Size Control and Enhanced Stability of Silver Nanoparticles by Cyclic Poly(ethylene glycol)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Synthesis of c-PEG
2.4. Synthesis of MeO–PEG–OMe
2.5. Synthesis of AgNPs through the Tollens’ Process
3. Results
3.1. Synthesis of c-PEG
3.2. Synthesis of AgNPs
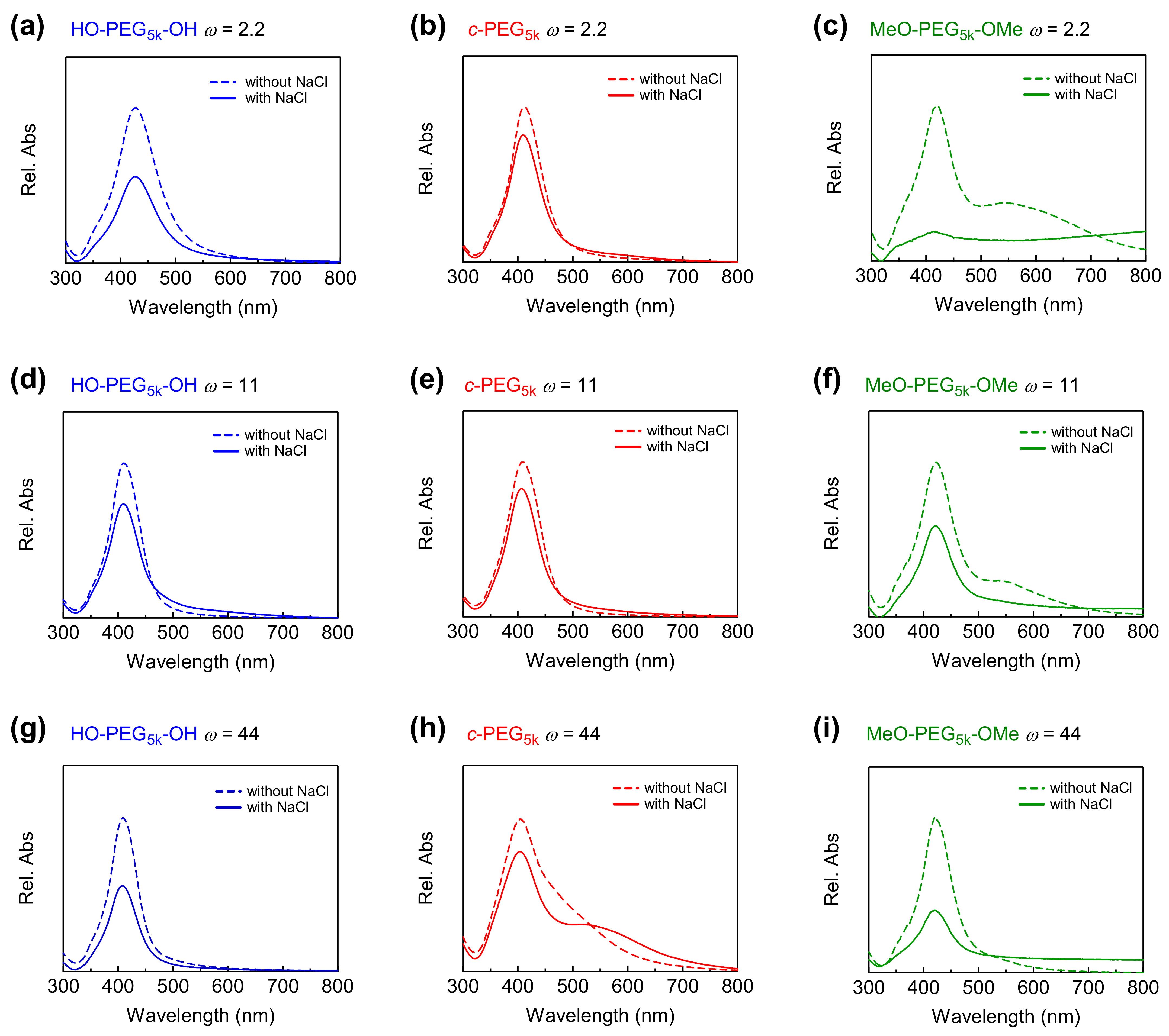
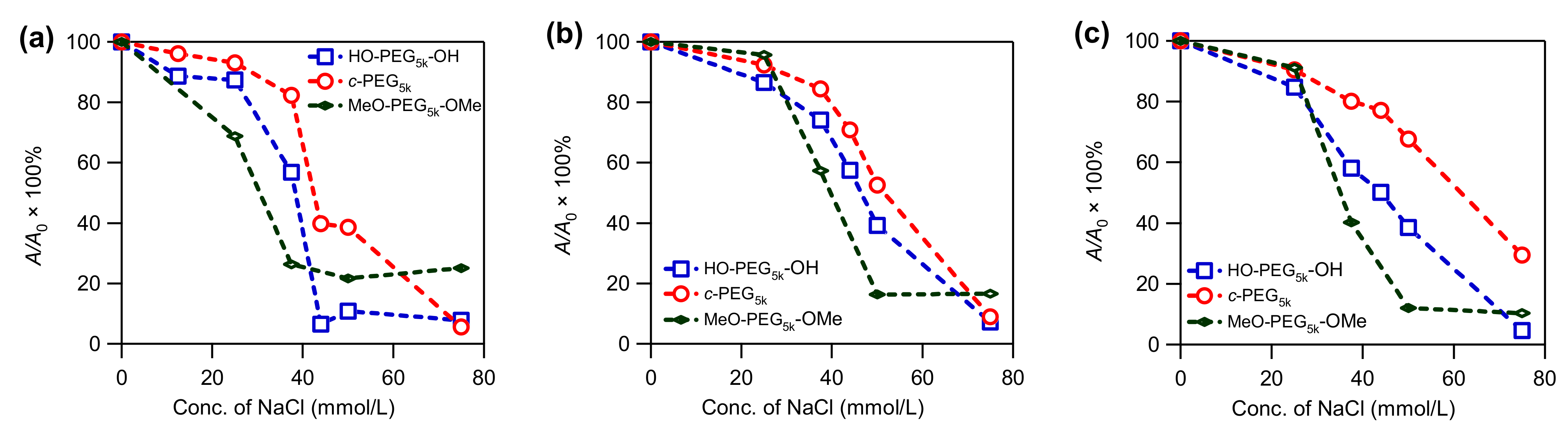
3.3. Stability of AgNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kästner, C.; Thünemann, A.F. Catalytic reduction of 4-nitrophenol using silver nanoparticles with adjustable activity. Langmuir 2016, 32, 7383–7391. [Google Scholar] [CrossRef] [PubMed]
- Beqa, L.; Singh, A.K.; Khan, S.A.; Senapati, D.; Arumugam, S.R.; Ray, P.C. Gold nanoparticle-based simple colorimetric and ultrasensitive dynamic light scattering assay for the selective detection of Pb(II) from paints, plastics, and water samples. ACS Appl. Mater. Interfaces 2011, 3, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, A.S.; Kinnan, M.K.; Chumanov, G. Multipole plasmon resonances of submicron silver particles. J. Am. Chem. Soc. 2005, 127, 12444–12445. [Google Scholar] [CrossRef]
- Hu, M.; Chen, J.Y.; Li, Z.Y.; Au, L.; Hartland, G.V.; Li, X.D.; Marquez, M.; Xia, Y.N. Gold nanostructures: Engineering their plasmonic properties for biomedical applications. Chem. Soc. Rev. 2006, 35, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Wiley, B.J.; Chen, Y.C.; McLellan, J.M.; Xiong, Y.J.; Li, Z.Y.; Ginger, D.; Xia, Y.N. Synthesis and optical properties of silver nanobars and nanorice. Nano Lett. 2007, 7, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Mittelman, A.M.; Fortner, J.D.; Pennell, K.D. Effects of ultraviolet light on silver nanoparticle mobility and dissolution. Environ. Sci. Nano 2015, 2, 683–691. [Google Scholar] [CrossRef]
- Qu, Y.Q.; Yang, H.B.; Yang, N.; Fan, Y.Z.; Zhu, H.Y.; Zou, G.T. The effect of reaction temperature on the particle size, structure and magnetic properties of coprecipitated CoFe2O4 nanoparticles. Mater. Lett. 2006, 60, 3548–3552. [Google Scholar] [CrossRef]
- Hu, S.Q.; Huang, P.J.J.; Wang, J.X.; Liu, J.W. Dissecting the effect of salt for more sensitive label-free colorimetric detection of DNA using gold nanoparticles. Anal. Chem. 2020, 92, 13354–13360. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Chen, B.; Banfield, J.F. Particle size and pH effects on nanoparticle dissolution. J. Phys. Chem. C 2010, 114, 14876–14884. [Google Scholar] [CrossRef]
