PVP/CS/Phyllanthus emblica Nanofiber Membranes for Dry Facial Masks: Manufacturing Process and Evaluations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Extraction of P. emblica
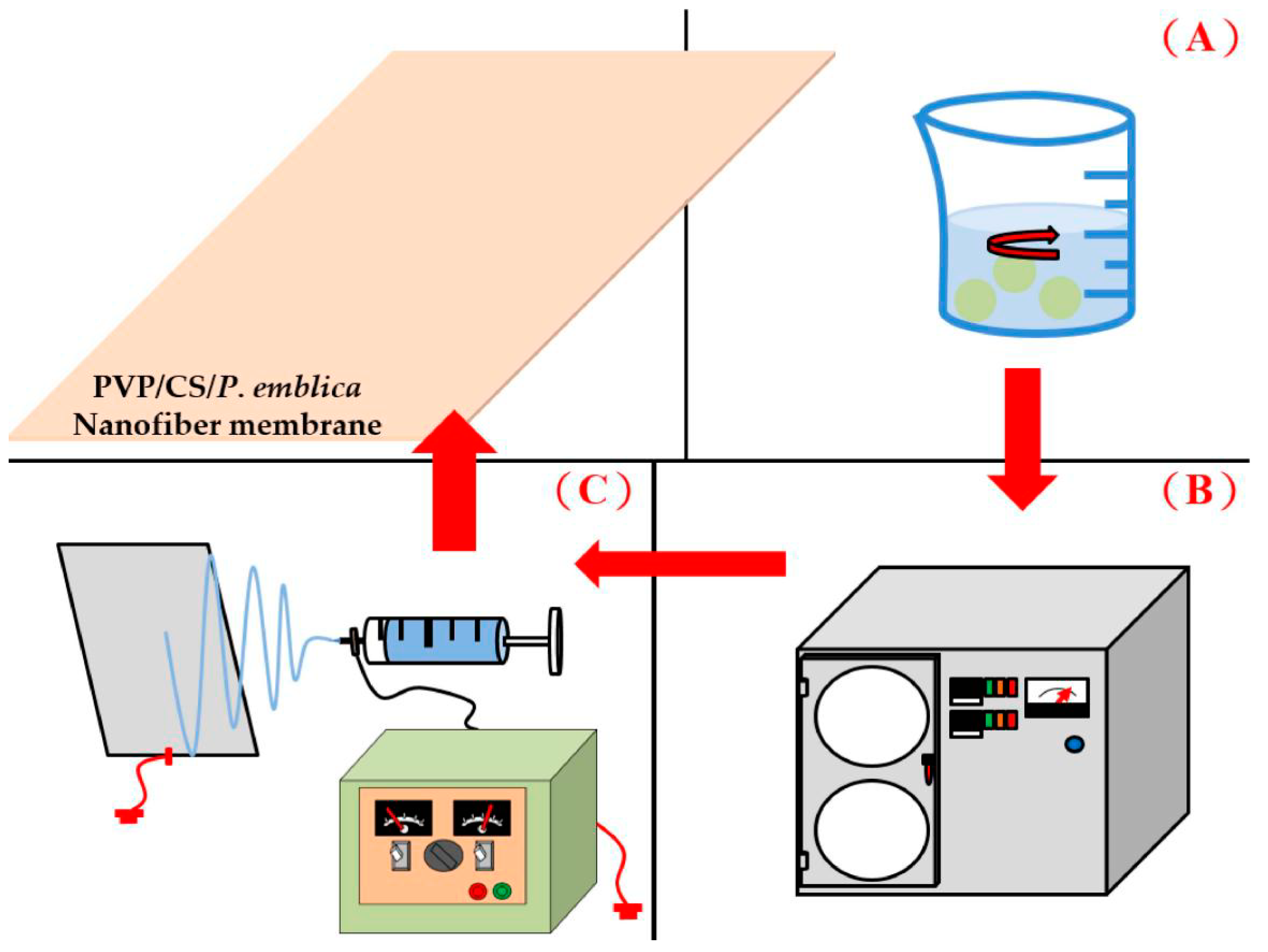
2.2.2. Preparation of Nanofiber Membranes
2.3. Characterizations
2.3.1. Extraction Rate Measurement
2.3.2. Scanning Electron Microscopy
2.3.3. Water Contact Angle Test
2.3.4. Release Rate Measurement
2.3.5. Tyrosinase Inhibitor Assay
2.3.6. Biocompatibility Measurement
2.3.7. Anti-Inflammatory Capacity Measurement
3. Results
3.1. Calibration Analysis of P. emblica Powders
3.2. Extraction Rate of P. emblica Powders
3.3. Tyrosinase Inhibition Assay for P. emblica Powders
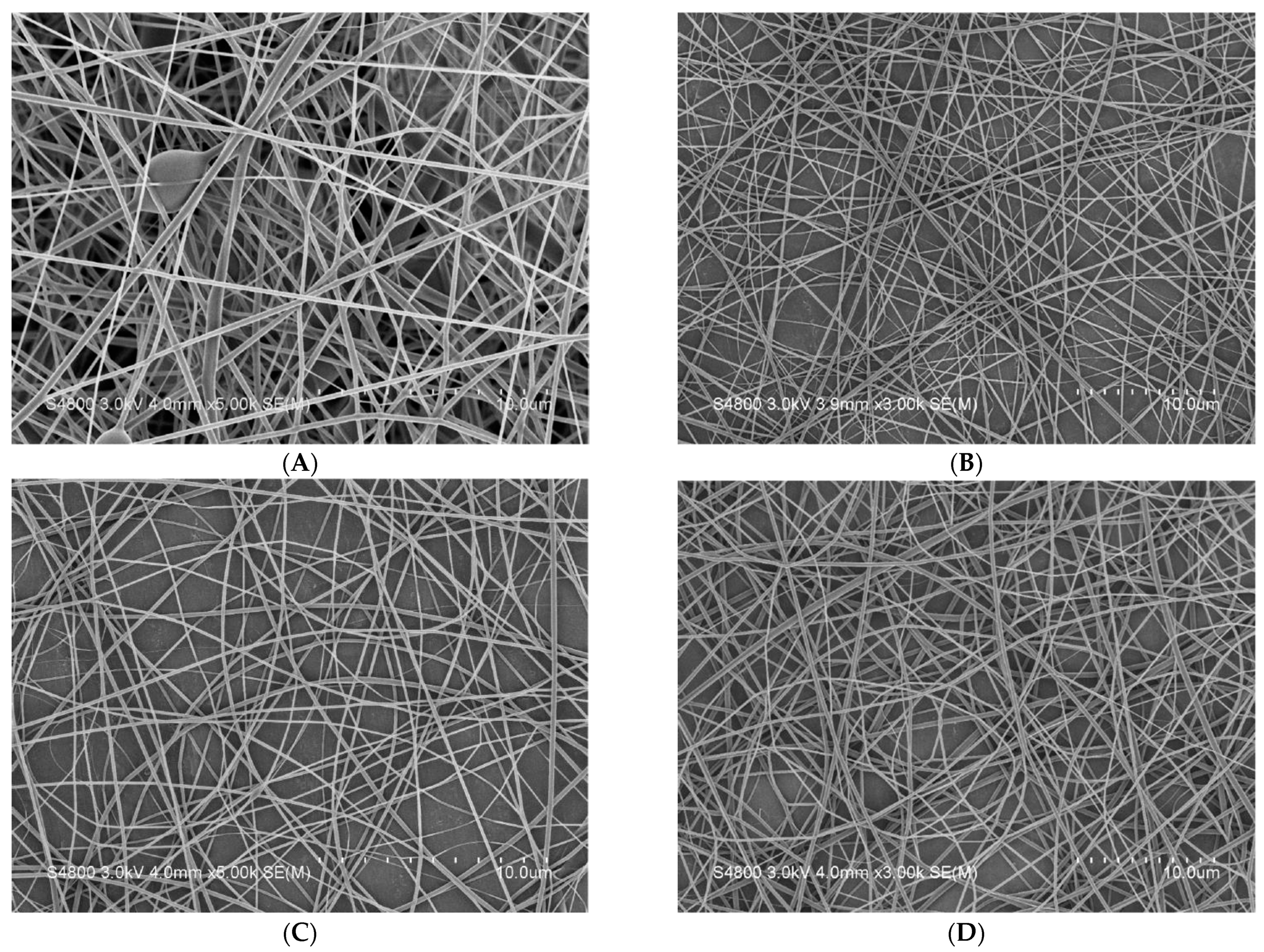
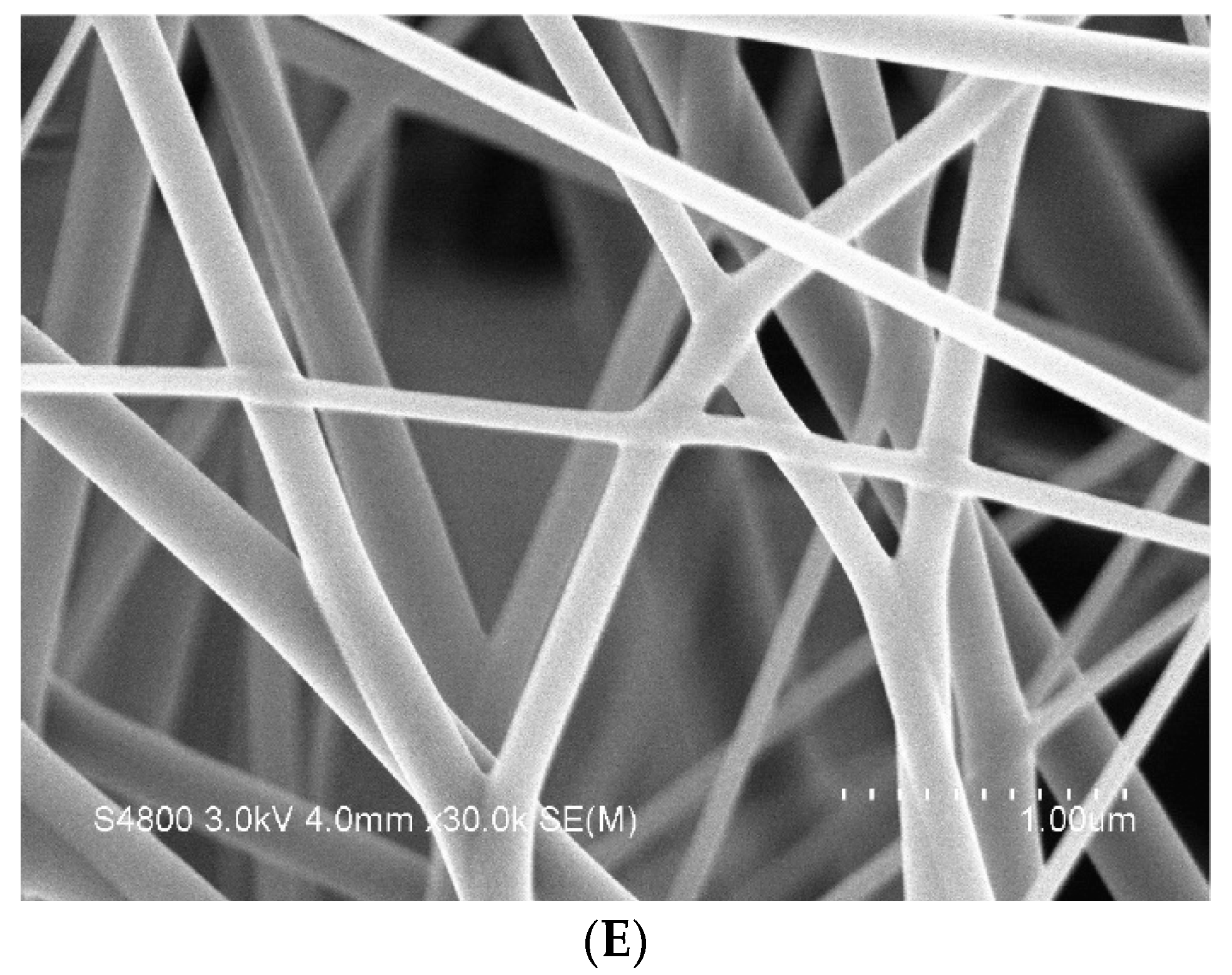
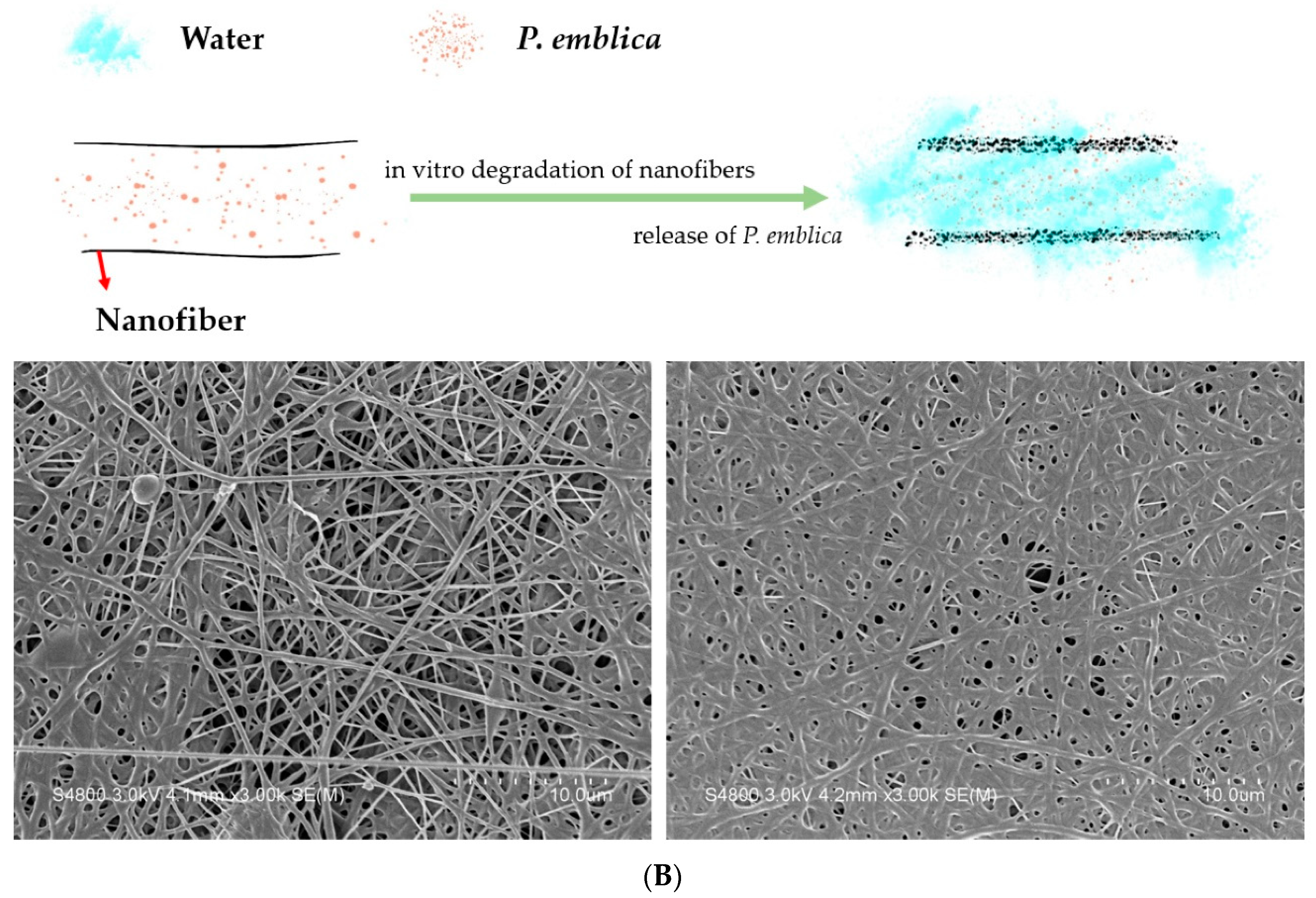
3.4. SEM Observation of Nanofiber Membranes
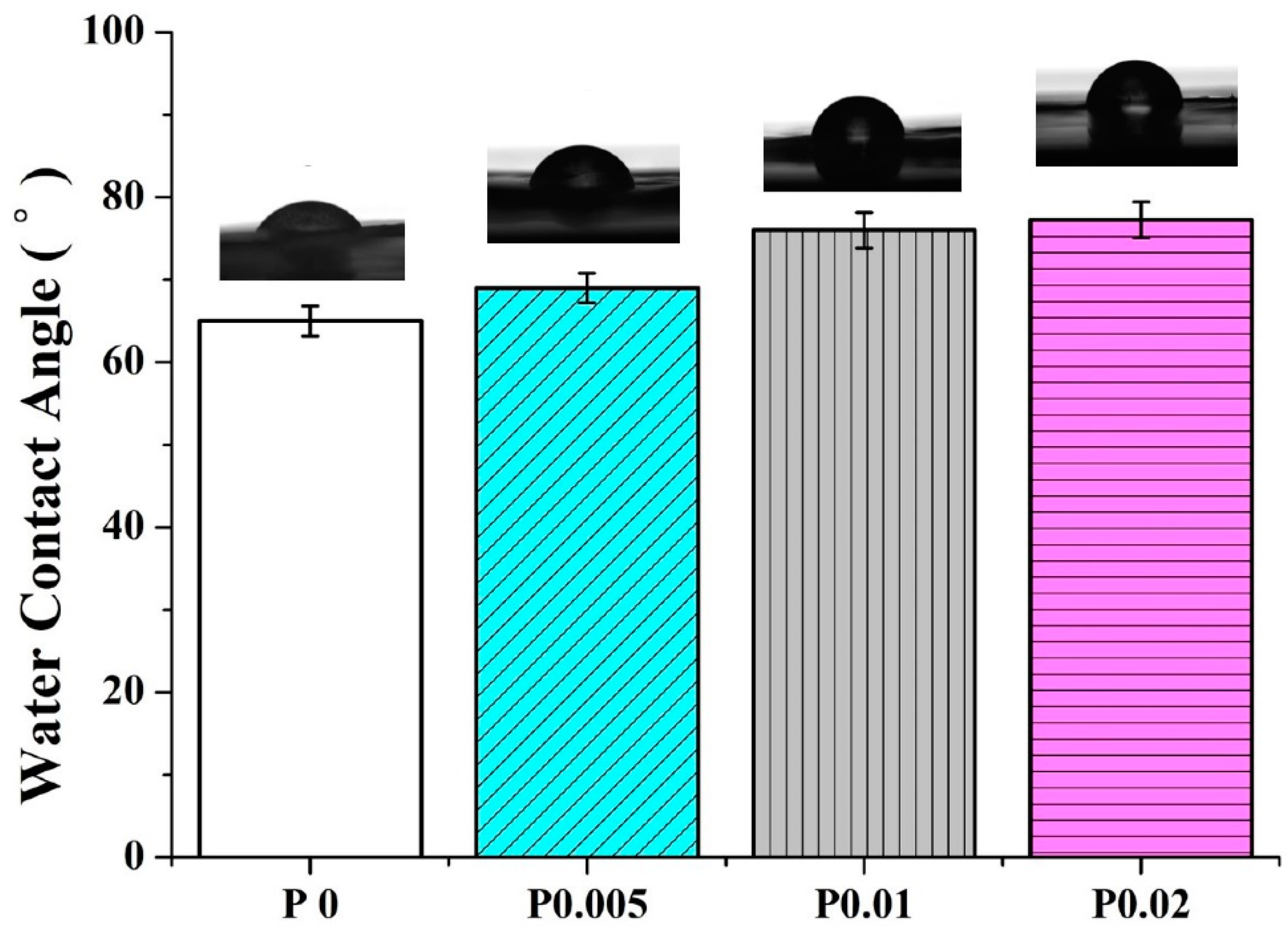
3.5. Hydrophilicity of Nanofiber Membranes
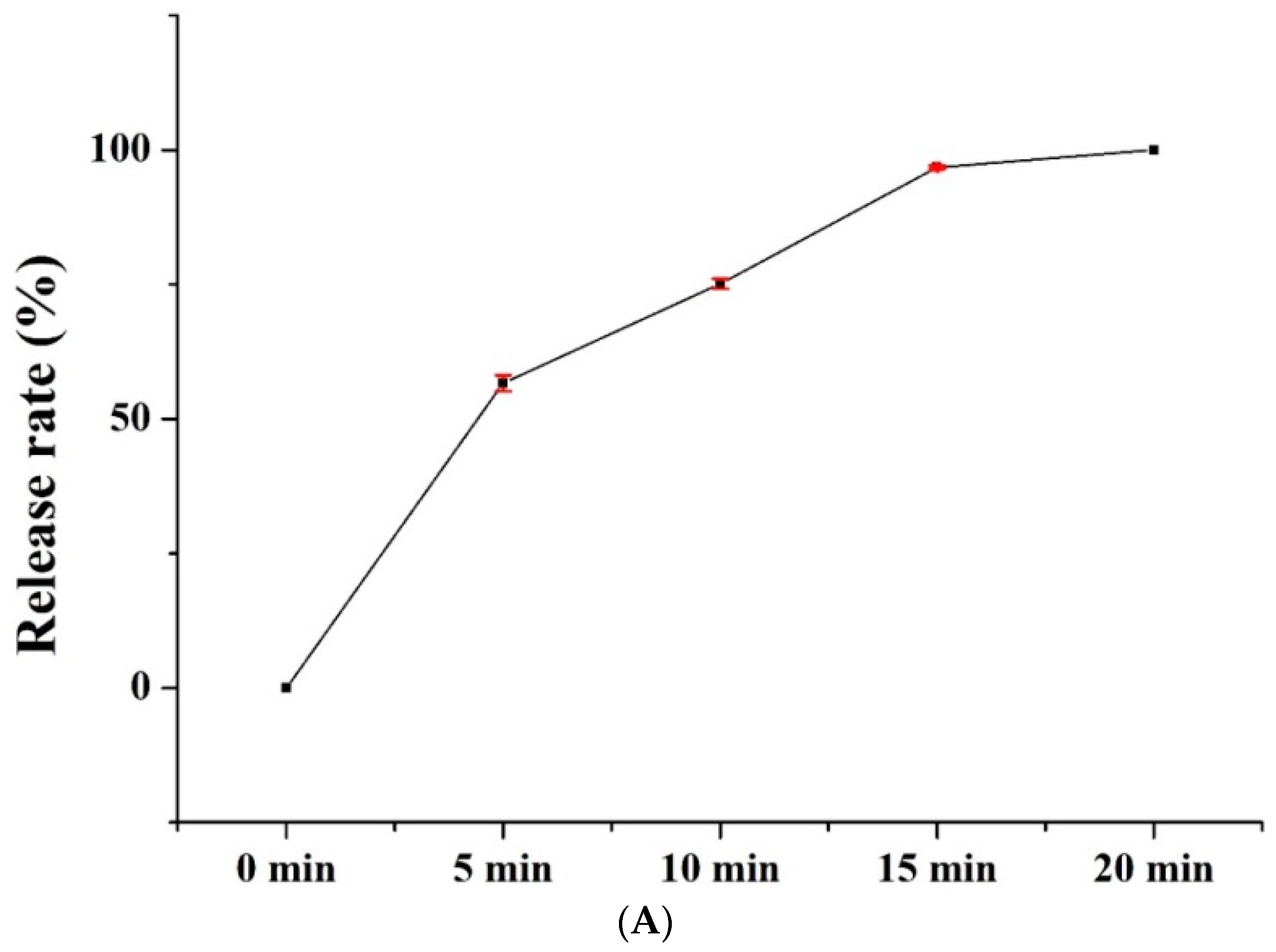
3.6. Release Rate of Nanofiber Membranes
3.7. Tyrosinase Inhibition Assay of Nanofiber Membranes
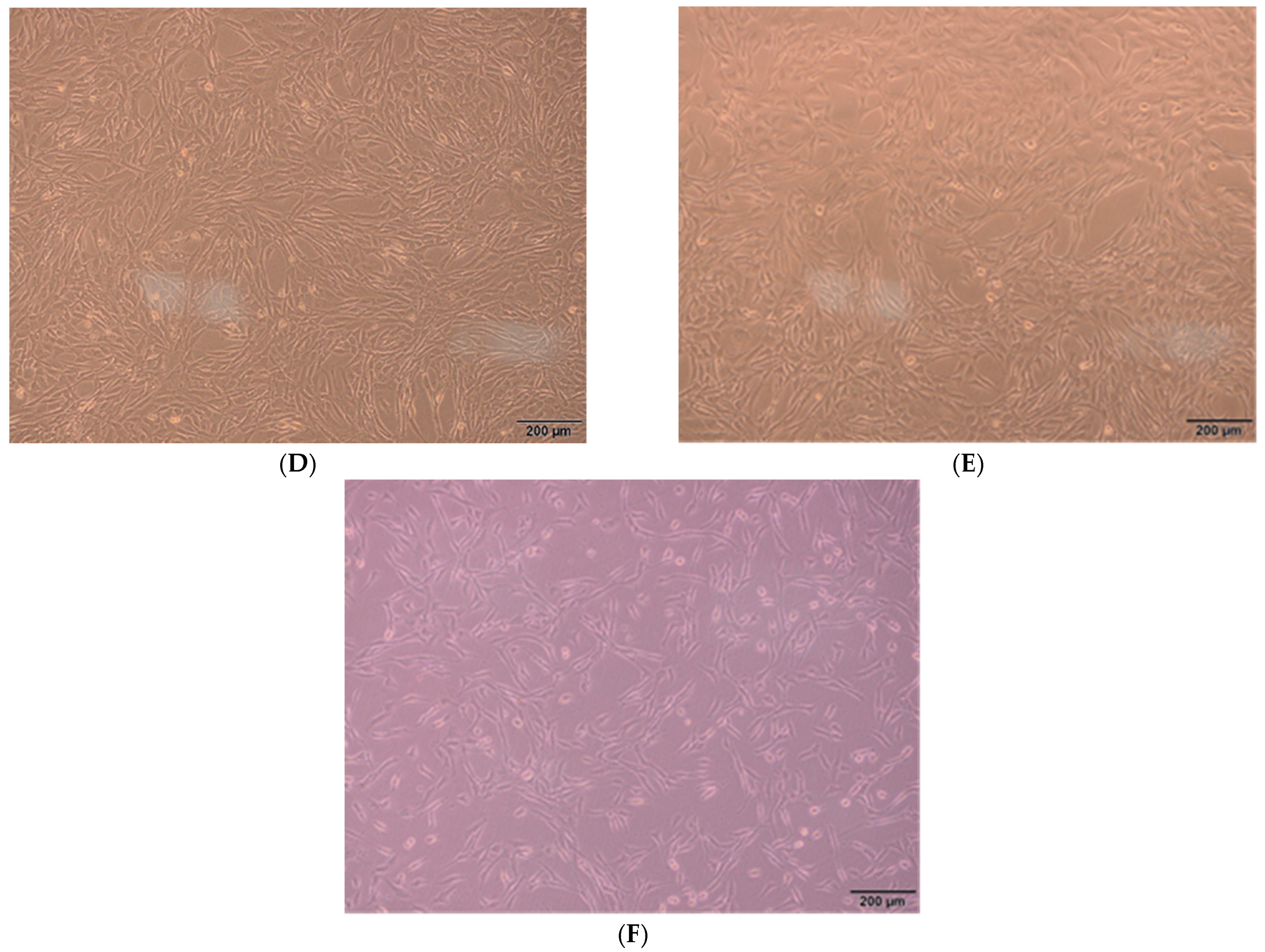
3.8. Biocompatibility of Nanofiber Membranes
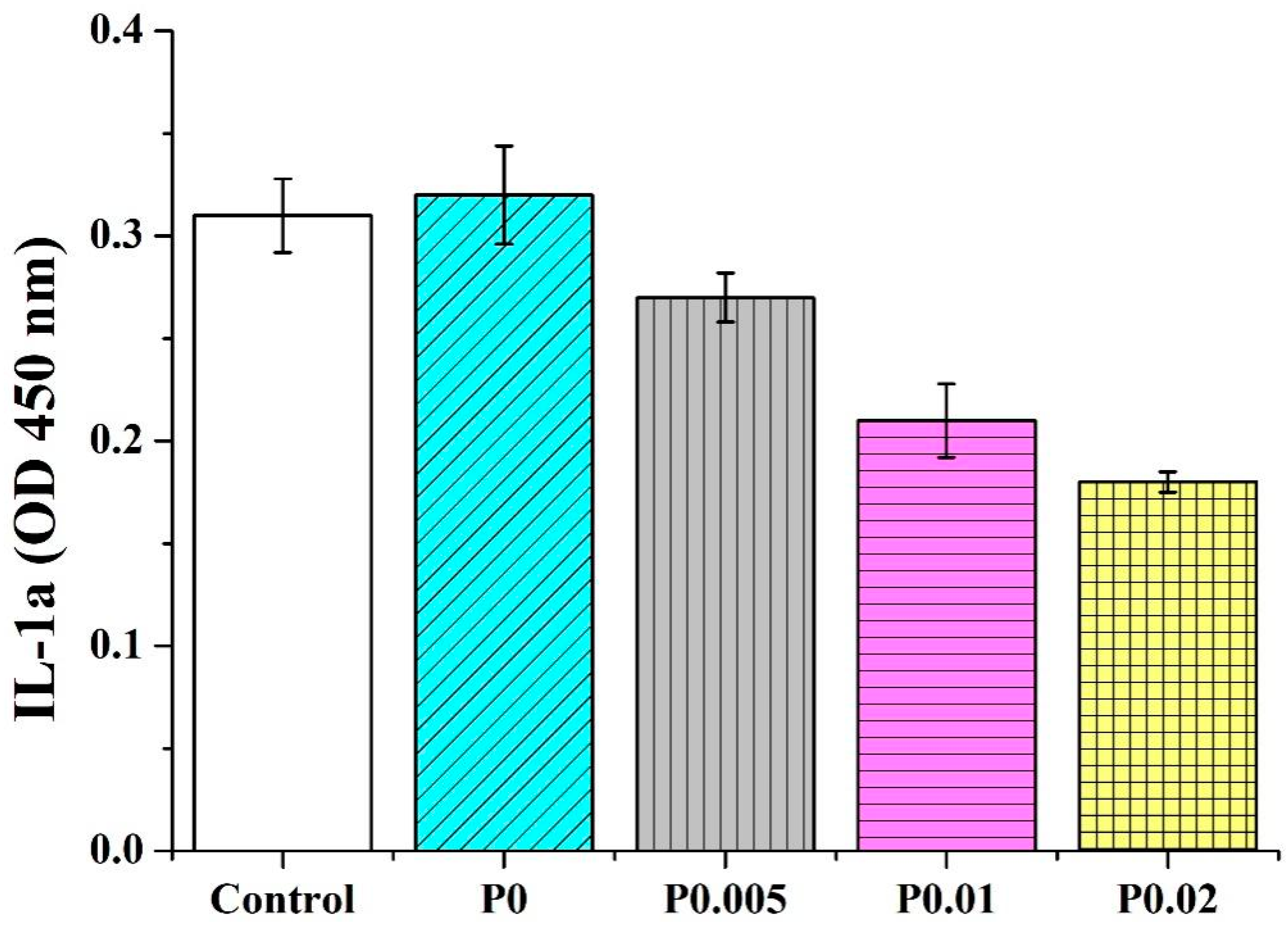
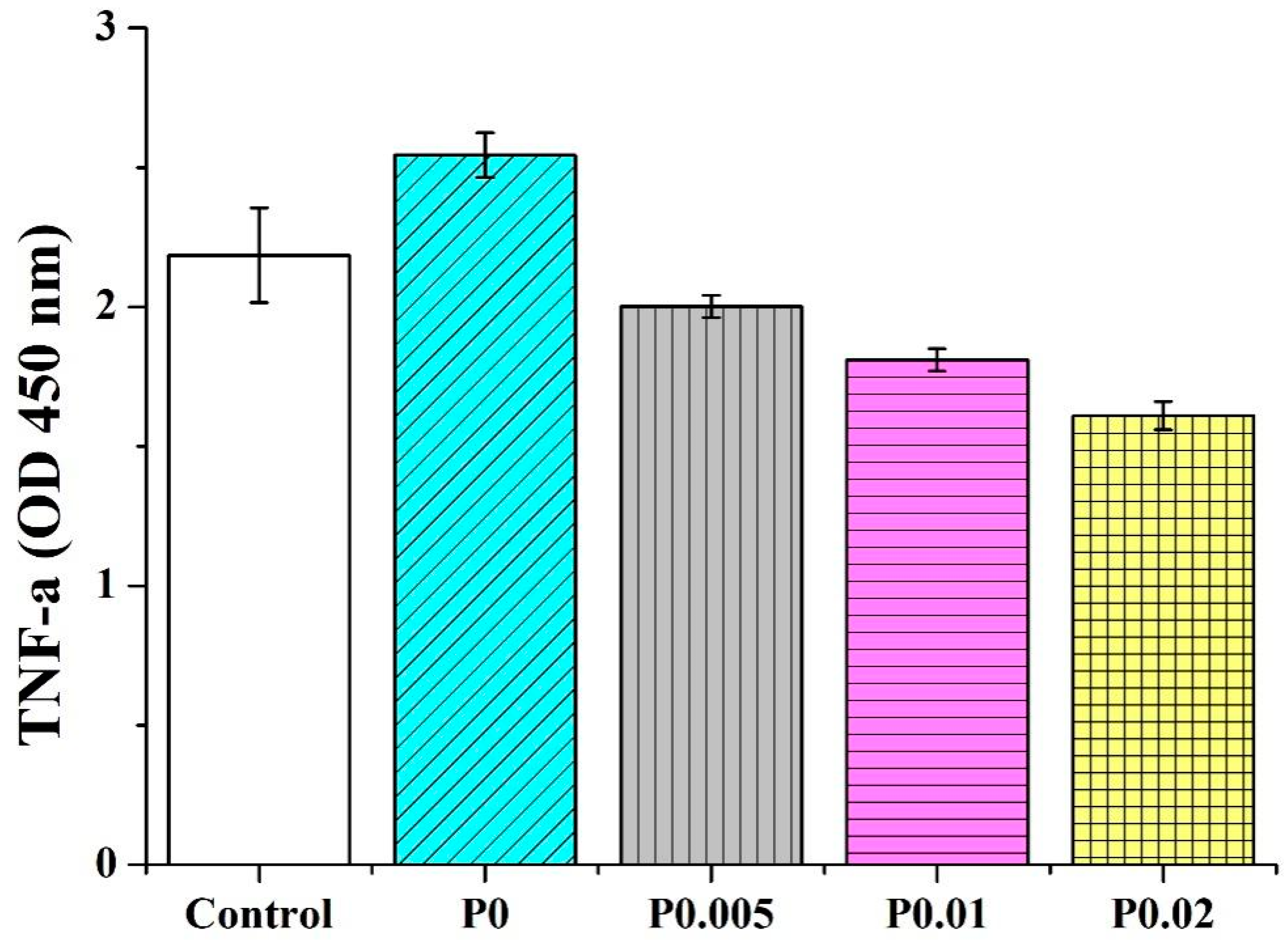
3.9. Anti-Inflammatory Capacity of Nanofiber Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savary, G.; Grisel, M.; Picard, C. Cosmetics and personal care products. In Natural Polymers; Springer: Berlin/Heidelberg, Germany, 2016; pp. 219–261. [Google Scholar]
- Meftahi, A.; Samyn, P.; Geravand, S.A.; Khajavi, R.; Alibkhshi, S.; Bechelany, M.; Barhoum, A.J.C.P. Nanocelluloses as skin biocompatible materials for skincare, cosmetics, and healthcare: Formulations, regulations, and emerging applications. Carbohydr. Polym. 2022, 278, 118956. [Google Scholar] [CrossRef] [PubMed]
