Environmental Properties and Applications of Biodegradable Starch-Based Nanocomposites
Abstract
:1. Introduction
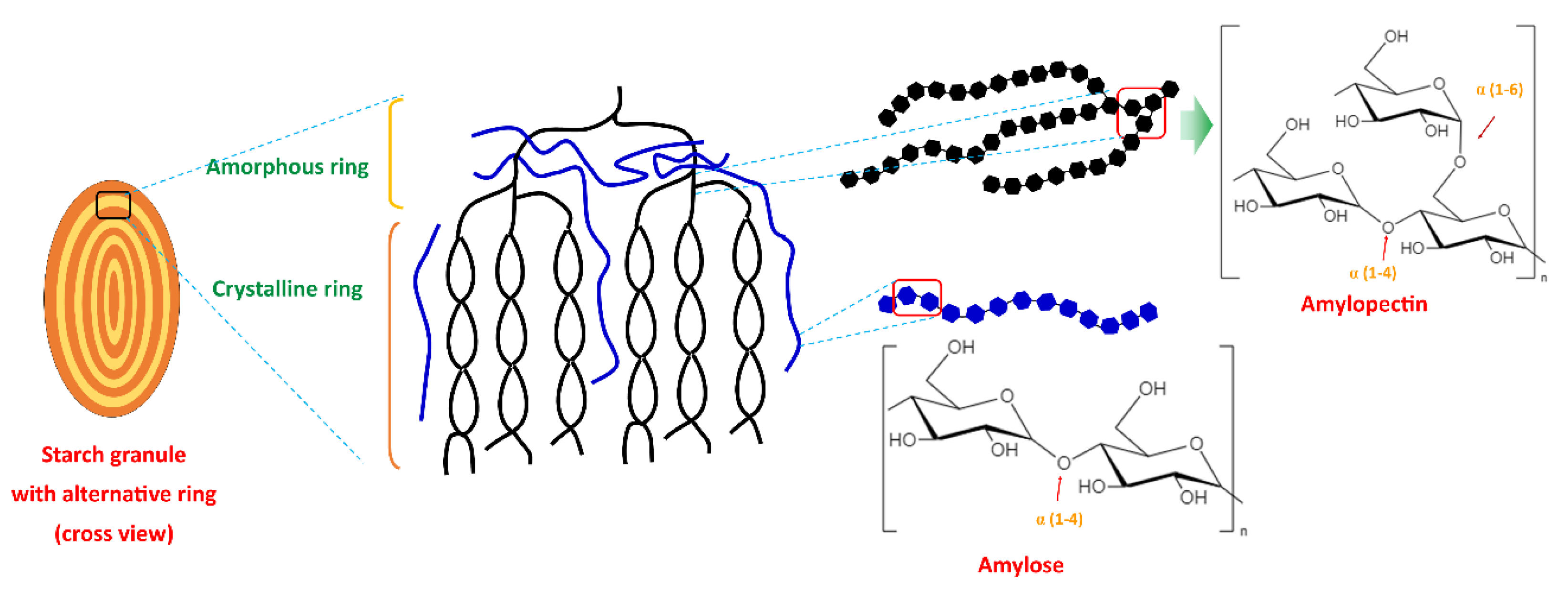
2. Starch
3. Nanomaterials and Nanocomposites
4. Starch Nanoparticles (SNPs)
5. Starch-Based Nanocomposites
5.1. Starch/Nanocellulose Composite
5.2. Starch/Chitin Nanoparticles Composites
5.3. Starch/Nanoclay Nanocomposites
5.4. Starch/Carbonaceous Nanocomposites
6. Applications of Biodegradable Starch-Based Nanocomposites
6.1. Agriculture
6.2. Packaging
6.3. Biomedical
6.4. Environment
6.5. Other Applications
7. Lifecycle Analysis of Nanocomposites
| Impact Category | Ozone Depletion (kg CFC-11 eq.) | Global Warming Potential/Greenhouse Gas Emissions (kg CO2 eq.) | Smog (kg O3 eq.) | Acidification (kg SO2 eq.) | Eutrophication (kg N eq.) | Human Toxicity, Carcinogen (CTUh) | Human Toxicity, Noncarcinogen (CTUh) | Respiratory Effects (kg PM2.5 eq.) | Ecotoxicity (CTUe) | Water Consumption (Kg) | Agricultural Land Use (m2yr/kg) | Fossil Fuel Depletion/Non-Renewable Energy Use (MJ Surplus) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PE waste management | 1.28 × 10−5 | 3.82 × 103 | 5.77 × 10 | 1.39 × 10 c | 1.05 × 10−4 | [143] | |||||||
| Starch-based polymers production with PBS, PLA/PBAT, PHB, PLA, PBS/fiber, and recycled-PLA | 1.8–3.7 | 1.2–1.9 d | 0.3–1.3 | 33–72 | [141] | ||||||||
| Starch-stabilized Ag NPs manufacturing via microwave-assisted heating | 1.24 × 10−7 | 8.44 × 10−2 | 2.37 × 10−1 | 2.51 × 10−1 | 1.21 × 10−1 | 4.44 × 10−6 | 8.02 × 10−4 | 6.41 × 102 | 5.85 × 105 | 7.08 × 102 | [137] | ||
| Starch nanofiller preparation using various process | 0.00 | 7.95–13.07 | 0.5–0.6 a | 8.78–15.51 b | 0.16–0.23 | 0.9–0.16 e | 2216.99–3747.76 f | 0.02 | 33.15–115.82 h | 16–19 | [132] | ||
| Nanofiller OMMT | 1.52 | 1.139 g | 40.079 | [144] |
8. Biodegradation of Starch
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, Structures, and Advanced Applications of Nanocomposites from Biorenewable Resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef] [PubMed]
- Saad, E.M.; Elshaarawy, R.F.; Mahmoud, S.A.; El-Moselhy, K.M. New Ulva Lactuca Algae Based Chitosan Bio-Composites for Bioremediation of Cd(II) Ions. J. Bioresour. Bioprod. 2021, 6, 223–242. [Google Scholar] [CrossRef]
- Puiggalí, J.; Katsarava, R. Chapter 7—Bionanocomposites. In Clay-Polymer Nanocomposites; Jlassi, K., Chehimi, M.M., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 239–272. ISBN 978-0-323-46153-5. [Google Scholar]
- Madhumitha, G.; Fowsiya, J.; Mohana Roopan, S.; Thakur, V.K. Recent Advances in Starch–Clay Nanocomposites. Int. J. Polym. Anal. Charact. 2018, 23, 331–345. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Geethamma, V.G.; Gopi, S.; Thomas, M.G.; Kunaver, M.; Huskić, M.; Kalarikkal, N.; Volova, T.; Rouxel, D.; Thomas, S. Thermal, Biodegradation and Theoretical Perspectives on Nanoscale Confinement in Starch/Cellulose Nanocomposite Modified via Green Crosslinker. Int. J. Biol. Macromol. 2019, 134, 781–790. [Google Scholar] [CrossRef] [Green Version]
- Arora, B.; Bhatia, R.; Attri, P. 28—Bionanocomposites: Green Materials for a Sustainable Future. In New Polymer Nanocomposites for Environmental Remediation; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: Wilmington, NC, USA, 2018; pp. 699–712. ISBN 978-0-12-811033-1. [Google Scholar]
- García, N.L.; Famá, L.; D’Accorso, N.B.; Goyanes, S. Biodegradable Starch Nanocomposites. In Eco-friendly Polymer Nanocomposites: Processing and Properties; Thakur, V.K., Thakur, M.K., Eds.; Advanced Structured Materials; Springer: New Delhi, India, 2015; pp. 17–77. ISBN 978-81-322-2470-9. [Google Scholar]
- Mohammad, F.; Arfin, T.; Bwatanglang, I.B.; Al-lohedan, H.A. Starch-Based Nanocomposites: Types and Industrial Applications. In Bio-Based Polymers and Nanocomposites: Preparation, Processing, Properties & Performance; Sanyang, M.L., Jawaid, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 157–181. ISBN 978-3-030-05825-8. [Google Scholar]
- BeMiller, J.N. Chapter 5—Physical Modification of Starch. In Starch in Food, 2nd ed.; Sjöö, M., Nilsson, L., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2018; pp. 223–253. ISBN 978-0-08-100868-3. [Google Scholar]
- Gunawardene, O.H.P.; Gunathilake, C.A.; Amaraweera, A.P.S.M.; Fernando, N.M.L.; Manipura, A.; Manamperi, W.A.; Kulatunga, K.M.A.K.; Rajapaksha, S.M.; Gamage, A.; Dassanayake, R.S.; et al. Removal of Pb(II) Ions from Aqueous Solution Using Modified Starch. J. Compos. Sci. 2021, 5, 46. [Google Scholar] [CrossRef]
- Okoli, C.P.; Ofomaja, A.E. Degree of Time Dependency of Kinetic Coefficient as a Function of Adsorbate Concentration; New Insights from Adsorption of Tetracycline onto Monodispersed Starch-Stabilized Magnetic Nanocomposite. J. Environ. Manag. 2018, 218, 139–147. [Google Scholar] [CrossRef]
- Zarski, A.; Bajer, K.; Kapuśniak, J. Review of the Most Important Methods of Improving the Processing Properties of Starch toward Non-Food Applications. Polymers 2021, 13, 832. [Google Scholar] [CrossRef]
- Le Corre, D.; Angellier-Coussy, H. Preparation and Application of Starch Nanoparticles for Nanocomposites: A Review. React. Funct. Polym. 2014, 85, 97–120. [Google Scholar] [CrossRef]
- Cano, A.I.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Biodegradation Behavior of Starch-PVA Films as Affected by the Incorporation of Different Antimicrobials. Polym. Degrad. Stab. 2016, 132, 11–20. [Google Scholar] [CrossRef]
- Mohan, T.; Devchand, K.; Kanny, K. Barrier and Biodegradable Properties of Corn Starch-Derived Biopolymer Film Filled with Nanoclay Fillers. J. Plast. Film Sheeting 2017, 33, 309–336. [Google Scholar] [CrossRef]
- Venkatesh, G.; Nyflött, Å.; Bonnerup, C.; Lestelius, M. An Economic-Environmental Analysis of Selected Barrier-Coating Materials Used in Packaging Food Products: A Swedish Case Study. Env. Dev. Sustain. 2018, 20, 1483–1497. [Google Scholar] [CrossRef] [Green Version]
