Comparative Study of Octavinyl Oligomeric Sesquisiloxane Nanomaterial-Modified Asphalt Using Molecular Dynamics Method
Abstract
1. Introduction
2. Simulation Models and Methods
2.1. Molecular Models of Asphalt
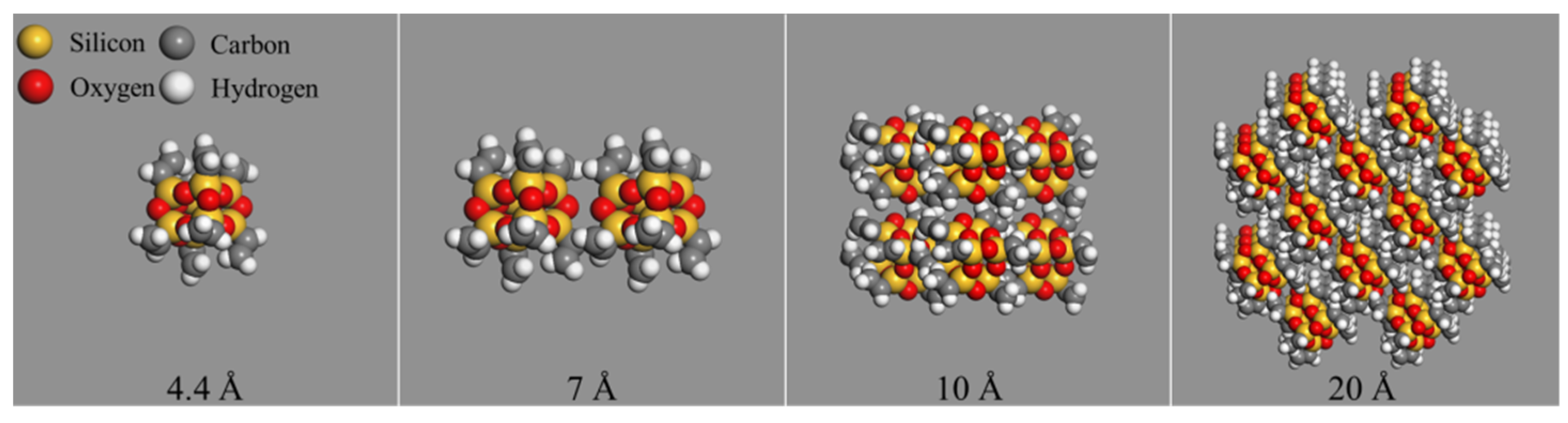
2.2. Molecular Models of Nanomaterials
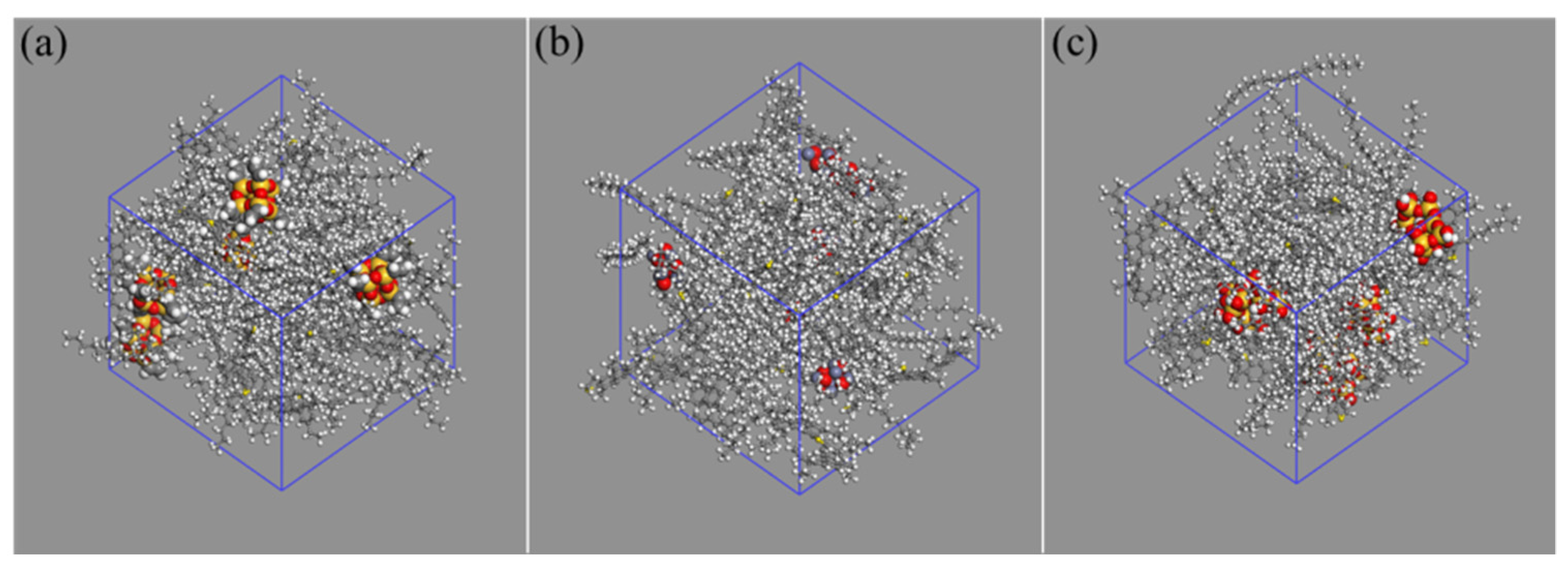
2.3. Construction of the Modified Asphalt Models
2.4. Simulation Process
2.5. Molecular Dynamics Simulation Theories
2.5.1. Structural Parameters
2.5.2. Compatibility
3. Results and Discussion
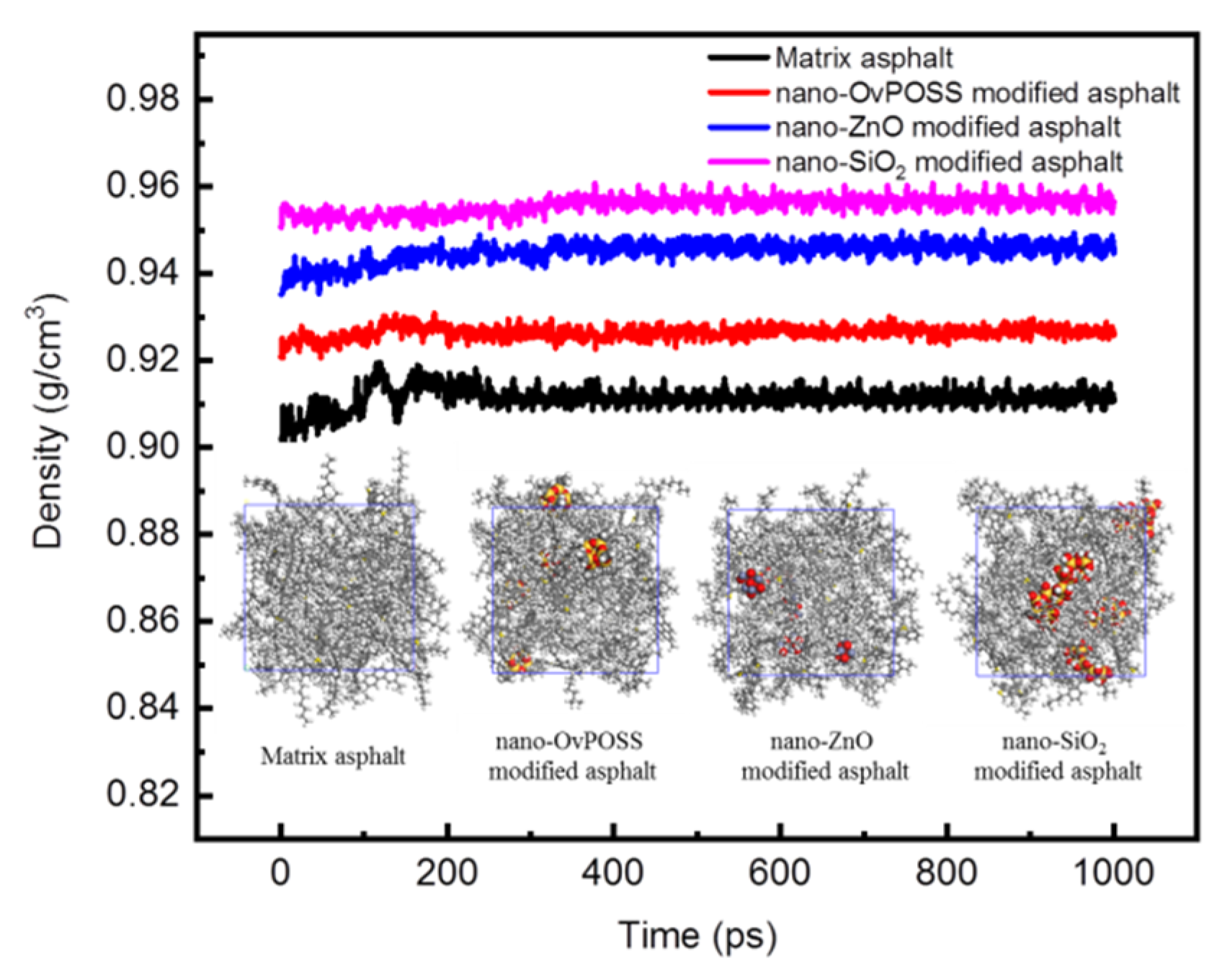