- Martín-Pozo, L.; del Carmen Gómez-Regalado, M.; Moscoso-Ruiz, I.; Zafra-Gómez, A.J.T. Analytical methods for the determination of endocrine disrupting chemicals in cosmetics and personal care products: A review. Talanta 2021, 234, 122642. [Google Scholar] [CrossRef]
- Srinivasulu, M.; Chandra, M.S.; Gooty, J.M.; Madhavi, A. Personal care products—fragrances, cosmetics, and sunscreens—in the environment. In Environmental Micropollutants; Elsevier: Amsterdam, The Netherlands, 2022; pp. 131–149. [Google Scholar]
- Alvarez-Rivera, G.; Llompart, M.; Lores, M.; Garcia–Jares, C. Preservatives in cosmetics: Regulatory aspects and analytical methods. In Analysis of Cosmetic Products; Elsevier: Abingdon, UK, 2018; pp. 175–224. [Google Scholar]
- Du, X.; Zheng, H.; Zhang, Y.; Zhao, N.; Chen, M.; Huang, Q. Pore structure design and optimization of electrospun PMIA nanofiber membrane. J. Taiwan Inst. Chem. Eng. 2022, 139, 104512. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L.J.P. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Liao, Y.; Loh, C.-H.; Tian, M.; Wang, R.; Fane, A.G. Progress in electrospun polymeric nanofibrous membranes for water treatment: Fabrication, modification and applications. Prog. Polym. Sci. 2018, 77, 69–94. [Google Scholar] [CrossRef]
- Wang, Z.; Crandall, C.; Sahadevan, R.; Menkhaus, T.J.; Fong, H. Microfiltration performance of electrospun nanofiber membranes with varied fiber diameters and different membrane porosities and thicknesses. Polymer 2017, 114, 64–72. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Min, L.-L.; Yuan, Z.-H.; Zhong, L.-B.; Liu, Q.; Wu, R.-X.; Zheng, Y.-M. Preparation of chitosan based electrospun nanofiber membrane and its adsorptive removal of arsenate from aqueous solution. Chem. Eng. J. 2015, 267, 132–141. [Google Scholar] [CrossRef]