- Wani, A.A.; Singh, P. Application of Life Cycle Assessment for Starch and Starch Blends. In Starch-Based Polymeric Materials and Nanocomposites; Ahmed, J., Tiwari, B.K., Imam, S.H., Rao, M.A., Eds.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-0-429-10818-1. [Google Scholar]
- Kakadellis, S.; Harris, Z.M. Don’t Scrap the Waste: The Need for Broader System Boundaries in Bioplastic Food Packaging Life-Cycle Assessment—A Critical Review. J. Clean. Prod. 2020, 274, 122831. [Google Scholar] [CrossRef]
- Bertolini, A. (Ed.) Starches: Characterization, Properties, and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978-0-429-14172-0. [Google Scholar]
- Govindaraju, I.; Zhuo, G.-Y.; Chakraborty, I.; Melanthota, S.K.; Mal, S.S.; Sarmah, B.; Baruah, V.J.; Mahato, K.K.; Mazumder, N. Investigation of Structural and Physico-Chemical Properties of Rice Starch with Varied Amylose Content: A Combined Microscopy, Spectroscopy, and Thermal Study. Food Hydrocoll. 2022, 122, 107093. [Google Scholar] [CrossRef]
- Pérez, S.; Baldwin, P.M.; Gallant, D.J. Chapter 5—Structural Features of Starch Granules I. In Starch, 3rd ed.; BeMiller, J., Whistler, R., Eds.; Food Science and Technology; Academic Press: San Diego, CA, USA, 2009; pp. 149–192. ISBN 978-0-12-746275-2. [Google Scholar]
- Alves, Z.; Abreu, B.; Ferreira, N.M.; Marques, E.F.; Nunes, C.; Ferreira, P. Enhancing the Dispersibility of Multiwalled Carbon Nanotubes within Starch-Based Films by the Use of Ionic Surfactants. Carbohydr. Polym. 2021, 273, 118531. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of Starch Type on the Physico-Chemical Properties of Edible Films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Vijayakumar, R.P. Synthesis of Polystyrene/Starch/CNT Composite and Study on Its Biodegradability. J. Polym. Res. 2020, 27, 187. [Google Scholar] [CrossRef]
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and Functionality of Starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Cornejo-Ramírez, Y.I.; Martínez-Cruz, O.; Del Toro-Sánchez, C.L.; Wong-Corral, F.J.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J. The Structural Characteristics of Starches and Their Functional Properties. CyTA—J. Food 2018, 16, 1003–1017. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; de Oliveira, R.A. Extraction and Characterization of Arrowroot (Maranta Arundinaceae L.) Starch and Its Application in Edible Films. Carbohydr. Polym. 2018, 186, 64–72. [Google Scholar] [CrossRef]
- Lemos, P.V.F.; Barbosa, L.S.; Ramos, I.G.; Coelho, R.E.; Druzian, J.I. The Important Role of Crystallinity and Amylose Ratio in Thermal Stability of Starches. J. Anal. Calorim. 2018, 131, 2555–2567. [Google Scholar] [CrossRef]
- Li, Z.; Guo, K.; Lin, L.; He, W.; Zhang, L.; Wei, C. Comparison of Physicochemical Properties of Starches from Flesh and Peel of Green Banana Fruit. Molecules 2018, 23, 2312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanyapanich, N.; Jimtaisong, A.; Rawdkuen, S. Functional Properties of Banana Starch (Musa Spp.) and Its Utilization in Cosmetics. Molecules 2021, 26, 3637. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.; Höchstötter, A.; Jekle, M.; Arendt, E.; Becker, T. Physicochemical and Morphological Characterization of Different Starches with Variable Amylose/Amylopectin Ratio. Food Hydrocoll. 2013, 32, 52–63. [Google Scholar] [CrossRef]
- Chisenga, S.M.; Workneh, T.S.; Bultosa, G.; Alimi, B.A. Progress in Research and Applications of Cassava Flour and Starch: A Review. J. Food Sci. Technol. 2019, 56, 2799–2813. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Hou, J.; Yang, N.; Zhang, Y.; Chen, H.; Zhang, Z.; Shen, Y.; Huang, S.; Guo, S. Insight on the Changes of Cassava and Potato Starch Granules during Gelatinization. Int. J. Biol. Macromol. 2019, 126, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liu, L.; Qu, J.; Blennow, A.; Hansen, A.R.; Wu, Y.; Guo, D.; Liu, X. Amylose Content and Specific Fine Structures Affect Lamellar Structure and Digestibility of Maize Starches. Food Hydrocoll. 2020, 108, 105994. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, P.; Sui, Z.; Bao, J. Physicochemical Properties of Starches from Diverse Rice Cultivars Varying in Apparent Amylose Content and Gelatinisation Temperature Combinations. Food Chem. 2015, 172, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yang, J.; Hong, Y.; Liu, G.; Zheng, J.; Gu, Z.; Zhang, P. Impact of Amylose Content on Starch Physicochemical Properties in Transgenic Sweet Potato. Carbohydr. Polym. 2015, 122, 417–427. [Google Scholar] [CrossRef]
- Biduski, B.; da Silva, W.M.F.; Colussi, R.; Halal, S.L.; De, M.E.; Lim, L.-T.; Dias, Á.R.G.; da Zavareze, E.R. Starch Hydrogels: The Influence of the Amylose Content and Gelatinization Method. Int. J. Biol. Macromol. 2018, 113, 443–449. [Google Scholar] [CrossRef]
- Singh, S.; Singh, N.; Isono, N.; Noda, T. Relationship of Granule Size Distribution and Amylopectin Structure with Pasting, Thermal, and Retrogradation Properties in Wheat Starch. J. Agric. Food Chem. 2010, 58, 1180–1188. [Google Scholar] [CrossRef]
- Bahrami, B.; Behzad, T.; Salehinik, F.; Zamani, A.; Heidarian, P. Incorporation of Extracted Mucor Indicus Fungus Chitin Nanofibers into Starch Biopolymer: Morphological, Physical, and Mechanical Evaluation. Starch—Stärke 2021, 73, 2000218. [Google Scholar] [CrossRef]
- Ngô, C.; Van de Voorde, M.H. Nanomaterials: Doing More with Less. In Nanotechnology in a Nutshell: From Simple to Complex Systems; Ngô, C., Van de Voorde, M., Eds.; Atlantis Press: Paris, France, 2014; pp. 55–70. ISBN 978-94-6239-012-6. [Google Scholar]