3.1. Basic Information
3.1.1. The RDF of the Three Nanomaterials
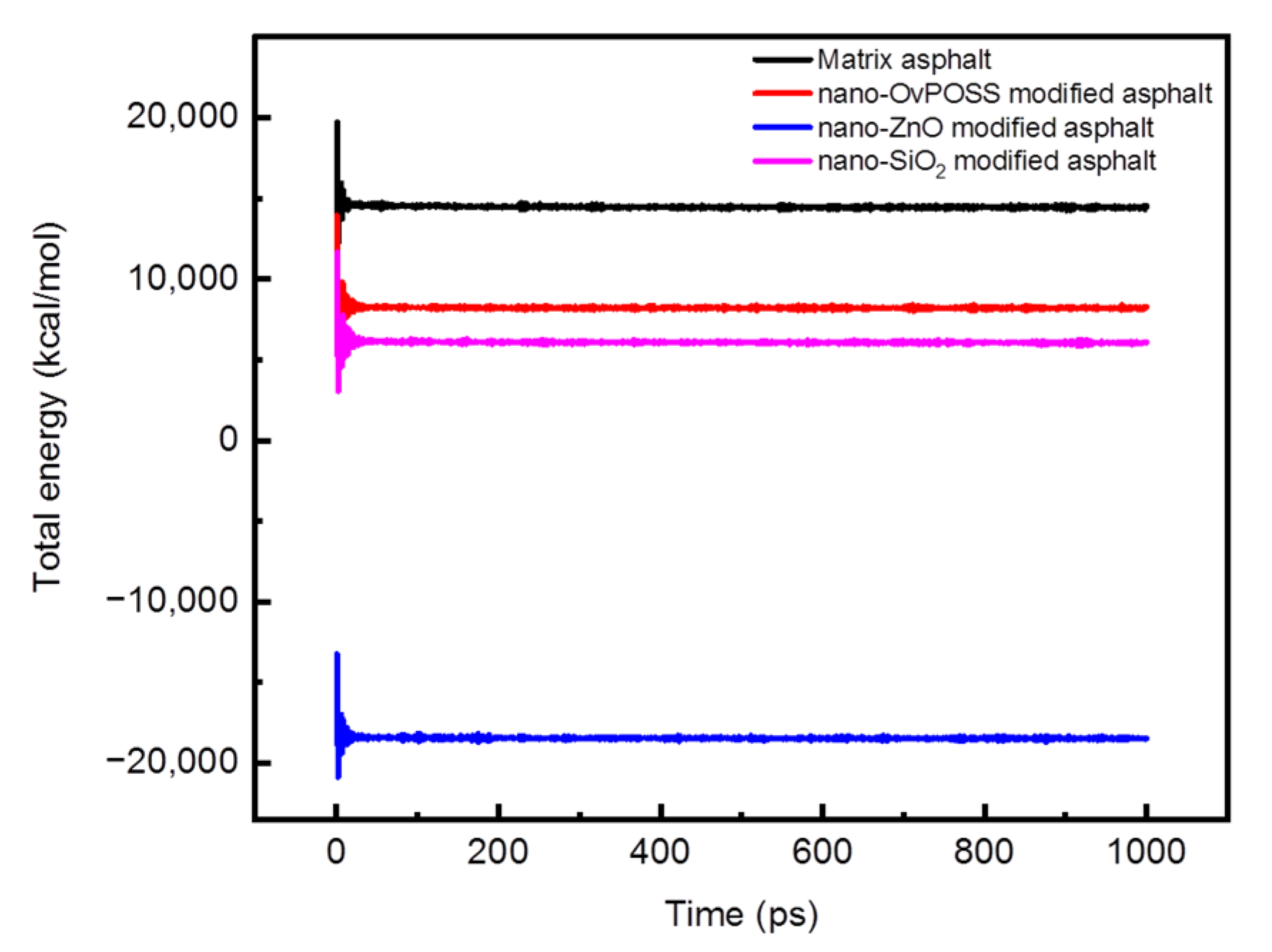
3.1.2. Model Validation
3.2. Compatibility Analysis
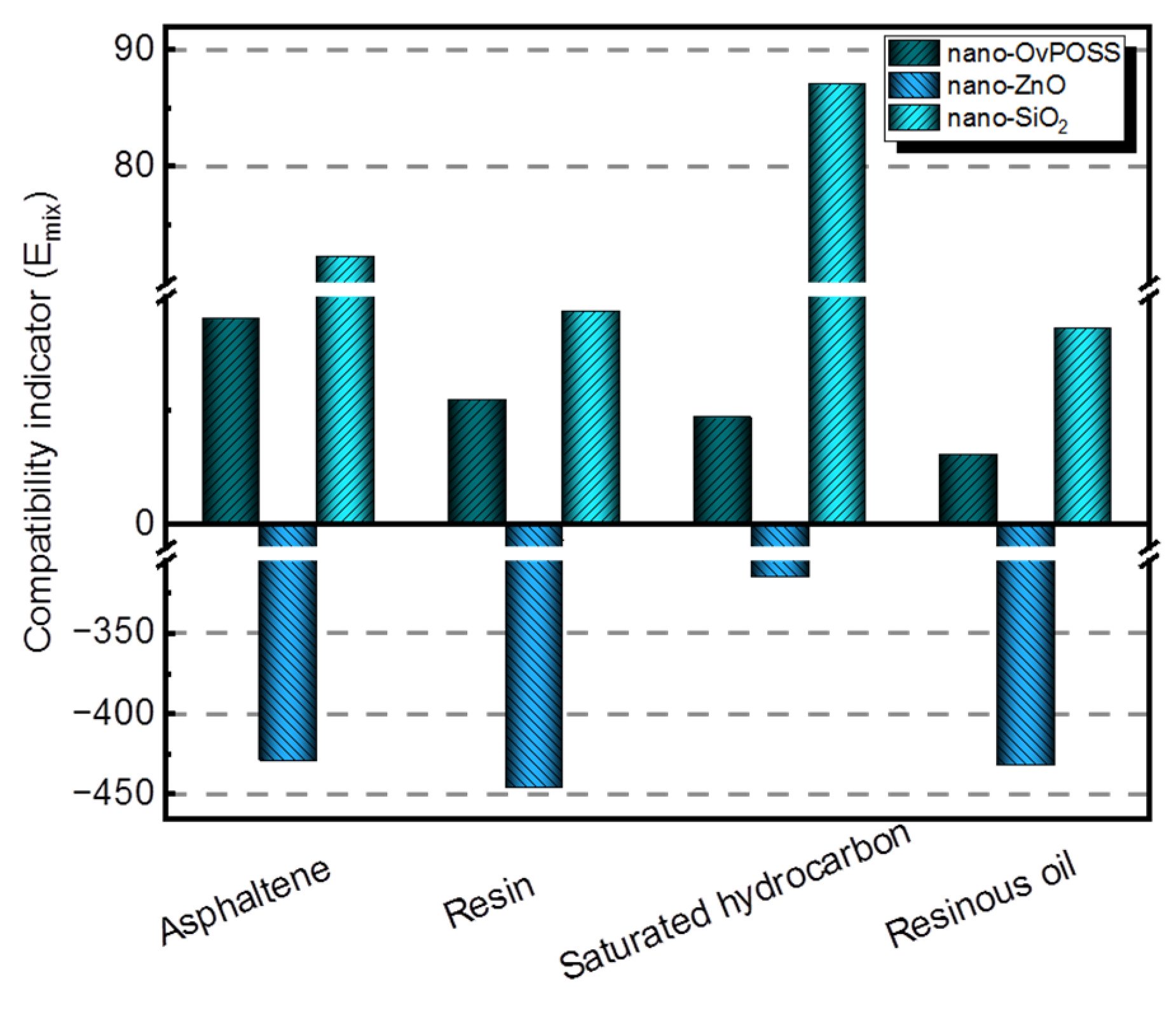
3.2.1. The Mixing Energy of Asphalt Molecules with Nano-OvPOSS, Nano-ZnO, and Nano-SiO2, Respectively
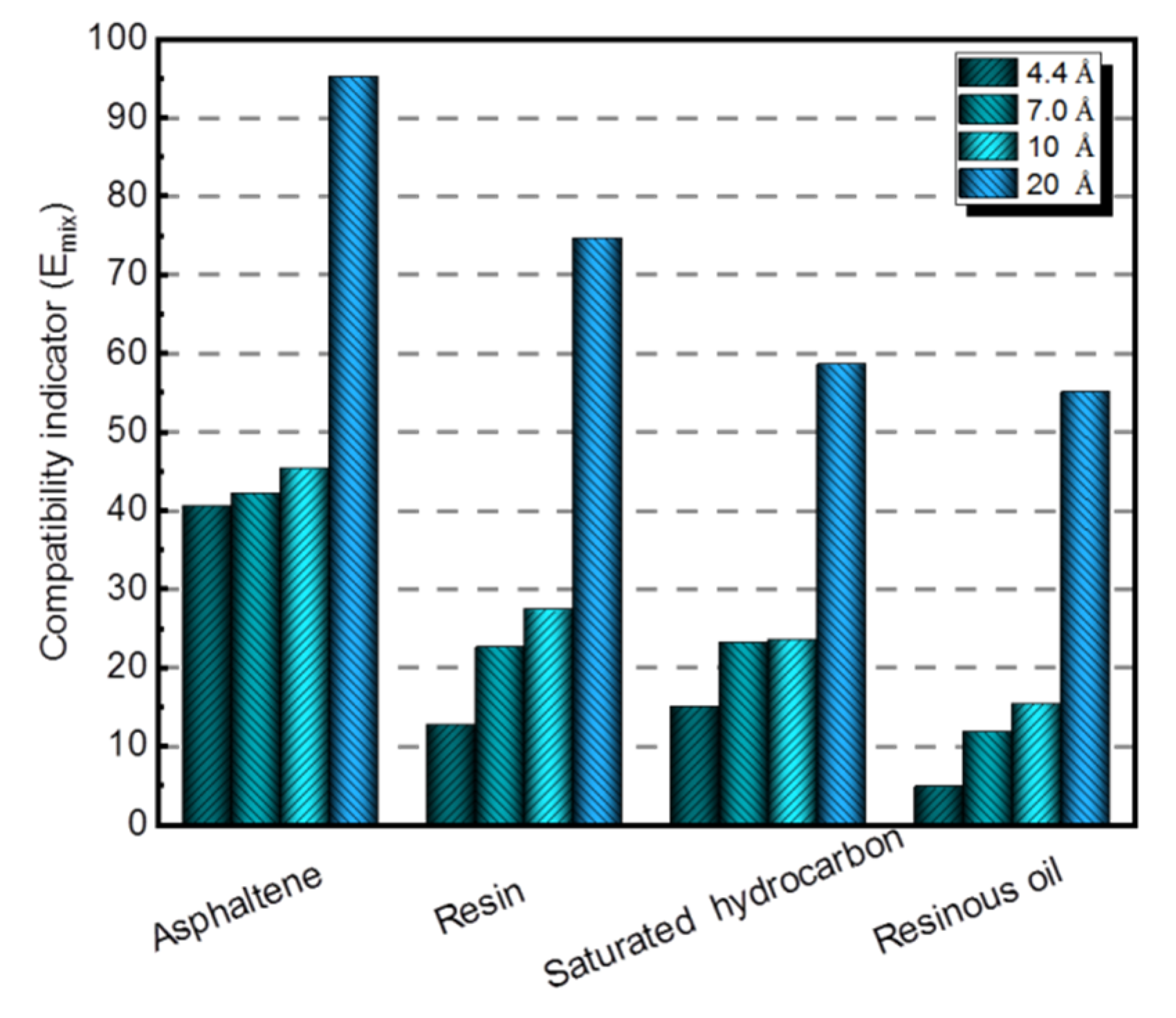
3.2.2. The Mixing Energy between Four Asphalt Components and Nano-OvPOSS with Different Sizes
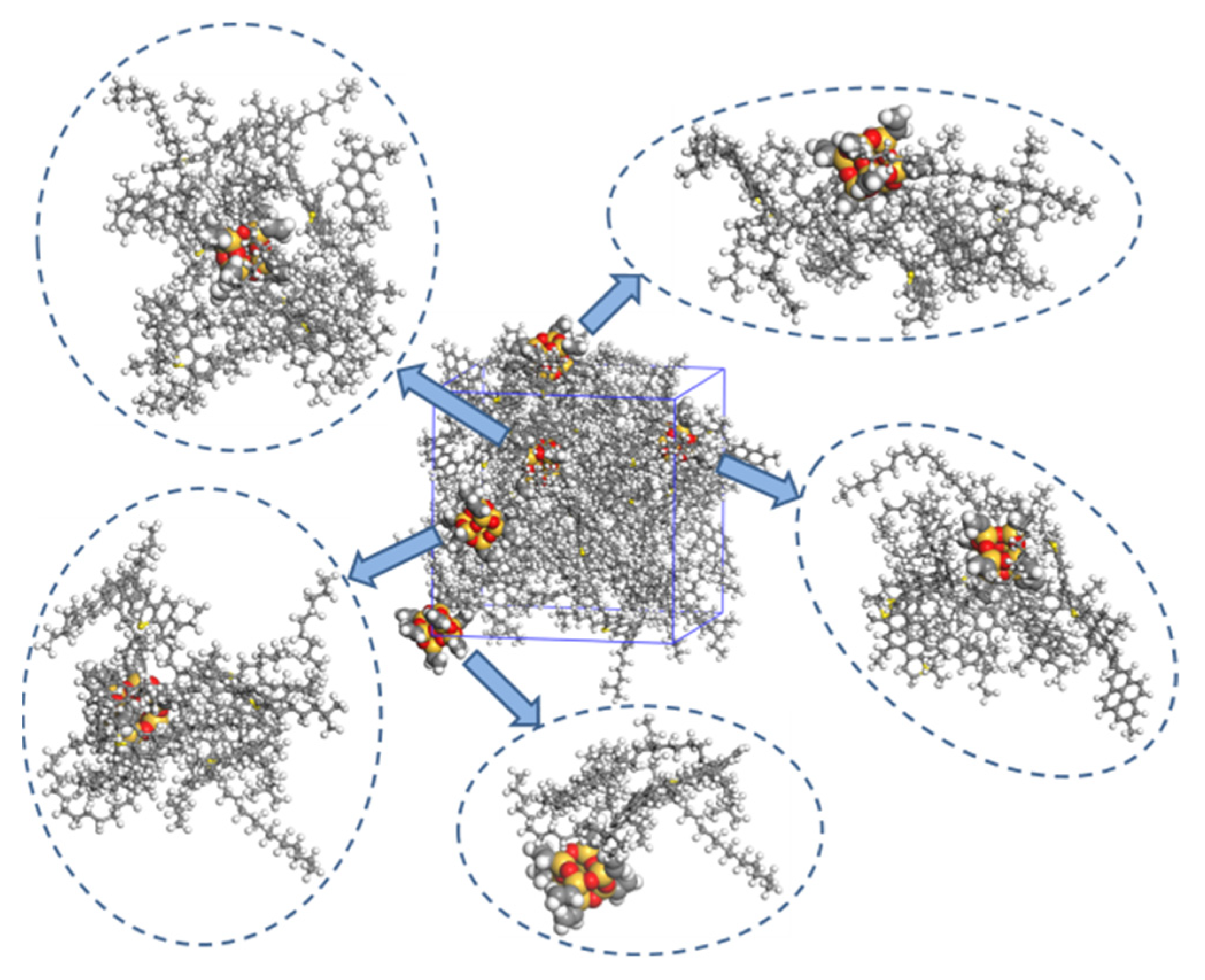
3.3. Distribution of Asphalt Molecule around Nanomaterial
3.4. Properties Comparison
3.4.1. Aggregation Behavior
3.4.2. The Free Volume of Asphalt Systems
3.4.3. Temperature Stability
3.4.4. Resistance to Deformation
4. Conclusions
- The three nanomaterials exhibit different levels of compatibility with asphalt, among which nano-OvPOSS performs the best compatibility with asphalt, followed by nano-SiO2 and nano-ZnO subsequently. Nano-OvPOSS exhibits the most favorable compatibility with resinous oil out of the four asphalt components. The size of nano-OvPOSS determines its compatibility with asphalt. The smaller the particle size of nano-OvPOSS, the better its compatibility with asphalt. Therefore, of all the four sizes of nano-OvPOSS (4.4 Å, 7 Å, 10 Å, and 20 Å) adopted in this study, the 4.4 Å nano-OvPOSS exhibits the best compatibility with asphalt.
- Nano-OvPOSS is able to attract a larger and closer distribution of asphalt molecules around it than nano-SiO2 and nano-ZnO thanks to its distinctive characteristic of possessing eight organophilic groups, thus making it more compatible with asphalt and helping to form a more stable asphalt structure.