- Min, L.-L.; Yang, L.-M.; Wu, R.-X.; Zhong, L.-B.; Yuan, Z.-H.; Zheng, Y.-M. Enhanced adsorption of arsenite from aqueous solution by an iron-doped electrospun chitosan nanofiber mat: Preparation, characterization and performance. J. Colloid Interface Sci. 2019, 535, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Wan, X.; He, S.; Yang, Q.; He, Y. Design of durable and efficient poly (arylene ether nitrile)/bioinspired polydopamine coated graphene oxide nanofibrous composite membrane for anionic dyes separation. Chem. Eng. J. 2018, 333, 132–145. [Google Scholar] [CrossRef]
- Mohammadalizadeh, Z.; Bahremandi-Toloue, E.; Karbasi, S.J.R.; Polymers, F. Recent advances in modification strategies of pre-and post-electrospinning of nanofiber scaffolds in tissue engineering. React. Funct. Polym. 2022, 172, 105202. [Google Scholar] [CrossRef]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M.J. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Munir, N.; McDonald, A.; Callanan, A.J.B. A combinatorial approach: Cryo-printing and electrospinning hybrid scaffolds for cartilage tissue engineering. Bioprinting 2019, 16, e00056. [Google Scholar] [CrossRef]
- Sánchez, L.D.; Brack, N.; Postma, A.; Pigram, P.J.; Meagher, L.J.B. Surface modification of electrospun fibres for biomedical applications: A focus on radical polymerization methods. Biomaterials 2016, 106, 24–45. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; He, M.; Liu, H.; Niu, Y.; Crawford, A.; Coates, P.D.; Chen, D.; Shi, R.; Zhang, L.J.B. Drug loaded homogeneous electrospun PCL/gelatin hybrid nanofiber structures for anti-infective tissue regeneration membranes. Biomaterials 2014, 35, 9395–9405. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Yoo, H.S. MMPs-responsive release of DNA from electrospun nanofibrous matrix for local gene therapy: In vitro and in vivo evaluation. J. Control. Release 2010, 145, 264–271. [Google Scholar] [CrossRef]
- Rad, Z.P.; Mokhtari, J.; Abbasi, M.J. Calendula officinalis extract/PCL/Zein/Gum arabic nanofibrous bio-composite scaffolds via suspension, two-nozzle and multilayer electrospinning for skin tissue engineering. Int. J. Biol. Macromol. 2019, 135, 530–543. [Google Scholar]
- Bao, X.; Zhu, Q.; Chen, Y.; Tang, H.; Deng, W.; Guo, H.; Zeng, L. Antibacterial and antioxidant films based on HA/Gr/TA fabricated using electrospinning for wound healing. Int. J. Pharm. 2022, 626, 122139. [Google Scholar] [CrossRef]
- Bombin, A.D.J.; Dunne, N.J.; McCarthy, H.O. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater. Sci. Eng. C 2020, 114, 110994. [Google Scholar] [CrossRef]
- Cai, Y.; Yan, L.; Liu, G.; Yuan, H.; Xiao, D. In-situ synthesis of fluorescent gold nanoclusters with electrospun fibrous membrane and application on Hg (II) sensing. Biosens. Bioelectron. 2013, 41, 875–879. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Yu, J.; Wang, M. Engineering biomimetic superhydrophobic surfaces of electrospun nanomaterials. Nano Today 2011, 6, 510–530. [Google Scholar] [CrossRef]
- Sung, H.J.; Khan, M.F.; Kim, Y.H. Recombinant lignin peroxidase-catalyzed decolorization of melanin using in-situ generated H2O2 for application in whitening cosmetics. Int. J. Biol. Macromol. 2019, 136, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiang, H.; Dong, P.; Zhang, T.; Lu, C.; Jin, T.; Chai, K. Pegylated azelaic acid: Synthesis, tyrosinase inhibitory activity, antibacterial activity and cytotoxic studies. J. Mol. Struct. 2021, 1224, 129234. [Google Scholar] [CrossRef]
