains Isolated from French Polynesia Marine Environments. Mar. Drugs 2021, 19, 522. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Salem, D.R.; Sani, R.K. Extremophilic exopolysaccharides: A review and new perspectives on engineering strategies and applications. Carbohydr. Polym. 2019, 205, 8–26. [Google Scholar] [CrossRef]
- Zarandona, I.; Estupiñán, M.; Pérez, C.; Alonso-Sáez, L.; Guerrero, P.; De La Caba, K. Chitosan Films Incorporated with Exopolysaccharides from Deep Seawater Alteromonas sp. Mar. Drugs 2020, 18, 447. [Google Scholar] [CrossRef] [PubMed]
- Carreau, P.J. Rheological Equations from Molecular Network Theories. J. Rheol. 1972, 16, 99–127. [Google Scholar] [CrossRef]
- López, O.V.; García, M.A. Starch films from a novel (Pachyrhizus ahipa) and conventional sources: Development and characterization. Mater. Sci. Eng. C 2012, 32, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Piermaria, J.A.; Pinotti, A.; Garcia, M.A.; Abraham, A.G. Films based on kefiran, an exopolysaccharide obtained from kefir grain: Development and characterization. Food Hydrocoll. 2009, 23, 684–690. [Google Scholar] [CrossRef]
- Vivek, N.; Gopalan, N.; Das, S.; Sasikumar, K.; Sindhu, R.; Nampoothiri, K.M.; Pandey, A.; Binod, P. Synthesis and Characterization of Transparent Biodegradable Chitosan: Exopolysaccharide Composite Films Plasticized by Bio-Derived 1,3-Propanediol. Sustain. Chem. 2021, 2, 49–62. [Google Scholar] [CrossRef]
- Rodriguez, S.; Torres, F.G.; López, D. Preparation and Characterization of Polysaccharide Films from the Cyanobacteria Nostoc commune. Polym. Renew. Resour. 2017, 8, 133–150. [Google Scholar] [CrossRef]
- Silva, F.M.; Silva, C.L.M. Colour changes in thermally processed cupuaçu (Theobroma grandiflorum) puree: Critical times and kinetics modelling. Int. J. Food Sci. Technol. 1999, 34, 87–94. [Google Scholar] [CrossRef]
- Tomé, L.C.; Silva, N.H.C.S.; Soares, H.R.; Coroadinha, A.S.; Sadocco, P.; Marrucho, I.M.; Freire, C.S.R. Bioactive transparent films based on polysaccharides and cholinium carboxylate ionic liquids. Green Chem. 2015, 17, 4291–4299. [Google Scholar] [CrossRef]
- Pagano, C.; Puglia, D.; Luzi, F.; Michele, A.D.; Scuota, S.; Primavilla, S.; Ceccarini, M.R.; Beccari, T.; Iborra, C.A.V.; Ramella, D.; et al. Development and Characterization of Xanthan Gum and Alginate Based Bioadhesive Film for Pycnogenol Topical Use in Wound Treatment. Pharmaceutics 2021, 13, 324. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yang, J.; Duan, A.; Li, X. Pectin/sodium alginate/xanthan gum edible composite films as the fresh-cut package. Int. J. Biol. Macromol. 2021, 181, 1003–1009. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Gouveia, A.R.; Pinheiro, C.; Torres, C.A.V.; Grandfils, C.; Reis, M.A.M. Controlled Production of Exopolysaccharides from Enterobacter A47 as a Function of Carbon Source with Demonstration of Their Film and Emulsifying Abilities. Appl. Biochem. Biotechnol. 2014, 172, 641–657. [Google Scholar] [CrossRef] [PubMed]
