One-Pot Synthesis of Hyperbranched Polyurethane-Triazoles with Controlled Structural, Molecular Weight and Hydrodynamic Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
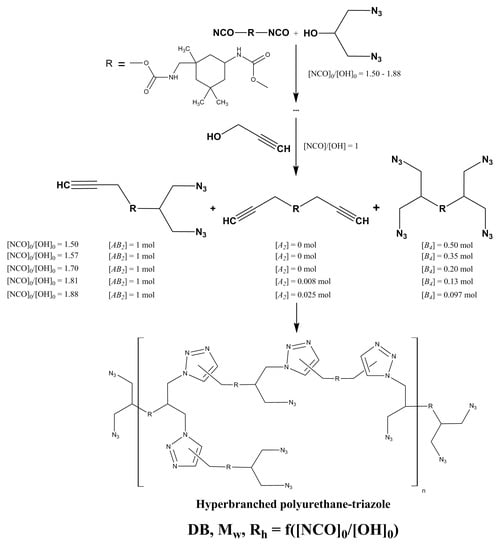
2.3. One-Pot Synthesis of Hyperbranched Polyurethane-Triazoles
3. Results and Discussion
3.1. Influence of [NCO]0/[OH]0 Ratio on the Composition of AB2 + A2 + B4 Monomer Mixture and Structural-Kinetic Model of Their Polyaddition
3.2. Synthesis of Hyperbranched Azide-Containing Polyurethane-Triazoles
3.3. Structural, Molecular Weight, Hydrodynamic and Thermal Characteristics of Hyperbranched Polyurethane-Triazoles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaiser, T.; Frey, H. Hyperbranched Polymer Architectures: From Flory’s AB(f-1) Polycondensates to Controlled Structures. Polymer 2020, 211, 123113. [Google Scholar] [CrossRef]
- Hao, M.; Wu, T.; Chen, Q.; Lian, X.; Wu, H.; Shi, B. Hyperbranched Polyglycerols as Robust Up-Conversion Nanoparticle Coating Layer for Feasible Cell Imaging. Polymers 2020, 12, 2592. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.A.; Soldatova, A.E.; Tsegel’skaya, A.Y.; Semenova, G.K. Synthesis of Branched Polyimides of Different Topological Structure. Polym. Sci. Ser. C 2020, 62, 21. [Google Scholar] [CrossRef]
- Migulin, D.; Milenin, S.; Cherkaev, G.; Zezin, A.; Zezina, E.; Muzafarov, A. Sodium 3-Azidopropyldialkoxysilanolate—A Versatile Route towards New Functional 1,2,3–Triazole Based Hyperbranched Polyorganoalkoxysiloxanes. React. Funct. Polym. 2020, 154, 104648. [Google Scholar] [CrossRef]
- Chen, C. Preparation and Characteristics of an Environmentally Friendly Hyperbranched Flame-Retardant Polyurethane Hybrid Containing Nitrogen, Phosphorus, and Silicon. Polymers 2019, 11, 720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabae, Y.; Kakimoto, M. Design and Synthesis of Hyperbranched Aromatic Polymers for Catalysis. Polymers 2018, 10, 1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pouyan, P.; Cherri, M.; Haag, R. Polyglycerols as Multi-Functional Platforms: Synthesis and Biomedical Applications. Polymers 2022, 14, 2684. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, S.R.; Stimpson, A.; Moon, R.; Cowie, L.; Aragrag, N.; Filip, S.V.; Smith, A.G.; Irvine, D.J. Facile Synthesis of Functionalised Hyperbranched Polymers for Application as Novel, Low Viscosity Lubricant Formulation Components. Polymers 2022, 14, 3841. [Google Scholar] [CrossRef]
- Chen, J.; Yan, Z.; Li, Z.; Dai, S. Direct Synthesis of Chain-End Toluene Functionalized Hyperbranched Ethylene Oligomers. Polymers 2022, 14, 3049. [Google Scholar] [CrossRef]
- Babaei, N.; Yeganeh, H.; Gharibi, R. Anticorrosive and Self-Healing Waterborne Poly(Urethane-Triazole) Coatings Made through a Combination of Click Polymerization and Cathodic Electrophoretic Deposition. Eur. Polym. J. 2019, 112, 636–647. [Google Scholar] [CrossRef]
