Stimuli-Responsive Polysaccharide Hydrogels and Their Composites for Wound Healing Applications
Abstract
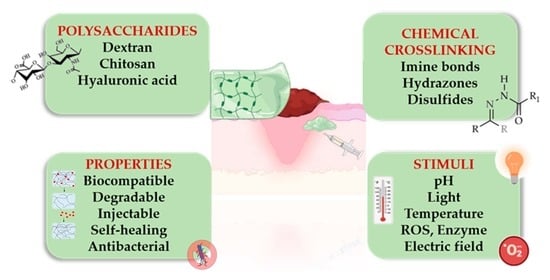
1. Introduction
2. Chemical Functionalization of Polysaccharides
2.1. Oxidation of Polysaccharides
2.2. Modification of Chitin and Chitosan
2.2.1. Quaternization of Chitosan

2.2.2. Carboxyalkylation of Chitosan
2.2.3. Etherification of Chitosan
3. Stimuli-Responsive Polysaccharide-Based Hydrogels
3.1. pH-Responsive Polysaccharide Hydrogels
3.2. Redox-Responsive Polysaccharide Hydrogels
3.3. Photo-Responsive Polysaccharide Hydrogels
3.4. Dual Responsive Hydrogels
3.4.1. pH- and Temperature-Responsive Polysaccharide Hydrogels
3.4.2. pH- and ROS-Responsive Polysaccharide Hydrogels
3.4.3. pH- and Electro-Responsive Polysaccharide Hydrogels
4. Polysaccharide-Peptide Composites with Cell Instructive/Responsive Properties
5. Applications in Wound Healing/Dressings
5.1. pH-Responsive Polysaccharide Hydrogels
5.2. Thermoresponsive Polysaccharide Hydrogels
5.3. Enzyme-Responsive Polysaccharide Hydrogels
5.4. Redox-Responsive Polysaccharide Hydrogels
5.5. Photo-Responsive Polysaccharide Hydrogels
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Name |
| ROS | reactive oxygen species |
| GSH | glutathione |
| CS | chitosan |
| QCS | quaternized chitosan |
| HBCS | hydroxybutyl chitosan |
| CMCS | carboxymethyl chitosan |
| GTMAC | glycidyl trimethyl ammonium chloride |
| OHA | oxidized hyaluronic acid |
| PEG | poly(ethylene glycol) |
| HPC | hydroxypropyl cellulose |
| OCAPS | octa (γ-chloroammoniumpropyl) silsesquioxane |
| rGO | reduced graphene oxide |
| TEOS | tetraethyl orthosilicate |
| OD | oxidized dextran |
| HTCC | N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride |
| HA-ADH | dihydrazide hyaluronic acid |
| BBH | berberine |
| EGF | epidermal growth factor |
| PEDOT: PSS | poly(3, 4-ethylenedioxythiophene):poly(styrene sulfonate) |
| PF127-CHO | aldehyde pluronic F127 |
| OD-DA | oxidized dextran |
| DFO | deferoxamine |
| CECS | carboxyethyl chitosan |
| ADH | adipic acid dihydrazide |
| AEDa | Aminoethyl disulfide |
| β-CD | β-cyclodextrin |
| HA-CD | HA conjugated with β-cyclodextrin |
| HA-Azo | HA conjugated with trans-azobenzene |
| APu | aldehyde pullulan |
| PEI | polyethylenimine |
| ADSC | adipose mesenchymal stem cell |
| HPCH | hydroxypropyl chitin |
| TA | tannic acid |
| ALG-BA | alginate backbone |
| CHOL | cholesterol |
| CP | chitosan-g-polyaniline |
| RGD | arginine-glycine-aspartate |
| CM-TM-CS | carboxymethyl-trimethyl chitosan |
| GelMA | gelatin methacrylamide |
| MMPs | matrix metalloproteinases |
| HIF-1α | factor-1 alpha |
| VEGF | vascular endothelial growth factor |
| SS | sisomicin sulfate |
| AgNPs | silver nanoparticles |
| PB | poly(vinyl alcohol)-borax |
| OCMC-DA | dopamine grafted oxidized carboxymethyl cellulose |
| EPL | ε-poly-L-lysine |
| KGF | keratinocyte growth factor |
| HP | heparin-modified poloxamer |
| FEP | fluorinated ethylene propylene |
| EDTA | ethylenediaminetetraacetic acid |
| ZIF-8 | zeolite imidazole framework-8 |
| PEG-TK | PEG-thioketal |
| BA | boric acid |
| HA | hyaluronic acid |
| NIR | Near infrared |
| WS2-NS2 | tungsten disulphide nanosheets |
References
- Reinke, J.M.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Kruse, C.R.; Nuutila, K.; Lee, C.C.Y.; Kiwanuka, E.; Singh, M.; Caterson, E.J.; Eriksson, E.; Sørensen, J.A. The External Microenvironment of Healing Skin Wounds. Wound Repair Regen. 2015, 23, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Laurens, N.; Koolwijk, P.; De Maat, M.P.M. Fibrin Structure and Wound Healing. J. Thromb. Haemost. 2006, 4, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, W.; Li, N.; Wang, M.; Liang, B.; Ullah, I.; Luis Neve, A.; Feng, Y.; Chen, H.; Shi, C. Design and Development of Polysaccharide Hemostatic Materials and Their Hemostatic Mechanism. Biomater. Sci. 2017, 5, 2357–2368. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive Oxygen Species (ROS) and Wound Healing: The Functional Role of ROS and Emerging ROS-Modulating Technologies for Augmentation of the Healing Process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Gao, Z.; Golland, B.; Tronci, G.; Thornton, P.D. A Redox-Responsive Hyaluronic Acid-Based Hydrogel for Chronic Wound Management. J. Mater. Chem. B 2019, 7, 7494–7501. [Google Scholar] [CrossRef]