- Henglein, A.; Giersig, M. Formation of colloidal silver nanoparticles: Capping action of citrate. J. Phys. Chem. B 1999, 103, 9533–9539. [Google Scholar] [CrossRef]
- Fenger, R.; Fertitta, E.; Kirmse, H.; Thunemann, A.F.; Rademann, K. Size dependent catalysis with CTAB-stabilized gold nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9343–9349. [Google Scholar] [CrossRef]
- Tejamaya, M.; Romer, I.; Merrifield, R.C.; Lead, J.R. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef]
- Hussain, I.; Brust, M.; Papworth, A.J.; Cooper, A.I. Preparation of acrylate-stabilized gold and silver hydrosols and gold-polymer composite films. Langmuir 2003, 19, 4831–4835. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, Y.; Zeng, X.; Zeng, Y.; Wang, Y. The role of poly(ethylene glycol) in the formation of silver nanoparticles. J. Colloid Interface Sci. 2005, 288, 444–448. [Google Scholar] [CrossRef]
- Xia, X.; Yang, M.; Wang, Y.; Zheng, Y.; Li, Q.; Chen, J.; Xia, Y. Quantifying the coverage density of poly(ethylene glycol) chains on the surface of gold nanostructures. ACS Nano 2012, 6, 512–522. [Google Scholar] [CrossRef]
- Li, Y.; Lin, H.; Zhou, W.; Sun, L.; Samanta, D.; Mirkin, C. Corner-, edge-, and facet-controlled growth of nanocrystals. Sci. Adv. 2021, 7, eabf1410. [Google Scholar] [CrossRef]
- Papastefanaki, F.; Jakovcevski, I.; Poulia, N.; Djogo, N.; Schulz, F.; Martinovic, T.; Ciric, D.; Loers, G.; Vossmeyer, T.; Weller, H.; et al. Intraspinal delivery of polyethylene glycol-coated gold nanoparticles promotes functional recovery after spinal cord injury. Mol. Ther. 2015, 23, 993–1002. [Google Scholar] [CrossRef]
- Li, X.; Lenhart, J.J.; Walker, H.W. Aggregation kinetics and dissolution of coated silver nanoparticles. Langmuir 2012, 28, 1095–1104. [Google Scholar] [CrossRef]
- Li, X.A.; Lenhart, J.J.; Walker, H.W. Dissolution-accompanied aggregation kinetics of silver nanoparticles. Langmuir 2010, 26, 16690–16698. [Google Scholar] [CrossRef]
- Zhang, X.; Servos, M.R.; Liu, J. Ultrahigh nanoparticle stability against salt, pH, and solvent with retained surface accessibility via depletion stabilization. J. Am. Chem. Soc. 2012, 134, 9910–9913. [Google Scholar] [CrossRef] [PubMed]
- Radziuk, D.; Skirtach, A.; Sukhorukov, G.; Shchukin, D.; Möhwald, H. Stabilization of silver nanoparticles by polyelectrolytes and poly(ethylene glycol). Macromol. Rapid Commun. 2007, 28, 848–855. [Google Scholar] [CrossRef]