- He, M.; Fan, M.; Liu, W.; Li, Y.; Wang, G. Design, synthesis, molecular modeling, and biological evaluation of novel kojic acid derivatives containing bioactive heterocycle moiety as inhibitors of tyrosinase and antibrowning agents. Food Chem. 2021, 362, 130241. [Google Scholar] [CrossRef]
- Elinson, M.N.; Ryzhkova, Y.E.; Ryzhkov, F.V.; Fakhrutdinov, A.N. Kojic acid aldol adduct with isatin as inhibitors of pyruvate dehydrogenase kinase. J. Heterocycl. Chem. 2022, 59, 760–770. [Google Scholar] [CrossRef]
- Mikayoulou, M.; Mayr, F.; Temml, V.; Pandian, A.; Vermaak, I.; Chen, W.; Komane, B.; Stuppner, H.; Viljoen, A. Anti-tyrosinase activity of South African Aloe species and isolated compounds plicataloside and aloesin. Fitoterapia 2021, 150, 104828. [Google Scholar] [CrossRef]
- Baruah, A.; Bordoloi, M.; Baruah, H. Aloe vera: A multipurpose industrial crop. Ind. Crops Prod. 2016, 94, 951–963. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, K.-J.; Lee, M.-W.; Lee, S.-Y.; Yun, Y.-H.; Shim, W.-G.; Yoon, S.-D. Preparation and release properties of arbutin imprinted inulin/polyvinyl alcohol biomaterials. Int. J. Biol. Macromol. 2020, 161, 763–770. [Google Scholar] [CrossRef]
- Huang, H.; Tan, P.; Li, M.; Tan, Q.; Gao, J.; Bao, X.; Fan, S.; Mo, T.; Mao, W.; Lin, F. Quality analysis combined with mass spectrometry imaging reveal the difference between wild and cultivated Phyllanthus emblica Linn.: From chemical composition to molecular mechanism. Arab. J. Chem. 2022, 15, 103790. [Google Scholar] [CrossRef]