- Saleh, T.A. Nanomaterials: Classification, Properties, and Environmental Toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Singh, V.; Yadav, P.; Mishra, V. Recent Advances on Classification, Properties, Synthesis, and Characterization of Nanomaterials. In Green Synthesis of Nanomaterials for Bioenergy Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 83–97. ISBN 978-1-119-57678-5. [Google Scholar]
- Zafar, R.; Zia, K.M.; Tabasum, S.; Jabeen, F.; Noreen, A.; Zuber, M. Polysaccharide Based Bionanocomposites, Properties and Applications: A Review. Int. J. Biol. Macromol. 2016, 92, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Turan, D.; Gunes, G.; Kilic, A. Perspectives of Bio-Nanocomposites for Food Packaging Applications. In Bionanocomposites for Packaging Applications; Jawaid, M., Swain, S.K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–32. ISBN 978-3-319-67319-6. [Google Scholar]
- Sandhu, K.S.; Nain, V. Starch Nanoparticles: Their Preparation and Applications. In Plant Biotechnology: Recent Advancements and Developments; Gahlawat, S.K., Salar, R.K., Siwach, P., Duhan, J.S., Kumar, S., Kaur, P., Eds.; Springer: Singapore, 2017; pp. 213–232. ISBN 978-981-10-4732-9. [Google Scholar]
- Santana, J.S.; de Carvalho Costa, É.K.; Rodrigues, P.R.; Correia, P.R.C.; Cruz, R.S.; Druzian, J.I. Morphological, Barrier, and Mechanical Properties of Cassava Starch Films Reinforced with Cellulose and Starch Nanoparticles. J. Appl. Polym. Sci. 2019, 136, 47001. [Google Scholar] [CrossRef]
- Campelo, P.H.; Sant’Ana, A.S.; Pedrosa Silva Clerici, M.T. Starch Nanoparticles: Production Methods, Structure, and Properties for Food Applications. Curr. Opin. Food Sci. 2020, 33, 136–140. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Tarté, R.; Acevedo, N.C. Synergistic Effects of Starch Nanoparticles and Chitin Nanofibers on the Stability of Oil-in-Water Pickering Emulsions. Food Chem. 2021, 363, 130301. [Google Scholar] [CrossRef]
- Lin, Q.; Ji, N.; Li, M.; Dai, L.; Xu, X.; Xiong, L.; Sun, Q. Fabrication of Debranched Starch Nanoparticles via Reverse Emulsification for Improvement of Functional Properties of Corn Starch Films. Food Hydrocoll. 2020, 104, 105760. [Google Scholar] [CrossRef]
- Amirsoleimani, M.; Khalilzadeh, M.A.; Sadeghifar, F.; Sadeghifar, H. Surface Modification of Nanosatrch Using Nano Silver: A Potential Antibacterial for Food Package Coating. J. Food Sci. Technol. 2018, 55, 899–904. [Google Scholar] [CrossRef]
- Krystyjan, M.; Khachatryan, G.; Khachatryan, K.; Konieczna-Molenda, A.; Grzesiakowska, A.; KuchtaGładysz, M.; Kawecka, A.; Grzebieniarz, W.; Nowak, N. The Functional and Application Possibilities of Starch/Chitosan Polymer Composites Modified by Graphene Oxide. Int. J. Mol. Sci. 2022, 23, 5956. [Google Scholar] [CrossRef]
- Othman, S.H. Bio-Nanocomposite Materials for Food Packaging Applications: Types of Biopolymer and Nano-Sized Filler. Agric. Agric. Sci. Procedia 2014, 2, 296–303. [Google Scholar] [CrossRef]
- Nasir, M.; Hashim, R.; Sulaiman, O.; Asim, M. 11—Nanocellulose: Preparation Methods and Applications. In Cellulose-Reinforced Nanofibre Composites; Jawaid, M., Boufi, S., Hps, A.K., Eds.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Cambridge, UK, 2017; pp. 261–276. ISBN 978-0-08-100957-4. [Google Scholar]
- Dufresne, A. Nanocellulose: A New Ageless Bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Paul, S.A.; Madhavan, A.; Sindhu, R.; Binod, P.; Pandey, A.; Sirohi, R. Nanocellulose-Based Products for Sustainable Applications-Recent Trends and Possibilities. Rev. Env. Sci. Biotechnol. 2020, 19, 779–806. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and Application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.H.; Nordin, N.; Azman, N.A.A.; Tawakkal, I.S.M.A.; Basha, R.K. Effects of Nanocellulose Fiber and Thymol on Mechanical, Thermal, and Barrier Properties of Corn Starch Films. Int. J. Biol. Macromol. 2021, 183, 1352–1361. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Andrade-Mahecha, M.M.; do Sobral, P.J.A.; Menegalli, F.C. Nanocomposites Based on Banana Starch Reinforced with Cellulose Nanofibers Isolated from Banana Peels. J. Colloid Interface Sci. 2017, 505, 154–167. [Google Scholar] [CrossRef]
- Slavutsky, A.M.; Bertuzzi, M.A. Water Barrier Properties of Starch Films Reinforced with Cellulose Nanocrystals Obtained from Sugarcane Bagasse. Carbohydr. Polym. 2014, 110, 53–61. [Google Scholar] [CrossRef]
- Heidari, M.; Khomeiri, M.; Yousefi, H.; Rafieian, M.; Kashiri, M. Chitin Nanofiber-Based Nanocomposites Containing Biodegradable Polymers for Food Packaging Applications. J. Consum. Prot. Food Saf. 2021, 16, 237–246. [Google Scholar] [CrossRef]
- Thomas, M.S.; Koshy, R.R.; Mary, S.K.; Thomas, S.; Pothan, L.A. Starch, Chitin and Chitosan Based Composites and Nanocomposites; SpringerBriefs in Molecular Science; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-03157-2. [Google Scholar]
- Yang, X.; Liu, J.; Pei, Y.; Zheng, X.; Tang, K. Recent Progress in Preparation and Application of Nano-Chitin Materials. Energy Environ. Mater. 2020, 3, 492–515. [Google Scholar] [CrossRef]
- Chang, P.R.; Jian, R.; Yu, J.; Ma, X. Starch-Based Composites Reinforced with Novel Chitin Nanoparticles. Carbohydr. Polym. 2010, 80, 420–425. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-Layered Silicate Nanocomposites: Preparation, Properties and Uses of a New Class of Materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Mansour, G.; Zoumaki, M.; Marinopoulou, A.; Raphaelides, S.N.; Tzetzis, D.; Zoumakis, N. Investigation on the Effects of Glycerol and Clay Contents on the Structure and Mechanical Properties of Maize Starch Nanocomposite Films. Starch—Stärke 2020, 72, 1900166. [Google Scholar] [CrossRef]