- Mallinjoud, P.; Villemin, J.-P.; Mortada, H.; Polay Espinoza, M.; Desmet, F.-O.; Samaan, S.; Chautard, E.; Tranchevent, L.-C.; Auboeuf, D. Endothelial, Epithelial, and Fibroblast Cells Exhibit Specific Splicing Programs Independently of Their Tissue of Origin. Genome Res. 2014, 24, 511–521. [Google Scholar] [CrossRef]
- Farasati Far, B.; Naimi-Jamal, M.R.; Safaei, M.; Zarei, K.; Moradi, M.; Yazdani Nezhad, H. A Review on Biomedical Application of Polysaccharide-Based Hydrogels with a Focus on Drug Delivery Systems. Polymers 2022, 14, 5432. [Google Scholar] [CrossRef]
- Ribeiro, D.M.L.; Júnior, A.R.C.; de Macedo, G.H.R.V.; Chagas, V.L.; Silva, L.D.S.; da Silva Cutrim, B.; Santos, D.M.; Soares, B.L.L.; Zagmignan, A.; de Miranda, R.d.C.M.; et al. Polysaccharide-Based Formulations for Healing of Skin-Related Wound Infections: Lessons from Animal Models and Clinical Trials. Biomolecules 2020, 10, 63. [Google Scholar] [CrossRef]
- Kaliva, M.; Kavasi, R.; Chatzinikolaidou, M.; Vamvakaki, M. Book chapter 4.01 Polysaccharides and Applications in Regenerative Medicine. In Comprehensive Glycoscience (Second Edition); Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–33. ISBN 9780128194751. [Google Scholar] [CrossRef]
- Farahani, M.; Shafiee, A. Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021, 10, 2100477. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Xu, F.J. Rational Design and Latest Advances of Polysaccharide-Based Hydrogels for Wound Healing. Biomater. Sci. 2020, 8, 2084–2101. [Google Scholar] [CrossRef] [PubMed]
- Gholamali, I. Stimuli-Responsive Polysaccharide Hydrogels for Biomedical Applications: A Review. Regen. Eng. Transl. Med. 2021, 7, 91–114. [Google Scholar] [CrossRef]
- Mukherjee, I. Recent Development of Polysaccharide-Derived Hydrogel: Properties, Stimuli-Responsiveness and Bioapplications. ChemRxiv. Cambridge Cambridge Open Engag. 2022. [Google Scholar] [CrossRef]
- Rial-Hermida, M.I.; Rey-Rico, A.; Blanco-Fernandez, B.; Carballo-Pedrares, N.; Byrne, E.M.; Mano, J.F. Recent Progress on Polysaccharide-Based Hydrogels for Controlled Delivery of Therapeutic Biomolecules. ACS Biomater. Sci. Eng. 2021, 7, 4102–4127. [Google Scholar] [CrossRef]
- Taylor, D.L.; in het Panhuis, M. Self-Healing Hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Li, T.; Zhang, J.; Tian, H. Stimuli-Responsive Hydrogels: Fabrication and Biomedical Applications. VIEW 2022, 3, 20200112. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Sun, Y.; Zhao, S.; Feng, M.; Xu, G.; Zhu, H.; Ji, P.; Mao, H.; He, Y.; et al. A Double-Network Polysaccharide-Based Composite Hydrogel for Skin Wound Healing. Carbohydr. Polym. 2021, 261, 117870. [Google Scholar] [CrossRef]
- Wang, X.; Xu, P.; Yao, Z.; Fang, Q.; Feng, L.; Guo, R.; Cheng, B. Preparation of Antimicrobial Hyaluronic Acid/Quaternized Chitosan Hydrogels for the Promotion of Seawater-Immersion Wound Healing. Front. Bioeng. Biotechnol. 2019, 7, 360. [Google Scholar] [CrossRef]
- Mao, Q.; Hoffmann, O.; Yu, K.; Lu, F.; Lan, G.; Dai, F.; Shang, S.; Xie, R. Self-Contracting Oxidized Starch/Gelatin Hydrogel for Noninvasive Wound Closure and Wound Healing. Mater. Des. 2020, 194, 108916. [Google Scholar] [CrossRef]