- Chekdaengphanao, P.; Jaiseri, D.; Sriraj, P.; Aukkanimart, R.; Prathumtet, J.; Udonsan, P.; Boonmars, T. Anticancer activity of Terminalia chebula, Terminalia bellirica, and Phyllanthus emblica extracts on cholangiocarcinoma cell proliferation and induction of apoptosis. J. Herb. Med. 2022, 35, 100582. [Google Scholar] [CrossRef]
- Dhar, S.A.; Chowdhury, R.A.; Das, S.; Nahian, M.K.; Islam, D.; Gafur, M. Plant-mediated green synthesis and characterization of silver nanoparticles using Phyllanthus emblica fruit extract. Mater. Today Proc. 2021, 42, 1867–1871. [Google Scholar] [CrossRef]
- Chauhan, P.; Kumar, R.R.; Mendiratta, S.K.; Talukder, S.; Gangwar, M.; Sakunde, D.T.; Meshram, S.K. In-vitro functional efficacy of extracts from Phyllanthus emblica, Eucalyptus globulus, Tinospora cordifolia as pancreatic lipase inhibitor and source of anti-oxidant in goat meat nuggets. Food Chem. 2021, 348, 129087. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Sharma, N.; Oladeji, O.S.; Sourirajan, A.; Dev, K.; Zengin, G.; El-Shazly, M.; Kumar, V.J. Traditional uses, bioactive composition, pharmacology, and toxicology of Phyllanthus emblica fruits: A comprehensive review. J. Ethnopharmacol. 2022, 282, 114570. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yaseen, A.; Chen, B.; Li, F.; Wang, L.; Hu, W.; Wang, M.J. Chemical constituents from the fruits of Phyllanthus emblica L. Biochem. Syst. Ecol. 2020, 92, 104122. [Google Scholar] [CrossRef]
- Sriwatcharakul, S.J. Evaluation of bioactivities of Phyllanthus emblica seed. Energy Rep. 2020, 6, 442–447. [Google Scholar] [CrossRef]
- Bramanti, E.; Bonaccorsi, L.; Campanella, B.; Ferrari, C.; Malara, A.; Freni, A.J. Structural characterization of electrospun tetraethylortosilicate (TEOS)/Polyvinylpyrrolidone (PVP) microfibres. Mater. Chem. Phys. 2022, 287, 126248. [Google Scholar] [CrossRef]