- Abulyazied, D.E.; Ene, A. An Investigative Study on the Progress of Nanoclay-Reinforced Polymers: Preparation, Properties, and Applications: A Review. Polymers 2021, 13, 4401. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Aryana, S.; Han, Y.; Jiao, Y. A Review of the Synthesis and Applications of Polymer–Nanoclay Composites. Appl. Sci. 2018, 8, 1696. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, A.R.; Savadkoohi, B.; Zahedi, Y.; Hatami, M.; Ako, K. Fabrication and Characterization of Hybrid Sodium Montmorillonite/TiO2 Reinforced Cross-Linked Wheat Starch-Based Nanocomposites. Int. J. Biol. Macromol. 2019, 131, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Garrido-Miranda, K.A.; Pereira, E.D. Release of Essential Oil Constituent from Thermoplastic Starch/Layered Silicate Bionanocomposite Film as a Potential Active Packaging Material. Eur. Polym. J. 2018, 109, 64–71. [Google Scholar] [CrossRef]
- Shayan, M.; Azizi, H.; Ghasemi, I.; Karrabi, M. Effect of Modified Starch and Nanoclay Particles on Biodegradability and Mechanical Properties of Cross-Linked Poly Lactic Acid. Carbohydr. Polym. 2015, 124, 237–244. [Google Scholar] [CrossRef]
- Iamareerat, B.; Singh, M.; Sadiq, M.B.; Anal, A.K. Reinforced Cassava Starch Based Edible Film Incorporated with Essential Oil and Sodium Bentonite Nanoclay as Food Packaging Material. J. Food Sci. Technol. 2018, 55, 1953–1959. [Google Scholar] [CrossRef]
- Dang, K.M.; Yoksan, R.; Pollet, E.; Avérous, L. Morphology and Properties of Thermoplastic Starch Blended with Biodegradable Polyester and Filled with Halloysite Nanoclay. Carbohydr. Polym. 2020, 242, 116392. [Google Scholar] [CrossRef]
- Schmitt, H.; Prashantha, K.; Soulestin, J.; Lacrampe, M.F.; Krawczak, P. Preparation and Properties of Novel Melt-Blended Halloysite Nanotubes/Wheat Starch Nanocomposites. Carbohydr. Polym. 2012, 89, 920–927. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y. Biodegradable and Water Resistant Poly(Vinyl) Alcohol (PVA)/Starch (ST)/Glycerol (GL)/Halloysite Nanotube (HNT) Nanocomposite Films for Sustainable Food Packaging. Front. Mater. 2019, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Ambika; Singh, P.P. Advances in Carbon Nanomaterial-Based Green Nanocomposites. In Emerging Carbon-Based Nanocomposites for Environmental Applications; Mishra, A.K., Hussain, C.M., Mishra, S.B., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 175–201. ISBN 978-1-119-55488-2. [Google Scholar]
- Domene-López, D.; Delgado-Marín, J.J.; García-Quesada, J.C.; Martín-Gullón, I.; Montalbán, M.G. Electroconductive Starch/Multi-Walled Carbon Nanotube Films Plasticized by 1-Ethyl-3-Methylimidazolium Acetate. Carbohydr. Polym. 2020, 229, 115545. [Google Scholar] [CrossRef] [PubMed]
- Famá, L.; Rojo, P.G.; Bernal, C.; Goyanes, S. Biodegradable Starch Based Nanocomposites with Low Water Vapor Permeability and High Storage Modulus. Carbohydr. Polym. 2012, 87, 1989–1993. [Google Scholar] [CrossRef]
- Xu, D. Carbon Nanotubes (CNTs) Composite Materials and Food Packaging. In Composites Materials for Food Packaging; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 235–249. ISBN 978-1-119-16024-3. [Google Scholar]
- Mollik, S.I.; Alam, R.B.; Islam, M.R. Significantly Improved Dielectric Properties of Bio-Compatible Starch/Reduced Graphene Oxide Nanocomposites. Synth. Met. 2021, 271, 116624. [Google Scholar] [CrossRef]
- Solati, M.; Saeidi, A.; Ghasemi, I. The Effect of Graphene Nanoplatelets on Dynamic Properties, Crystallization, and Morphology of a Biodegradable Blend of Poly(Lactic Acid)/Thermoplastic Starch. Iran Polym. J. 2019, 28, 649–658. [Google Scholar] [CrossRef]
- Kalia, A.; Sharma, S.P.; Kaur, H.; Kaur, H. Chapter 5—Novel Nanocomposite-Based Controlled-Release Fertilizer and Pesticide Formulations: Prospects and Challenges. In Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems; Abd-Elsalam, K.A., Ed.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 99–134. ISBN 978-0-12-821354-4. [Google Scholar]
- Merino, D.; Gutiérrez, T.J.; Mansilla, A.Y.; Casalongué, C.A.; Alvarez, V.A. Critical Evaluation of Starch-Based Antibacterial Nanocomposites as Agricultural Mulch Films: Study on Their Interactions with Water and Light. ACS Sustain. Chem. Eng. 2018, 6, 15662–15672. [Google Scholar] [CrossRef] [Green Version]
- Salimi, M.; Motamedi, E.; Motesharezedeh, B.; Hosseini, H.M.; Alikhani, H.A. Starch-g-Poly(Acrylic Acid-Co-Acrylamide) Composites Reinforced with Natural Char Nanoparticles toward Environmentally Benign Slow-Release Urea Fertilizers. J. Environ. Chem. Eng. 2020, 8, 103765. [Google Scholar] [CrossRef]
- Gamage, A.; Liyanapathiranage, A.; Manamperi, A.; Gunathilake, C.; Mani, S.; Merah, O.; Madhujith, T. Applications of Starch Biopolymers for a Sustainable Modern Agriculture. Sustainability 2022, 14, 6085. [Google Scholar] [CrossRef]
- Merino, D.; Gutiérrez, T.J.; Alvarez, V.A. Structural and Thermal Properties of Agricultural Mulch Films Based on Native and Oxidized Corn Starch Nanocomposites. Starch—Stärke 2019, 71, 1800341. [Google Scholar] [CrossRef]
- Pereira, E.I.; Giroto, A.S.; Bortolin, A.; Yamamoto, C.F.; Marconcini, J.M.; de Campos Bernardi, A.C.; Ribeiro, C. Perspectives in Nanocomposites for the Slow and Controlled Release of Agrochemicals: Fertilizers and Pesticides. In Nanotechnologies in Food and Agriculture; Rai, M., Ribeiro, C., Mattoso, L., Duran, N., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 241–265. ISBN 978-3-319-14024-7. [Google Scholar]
- Pimsen, R.; Porrawatkul, P.; Nuengmatcha, P.; Ramasoot, S.; Chanthai, S. Efficiency Enhancement of Slow Release of Fertilizer Using Nanozeolite–Chitosan/Sago Starch-Based Biopolymer Composite. J. Coat. Technol. Res. 2021, 18, 1321–1332. [Google Scholar] [CrossRef]
- Zafar, N.; Niazi, M.B.K.; Sher, F.; Khalid, U.; Jahan, Z.; Shah, G.A.; Zia, M. Starch and Polyvinyl Alcohol Encapsulated Biodegradable Nanocomposites for Environment Friendly Slow Release of Urea Fertilizer. Chem. Eng. J. Adv. 2021, 7, 100123. [Google Scholar] [CrossRef]