- Kang, M.; Oderinde, O.; Han, X.; Fu, G.; Zhang, Z. Development of Oxidized Hydroxyethyl Cellulose-Based Hydrogel Enabling Unique Mechanical, Transparent and Photochromic Properties for Contact Lenses. Int. J. Biol. Macromol. 2021, 183, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Rahmat, D.; Müller, C.; Barthelmes, J.; Shahnaz, G.; Martien, R.; Bernkop-Schnürch, A. Thiolated Hydroxyethyl Cellulose: Design and in Vitro Evaluation of Mucoadhesive and Permeation Enhancing Nanoparticles. Eur. J. Pharm. Biopharm. 2013, 83, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wang, C.; Lai, R.C.; Su, K.; Zhang, F.; Wang, D. An Improved Injectable Polysaccharide Hydrogel: Modified Gellan Gum for Long-Term Cartilage Regenerationin Vitro. J. Mater. Chem. 2009, 19, 1968–1977. [Google Scholar] [CrossRef]
- Bacelar, A.H.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Recent Progress in Gellan Gum Hydrogels Provided by Functionalization Strategies. J. Mater. Chem. B 2016, 4, 6164–6174. [Google Scholar] [CrossRef]
- Ijaz, M.; Matuszczak, B.; Rahmat, D.; Mahmood, A.; Bonengel, S.; Hussain, S.; Huck, C.W.; Bernkop-Schnürch, A. Synthesis and Characterization of Thiolated β-Cyclodextrin as a Novel Mucoadhesive Excipient for Intra-Oral Drug Delivery. Carbohydr. Polym. 2015, 132, 187–195. [Google Scholar] [CrossRef]
- Kobayashi, M.; Urayama, T.; Suzawa, I.; Takagi, S.; Matsuda, K.; Ichishima, E. Cyclodextrin–Dialdehyde Prepared by Periodate Oxidation. Agric. Biol. Chem. 1988, 52, 2695–2702. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial Anti-Oxidant Electroactive Injectable Hydrogel as Self-Healing Wound Dressing with Hemostasis and Adhesiveness for Cutaneous Wound Healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Sun, M.; Wang, T.; Pang, J.; Chen, X.; Liu, Y. Hydroxybutyl Chitosan Centered Biocomposites for Potential Curative Applications: A Critical Review. Biomacromolecules 2020, 21, 1351–1367. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, W.; Zhang, H.; Zheng, W.; Zhou, Q. Carboxymethyl Chitosan-Based Hydrogels Containing Fibroblast Growth Factors for Triggering Diabetic Wound Healing. Carbohydr. Polym. 2022, 287, 119336. [Google Scholar] [CrossRef]
- Li, X.; Weng, Y.; Kong, X.; Zhang, B.; Li, M.; Diao, K.; Zhang, Z.; Wang, X.; Chen, H. A Covalently Crosslinked Polysaccharide Hydrogel for Potential Applications in Drug Delivery and Tissue Engineering. J. Mater. Sci. Mater. Med. 2012, 23, 2857–2865. [Google Scholar] [CrossRef]
- Xue, C.; Xu, X.; Zhang, L.; Liu, Y.; Liu, S.; Liu, Z.; Wu, M.; Shuai, Q. Self-Healing/PH-Responsive/Inherently Antibacterial Polysaccharide-Based Hydrogel for a Photothermal Strengthened Wound Dressing. Colloids Surf. B Biointerfaces 2022, 218, 112738. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, B.; Li, M.; He, J.; Yin, Z.; Guo, B. Injectable Antimicrobial Conductive Hydrogels for Wound Disinfection and Infectious Wound Healing. Biomacromolecules 2020, 21, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Andreica, B.-I.; Cheng, X.; Marin, L. Quaternary Ammonium Salts of Chitosan. A Critical Overview on the Synthesis and Properties Generated by Quaternization. Eur. Polym. J. 2020, 139, 110016. [Google Scholar] [CrossRef]
- Jain, A.; Gulbake, A.; Shilpi, S.; Jain, A.; Hurkat, P.; Jain, S.K. A New Horizon in Modifications of Chitosan: Syntheses and Applications. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30, 91–181. [Google Scholar] [CrossRef] [PubMed]
- Mourya, V.K.; Inamdara, N.; Ashutosh Tiwari, N. Carboxymethyl Chitosan And Its Applications. Adv. Mater. Lett. 2010, 1, 11–33. [Google Scholar] [CrossRef]
- Li, J.; Yu, F.; Chen, G.; Liu, J.; Li, X.-L.; Cheng, B.; Mo, X.-M.; Chen, C.; Pan, J.-F. Moist-Retaining, Self-Recoverable, Bioadhesive, and Transparent in Situ Forming Hydrogels To Accelerate Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 2023–2038. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Liu, H.; Ren, M.; Wang, Z.; Wang, X.; Liu, H.; Feng, Y.; Lin, Q.; Wang, C.; et al. PH-Responsive Hydrogel Loaded with Insulin as a Bioactive Dressing for Enhancing Diabetic Wound Healing. Mater. Des. 2021, 210, 110104. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable Conductive Injectable Hydrogels as Novel Antibacterial, Anti-Oxidant Wound Dressings for Wound Healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Biagini, G.; Bertani, A.; Muzzarelli, R.; Damadei, A.; DiBenedetto, G.; Belligolli, A.; Riccotti, G.; Zucchini, C.; Rizzoli, C. Wound Management with N-Carboxybutyl Chitosan. Biomaterials 1991, 12, 281–286. [Google Scholar] [CrossRef]