- Saeedi, M.; Vahidi, O.; Moghbeli, M.; Ahmadi, S.; Asadnia, M.; Akhavan, O.; Seidi, F.; Rabiee, M.; Saeb, M.R.; Webster, T.J. Customizing nano-chitosan for sustainable drug delivery. J. Control. Release 2022, 350, 175–192. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Fathi, M.; Kadivar, M.J. Production and characterization of chitosan-gelatin nanofibers by nozzle-less electrospinning and their application to enhance edible film’s properties. Food Packag. Shelf Life 2019, 22, 100387. [Google Scholar] [CrossRef]
- Dlamini, Z.W.; Vallabhapurapu, S.; Wu, S.; Mahule, T.S.; Srivivasan, A.; Vallabhapurapu, V.S. Resistive switching memory based on chitosan/polyvinylpyrrolidone blend as active layers. Solid State Commun. 2022, 345, 114677. [Google Scholar] [CrossRef]
- Kalanidhi, K.; Nagaraaj, P. N-doped carbon dots incorporated chitosan/polyvinylpyrrolidone based polymer film for advanced packaging applications. Chem. Phys. Lett. 2022, 805, 139960. [Google Scholar] [CrossRef]
- Siefen, T.; Bjerregaard, S.; Plaksin, D.; Lokhnauth, J.; Liang, A.; Larsen, C.C.; Lamprecht, A.J. Co-formulations of adalimumab with hyaluronic acid/polyvinylpyrrolidone to combine intraarticular drug delivery and viscosupplementation. Eur. J. Pharm. Biopharm. 2022, 177, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Haung, X.-Y.; Chiu, C.-C.; Lin, M.-Y.; Lin, W.-H.; Chang, W.-T.; Tseng, C.-C.; Wang, H.-M. Inhibitions of melanogenesis via Phyllanthus emblica fruit extract powder in B16F10 cells. Food Biosci. 2019, 28, 177–182. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Chen, R.; Li, Y.; Miao, J.; Liu, G.; Lan, Y.; Chen, Y.; Cao, Y. HPLC fingerprint analysis of Phyllanthus emblica ethanol extract and their antioxidant and anti-inflammatory properties. J. Ethnopharmacol. 2020, 254, 112740. [Google Scholar] [CrossRef] [PubMed]