- Jain, S.K.; Dutta, A.; Kumar, J.; Shakil, N.A. Preparation and Characterization of Dicarboxylic Acid Modified Starch-Clay Composites as Carriers for Pesticide Delivery. Arab. J. Chem. 2020, 13, 7990–8002. [Google Scholar] [CrossRef]
- Galstyan, V.; Bhandari, M.P.; Sberveglieri, V.; Sberveglieri, G.; Comini, E. Metal Oxide Nanostructures in Food Applications: Quality Control and Packaging. Chemosensors 2018, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Oleyaei, S.A.; Zahedi, Y.; Ghanbarzadeh, B.; Moayedi, A.A. Modification of Physicochemical and Thermal Properties of Starch Films by Incorporation of TiO2 Nanoparticles. Int. J. Biol. Macromol. 2016, 89, 256–264. [Google Scholar] [CrossRef]
- Pirsa, S.; Karimi Sani, I.; Khodayvandi, S. Design and Fabrication of Starch-Nano Clay Composite Films Loaded with Methyl Orange and Bromocresol Green for Determination of Spoilage in Milk Package. Polym. Adv. Technol. 2018, 29, 2750–2758. [Google Scholar] [CrossRef]
- Patil, S.; Bharimalla, A.K.; Nadanathangam, V.; Dhakane-Lad, J.; Mahapatra, A.; Jagajanantha, P.; Saxena, S. Nanocellulose Reinforced Corn Starch-Based Biocomposite Films: Composite Optimization, Characterization and Storage Studies. Food Packag. Shelf Life 2022, 33, 100860. [Google Scholar] [CrossRef]
- Babaei-Ghazvini, A.; Shahabi-Ghahfarrokhi, I.; Goudarzi, V. Preparation of UV-Protective Starch/Kefiran/ZnO Nanocomposite as a Packaging Film: Characterization. Food Packag. Shelf Life 2018, 16, 103–111. [Google Scholar] [CrossRef]
- Sharmin, E.; Kafyah, M.T.; Alzaydi, A.A.; Fatani, A.A.; Hazazzi, F.A.; Babgi, S.K.; Alqarhi, N.M.; Sindi, A.A.H.; Akram, D.; Alam, M.; et al. Synthesis and Characterization of Polyvinyl Alcohol/Corn Starch/Linseed Polyol-Based Hydrogel Loaded with Biosynthesized Silver Nanoparticles. Int. J. Biol. Macromol. 2020, 163, 2236–2247. [Google Scholar] [CrossRef]
- Mohanty, F.; Swain, S.K. Nano Silver Embedded Starch Hybrid Graphene Oxide Sandwiched Poly(Ethylmethacrylate) for Packaging Application. Nano-Struct. Nano-Objects 2019, 18, 100300. [Google Scholar] [CrossRef]
- Jha, P.; Dharmalingam, K.; Nishizu, T.; Katsuno, N.; Anandalakshmi, R. Effect of Amylose–Amylopectin Ratios on Physical, Mechanical, and Thermal Properties of Starch-Based Bionanocomposite Films Incorporated with CMC and Nanoclay. Starch—Stärke 2020, 72, 1900121. [Google Scholar] [CrossRef]
- de Souza, A.G.; dos Santos, N.M.A.; da Silva Torin, R.F.; dos Santos Rosa, D. Synergic Antimicrobial Properties of Carvacrol Essential Oil and Montmorillonite in Biodegradable Starch Films. Int. J. Biol. Macromol. 2020, 164, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Issa, A.T.; Schimmel, K.A.; Worku, M.; Shahbazi, A.; Ibrahim, S.A.; Tahergorabi, R. Sweet Potato Starch-Based Nanocomposites: Development, Characterization, and Biodegradability. Starch—Stärke 2018, 70, 1700273. [Google Scholar] [CrossRef]
- Barzegar, H.; Azizi, M.H.; Barzegar, M.; Hamidi-Esfahani, Z. Effect of Potassium Sorbate on Antimicrobial and Physical Properties of Starch–Clay Nanocomposite Films. Carbohydr. Polym. 2014, 110, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, Z.; Das, R.; Jiang, Q. Starch-Based Carbon Nanotubes and Graphene: Preparation, Properties and Applications. ES Food Agrofor. 2020, 2, 13–21. [Google Scholar] [CrossRef]
- Gomes, M.E.; Godinho, J.S.; Tchalamov, D.; Cunha, A.M.; Reis, R.L. Alternative Tissue Engineering Scaffolds Based on Starch: Processing Methodologies, Morphology, Degradation and Mechanical Properties. Mater. Sci. Eng. C 2002, 20, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.R.; Xiao, C.M.; Xu, S.J. Starch-Based Completely Biodegradable Polymer Materials. Express Polym. Lett. 2009, 3, 366–375. [Google Scholar] [CrossRef]
- Taherimehr, M.; Bagheri, R.; Taherimehr, M. In-Vitro Evaluation of Thermoplastic Starch/ Beta-Tricalcium Phosphate Nano-Biocomposite in Bone Tissue Engineering. Ceram. Int. 2021, 47, 15458–15463. [Google Scholar] [CrossRef]
- Waghmare, V.S.; Wadke, P.R.; Dyawanapelly, S.; Deshpande, A.; Jain, R.; Dandekar, P. Starch Based Nanofibrous Scaffolds for Wound Healing Applications. Bioact. Mater. 2018, 3, 255–266. [Google Scholar] [CrossRef]
- Sadjadi, M.S.; Meskinfam, M.; Jazdarreh, H. Hydroxyapatite—Starch Nano Biocomposites Synthesis and Characterization. Int. J. Nano Dimens. 2010, 1, 57–63. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; Al-Deyab, S.S. Antimicrobial Activity of Silver/Starch/Polyacrylamide Nanocomposite. Int. J. Biol. Macromol. 2014, 68, 33–38. [Google Scholar] [CrossRef]
- Batool, S.; Hussain, Z.; Niazi, M.B.K.; Liaqat, U.; Afzal, M. Biogenic Synthesis of Silver Nanoparticles and Evaluation of Physical and Antimicrobial Properties of Ag/PVA/Starch Nanocomposites Hydrogel Membranes for Wound Dressing Application. J. Drug Deliv. Sci. Technol. 2019, 52, 403–414. [Google Scholar] [CrossRef]
- Davachi, S.M.; Shiroud Heidari, B.; Hejazi, I.; Seyfi, J.; Oliaei, E.; Farzaneh, A.; Rashedi, H. Interface Modified Polylactic Acid/Starch/Poly ε-Caprolactone Antibacterial Nanocomposite Blends for Medical Applications. Carbohydr. Polym. 2017, 155, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Khodadadzadeh, L. Ultrasonic-Assisted Fabrication of Starch/MWCNT-Glucose Nanocomposites for Drug Delivery. Ultrason. Sonochem. 2018, 40, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wei, L.; Yan, H.; Xu, B. Green Synthesis and Characteristic of Core-Shell Structure Silver/Starch Nanoparticles. Mater. Lett. 2011, 65, 2963–2965. [Google Scholar] [CrossRef]
- Nezami, S.; Sadeghi, M.; Mohajerani, H. A Novel PH-Sensitive and Magnetic Starch-Based Nanocomposite Hydrogel as a Controlled Drug Delivery System for Wound Healing. Polym. Degrad. Stab. 2020, 179, 109255. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, D.; Liu, M.; Fu, L.; Wan, Q.; Mao, L.; Dai, Y.; Wen, Y.; Zhang, X.; Wei, Y. Room Temperature Preparation of Fluorescent Starch Nanoparticles from Starch-Dopamine Conjugates and Their Biological Applications. Mater. Sci. Eng. C 2018, 82, 204–209. [Google Scholar] [CrossRef]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef] [Green Version]