- Xue, X.; Yang, J.; Huang, W.; Yang, H.; Jiang, B. Synthesis of Hyperbranched Poly(ε-Caprolactone) Containing Terminal Azobenzene Structure via Combined Ring-Opening Polymerization and “Click” Chemistry. Polymers 2015, 7, 1248–1268. [Google Scholar] [CrossRef] [Green Version]
- Flory, P.J. Molecular size distribution in three dimensional polymers. VI. Branched polymers containing A—R—Bf-1 type units. J. Am. Chem. Soc. 1952, 74, 2718–2723. [Google Scholar] [CrossRef]
- Voit, B.I.; Lederer, A. Hyperbranched and Highly Branched Polymer Architectures—Synthetic Strategies and Major Characterization Aspects. Chem. Rev. 2009, 109, 5924–5973. [Google Scholar] [CrossRef]
- Chen, H.; Kong, J.; Tian, W.; Fan, X.-D. Intramolecular Cyclization in A2 + B3 Polymers via Step-Wise Polymerization Resulting in a Highly Branched Topology: Quantitative Determination of Cycles by Combined NMR and SEC Analytics. Macromolecules 2012, 45, 6185–6195. [Google Scholar] [CrossRef]
- Armelin, E.; Whelan, R.; Martínez-Triana, Y.M.; Alemán, C.; Finn, M.G.; Díaz, D.D. Protective Coatings for Aluminum Alloy Based on Hyperbranched 1,4-Polytriazoles. ACS Appl. Mater. Interfaces 2017, 9, 4231–4243. [Google Scholar] [CrossRef]
- Mao, L.; Ren, X.; Feng, B.; Zhang, Y.; Zhang, J.; Huang, W. Sydnone-Maleimide Based Cascading Double 1,3-Dipolar Cycloaddition for Synthesis of “A(A′) + B3” Type Hyperbranched Polyimide. React. Funct. Polym. 2022, 174, 105246. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched Polymers: Advances from Synthesis to Applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef]
- Cao, X.; Shi, Y.; Wang, X.; Graff, R.W.; Gao, H. Design a Highly Reactive Trifunctional Core Molecule To Obtain Hyperbranched Polymers with over a Million Molecular Weight in One-Pot Click Polymerization. Macromolecules 2016, 49, 760–766. [Google Scholar] [CrossRef]
- Karpov, S.V.; Perepelitsina, E.O.; Malkov, G.V. Synthesis of New Branched Urethane-Triazole Polymers. Polym. Sci. Ser. B 2014, 56, 298–306. [Google Scholar] [CrossRef]
- Karpov, S.V.; Lodygina, V.P.; Komratova, V.V.; Dzhalmukhanova, A.S.; Malkov, G.V.; Badamshina, E.R. Kinetics of Urethane Formation from Isophorone Diisocyanate: The Alcohol Nature Effect. Kinet. Catal. 2016, 57, 319–325. [Google Scholar] [CrossRef]
- Karpov, S.V.; Lodygina, V.P.; Komratova, V.V.; Dzhalmukhanova, A.S.; Malkov, G.V.; Badamshina, E.R. Kinetics of Urethane Formation from Isophorone Diisocyanate: The Catalyst and Solvent Effects. Kinet. Catal. 2016, 57, 422–428. [Google Scholar] [CrossRef]
- Stagg, H.E. A Method for the Determination of Isocyanates. Analyst 1946, 71, 557. [Google Scholar] [CrossRef] [PubMed]
- Vander Werf, C.A.; Heisler, R.V.; McEwen, W.E. The Reaction of Sodium Azide with Some Representative Epoxides. J. Am. Chem. Soc. 1954, 76, 1231–1235. [Google Scholar] [CrossRef]
- Weissberger, A.; Proskauer, E.; Riddick, J.; Toops, E. Organic solvents. Physical Properties and Method of Purification; Interscience Publishers: New York, NY, USA, 1955; Volume VII. [Google Scholar]