- Cai, Y.; Zhong, Z.; He, C.; Xia, H.; Hu, Q.; Wang, Y.; Ye, Q.; Zhou, J. Homogeneously Synthesized Hydroxybutyl Chitosans in Alkali/Urea Aqueous Solutions as Potential Wound Dressings. ACS Appl. Bio Mater. 2019, 2, 4291–4302. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Kong, M.; An, Y.; Liu, Y.; Li, J.J.; Zhou, X.; Feng, C.; Li, J.; Jiang, S.Y.; Cheng, X.J.; et al. Hydroxybutyl Chitosan Thermo-Sensitive Hydrogel: A Potential Drug Delivery System. J. Mater. Sci. 2013, 48, 5614–5623. [Google Scholar] [CrossRef]
- Bi, S.; Hu, S.; Zhou, Z.; Kong, M.; Liu, Y.; Feng, C.; Cheng, X.; Chen, X. The Green and Stable Dissolving System Based on KOH/Urea for Homogeneous Chemical Modification of Chitosan. Int. J. Biol. Macromol. 2018, 120, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Bi, B.; Ma, M.; Lv, S.; Zhuo, R.; Jiang, X. In-Situ Forming Thermosensitive Hydroxypropyl Chitin-Based Hydrogel Crosslinked by Diels-Alder Reaction for Three Dimensional Cell Culture. Carbohydr. Polym. 2019, 212, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhong, Y.; Jiang, X. Thermosensitive and PH-Responsive Tannin-Containing Hydroxypropyl Chitin Hydrogel with Long-Lasting Antibacterial Activity for Wound Healing. Carbohydr. Polym. 2020, 236, 116096. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Bi, B.; Huang, J.; Zhuo, R.; Jiang, X. Thermosensitive and Photocrosslinkable Hydroxypropyl Chitin-Based Hydrogels for Biomedical Applications. Carbohydr. Polym. 2018, 192, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Gao, M.; Boakye-Yiadom, K.O.; Ho, W.; Yu, W.; Xu, X.; Zhang, X.Q. An Intrinsically Bioactive Hydrogel with On-Demand Drug Release Behaviors for Diabetic Wound Healing. Bioact. Mater. 2021, 6, 4592–4606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Meng, Y.; Shen, W.; Dou, J.; Liu, R.; Jin, Q.; Fang, S. PH-Responsive Injectable Polysaccharide Hydrogels with Self-Healing, Enhanced Mechanical Properties Based on POSS. React. Funct. Polym. 2021, 158, 104773. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Abd Razaq, S.I.; Mehboob, H.; Rehman, S.; Al-Arjan, W.S.; Amin, R. Antibacterial and Hemocompatible Ph-Responsive Hydrogel for Skin Wound Healing Application: In Vitro Drug Release. Polymers 2021, 13, 3703. [Google Scholar] [CrossRef]
- Hoque, J.; Prakash, R.G.; Paramanandham, K.; Shome, B.R.; Haldar, J. Biocompatible Injectable Hydrogel with Potent Wound Healing and Antibacterial Properties. Mol. Pharm. 2017, 14, 1218–1230. [Google Scholar] [CrossRef]
- Guan, S.; Li, Y.; Cheng, C.; Gao, X.; Gu, X.; Han, X.; Ye, H. Manufacture of PH- and HAase-Responsive Hydrogels with on-Demand and Continuous Antibacterial Activity for Full-Thickness Wound Healing. Int. J. Biol. Macromol. 2020, 164, 2418–2431. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial Adhesive Injectable Hydrogels with Rapid Self-Healing, Extensibility and Compressibility as Wound Dressing for Joints Skin Wound Healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Long, L.; Cao, J.; Zhang, S.; Wang, Y. Dual-Crosslinked Mussel-Inspired Smart Hydrogels with Enhanced Antibacterial and Angiogenic Properties for Chronic Infected Diabetic Wound Treatment via PH-Responsive Quick Cargo Release. Chem. Eng. J. 2021, 411, 128564. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Y.; Zhang, X.; Zhang, R.; Hu, Y.; Boyer, C.; Xu, F.J. Photo-Responsive Supramolecular Hyaluronic Acid Hydrogels for Accelerated Wound Healing. J. Control. Release 2020, 323, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Klouda, L.; Mikos, A.G. Thermoresponsive Hydrogels in Biomedical Applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, C.; Chen, M.; Xi, Y.; Cheng, W.; Mao, C.