- Tomé, L.C.; Gonçalves, C.M.B.; Boaventura, M.; Brandão, L.; Mendes, A.M.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A.; Freire, C.S.R.; Marrucho, I.M. Preparation and evaluation of the barrier properties of cellophane membranes modified with fatty acids. Carbohydr. Polym. 2011, 83, 836–842. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Polysaccharides: Sources, Characteristics, Properties, and Their Application in Biodegradable Films. Polysaccharides 2022, 3, 480–501. [Google Scholar] [CrossRef]
- Rodríguez, S.; Gatto, F.; Pesce, L.; Canale, C.; Pompa, P.P.; Bardi, G.; Lopez, D.; Torres, F.G. Monitoring cell substrate interactions in exopolysaccharide-based films reinforced with chitin whiskers and starch nanoparticles used as cell substrates. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 333–339. [Google Scholar] [CrossRef]
- Yang, L.; Paulson, A.T.; Nickerson, M.T. Mechanical and physical properties of calcium-treated gellan films. Food Res. Int. 2010, 43, 1439–1443. [Google Scholar] [CrossRef]
- Olivas, G.I.; Barbosa-Cánovas, G.V. Alginate–calcium films: Water vapor permeability and mechanical properties as affected by plasticizer and relative humidity. LWT Food Sci. Technol. 2008, 41, 359–366. [Google Scholar] [CrossRef]
- Kim, S.; Cho, D.-H.; Kweon, D.-K.; Jang, E.-H.; Hong, J.-Y.; Lim, S.-T. Improvement of mechanical properties of orodispersible hyaluronic acid film by carboxymethyl cellulose addition. Food Sci. Biotechnol. 2020, 29, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Grumezescu, A.M. An Up-to-Date Review of Biomaterials Application in Wound Management. Polymers 2022, 14, 421. [Google Scholar] [CrossRef] [PubMed]
- Lagaron, J.M.; Catalá, R.; Gavara, R. Structural characteristics defining high barrier properties in polymeric materials. Mater. Sci. Technol. 2004, 20, 1–7. [Google Scholar] [CrossRef]
- Salvada, J.; Alke, B.; Brazinha, C.; Alves, V.D.; Coelhoso, I.M. Development and Characterisation of Arabinoxylan-Based Composite Films. Coatings 2022, 12, 813. [Google Scholar] [CrossRef]
- Bastarrachea, L.; Dhawan, S.; Sablani, S.S. Engineering Properties of Polymeric-Based Antimicrobial Films for Food Packaging: A Review. Food Eng. Rev. 2011, 3, 79–93. [Google Scholar] [CrossRef]
 ), B (
), B ( ), C (
), C ( ), D (
), D ( ), E (
), E ( ), and F (
), and F (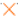 ), produced by Alteromonas strains isolated from French Polynesia (1.5 wt.% EPS, 30 wt.% glycerol for all solutions, except EPS A that was prepared with 60 wt.% glycerol).
), produced by Alteromonas strains isolated from French Polynesia (1.5 wt.% EPS, 30 wt.% glycerol for all solutions, except EPS A that was prepared with 60 wt.% glycerol).
 ), B (
), B ( ), C (
), C ( ), D (
), D ( ), E (
), E ( ), and F (
), and F ( ), produced by Alteromonas strains isolated from French Polynesia (1.5 wt.% EPS, 30 wt.% glycerol for all solutions, except EPS A that was prepared with 60 wt.% glycerol).
), produced by Alteromonas strains isolated from French Polynesia (1.5 wt.% EPS, 30 wt.% glycerol for all solutions, except EPS A that was prepared with 60 wt.% glycerol).