- Kvítek, L.; Prucek, R.; Panáček, A.; Novotny, R.; Hrbáč, J.; Zbořil, R. The influence of complexing agent concentration on particle size in the process of SERS active silver colloid synthesis. J. Mater. Chem. 2005, 15, 1099–1105. [Google Scholar] [CrossRef]
- Bastakoti, B.P.; Guragain, S.; Yusa, S.; Nakashima, K. Novel synthesis route for Ag@SiO2 core-shell nanoparticles via micelle template of double hydrophilic block copolymer. Rsc Adv. 2012, 2, 5938–5940. [Google Scholar] [CrossRef]
- Tezuka, Y.; Oike, H. Topological polymer chemistry. Prog. Polym. Sci. 2002, 27, 1069–1122. [Google Scholar] [CrossRef]
- Zhang, L.H.; Elupula, R.; Grayson, S.M.; Torkelson, J.M. Major impact of cyclic chain topology on the T-g-confinement effect of supported thin films of polystyrene. Macromolecules 2016, 49, 257–268. [Google Scholar] [CrossRef]
- Kricheldorf, H.R. Cyclic Polymers: Synthetic strategies and physical properties. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 251–284. [Google Scholar] [CrossRef]
- Zhang, S.S.; Cheng, X.X.; Wang, J.Z.; Zhang, Z.B.; Zhang, W.; Zhu, X.L. Synthesis of a cyclic-brush polymer with a high grafting density using activated ester chemistry via the “grafting onto” approach. Polym. Chem. 2018, 9, 5155–5163. [Google Scholar] [CrossRef]
- Jeong, Y.; Jin, Y.; Chang, T.; Uhlik, F.; Roovers, J. Intrinsic viscosity of cyclic polystyrene. Macromolecules 2017, 50, 7770–7776. [Google Scholar] [CrossRef]
- Honda, S.; Yamamoto, T.; Tezuka, Y. Topology-directed control on thermal stability: Micelles formed from linear and cyclized amphiphilic block copolymers. J. Am. Chem. Soc. 2010, 132, 10251–10253. [Google Scholar] [CrossRef]
- Honda, S.; Yamamoto, T.; Tezuka, Y. Tuneable enhancement of the salt and thermal stability of polymeric micelles by cyclized amphiphiles. Nat. Commun. 2013, 4, 1574. [Google Scholar] [CrossRef]
- Watanabe, T.; Chimura, S.; Wang, Y.B.; Ono, T.; Isono, T.; Tajima, K.; Satoh, T.; Sato, S.; Ida, D.; Yamamoto, T. Cyclization of PEG and pluronic surfactants and the effects of the topology on their interfacial activity. Langmuir 2021, 37, 6974–6984. [Google Scholar] [CrossRef]
- Wang, Y.; Quinsaat, J.E.Q.; Ono, T.; Maeki, M.; Tokeshi, M.; Isono, T.; Tajima, K.; Satoh, T.; Sato, S.; Miura, Y.; et al. Enhanced dispersion stability of gold nanoparticles by the physisorption of cyclic poly(ethylene glycol). Nat. Commun. 2020, 11, 6089. [Google Scholar] [CrossRef]