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; et al. Efficient Angiogenesis-Based Diabetic Wound Healing/Skin Reconstruction through Bioactive Antibacterial Adhesive Ultraviolet Shielding Nanodressing with Exosome Release. ACS Nano 2019, 13, 10279–10293. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, F.; Long, L.; Kong, Q.; Luo, R.; Wang, Y. Dual-Responsive Injectable Hydrogels Encapsulating Drug-Loaded Micelles for on-Demand Antimicrobial Activity and Accelerated Wound Healing. J. Control. Release 2020, 324, 204–217. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable Antibacterial Conductive Hydrogels with Dual Response to an Electric Field and PH for Localized “Smart” Drug Release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef]
- Deidda, G.; Jonnalagadda, S.V.R.; Spies, J.W.; Ranella, A.; Mossou, E.; Forsyth, V.T.; Mitchell, E.P.; Bowler, M.W.; Tamamis, P.; Mitraki, A. Self-Assembled Amyloid Peptides with Arg-Gly-Asp (RGD) Motifs As Scaffolds for Tissue Engineering. ACS Biomater. Sci. Eng. 2017, 3, 1404–1416. [Google Scholar] [CrossRef]
- Kumar, V.B.; Tiwari, O.S.; Finkelstein-Zuta, G.; Rencus-Lazar, S.; Gazit, E. Design of Functional RGD Peptide-Based Biomaterials for Tissue Engineering. Pharmaceutics 2023, 15, 345. [Google Scholar] [CrossRef]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate Hydrogels as Synthetic Extracellular Matrix Materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Hansson, A.; Hashom, N.; Falson, F.; Rousselle, P.; Jordan, O.; Borchard, G. In Vitro Evaluation of an RGD-Functionalized Chitosan Derivative for Enhanced Cell Adhesion. Carbohydr. Polym. 2012, 90, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Oki, Y.; Kirita, K.; Ohta, S.; Ohba, S.; Horiguchi, I.; Sakai, Y.; Ito, T. Switching of Cell Proliferation/Differentiation in Thiol-Maleimide Clickable Microcapsules Triggered by in Situ Conjugation of Biomimetic Peptides. Biomacromolecules 2019, 20, 2350–2359. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Pouraghaei Sevari, S.; Chen, C.; Sarrion, P.; Moshaverinia, A. RGD-Modified Alginate-GelMA Hydrogel Sheet Containing Gingival Mesenchymal Stem Cells: A Unique Platform for Wound Healing and Soft Tissue Regeneration. ACS Biomater. Sci. Eng. 2021, 7, 3774–3782. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, K.B.; Bidarra, S.J.; Oliveira, M.J.; Granja, P.L.; Barrias, C.C. Molecularly Designed Alginate Hydrogels Susceptible to Local Proteolysis as Three-Dimensional Cellular Microenvironments. Acta Biomater. 2011, 7, 1674–1682. [Google Scholar] [CrossRef]
- Suo, H.; Hussain, M.; Wang, H.; Zhou, N.; Tao, J.; Jiang, H.; Zhu, J. Injectable and PH-Sensitive Hyaluronic Acid-Based Hydrogels with On-Demand Release of Antimicrobial Peptides for Infected Wound Healing. Biomacromolecules 2021, 22, 3049–3059. [Google Scholar] [CrossRef]
- Gao, L.; Chen, J.; Feng, W.; Song, Q.; Huo, J.; Yu, L.; Liu, N.; Wang, T.; Li, P.; Huang, W. A Multifunctional Shape-Adaptive and Biodegradable Hydrogel with Hemorrhage Control and Broad-Spectrum Antimicrobial Activity for Wound Healing. Biomater. Sci. 2020, 8, 6930–6945. [Google Scholar] [CrossRef]
- Bennison, L.R.; Miller, C.N.; Summers, R.J.; Minnis, A.M.B.; Sussman, G.; McGuiness, W. The PH of Wounds during Healing and Infection: A Descriptive Literature Review. Wound Pract. Res. J. Aust. Wound Manag. Assoc. 2017, 25, 63–69. [Google Scholar]
- Zheng, C.; An, X.; Gong, J. Novel PH Sensitive N-Doped Carbon Dots with Both Long Fluorescence Lifetime and High Quantum Yield. RSC Adv. 2015, 5, 32319–32322. [Google Scholar] [CrossRef]
- Omidi, M.; Yadegari, A.; Tayebi, L. Wound Dressing Application of PH-Sensitive Carbon Dots/Chitosan Hydrogel. RSC Adv. 2017, 7, 10638–10649. [Google Scholar] [CrossRef]