- The eight vinyl groups of nano-OvPOSS reinforce the interaction between molecules in the modified asphalt system, which enables nano-OvPOSS to disperse evenly in the modified asphalt system without the occurrence of aggregation. By contrast, nano-SiO2 and nano-ZnO, not having such special structure, are prone to aggregate in the modified asphalt system.
- The separate addition of nano-OvPOSS, nano-ZnO, and nano-SiO2 all leads to the shrinking of free movement space of molecules in the matrix asphalt system, yet nano-OvPOSS is able to reduce the largest percentage of the free volume of the matrix asphalt system. Such reduced RFV of matrix asphalt, caused by the addition of nano-OvPOSS, warrants the structural stability and the deformation resistance of matrix asphalt.
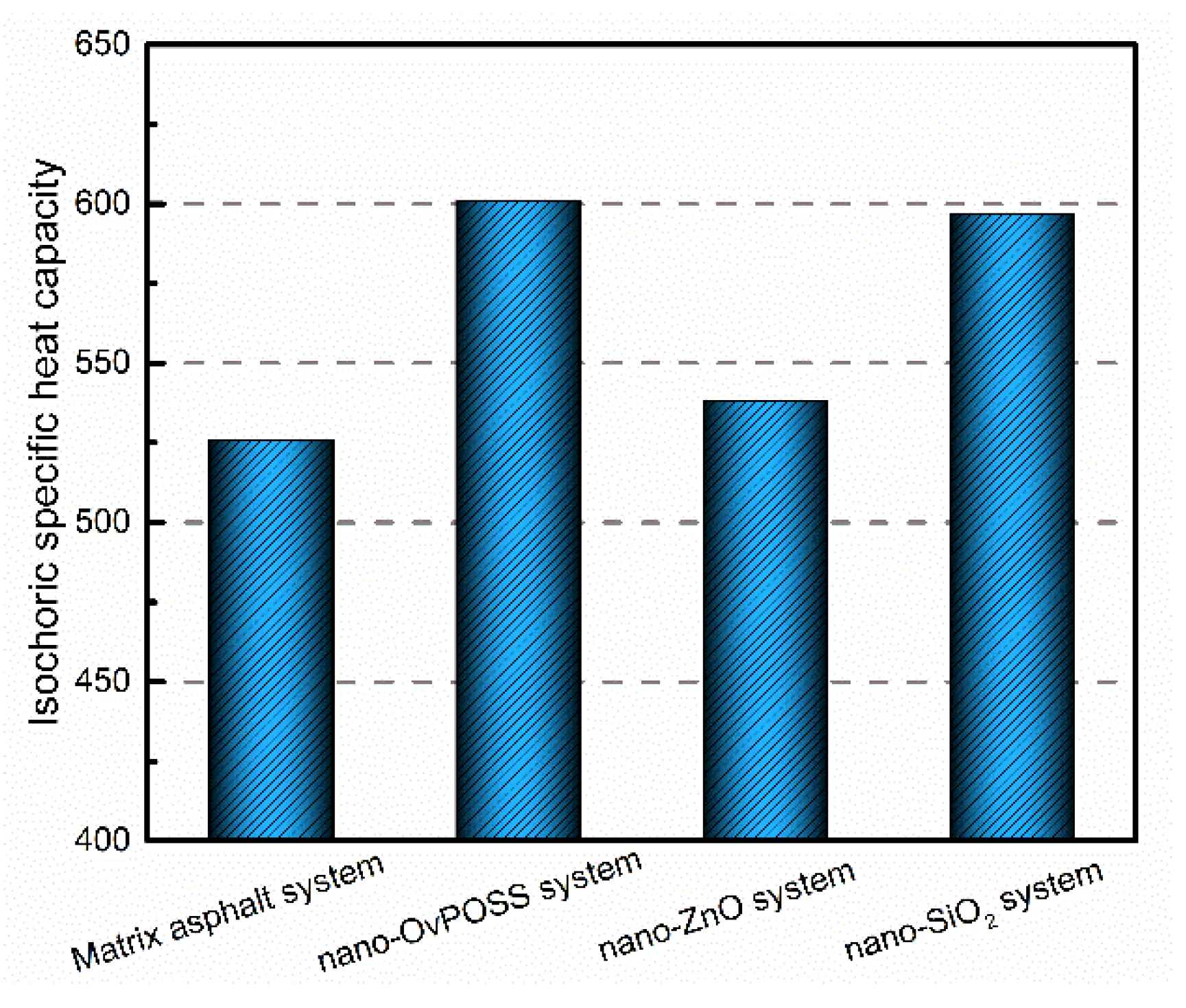
- Nano-OvPOSS and nano-SiO2 perform better in improving the Cv of matrix asphalt than nano-ZnO. Therefore, either the addition of nano-OvPOSS or that of nano-SiO2 will effectively slow down the rising of the temperature of the asphalt system and therefore reduce the negative impact of temperature rise on the rheological properties of the asphalt system.