| Solution | A | B | C | D |
|---|---|---|---|---|
| Tyrosine | 100 | 100 | 100 | 100 |
| Extract | - | - | 80 | 80 |
| Water/Ethanol (solvent) | 80 | 100 | - | 20 |
| Tyrosinase | 20 | - | 20 | - |
| Total | 200 | 200 | 200 | 200 |
| Time (h) | 6 | 12 | 24 | 48 |
|---|---|---|---|---|
| weight (g) before extraction | 10 | 10 | 10 | 10 |
| weight (g) after extraction | 0.58 | 2.9 | 2.53 | 2.5 |
| extraction rate (%) | 5.8 | 29.0 | 25.3 | 25.0 |
| Fiber Diameter (nm) | Viscosity (cP) | Conductivity (μS) | |
|---|---|---|---|
| P. emblica 0 | 214.27 ± 74.51 | 351.8 | 657 |
| P. emblica 0.005 | 107.95 ± 27.74 | 397.1 | 1084 |
| P. emblica 0.01 | 90.32 ± 29.84 | 421.6 | 1336 |
| P. emblica 0.02 | 165.33 ± 51.66 | 467.3 | 1609 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-H.; Shiu, B.-C.; Hsu, P.-W.; Lou, C.-W.; Lin, J.-H. PVP/CS/Phyllanthus emblica Nanofiber Membranes for Dry Facial Masks: Manufacturing Process and Evaluations. Polymers 2022, 14, 4470. https://doi.org/10.3390/polym14214470
Lin J-H, Shiu B-C, Hsu P-W, Lou C-W, Lin J-H. PVP/CS/Phyllanthus emblica Nanofiber Membranes for Dry Facial Masks: Manufacturing Process and Evaluations. Polymers. 2022; 14(21):4470. https://doi.org/10.3390/polym14214470
Chicago/Turabian StyleLin, Jian-Hong, Bing-Chiuan Shiu, Po-Wen Hsu, Ching-Wen Lou, and Jia-Horng Lin. 2022. "PVP/CS/Phyllanthus emblica Nanofiber Membranes for Dry Facial Masks: Manufacturing Process and Evaluations" Polymers 14, no. 21: 4470. https://doi.org/10.3390/polym14214470
APA StyleLin, J.-H., Shiu, B.-C., Hsu, P.-W., Lou, C.-W., & Lin, J.-H. (2022). PVP/CS/Phyllanthus emblica Nanofiber Membranes for Dry Facial Masks: Manufacturing Process and Evaluations. Polymers, 14(21), 4470. https://doi.org/10.3390/polym14214470