- Ji, H.; Dong, K.; Yan, Z.; Ding, C.; Chen, Z.; Ren, J.; Qu, X. Bacterial Hyaluronidase Self-Triggered Prodrug Release for Chemo-Photothermal Synergistic Treatment of Bacterial Infection. Small 2016, 12, 6200–6206. [Google Scholar] [CrossRef]
- Fouda, M.M.G.; Abdel-Mohsen, A.M.; Ebaid, H.; Hassan, I.; Al-Tamimi, J.; Abdel-Rahman, R.M.; Metwalli, A.; Alhazza, I.; Rady, A.; El-Faham, A.; et al. Wound Healing of Different Molecular Weight of Hyaluronan; in-Vivo Study. Int. J. Biol. Macromol. 2016, 89, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Seidi, F.; Li, C.; Wan, Z.; Jin, Y.; Song, J.; Xiao, H. Antimicrobial/Biocompatible Hydrogels Dual-Reinforced by Cellulose as Ultrastretchable and Rapid Self-Healing Wound Dressing. Biomacromolecules 2021, 22, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-L.; Xu, J.; Shen, B.-X.; Zhang, S.-S.; Jin, B.-H.; Zhu, Q.-Y.; ZhuGe, D.-L.; Wu, X.-Q.; Xiao, J.; Zhao, Y.-Z. Dual Regulations of Thermosensitive Heparin–Poloxamer Hydrogel Using ε-Polylysine: Bioadhesivity and Controlled KGF Release for Enhancing Wound Healing of Endometrial Injury. ACS Appl. Mater. Interfaces 2017, 9, 29580–29594. [Google Scholar] [CrossRef]
- Wang, Q.; He, Y.; Zhao, Y.; Xie, H.; Lin, Q.; He, Z.; Wang, X.; Li, J.; Zhang, H.; Wang, C.; et al. A Thermosensitive Heparin-Poloxamer Hydrogel Bridges AFGF to Treat Spinal Cord Injury. ACS Appl. Mater. Interfaces 2017, 9, 6725–6745. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wildhaber, B.E.; Teitelbaum, D.H. Keratinocyte Growth Factor Improves Epithelial Function after Massive Small Bowel Resection. J. Parenter. Enter. Nutr. 2003, 27, 198–206. [Google Scholar] [CrossRef]
- Ernst, M.; John, T.; Guenther, M.; Wagner, C.; Schaefer, U.F.; Lehr, C.-M. A Model for the Transient Subdiffusive Behavior of Particles in Mucus. Biophys. J. 2017, 112, 172–179. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic Acid with Antiviral and Antibacterial Activity as A Promising Component of Biomaterials—A Minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef]
- Fan, H.; Wang, J.; Zhang, Q.; Jin, Z. Tannic Acid-Based Multifunctional Hydrogels with Facile Adjustable Adhesion and Cohesion Contributed by Polyphenol Supramolecular Chemistry. ACS Omega 2017, 2, 6668–6676. [Google Scholar] [CrossRef]
- Qi, X.; Mao, W.; Gao, Y.; Chen, Y.; Chen, Y.; Zhao, C.; Li, N.; Wang, C.; Yan, M.; Lin, C.; et al. Chemical Characteristic of an Anticoagulant-Active Sulfated Polysaccharide from Enteromorpha Clathrata. Carbohydr. Polym. 2012, 90, 1804–1810. [Google Scholar] [CrossRef]
- Guo, S.-C.; Tao, S.-C.; Yin, W.-J.; Qi, X.; Yuan, T.; Zhang, C.-Q. Exosomes Derived from Platelet-Rich Plasma Promote the Re-Epithelization of Chronic Cutaneous Wounds via Activation of YAP in a Diabetic Rat Model. Theranostics 2017, 7, 81–96. [Google Scholar] [CrossRef]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes Derived from Human Adipose Mensenchymal Stem Cells Accelerates Cutaneous Wound Healing via Optimizing the Characteristics of Fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef]
- Baek, K.; Liang, J.; Lim, W.T.; Zhao, H.; Kim, D.H.; Kong, H. In Situ Assembly of Antifouling/Bacterial Silver Nanoparticle-Hydrogel Composites with Controlled Particle Release and Matrix Softening. ACS Appl. Mater. Interfaces 2015, 7, 15359–15367. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Luu, Y.K.; Chang, C.; Fang, D.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Incorporation and Controlled Release of a Hydrophilic Antibiotic Using Poly(Lactide-Co-Glycolide)-Based Electrospun Nanofibrous Scaffolds. J. Control. Release 2004, 98, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Qiu, X.; Yuan, P.; Lei, K.; Wang, L.; Bai, Y.; Liu, S.; Chen, X. Fabrication of Self-Healing Hydrogels with On-Demand Antimicrobial Activity and Sustained Biomolecule Release for Infected Skin Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 17018–17027. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, X.; Ji, S.; Tian, S.; Wu, H.; Luo, P.; Fang, H.; Wang, L.; Wu, G.; Xiao, S.; et al. Mepenzolate Bromide Promotes Diabetic Wound Healing by Modulating Inflammation and Oxidative Stress. Am. J. Transl. Res. 2016, 8, 2738–2747. [Google Scholar] [PubMed]