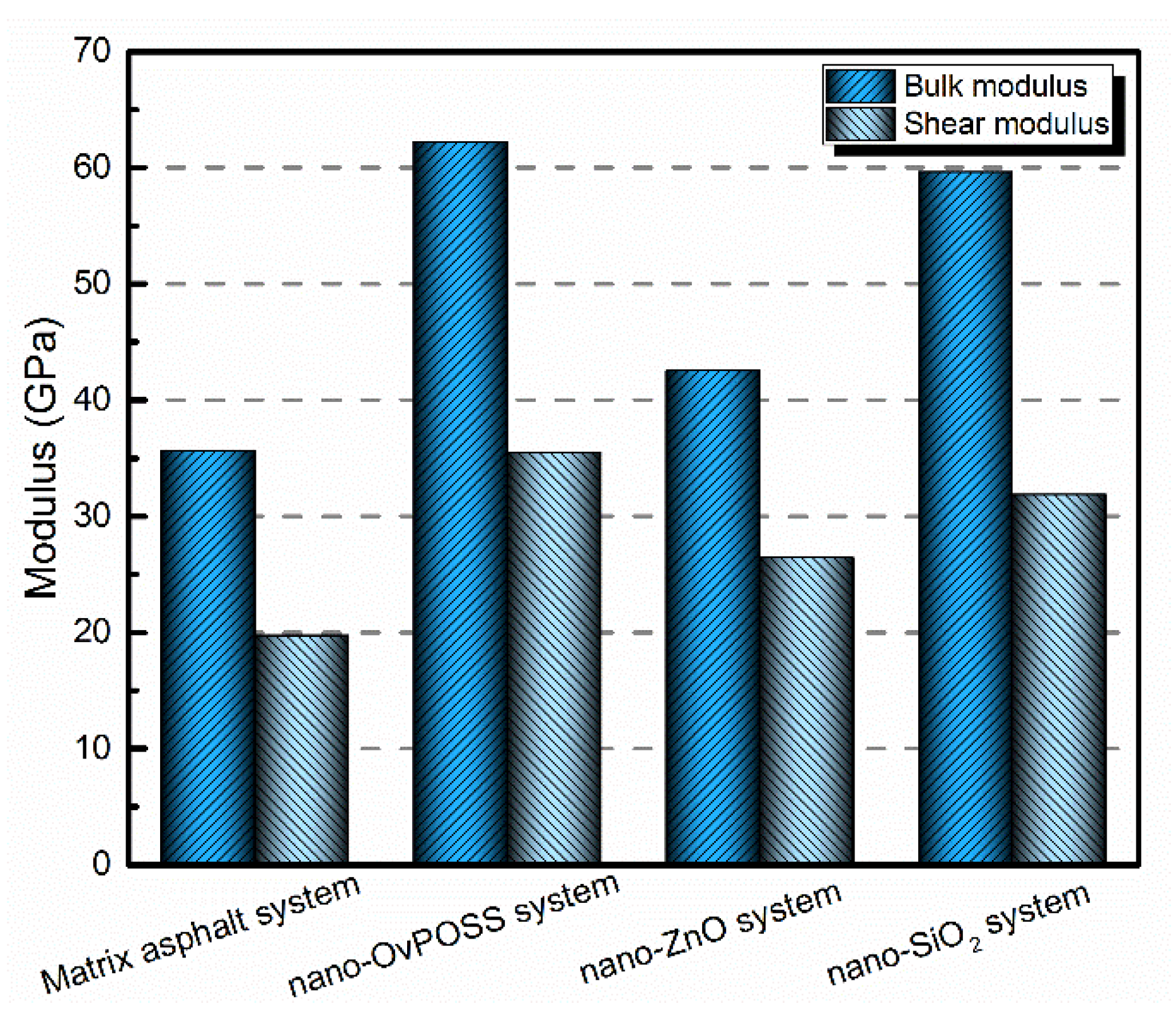
- Nano-OvPOSS, nano-SiO2, and nano-ZnO all positively affect the mechanical properties of matrix asphalt, yet nano-OvPOSS results in the largest increase in the bulk modulus and shear modulus of matrix asphalt, which means nano-OvPOSS does best in improving the mechanical properties of matrix asphalt. Therefore, nano-OvPOSS-modified asphalt shows the most desirable resistance to deformation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Abdelmagid, A.A.A.; Qiu, Y.; Yang, E.; Chen, Y. Study on the aging mechanism and microstructure analysis of rice-husk-ash- and crumb-rubber-powder-modified asphalt. Polymers 2022, 14, 1969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wu, H.; Li, P.; Qiu, J.; Nian, T. Pavement properties and predictive durability analysis of asphalt mixtures. Polymers 2022, 14, 803. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, J.; Liu, Z.; Li, X.; Zhang, F.; Shan, B. Rheological properties of styrene-butadiene-styrene asphalt mastic containing high elastic polymer and snow melting salt. Polymers 2022, 14, 3651. [Google Scholar] [CrossRef]
- Yuan, D.; Xing, C.; Jiang, W.; Xiao, J.; Wu, W.; Li, P.; Li, Y. Viscoelastic behavior and phase structure of high-content SBS-modified asphalt. Polymers 2022, 14, 2476. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Kang, A.; Li, B.; Xiao, P.; Ding, H. Effect of fiber characteristic parameters on the high and low temperature rheological properties of basalt fiber modified asphalt mortar. Case Stud. Constr. Mater. 2022, 17, e01247. [Google Scholar] [CrossRef]
- Han, Y.; Tian, J.; Ding, J.; Shu, L.; Ni, F. Evaluating the storage stability of SBR-modified asphalt binder containing polyphosphoric acid (PPA). Case Stud. Constr. Mater. 2022, 17, e01214. [Google Scholar] [CrossRef]
- Cai, M.; Zhao, X.; Han, X.; Du, P.; Su, Y.; Cheng, C. Effect of thermal oxygen aging mode on rheological properties and compatibility of lignin-modified asphalt binder by dynamic shear rheometer. Polymers 2022, 14, 3572. [Google Scholar] [CrossRef]
- Muslihati, A.; Basri, H.; Hartatik, N. A review of nanomaterial mixtures on asphalt pavement application. Mater. Sci. Forum 2021, 6248, 119–125. [Google Scholar] [CrossRef]
- Ashish, P.K.; Singh, D. Use of nanomaterial for asphalt binder and mixtures: A comprehensive review on development, prospect, and challenges. Road Mater. Pavement Des. 2021, 22, 492–538. [Google Scholar] [CrossRef]
- Ali, S.I.A.; Abubaker, A.; Alshetwi, A.B.Z. Characterization of asphalt binders blended with nanomaterial and polymer. IOP Conf. Ser. Mater. Sci. Eng. 2020, 800, 012002. [Google Scholar] [CrossRef]
- Abandansari, H.F.; Modarres, A. Investigating effects of using nanomaterial on moisture susceptibility of hot-mix asphalt using mechanical and thermodynamic methods. Constr. Build. Mater. 2017, 131, 667–675. [Google Scholar] [CrossRef]
- Fakhri, M.; Shahryari, E. The effects of nano zinc oxide (ZnO) and nano reduced graphene oxide (RGO) on moisture susceptibility property of stone mastic asphalt (SMA). Case Stud. Constr. Mater. 2021, 15, e00655. [Google Scholar] [CrossRef]
- Mashaan, N.; Chegenizadeh, A.; Nikraz, H. Performance of PET and nano-silica modified stone mastic asphalt mixtures. Case Stud. Constr. Mater. 2022, 16, e01044. [Google Scholar] [CrossRef]
- Bala, N.; Napiah, M.; Kamaruddin, I. Effect of nanosilica particles on polypropylene polymer modified asphalt mixture performance. Case Stud. Constr. Mater. 2018, 8, 447–454. [Google Scholar] [CrossRef]
- Mallakpour, S.; Nouruzi, N. Modification of morphological, mechanical, optical and thermal properties in polycaprolactone-based nanocomposites by the incorporation of diacid-modified ZnO nanoparticles. J. Mater. Sci. 2016, 51, 6400–6410. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C. Nano-network MnO2/polyaniline composites with enhanced electrochemical properties for supercapacitors. Mater. Des. 2016, 97, 512–518. [Google Scholar] [CrossRef]
- Zheng, F.; Wang, S.; Wen, S.; Shen, M.; Zhu, M.; Shi, X. Characterization and antibacterial activity of amoxicillin-loaded electrospun nano-hydroxyapatite/poly (lactic-co-glycolic acid) composite nanofibers. Biomaterials 2013, 34, 1402–1412. [Google Scholar] [CrossRef]
- Lam, C.K.; Lau, K.T. Tribological behavior of nanoclay/epoxy composites. Mater. Lett. 2007, 61, 3863–3866. [Google Scholar] [CrossRef]
- Khattak, M.J.; Khattab, A.; Rizvi, H.R.; Zhang, P. The impact of carbon nano-fiber modification on asphalt binder rheology. Constr. Build. Mater. 2012, 30, 257–264. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, C.; Wei, C.; Duan, H.; Yu, J. Application of functionalized nanomaterials in asphalt road construction materials. In Handbook of Functionalized Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 865–907. [Google Scholar]