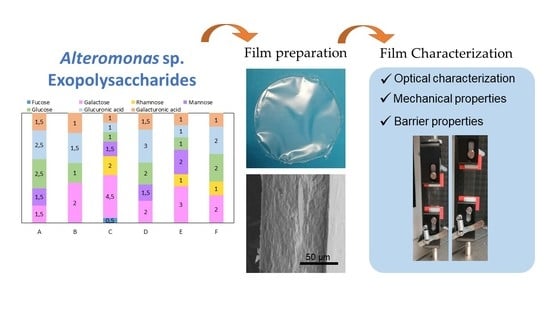
| EPS | Monosaccharide Composition (Molar Ratio) | Acyl Groups (wt%) | Sulphate (wt%) | Protein (wt%) | Inorganic Content (wt%) | Mw (MDa) | PDI | Tdeg (°C) |
|---|---|---|---|---|---|---|---|---|
| A | Glc:GlcA:Man:Gal:GalA (2.5:2.5:1.5:1.5:1.5) | Ac (0.5 ± 0.1) Pyr (4.9 ± 0.1) | 2.8 ± 0.1 | 2.8 ± 0.4 | 7.7 ± 1.5 | 1.6 4.6 1 | 1.3 1.3 1 | 269 |
| B | Gal:GlcA:Glc:GalA (2:1.5:1:1) | Ac (0.5 ± 0.0) Pyr (0.2 ± 0.0) Suc (1.8 ± 0.1) | 3.3 ± 0.3 | 3.5 ± 0.1 | 20.0 ± 1.7 | 4.6 | 1.4 | 265 |
| C | Gal:Rha:Man:Glc:GlcA:GalA:Fuc (4.5:2:1.5:1:1:1:0.5) | Pyr (1.1 ± 0.2) | 3.4 ±0.0 | 2.6 ± 1.0 | 34.7 ± 0.2 | 1.2 | 1.4 | 268 |
| D | GlcA:Glc:Gal:Man:GalA (3:2:2:1.5:1.5) | Ac (2.1 ± 0.0) | 2.0 ± 0.0 | 6.8 ± 0.2 | 11.4 ± 0.3 | 1.4 | 1.5 | 262 |
| E | Gal:Man:Rha:Glc:GlcA:GalA (3:2:1:1:1:1) | Suc (1.0 ± 0.0) | 2.9 ± 0.0 | 2.1 ± 0.3 | 15.0 ± 0.1 | 2.5 4.3 1 | 1.1 1.5 1 | 267 |
| F | Glc:Gal:GlcA:Rha:GalA (2:2:2:1:1) | Ac (0.7 ± 0.0) Pyr (5.5 ± 0.3) | 3.4 ± 0.0 | 1.7 ± 0.0 | 13.7 ± 0.3 | 3.2 | 1.2 | 260 |
| EPS | η0 (Pa.s) | n (-) | λ (s) | r2 |
|---|---|---|---|---|
| A | 59.2 ± 1.1 | 0.374 ± 0.036 | 22.6 ± 2.5 | 0.996 |
| B | 16.4 ± 0.3 | 0.329 ± 0.050 | 8.30 ± 1.00 | 0.995 |
| C | 8.06 ± 0.15 | 0.549 ± 0.008 | 25.3 ± 1.9 | 0.999 |
| D | 4.40 ± 0.08 | 0.474 ± 0.028 | 5.95 ± 0.69 | 0.996 |
| E | 21.9 ± 0.3 | 0.463 ± 0.014 | 27.2 ± 1.9 | 0.999 |
| F | 0.79 ± 0.01 | 0.667 ± 0.010 | 9.79 ± 1.04 | 0.998 |
| Colour | Parameter | EPS | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | ||
| White | a* | −0.80 ± 0.01 | −0.72 ± 0.02 | −0.80 ± 0.01 | −0.86 ± 0.05 | −0.84 ± 0.01 | −0.89 ± 0.07 |
| b* | 5.4 ± 0.1 | 4.6 ± 0.0 | 5.0 ± 0.0 | 5.0 ± 0.5 | 4.8 ± 0.1 | 5.4 ± 0.4 | |
| L* | 92.8 ± 0.1 | 93.1 ± 0.1 | 93.3 ± 0.1 | 93.4 ± 0.4 | 93.4 ± 0.0 | 92.9 ± 0.5 | |
| ΔEab | 2.5 ± 0.1 | 1.8 ± 0.1 | 1.9 ± 0.0 | 1.8 ± 0.7 | 1.7 ± 0.0 | 2.4 ± 0.7 | |
| Green | a* | −34.1 ± 0.0 | −33.9 ± 0.1 | −32.9 ± 0.3 | −33.8 ± 0.2 | −33.4 ± 0.0 | −33.7 ± 0.2 |
| b* | 32.7 ± 0.0 | 32.2 ± 0.0 | 31.3 ± 0.3 | 32.1 ± 0.2 | 31.9 ± 0.1 | 32.3 ± 0.1 | |
| L* | 69.2 ± 0.0 | 69.7 ± 0.0 | 70.1 ± 0.1 | 69.9 ± 0.1 | 70.2 ± 0.0 | 69.4 ± 0.1 | |
| ΔEab | 9.1 ± 0.0 | 9.6 ± 0.1 | 11.0 ± 0.4 | 9.8 ± 0.3 | 10.3 ± 0.1 | 9.7 ± 0.2 | |
| Blue | a* | −14.1 ± 0.1 | −13.5 ± 0.1 | −13.7 ± 0.0 | −13.9 ± 0.0 | −13.5 ± 0.1 | −13.7 ± 0.1 |
| b* | −14.8 ± 0.3 | −14.4 ± 0.1 | −14.1 ± 0.1 | −14.2 ± 0.0 | −14.0 ± 0.1 | −13.3 ± 0.1 | |