- Wu, C.; Belenda, C.; Leroux, J.-C.; Gauthier, M.A. Interplay of Chemical Microenvironment and Redox Environment on Thiol–Disulfide Exchange Kinetics. Chem.-A Eur. J. 2011, 17, 10064–10070. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, Z.; Liu, Y.; Zhang, W.; Geng, L.; Ni, T. Incorporation of ROS-Responsive Substance P-Loaded Zeolite Imidazolate Framework-8 Nanoparticles into a Ca2+-Cross-Linked Alginate/Pectin Hydrogel for Wound Dressing Applications. Int. J. Nanomed. 2020, 15, 333–346. [Google Scholar] [CrossRef]
- Bertoni, S.; Liu, Z.; Correia, A.; Martins, J.P.; Rahikkala, A.; Fontana, F.; Kemell, M.; Liu, D.; Albertini, B.; Passerini, N.; et al. PH and Reactive Oxygen Species-Sequential Responsive Nano-in-Micro Composite for Targeted Therapy of Inflammatory Bowel Disease. Adv. Funct. Mater. 2018, 28, 1806175. [Google Scholar] [CrossRef]
- Chung, M.-F.; Chia, W.-T.; Wan, W.-L.; Lin, Y.-J.; Sung, H.-W. Controlled Release of an Anti-Inflammatory Drug Using an Ultrasensitive ROS-Responsive Gas-Generating Carrier for Localized Inflammation Inhibition. J. Am. Chem. Soc. 2015, 137, 12462–12465. [Google Scholar] [CrossRef]
- Šoltés, L.; Mendichi, R.; Kogan, G.; Schiller, J.; Stankovská, M.; Arnhold, J. Degradative Action of Reactive Oxygen Species on Hyaluronan. Biomacromolecules 2006, 7, 659–668. [Google Scholar] [CrossRef]
- Yang, N.; Zhu, M.; Xu, G.; Liu, N.; Yu, C. A Near-Infrared Light-Responsive Multifunctional Nanocomposite Hydrogel for Efficient and Synergistic Antibacterial Wound Therapy and Healing Promotion. J. Mater. Chem. B 2020, 8, 3908–3917. [Google Scholar] [CrossRef] [PubMed]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2014, 5, 119–136. [Google Scholar] [CrossRef] [PubMed]
- McCarty, S.M.; Percival, S.L. Proteases and Delayed Wound Healing. Adv. Wound Care 2013, 2, 438–447. [Google Scholar] [CrossRef] [PubMed]











| Natural Polysaccharides | Advantages | Disadvantages |
|---|---|---|
| Chitosan/chitin | Facile modification methods, Versatility of functionalization, Inherent antibacterial activity, low cost | Performances of different batches greatly vary Non-soluble at neutral conditions Non-FDA approved Weak mechanical properties |
| Hyaluronic acid | Biocompatible, biodegradable, FDA-approved Inherent antioxidative properties Facile modification methods Versatility of functionalization High water-holding capacity | Weak mechanical properties Poor tissue-adhesion Fast degradation rates Expensive |
| Dextran | Biocompatible, biodegradable, FDA-approved Resistance to protein adsorption Facile modification methods Versatility of functionalization High water holding/absorbing capacity Low cost | Weak mechanical properties Poor tissue adhesion |
| Sodium alginate | Biocompatible, water-soluble Facile modification methods Low cost | Weak mechanical properties |
| Cellulose | Good biocompatibility, non-toxic, good mechanical properties | Solubility issues Non-adherent |
| Natural Polysaccharide | Composite/Active Agent | Synthetic Pathway/ Crosslinking | Stimuli- Responsiveness | Wound Healing Application | Clinical and Non-Clinical Application | Reference |
|---|---|---|---|---|---|---|
| Chitosan | Carbon dots | Solvent casting method | pH-monitoring | Antibacterial | In vivo (rats) | [70] |
| Quaternized chitosan and dopamine-oxidized dextran | Silver NPs and deferoxamine | Imine bonds and catechol-catechol adducts | pH-responsive | Antibacterial Angiogenic | In vivo (rats) | [53] |