- Oziri, O.J.; Wang, Y.B.; Watanabe, T.; Uno, S.; Maeki, M.; Tokeshi, M.; Isono, T.; Tajima, K.; Satoh, T.; Sato, S.I.; et al. PEGylation of silver nanoparticles by physisorption of cyclic poly(ethylene glycol) for enhanced dispersion stability, antimicrobial activity, and cytotoxicity. Nanoscale Adv. 2022, 4, 532–545. [Google Scholar] [CrossRef]
- Cooke, J.; Viras, K.; Yu, G.-E.; Sun, T.; Yonemitsu, T.; Ryan, A.J.; Price, C.; Booth, C. Large cyclic poly(oxyethylene)s: Chain folding in the crystalline state studied by Raman spectroscopy, X-ray scattering, and differential scanning calorimetry. Macromolecules 1998, 31, 3030–3039. [Google Scholar] [CrossRef]
- Panacek, A.; Prucek, R.; Hrbac, J.; Nevecna, T.; Steffkova, J.; Zboril, R.; Kvitek, L. Polyacrylate-assisted size control of silver nanoparticles and their catalytic activity. Chem. Mater. 2014, 26, 1332–1339. [Google Scholar] [CrossRef]
- Sun, T.; Yu, G.E.; Price, C.; Booth, C.; Cooke, J.; Ryan, A.J. Cyclic polyethers. J. Polymer 1995, 36, 3775–3778. [Google Scholar] [CrossRef]
- Hirose, Y.; Taira, T.; Sakai, K.; Sakai, H.; Endo, A.; Imura, T. Structures and surface properties of "Cyclic" polyoxyethylene alkyl ethers: Unusual behavior of cyclic surfactants in water. Langmuir 2016, 32, 8374–8382. [Google Scholar] [CrossRef]
- Lonsdale, D.E.; Bell, C.A.; Monteiro, M.J. Strategy for rapid and high-purity monocyclic polymers by CuAAC “Click” reactions. Macromolecules 2010, 43, 3331–3339. [Google Scholar] [CrossRef]
- Pressly, E.D.; Amir, R.J.; Hawker, C.J. Rapid synthesis of block and cyclic copolymers via click chemistry in the presence of copper nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 814–819. [Google Scholar] [CrossRef]
- Nam, S.; Parikh, D.V.; Condon, B.D.; Zhao, Q.; Yoshioka-Tarver, M. Importance of poly(ethylene glycol) conformation for the synthesis of silver nanoparticles in aqueous solution. J. Nanopart. Res. 2011, 13, 3755–3764. [Google Scholar] [CrossRef]
- Shin, H.S.; Yang, H.J.; Kim, S.B.; Lee, M.S. Mechanism of growth of colloidal silver nanoparticles stabilized by polyvinyl pyrrolidone in γ-irradiated silver nitrate solution. J. Colloid Interface Sci. 2004, 274, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Heo, K.; Kim, Y.Y.; Kitazawa, Y.; Kim, M.; Jin, K.S.; Yamamoto, T.; Ree, M. Structural characteristics of amphiphilic cyclic and linear block copolymer micelles in aqueous solutions. ACS Macro Lett. 2014, 3, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Ree, B.J.; Satoh, T.; Yamamoto, T. Micelle Structure Details and Stabilities of Cyclic Block Copolymer Amphiphile and Its Linear Analogues. Polymers 2019, 11, 163. [Google Scholar] [CrossRef]