- Irzhak, T.F.; Irzhak, V.I. Critical conversion in polymerization processes. Russ. Chem. Rev. 2010, 79, 921–944. [Google Scholar] [CrossRef]
- Rodionov, V.O.; Fokin, V.V.; Finn, M.G. Mechanism of the Ligand-Free CuI-Catalyzed Azide-Alkyne Cycloaddition Reaction. Angew. Chem. Int. Ed. 2005, 44, 2210–2215. [Google Scholar] [CrossRef]
- Pacini, A.; Nitti, A.; Sangiovanni, G.; Vitale, M.; Pasini, D. Clickable 2,2-Bis (Hydroxymethyl) Propionic Acid-Derived AB2 Monomers: Hyperbranched Polyesters through the CuAAC Cycloaddition (Click) Reaction. J. Polym. Sci. 2021, 59, 2014–2022. [Google Scholar] [CrossRef]
- Cao, X.; Shi, Y.; Gan, W.; Naguib, H.; Wang, X.; Graff, R.W.; Gao, H. Effect of Monomer Structure on the CuAAC Polymerization To Produce Hyperbranched Polymers. Macromolecules 2016, 49, 5342–5349. [Google Scholar] [CrossRef]
- Hölter, D.; Burgath, A.; Frey, H. Degree of Branching in Hyperbranched Polymers. Acta Polym. 1997, 48, 30–35. [Google Scholar] [CrossRef]









| [NCO]0/[OH]0 | [AB2]:[A2]:[B4] | DBexp (DBcalc) | MwLS | [η]·102 (dl/g) | Rhp (nm) | Tg (°C) | Td (°C) |
|---|---|---|---|---|---|---|---|
| 1.50 | 1:0:0.50 | 0.21 (0.25) | 10,100 | 3.5 | 1.9 | 40 | 230 |
| 1.57 | 1:0:0.35 | 0.28 (0.29) | 10,800 | 4.0 | 2.4 | 53 | 228 |
| 1.70 | 1:0:0.20 | 0.33 (0.35) | 15,900 | 5.6 | 3.0 | 69 | 231 |
| 1.81 | 1:0.008:0.13 | 0.39 (0.40) | 28,600 | 7.8 | 5.7 | 73 | 232 |
| 1.88 | 1:0.025:0.097 | 0.44 (0.45) | 174,700 | - | - | 86 | 230 |
| 1.93 | 1:0.042:0.083 | Cross-linking | 90 | 232 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpov, S.V.; Iakunkov, A.; Akkuratov, A.V.; Petrov, A.O.; Perepelitsina, E.O.; Malkov, G.V.; Badamshina, E.R. One-Pot Synthesis of Hyperbranched Polyurethane-Triazoles with Controlled Structural, Molecular Weight and Hydrodynamic Characteristics. Polymers 2022, 14, 4514. https://doi.org/10.3390/polym14214514
Karpov SV, Iakunkov A, Akkuratov AV, Petrov AO, Perepelitsina EO, Malkov GV, Badamshina ER. One-Pot Synthesis of Hyperbranched Polyurethane-Triazoles with Controlled Structural, Molecular Weight and Hydrodynamic Characteristics. Polymers. 2022; 14(21):4514. https://doi.org/10.3390/polym14214514
Chicago/Turabian StyleKarpov, Sergei V., Artem Iakunkov, Alexander V. Akkuratov, Artem O. Petrov, Eugenia O. Perepelitsina, Georgiy V. Malkov, and Elmira R. Badamshina. 2022. "One-Pot Synthesis of Hyperbranched Polyurethane-Triazoles with Controlled Structural, Molecular Weight and Hydrodynamic Characteristics" Polymers 14, no. 21: 4514. https://doi.org/10.3390/polym14214514
APA StyleKarpov, S. V., Iakunkov, A., Akkuratov, A. V., Petrov, A. O., Perepelitsina, E. O., Malkov, G. V., & Badamshina, E. R. (2022). One-Pot Synthesis of Hyperbranched Polyurethane-Triazoles with Controlled Structural, Molecular Weight and Hydrodynamic Characteristics. Polymers, 14(21), 4514. https://doi.org/10.3390/polym14214514