- García-Padilla, Á.; Moreno-Sader, K.A.; Realpe, Á.; Acevedo-Morantes, M.; Soares, J.B.P. Evaluation of Adsorption Capacities of Nanocomposites Prepared from Bean Starch and Montmorillonite. Sustain. Chem. Pharm. 2020, 17, 100292. [Google Scholar] [CrossRef]
- Mallakpour, S.; Nouruzi, N. Application of Vitamin B1-Coated Carbon Nanotubes for the Production of Starch Nanocomposites with Enhanced Structural, Optical, Thermal and Cd(II) Adsorption Properties. J. Polym. Environ. 2018, 26, 2954–2963. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, Cellulose, Pectin, Gum, Alginate, Chitin and Chitosan Derived (Nano)Materials for Sustainable Water Treatment: A Review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef]
- Orooji, Y.; Nezafat, Z.; Nasrollahzadeh, M.; Kamali, T.A. Polysaccharide-Based (Nano)Materials for Cr(VI) Removal. Int. J. Biol. Macromol. 2021, 188, 950–973. [Google Scholar] [CrossRef] [PubMed]
- del Orta, M.M.; Martín, J.; Santos, J.L.; Aparicio, I.; Medina-Carrasco, S.; Alonso, E. Biopolymer-Clay Nanocomposites as Novel and Ecofriendly Adsorbents for Environmental Remediation. Appl. Clay Sci. 2020, 198, 105838. [Google Scholar] [CrossRef]
- Russo, T.; Fucile, P.; Giacometti, R.; Sannino, F. Sustainable Removal of Contaminants by Biopolymers: A Novel Approach for Wastewater Treatment. Current State and Future Perspectives. Processes 2021, 9, 719. [Google Scholar] [CrossRef]
- Tripathy, T.; Kolya, H.; Jana, S.; Senapati, M. Green Synthesis of Ag-Au Bimetallic Nanocomposites Using a Biodegradable Synthetic Graft Copolymer; Hydroxyethyl Starch-g-Poly (Acrylamide-Co-Acrylic Acid) and Evaluation of Their Catalytic Activities. Eur. Polym. J. 2017, 87, 113–123. [Google Scholar] [CrossRef]
- Yang, C.; Ge, C.; Li, X.; Li, L.; Wang, B.; Lin, A.; Yang, W. Does Soluble Starch Improve the Removal of Cr(VI) by NZVI Loaded on Biochar? Ecotoxicol. Environ. Saf. 2021, 208, 111552. [Google Scholar] [CrossRef]
- Adeola, A.O.; Nomngongo, P.N. Advanced Polymeric Nanocomposites for Water Treatment Applications: A Holistic Perspective. Polymers 2022, 14, 2462. [Google Scholar] [CrossRef]
- Gomes, R.F.; de Azevedo, A.C.N.; Pereira, A.G.B.; Muniz, E.C.; Fajardo, A.R.; Rodrigues, F.H.A. Fast Dye Removal from Water by Starch-Based Nanocomposites. J. Colloid Interface Sci. 2015, 454, 200–209. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Ramin, S. Fabrication of Starch-Graft-Poly(Acrylamide)/Graphene Oxide/Hydroxyapatite Nanocomposite Hydrogel Adsorbent for Removal of Malachite Green Dye from Aqueous Solution. Int. J. Biol. Macromol. 2018, 106, 101–115. [Google Scholar] [CrossRef]
- Vaezi, K.; Asadpour, G.; Sharifi, S.H. Bio Nanocomposites Based on Cationic Starch Reinforced with Montmorillonite and Cellulose Nanocrystals: Fundamental Properties and Biodegradability Study. Int. J. Biol. Macromol. 2020, 146, 374–386. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Ali, S.S.; Matter, I.A.; Elsamahy, T.; Mahmoud, Y.A. Chapter Twenty—Enzymes Immobilization onto Magnetic Nanoparticles to Improve Industrial and Environmental Applications. In Methods in Enzymology; Kumar, C.V., Ed.; Nanoarmoring of Enzymes with Carbon Nanotubes and Magnetic Nanoparticles; Academic Press: Boca Raton, FL, USA, 2020; Volume 630, pp. 481–502. [Google Scholar]
- Sharmeen, S.; Rahman, S.; Islam, M.; Islam, S.; Shahruzzaman; Mallik, A.K.; Haque, P.; Rahman, M.M. 11—Application of Polysaccharides in Enzyme Immobilization. In Functional Polysaccharides for Biomedical Applications; Maiti, S., Jana, S., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 357–395. ISBN 978-0-08-102555-0. [Google Scholar]
- Liu, D.-M.; Dong, C. Recent Advances in Nano-Carrier Immobilized Enzymes and Their Applications. Process. Biochem. 2020, 92, 464–475. [Google Scholar] [CrossRef]
- Mehde, A.A. Development of Magnetic Cross-Linked Peroxidase Aggregates on Starch as Enhancement Template and Their Application for Decolorization. Int. J. Biol. Macromol. 2019, 131, 721–733. [Google Scholar] [CrossRef] [PubMed]
- LeCorre, D.; Hohenthal, C.; Dufresne, A.; Bras, J. Comparative Sustainability Assessment of Starch Nanocrystals. J. Polym. Environ. 2013, 21, 71–80. [Google Scholar] [CrossRef]
- Finnveden, G.; Moberg, Å. Environmental Systems Analysis Tools—An Overview. J. Clean. Prod. 2005, 13, 1165–1173. [Google Scholar] [CrossRef]
- Shen, L.; Patel, M.K. Life Cycle Assessment of Polysaccharide Materials: A Review. J. Polym. Environ. 2008, 16, 154–167. [Google Scholar] [CrossRef] [Green Version]
- Foroughi, F.; Rezvani Ghomi, E.; Morshedi Dehaghi, F.; Borayek, R.; Ramakrishna, S. A Review on the Life Cycle Assessment of Cellulose: From Properties to the Potential of Making It a Low Carbon Material. Materials 2021, 14, 714. [Google Scholar] [CrossRef] [PubMed]
- Klöpffer, W.; Grahl, B. Life Cycle Assessment (LCA): A Guide to Best Practice; John Wiley & Sons: Hoboken, NJ, USA, 2014; ISBN 978-3-527-65564-9. [Google Scholar]
- Bafana, A.; Kumar, S.V.; Temizel-Sekeryan, S.; Dahoumane, S.A.; Haselbach, L.; Jeffryes, C.S. Evaluating Microwave-Synthesized Silver Nanoparticles from Silver Nitrate with Life Cycle Assessment Techniques. Sci. Total Environ. 2018, 636, 936–943. [Google Scholar] [CrossRef]
- Bishop, G.; Styles, D.; Lens, P.N.L. Environmental Performance Comparison of Bioplastics and Petrochemical Plastics: A Review of Life Cycle Assessment (LCA) Methodological Decisions. Resour. Conserv. Recycl. 2021, 168, 105451. [Google Scholar] [CrossRef]