| L* | 58.7 ± 0.0 | 58.9 ± 0.0 | 58.7 ± 0.2 | 58.2 ± 0.3 | 59.2 ± 0.4 | 57.7 ± 0.4 | |
| ΔEab | 3.3 ± 0.3 | 4.0 ± 0.1 | 4.1 ± 0.1 | 3.9 ± 0.0 | 4.4 ± 0.2 | 4.8 ± 0.1 | |
| Yellow | a* | −5.8 ± 0.1 | −6.1 ± 0.0 | −5.9 ± 0.0 | −6.3 ± 0.1 | −6.1 ± 0.0 | −6.0 ± 0.2 |
| b* | 63.7 ± 0.0 | 63.1 ± 0.0 | 61.5 ± 0.1 | 63.5 ± 0.2 | 62.9 ± 0.1 | 63.3 ± 0.5 | |
| L* | 88.4 ± 0.2 | 89.0 ± 0.1 | 89.0 ± 0.1 | 89.2 ± 0.1 | 89.3 ± 0.0 | 88.7 ± 0.2 | |
| ΔEab | 9.2 ± 0.1 | 9.6 ± 0.0 | 11.2 ± 0.1 | 9.2 ± 0.2 | 9.8 ± 0.1 | 9.5 ± 0.5 | |
| Red | a* | 41.3 ± 0.2 | 40.7 ± 0.1 | 39.6 ± 0.1 | 40.9 ± 0.6 | 40.5 ± 0.2 | 40.7 ± 0.1 |
| b* | 24.8 ± 0.1 | 24.1 ± 0.0 | 23.1 ± 0.1 | 24.0 ± 0.3 | 23.8 ± 0.1 | 24.4 ± 0.2 | |
| L* | 59.3 ± 0.0 | 59.9 ± 0.0 | 60.5 ± 0.0 | 60.7 ± 0.8 | 60.4 ± 0.1 | 59.7 ± 0.2 | |
| ΔEab | 10.0 ± 0.2 | 11.0 ± 0.1 | 12.6 ± 0.2 | 11.2 ± 1.1 | 11.5 ± 0.2 | 10.8 ± 0.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Concórdio-Reis, P.; Pereira, J.R.; Alves, V.D.; Nabais, A.R.; Neves, L.A.; Marques, A.C.; Fortunato, E.; Moppert, X.; Guézennec, J.; Reis, M.A.M.; et al. Characterisation of Films Based on Exopolysaccharides from Alteromonas Strains Isolated from French Polynesia Marine Environments. Polymers 2022, 14, 4442. https://doi.org/10.3390/polym14204442
Concórdio-Reis P, Pereira JR, Alves VD, Nabais AR, Neves LA, Marques AC, Fortunato E, Moppert X, Guézennec J, Reis MAM, et al. Characterisation of Films Based on Exopolysaccharides from Alteromonas Strains Isolated from French Polynesia Marine Environments. Polymers. 2022; 14(20):4442. https://doi.org/10.3390/polym14204442
Chicago/Turabian StyleConcórdio-Reis, Patrícia, João R. Pereira, Vítor D. Alves, Ana R. Nabais, Luísa A. Neves, Ana C. Marques, Elvira Fortunato, Xavier Moppert, Jean Guézennec, Maria A.M. Reis, and et al. 2022. "Characterisation of Films Based on Exopolysaccharides from Alteromonas Strains Isolated from French Polynesia Marine Environments" Polymers 14, no. 20: 4442. https://doi.org/10.3390/polym14204442
APA StyleConcórdio-Reis, P., Pereira, J. R., Alves, V. D., Nabais, A. R., Neves, L. A., Marques, A. C., Fortunato, E., Moppert, X., Guézennec, J., Reis, M. A. M., & Freitas, F. (2022). Characterisation of Films Based on Exopolysaccharides from Alteromonas Strains Isolated from French Polynesia Marine Environments. Polymers, 14(20), 4442. https://doi.org/10.3390/polym14204442