- Quinsaat, J.E.Q.; Testino, A.; Pin, S.; Huthwelke, T.; Nuesch, F.A.; Bowen, P.; Hofmann, H.; Ludwig, C.; Opris, D.M. Continuous production of tailored silver nanoparticles by polyol synthesis and reaction yield measured by X-ray absorption spectroscopy: Toward a growth mechanism. J. Phys. Chem. C 2014, 118, 11093–11103. [Google Scholar] [CrossRef]
- Tadano, T.; Zhu, R.; Muroga, Y.; Hoshi, T.; Sasaki, D.; Yano, S.; Sawaguchi, T. A new mechanism for the silica nanoparticle dispersion-agglomeration transition in a poly(methyl methacrylate)/silica hybrid suspension. Polym. J. 2014, 46, 342–348. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, Z.; Zhang, H.; Zhang, Q. Control of heat integrated pressure-swing-distillation process for separating azeotropic mixture of tetrahydrofuran and methanol. Ind. Eng. Chem. Res. 2015, 54, 1646–1655. [Google Scholar] [CrossRef]

| Polymer | ω1 | TEM-Measured Size of AgNPs (nm) | ||
|---|---|---|---|---|
| HO–PEG–OH | c-PEG | MeO–PEG–OMe | ||
| PEG3k | 2.2 | 55 ± 8 | 52 ± 8 | - |
| 11 | 43 ± 8 | 45 ± 8 | - | |
| 44 | 33 ± 4 | 30 ± 7 | - | |
| PEG5k | 2.2 | 58 ± 13 | 42 ± 9 | 48 ± 6 |
| 11 | 36 ± 5 | 31 ± 4 | 44 ± 10 | |
| 44 | 40 ± 7 | 21 ± 4 | 48 ± 9 | |
| PEG10k | 2.2 | 50 ± 8 | 30 ± 3 | - |
| 11 | 48 ± 10 | 35 ± 4 | - | |
| 44 | 37 ± 7 | 26 ± 4 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Quinsaat, J.E.Q.; Li, F.; Isono, T.; Tajima, K.; Satoh, T.; Sato, S.-i.; Yamamoto, T. Size Control and Enhanced Stability of Silver Nanoparticles by Cyclic Poly(ethylene glycol). Polymers 2022, 14, 4535. https://doi.org/10.3390/polym14214535
Wang Y, Quinsaat JEQ, Li F, Isono T, Tajima K, Satoh T, Sato S-i, Yamamoto T. Size Control and Enhanced Stability of Silver Nanoparticles by Cyclic Poly(ethylene glycol). Polymers. 2022; 14(21):4535. https://doi.org/10.3390/polym14214535
Chicago/Turabian StyleWang, Yubo, Jose Enrico Quijano Quinsaat, Feng Li, Takuya Isono, Kenji Tajima, Toshifumi Satoh, Shin-ichiro Sato, and Takuya Yamamoto. 2022. "Size Control and Enhanced Stability of Silver Nanoparticles by Cyclic Poly(ethylene glycol)" Polymers 14, no. 21: 4535. https://doi.org/10.3390/polym14214535
APA StyleWang, Y., Quinsaat, J. E. Q., Li, F., Isono, T., Tajima, K., Satoh, T., Sato, S.-i., & Yamamoto, T. (2022). Size Control and Enhanced Stability of Silver Nanoparticles by Cyclic Poly(ethylene glycol). Polymers, 14(21), 4535. https://doi.org/10.3390/polym14214535