| Oxidized hyaluronic acid and dihydrazide hyaluronic acid | Sisomicin sulfate or quaternized chitosan | Imine and acylhydrazonebonds | pH and enzyme-responsive | Antimicrobial | In vivo (mice) | [51] |
| Dopamine-grafted oxidized carboxymethyl cellulose | Poly(vinyl alcohol)-borax, neomycin | Hydrogen, borate ester, and imine bonds | pH-responsive | Antibacterial antioxidant | In vitro | [73] |
| Heparin | Poloxamer, Keratinocyte growth factor, ε-polylysine | Electrostatic interactions | Thermo-responsive | Wound healing of endometrial injury | In vivo (rats) | [74] |
| Hydroxypropyl chitin | Tannic acidFe3+ ions | Hydrogen and coordination bonds | Thermo-responsive pH-responsive | Antibacterial | In vivo (mice) | [45] |
| Hyaluronic acid | Ethylenediaminetetraacetic acid (EDTA)−Fe3+ complexes Platelet-derived growth factor | Coordination interactions | Enzyme-responsive | Antibacterial cutaneous regeneration | In vivo (mice) | [85] |
| Hyaluronic acid | Glutathione, Aminoethyl disulfide | Amide bonds | Redox-responsive | Wound monitoringantioxidant fibroblast growth | In vitro | [7] |
| Sodium alginate pectin | ZIF-8 nanoparticles calcium chloride, neuro peptide (SP), PEG-thioketal | Ionic cross-linking | Redox responsive | Wound healing acceleration | In vivo (mice) | [88] |
| Hyaluronic acid-cholesterol and alginate-boronic acid | Naproxen, amikacin | Boronic ester | Redox and pH-responsive | Wound healing acceleration, antibacterial, anti- inflammation | In vivo (rats) | [57] |
| Chitosan | Tungsten disulfide nanosheets (WS2-NS2), ciprofloxacin | Imine bonds | Photo-responsive | Antimicrobial, hemostaticantioxidant | In vivo (mice) | [92] |
| Hyaluronic acid- β-cyclodextrin and hyaluronic acid-azobenze | Epidermal growth factor | Host–guest interactions | Photo-responsive | Angiogenesis, granulation tissue formation | In vivo (mice) | [54] |
| Quaternized chitosan, and oxidized hyaluronic acid | Epidermal growth factor PEDOT:PSS, berberine | Imine bonds | pH-responsive | Antibacterial, wound healing acceleration | In vivo (mice) | [32] |
| Quaternized chitosan | Pluronic®F127 (PF127-CHO), curcumin | Imine bonds | pH-responsive | Antibacterial, hemostatic | In vivo (mice) | [52] |
| N-narboxyethyl chitosan, oxidized hyaluronic acid | Adipic acid dihydrazide, insulin | Acylhydrazones and imine bonds | pH-responsive | Diabetic wound healing, Re-epithelization Angiogenesis, | In vivo (rats) | [38] |
| N-carboxyethyl chitosan and oxidized hyaluronic acid | Polyaniline, amoxicillin | Imine bonds | pH and electro-responsive | antibacterial, antioxidant, angiogenesis, collagen disposition | In vivo (mice) | [39] |
| Oxidized hyaluronic acid | Antimicrobial peptide KK(SLKL)3KK | Imine bonds | pH-responsive | Antibacterial | In vivo (mice) | [66] |
| Oxidized pullulan | Polyethyleneimine, Pluronic F127 | Imine bonds | pH and thermo responsive | Antibacterial, hemostatic, stimulate angiogenesis, remodelling and re-epithelialization | In vivo (mice) | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psarrou, M.; Mitraki, A.; Vamvakaki, M.; Kokotidou, C. Stimuli-Responsive Polysaccharide Hydrogels and Their Composites for Wound Healing Applications. Polymers 2023, 15, 986. https://doi.org/10.3390/polym15040986
Psarrou M, Mitraki A, Vamvakaki M, Kokotidou C. Stimuli-Responsive Polysaccharide Hydrogels and Their Composites for Wound Healing Applications. Polymers. 2023; 15(4):986. https://doi.org/10.3390/polym15040986
Chicago/Turabian StylePsarrou, Maria, Anna Mitraki, Maria Vamvakaki, and Chrysoula Kokotidou. 2023. "Stimuli-Responsive Polysaccharide Hydrogels and Their Composites for Wound Healing Applications" Polymers 15, no. 4: 986. https://doi.org/10.3390/polym15040986
APA StylePsarrou, M., Mitraki, A., Vamvakaki, M., & Kokotidou, C. (2023). Stimuli-Responsive Polysaccharide Hydrogels and Their Composites for Wound Healing Applications. Polymers, 15(4), 986. https://doi.org/10.3390/polym15040986