- Zhang, H.; Zhu, C.; Yan, K.; Yu, J. Effect of rectorite and its organic modification on properties of bitumen. J. Mater. Civ. Eng. 2015, 27, C4014002. [Google Scholar] [CrossRef]
- Shen, C.; Li, R.; Pei, J.; Cai, J.; Liu, T.; Li, Y. Preparation and the effect of surface-functionalized calcium carbonate nanoparticles on asphalt binder. Appl. Sci. 2020, 10, 91. [Google Scholar] [CrossRef]
- He, H.; Hu, J.; Li, R.; Shen, C.; Pei, J.; Zhou, B. Study on rheological properties of silica nanofluids modified asphalt binde. Constr. Build. Mater. 2021, 273, 122046. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, B.; Feng, W.; Wei, W.; Shen, W.; Fang, S.; Fan, K. Fully physically crosslinked POSS-based hydrogel with low swelling, high stretchable, self-healing, and conductive properties for human motion sensing. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 130016. [Google Scholar] [CrossRef]
- Kausar, A. State-of-the-art overview on polymer/POSS nanocomposite. Polym. Plast. Technol. Eng. 2017, 56, 1401–1420. [Google Scholar] [CrossRef]
- Wei, K.; Li, L.; Zheng, S.; Wang, G.; Liang, Q. Organic-inorganic random copolymers from methacrylate-terminated poly (ethylene oxide) with 3-methacryloxypropylheptaphenyl polyhedral oligomeric silsesquioxane: Synthesis via RAFT polymerization and self-assembly behavior. Soft Matter 2014, 10, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.W. Thermal rearrangement of branched-chain methylpolysiloxanes. J. Am. Chem. Soc. 1946, 68, 356–358. [Google Scholar] [CrossRef]
- Zhao, W.; Dong, Z.; Zhao, L. Radiation synthesis of polyhedral oligomeric silsesquioxanes (POSS) gel polymers. Radiat. Phys. Chem. 2022, 198, 110251. [Google Scholar] [CrossRef]
- Jeon, H.G.; Mather, P.T.; Haddad, T.S. Shape memory and nanostructure in poly (norbornyl-POSS) copolymers. Polym. Int. 2000, 49, 453–457. [Google Scholar] [CrossRef]
- Bicer, E.; Demir, G.K.; Kodal, M.; Ozkoc, G. Crosslinked low-density polyethylene/polyhedral oligomeric silsesquioxanes composites: Effects of crosslinker concentration on the mechanical, thermal, rheological, and shape memory properties. J. Macromol. Sci. Part B 2021, 60, 999–1024. [Google Scholar] [CrossRef]
- Lin, X.; Siew, W.H.; Liggat, J.; Given, M.; He, J. Octavinyl polyhedral oligomeric silsesquioxane on tailoring the DC electrical characteristics of polypropylene. High Volt. 2021, 7, 137–146. [Google Scholar] [CrossRef]
- Zeng, W.; Huang, H.; Song, L.; Jiang, X.; Zhang, X. A novel hydroxyl polyacrylate latex modified by OvPOSS and its application in two-component waterborne polyurethane coatings. J. Coat. Technol. Res. 2020, 17, 181–191. [Google Scholar] [CrossRef]
- Wu, J.; Haddad, T.S.; Mather, P.T. Vertex group effects in entangled polystyrene-polyhedral oligosilsesquioxane (POSS) copolymers. Macromolecules 2009, 42, 1142–1152. [Google Scholar] [CrossRef]
- Yu, H.; Ren, W.; Zhang, Y. Nonisothermal decomposition kinetics of nylon 1010/POSS composites. J. Appl. Polym. Sci. 2009, 113, 17–23. [Google Scholar] [CrossRef]
- Xu, H.; Yang, B.; Wang, J.; Guang, S.; Li, C. Preparation, thermal properties, and Tg increase mechanism of poly (acetoxystyrene-co-octavinyl-polyhedral oligomeric silsesquioxane) hybrid nanocomposites. Macromolecules 2010, 38, 10455–10460. [Google Scholar] [CrossRef]
- Yang, B.; Xu, H.; Wang, J.; Gang, S.; Li, C. Preparation and thermal property of hybrid nanocomposites by free radical copolymerization of styrene with octavinyl polyhedral oligomeric silsesquioxane. J. Appl. Polym. Sci. 2010, 106, 320–326. [Google Scholar] [CrossRef]
- Hao, L.; Chen, J.; Ma, T.; Cheng, J.; Zhang, J.; Zhao, F. Low dielectric and high performance of epoxy polymer via grafting POSS dangling chains. Eur. Polym. J. 2022, 173, 111313. [Google Scholar] [CrossRef]
- Hansen, J.S.; Lemarchand, C.A.; Nielsen, E.; Dyre, J.C.; Schroder, T. Four-component united-atom model of bitumen. J. Chem. Phys. 2013, 138, 094508. [Google Scholar] [CrossRef]
- Sun, H.; Ren, P.; Fried, J.R. The COMPASS force field: Parameterization and validation for phosphazenes. Comput. Theor. Polym. Sci. 1998, 8, 229–246. [Google Scholar] [CrossRef]
- Rigby, D.; Sun, H.; Eichinger, B.E. Computer simulations of poly (ethylene oxide): Force field, pvt diagram and cyclization behaviour. Polym. Int. 1997, 44, 311–330. [Google Scholar] [CrossRef]
- Nose’, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.P.; Postma, J.; Gunsteren, W.; Dinola, A.D.; Haak, J.R. Molecular-dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Huggins, M.L. Principles of polymer chemistry. J. Am. Chem. Soc. 1954, 76, 2854. [Google Scholar] [CrossRef]
- Bawendi, M.G.; Freed, K.F. A lattice model for self- and mutually avoiding semiflexible polymer chains. J. Chem. Phys. 1987, 86, 3720–3730. [Google Scholar] [CrossRef]