- Miseljic, M.; Olsen, S.I. LCA of Nanomaterials. In Life Cycle Assessment: Theory and Practice; Hauschild, M.Z., Rosenbaum, R.K., Olsen, S.I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 817–833. ISBN 978-3-319-56475-3. [Google Scholar]
- Nizam, N.U.M.; Hanafiah, M.M.; Woon, K.S. A Content Review of Life Cycle Assessment of Nanomaterials: Current Practices, Challenges, and Future Prospects. Nanomaterials 2021, 11, 3324. [Google Scholar] [CrossRef]
- Broeren, M.L.M.; Kuling, L.; Worrell, E.; Shen, L. Environmental Impact Assessment of Six Starch Plastics Focusing on Wastewater-Derived Starch and Additives. Resour. Conserv. Recycl. 2017, 127, 246–255. [Google Scholar] [CrossRef]
- Rojas-Bringas, P.M.; De-la-Torre, G.E.; Torres, F.G. Influence of the Source of Starch and Plasticizers on the Environmental Burden of Starch-Brazil Nut Fiber Biocomposite Production: A Life Cycle Assessment Approach. Sci. Total Environ. 2021, 769, 144869. [Google Scholar] [CrossRef]
- Aryan, Y.; Yadav, P.; Samadder, S.R. Life Cycle Assessment of the Existing and Proposed Plastic Waste Management Options in India: A Case Study. J. Clean. Prod. 2019, 211, 1268–1283. [Google Scholar] [CrossRef]
- Joshi, S. Can Nanotechnology Improve the Sustainability of Biobased Products? J. Ind. Ecol. 2008, 12, 474–489. [Google Scholar] [CrossRef]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Borowski, G.; Szczypiński, M.; Klepka, T.; Thakur, V.K. Recent Progress in Biodegradable Polymers and Nanocomposite-Based Packaging Materials for Sustainable Environment. Int. J. Polym. Anal. Charact. 2018, 23, 383–395. [Google Scholar] [CrossRef]
- Kochkina, N.E.; Butikova, O.A. Effect of Fibrous TiO2 Filler on the Structural, Mechanical, Barrier and Optical Characteristics of Biodegradable Maize Starch/PVA Composite Films. Int. J. Biol. Macromol. 2019, 139, 431–439. [Google Scholar] [CrossRef]
- Glaskova-Kuzmina, T.; Starkova, O.; Gaidukovs, S.; Platnieks, O.; Gaidukova, G. Durability of Biodegradable Polymer Nanocomposites. Polymers 2021, 13, 3375. [Google Scholar] [CrossRef] [PubMed]
- Bher, A.; Unalan, I.U.; Auras, R.; Rubino, M.; Schvezov, C.E. Graphene Modifies the Biodegradation of Poly(Lactic Acid)-Thermoplastic Cassava Starch Reactive Blend Films. Polym. Degrad. Stab. 2019, 164, 187–197. [Google Scholar] [CrossRef]
- Prusty, K.; Swain, S.K. Nano CaCO3 Imprinted Starch Hybrid Polyethylhexylacrylate\polyvinylalcohol Nanocomposite Thin Films. Carbohydr. Polym. 2016, 139, 90–98. [Google Scholar] [CrossRef]









| Starch Source | Amylose (%) | Reference |
|---|---|---|
| Arrowroot | 35.52 | [27] |
| Banana (pulp) | 16.36–26.2 | [28,29,30] |
| Banana (peel) | 25.7 | [29] |
| Barley (regular) | 24.7 | [31] |
| Cassava | 2.5–32.12 | [28,32,33] |
| Corn | 0–79.05 | [28,32] |
| Maize (normal) | 22.7–28.9 | [31,34] |
| Maize (waxy) | 0.18 | [34] |
| Maize (high amylose content) | 35.5–64.8 | [34] |
| Potato | 18.6–31.9 | [28,31,32,33] |
| Rice | 0.1–28.7 | [20,35] |
| Sweet potato (normal) | 30.4 | [36] |
| Wheat | 6.2–22.8 | [31,32] |
| Starch-Based Nanocomposites | Application | Properties | References |
|---|---|---|---|
| Native (TPS) or oxidized (TPS-ox) corn starch/chitosan (CS)/bentonite (Bent) | Mulch film | The addition of 4% CS/Bent improved water resistance (decreased water solubility), radiometric, and antibacterial properties. Decreased mechanical property (tensile strength and elastic modulus: TPS-ox > TPS-ox/CS/Bent > TPS > TPS/CS/Bent). | [83] |
| Native (TPS) or oxidized (TPS-ox) corn starch/chitosan (CS)/bentonite (Bent) | Mulch film | The addition of 4% CS/Bent increased the crystallinity (3.30 and 3.00%) and led to a slight increase in thermal stability (Tmax 139.2 and 126.9 °C) in TPS and TPS-ox, respectively. | [86] |
| Corn starch-g-poly(AA-co-AAm)/natural char nanoparticles (NCNPs)/urea | Bi-functional slow-release fertilizers | Provided improved biodegradability, soil water-retention capacity (35.6% and 33.2% at pH 4.5 and 5.5, respectively, after 6 days), water absorbency (215.1 g/g) along with the slow release of urea (73% in deionized water and 37% in NaCl). | [84] |
| Urea encapsulated with starch (10%)/PVA (5%) with crosslinker acrylic acid (2%) and citric acid (2%) | Slow release of fertilizer | Releasing efficiency of starch/PVA/acrylic acid and starch/PVA/citric acid were 70.10 and 50.74%, respectively. Improved the growth factors in spinach plants | [89] |
| Corn starch/Debranched starch NPs (DSNPs) | Food packaging | Addition of 5% DSNPs increased the tensile strength (from 0.95 to 1.73 MPa) and decreased the water vapor permeability (7.11 to 4.91 × 10−10 gPa−1h−1m−1) and oxygen transmission rate (394 to 81.61 cm3/m2⋅day) | [49] |
| Starch NPs/Ag NPs | Coating material for food packaging | Antibacterial activity against Staphylococcus aureus, Salmonella typhi, and Escherichia coli. | [50] |
| Cross-linked wheat starch (CLWS)/sodium montmorillonite (Na-MMT)/TiO2 NPs | Food packaging material | Showed exfoliated structure. Adding Na-MMT (5%) and TiO2 NPs (1%) into CLWS showed reduced water vapor permeability (from 9.1 to 4.8 × 10−5 g/m.d.Pa) and water solubility (100–50.35%), and increased thermal stability, tensile strength (2.49–5.56 MPa), and Young’s modulus (0.71–1.09 MPa) in comparison to native wheat starch. CLWS/Na-MMT/TiO2 NPs showed better UV-blocking properties than CLWS/Na-MMT. | [69] |
| Sweet potato starch (SPS)/montmorillonite (MMT)/thyme essential oil (TEO) | Food packaging | The addition of MMT improved the tensile (44.91%), Young’s modulus (135.69 MPa), and water vapor barrier (0.022 gm/m2/day) and hindered the biodegradability of SPS. The addition of TEO decreased the mechanical and water vapor barrier properties of SPS/MMT nanocomposites. The addition of MMT and TEO improved water resistance by 50%. | [100] |