- Fan, C.F.; Olafson, B.D.; Blanco, M.; Hsu, S.L. Application of molecular simulation to derive phase diagrams of binary mixtures. Macromolecules 1992, 25, 3667–3676. [Google Scholar] [CrossRef]
- Freed, K.F.; Bawendi, M.G. Lattice theories of polymeric fluids. J. Phys. Chem. 1989, 93, 2194–2203. [Google Scholar] [CrossRef]
- Choi, W.S.; Lee, H. Nanostructured materials for water purification: Adsorption of heavy metal ions and organic dyes. Polymers 2022, 14, 2183. [Google Scholar] [PubMed]
- Vu, D.; Ahn, K. Triboelectric enhancement of polyvinylidene fluoride membrane using magnetic nanoparticle for water-based energy harvesting. Polymers 2022, 14, 1547. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, H.; Quan, Y.; Ma, R.; Pentzer, E.B.; Green, M.J.; Wang, Q. Thermal stability and flammability studies of mxene-organic hybrid polystyrene nanocomposites. Polymers 2022, 14, 1213. [Google Scholar] [CrossRef]
- Coleman, M.M.; Painter, P.C. Hydrogen bonded polymer blends. Prog. Polym. Sci. 1995, 20, 1–59. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Wong, C.P. Study on mono-dispersed nano-size silica by surface modification for underfill applications. J. Colloid Interface Sci. 2005, 292, 436–444. [Google Scholar] [CrossRef]
- Vrentas, J.S.; Duda, J.L.; Ling, H.C. Free-volume theories for self-diffusion in polymer-solvent systems. I. Conceptual differences in theories. J. Polym. Sci. Part B Polym. Phys. Ed. 1985, 23, 275–288. [Google Scholar] [CrossRef]
- Rad, F.Y.; Sefidmazgi, N.R.; Bahia, H. Application of diffusion mechanism. Transp. Res. Rec. J. Transp. Res. Board 2014, 2444, 71–77. [Google Scholar] [CrossRef]
- Sadeghnejad, M.; Shafabakhsh, G. Use of Nano SiO2 and Nano TiO2 to improve the mechanical behaviour of stone mastic asphalt mixtures. Constr. Build. Mater. 2017, 157, 965–974. [Google Scholar] [CrossRef]
- Turan, D.; Sirin, H.; Ozkoc, G. Effects of POSS particles on the mechanical, thermal, and morphological properties of PLA and Plasticised PLA. J. Appl. Polym. Sci. 2011, 121, 1067–1075. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Guo, G.; Zhao, B.; Yu, J. Effects of ZnO particle size on properties of asphalt and asphalt mixture. Constr. Build. Mater. 2018, 159, 578–586. [Google Scholar] [CrossRef]










| Composition | Molecular Formula | Number |
|---|---|---|
| Asphaltene | C64H52S2 | 4 |
| Resin | C41H54S | 12 |
| Saturated hydrocarbon | C22H46 | 28 |
| Resinous oil | C24H28 | 20 |
| Nano-OvPOSS/Nano-ZnO/Nano-SiO2 | C16H24O12Si8/O6Zn6/H16O24Si8 | 5/6/5 |
| Type | Meaning | Equation | Explanation |
|---|---|---|---|
| RDF | The ratio of regional density to the average density. | The g(r) is radial distribution function; N is the total number of atoms; r is the distance; ρ is the density. | |
| RFV | The percentage of volume not occupied by molecules in system. | The Vf is free volume and Vo is the occupied volume. |
| Type | 1 | 2 | 3 | 4 | 5 | Average |
|---|---|---|---|---|---|---|
| Asphaltene | 1 | 1 | 1 | 0 | 1 | 0.8 |
| Resin | 4 | 2 | 2 | 1 | 1 | 2.0 |
| Saturated hydrocarbon | 3 | 5 | 5 | 3 | 6 | 4.4 |
| Resinous oil | 2 | 2 | 3 | 3 | 3 | 2.6 |
| Average distance/Å | 12.5 | 10.1 | 11.0 | 10.3 | 11.7 | 11.1 |
| Type | 1 | 2 | 3 | 4 | 5 | 6 | Average |
|---|---|---|---|---|---|---|---|
| Asphaltene | 1 | 0 | 1 | 1 | 0 | 1 | 0.7 |
| Resin | 2 | 2 | 0 | 1 | 1 | 0 | 1.0 |
| Saturated hydrocarbon | 2 | 2 | 4 | 3 | 3 | 2 | 2.7 |
| Resinous oil | 5 | 4 | 2 | 2 | 3 | 3 | 3.2 |
| Average distance/Å | 14.2 | 15.0 | 13.4 | 14.1 | 13.9 | 14.7 | 14.2 |
| Type | 1 | 2 | 3 | 4 | 5 | Average |
|---|---|---|---|---|---|---|
| Asphaltene | 0 | 1 | 0 | 1 | 1 | 0.6 |
| Resin | 2 | 2 | 2 | 2 | 1 | 1.8 |
| Saturated hydrocarbon | 4 | 1 | 3 | 4 | 4 | 3.2 |
| Resinous oil | 2 | 2 | 4 | 3 | 2 | 2.6 |
| Average distance/Å | 13.1 | 13.7 | 12.1 | 11.0 | 10.5 | 12.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Zhao, P.; Chen, T.; Jing, M. Comparative Study of Octavinyl Oligomeric Sesquisiloxane Nanomaterial-Modified Asphalt Using Molecular Dynamics Method. Polymers 2022, 14, 4577. https://doi.org/10.3390/polym14214577
Feng L, Zhao P, Chen T, Jing M. Comparative Study of Octavinyl Oligomeric Sesquisiloxane Nanomaterial-Modified Asphalt Using Molecular Dynamics Method. Polymers. 2022; 14(21):4577. https://doi.org/10.3390/polym14214577
Chicago/Turabian StyleFeng, Lei, Peng Zhao, Tongdan Chen, and Minghai Jing. 2022. "Comparative Study of Octavinyl Oligomeric Sesquisiloxane Nanomaterial-Modified Asphalt Using Molecular Dynamics Method" Polymers 14, no. 21: 4577. https://doi.org/10.3390/polym14214577
APA StyleFeng, L., Zhao, P., Chen, T., & Jing, M. (2022). Comparative Study of Octavinyl Oligomeric Sesquisiloxane Nanomaterial-Modified Asphalt Using Molecular Dynamics Method. Polymers, 14(21), 4577. https://doi.org/10.3390/polym14214577