| Starch (potato, wheat, and corn, high amylose corn) carboxyl methylcellulose (CMC)/Na-MMT | Food packaging | Corn starch/CMC/Na-MMT nanocomposite showed higher tensile strength, glass transition temperature, thermal stability, crystallinity, lower solubility, and water vapor permeability. | [98] |
| Cassava starch/glycerol/Na-bentonite nanoclay/cinnamon essential oil | Antimicrobial food packaging pork meatballs | Antibacterial activity against Escherichia coli, Salmonella typhimurium, and Staphylococcus aureus. Improved the antimicrobial efficacy in pork meatballs stored under ambient and refrigeration conditions. | [72] |
| Starch/polyvinyl alcohol (PVA)/cinnamaldehyde (Cin)/micro fibrillated cellulose (MFC) | Controlled-release active packaging film | MFC improved the tensile strength, crystallinity, hydrophobicity, and antimicrobial activity (against S. putrefaciens) with reduced flexibility. The oxygen and water vapor permeability reduced at 1 and 2.5% MFC and increased at higher concentrations. MFC at 1 and 7.5% controlled the release of Cin. | [102] |
| Corn starch (CS)/nanocellulose (NC)/glycerol (GL)/polyvinyl alcohol (PVOH) | Packaging material for edible oil | Optimum composition for CS-based nanocomposite: 0.89% NC, 2.53% GL, and 1.89% PVOH. Tensile strength 8.92 MPa, elongation at break 41.92%, bursting strength 556 kPa, and WVP 7.07 × 10−10 g/m.s.Pa, oxygen transmission rate 3.56 × 10−5 cm3/m2 d.Pa. Good heat salability. | [94] |
| Starch from unripe plantain bananas/cellulose nanofibers from banana peels | Food packaging | Homogenized nanocomposite at five times higher pressure increased the tensile strength (from 7.3–9.9 MP), Young’s modulus (478.6–663.1 MPa), decreased the elongation at break (32.2–20.7%), solubility (32.3–29.0%), WVP (10.7–6.0 × 10−11 g/m.s.Pa at low RH), sorption (2.73–2.20 × 10−7 mm2/s), and diffusion coefficient (0.42–0.27). | [59] |
| Corn starch (CS)/nanocellulose fiber (NCF)/thymol | Antioxidant and antimicrobial food packaging | Adding 1.5% of NCF improved the thermal stability, mechanical and water vapor, and oxygen barrier properties of corn starch film. CS/NCF/thymol composite reported improved thermal stability and flexibility with decreased tensile strength, Young’s modulus, and barrier properties. | [58] |
| Starch/cellulose nanocrystals (CNC) | Food packaging | Improved the tensile strength (2.8 to 17.4 MPa), Young’s modulus (112 to 520 MPa), water resistance (reduced solubility 26.6 to 18.5%), and water barrier properties and decreased surface hydrophilicity (contact angle 38.2 to 96.3°). | [60] |
| TPS/chitin nanofibers (CNF) from fungus Mucor indicus | Nanocomposite for food packaging and other applications. | Addition of 5 wt.% CNF enhanced Young’s modulus (239%) and tensile strength (by 180%) and reduced the elongation at break and moisture absorption compared to the TPS film. | [39] |
| PVA/starch/Ag NPs from Diospyros lotus fruit extract | Wound dressing applications | Increased swelling and moisture retention capacity, reduced water vapor transmission. Better antimicrobial activity against Escherichia coli and Staphylococcus aureus | [109] |
| Thermoplastic starch (TPS)/beta-tricalcium phosphate (β-TCP) NPs | Bone tissue engineering materials | Adding β-TCP at 10% improved the tensile strength (from 1.67 to 4.8 MPa) and Young’s modulus (from 66.54 to 390.5 MPa), and decreased elongation at break (78.56 to 18.03%) of TPS. Exhibited non-cytotoxicity effects and excellent biocompatibility. | [105] |
| Polylactic acid (PLA)/starch (S)/poly-ε-caprolactone (PCL)/nano hydroxyapatite (nHAp)/ | Controlled release of antibacterial triclosan | Incorporating nHA (3%) improved the hydrolytic hydrophilicity, hydrolytic degradation, antibacterial activity (against Escherichia coli and Staphylococcus aureus), and continuous drug release of PLA/S/PCL film. | [110] |
| Starch-itaconic acid/Fe3O4 NPs (St-IA/Fe3O4) | Controlled release of Guaifenesin (GFN) | The addition of magnetic Fe3O4 NPs at 0.83% enhanced the drug release percentage from 54.1 to 90.4% within 24 h in pH 7.4. Adding Fe3O4 NPs improved the wound healing ability in mice (healed after 10 days). Exhibited low cytotoxicity for human umbilical vein endothelial cells. | [113] |
| Graft copolymer hydroxyethyl starch-g-poly(acrylamide-co-acrylic acid)/Ag-Au bimetallic nanocomposite | Removal of toxic azo dyes from wastewater | Catalytic activities: reduction of 4-nitrophenol to 4-aminophenol and degradation by cleavage of −N = N-the bond of azo dyes (Congo red, Sudan-1, and methyl orange). | [122] |
| Starch-graft-poly(acrylamide) (PAM)/graphene oxide (GO)/hydroxyapatite NPs (nHAp) nanocomposite | Recyclable adsorbent for efficient removal of malachite green (MG) dye from aqueous solution | PAM/GO and nHAp at 1–5 wt.% reported excellent porosity (31–11%), degradability (41–11% after 15 days), the maximum adsorption capacity of 297 mg/g, excellent regeneration capacity after five consecutive adsorption-desorption cycle of dye (27–14% of MG dye was liberated after 5th cycle, i.e., 77–86% removal efficiency) | [126]. |
| Bean starch/sodium montmorillonite (Na-MMT) | Removal of Ni2+ from water | Adding Na-MMT improved the absorption yield for Ni2+ (from 72 to 97.1% at pH 4.5, initial concentration of 100 ppm) and Co2+ (74.2 to 78.03% at pH 6, initial concentration of 140) in comparison to the bean starch matrix. | [116] |
| MWCNT/starch plasticized with ionic liquid, 1-ethyl-3-methylimidazolium acetate ([emim+][Ac−]) | Packaging, lithium batteries, fuel cells, and dye-sensitized solar cells | MWCNT at 0.5 wt.% increased the tensile strength, Young’s modulus, and elongation at the break by 327%, 2484%, and 82%, respectively. Electrical conductivity increased with MWCNT content with the maximum (56.3 S/m) at 5 wt.% MWCNT. Starch plasticizer [emim+][Ac−] slightly decreased the thermal stability in comparison to glycerol in the MWCNT/starch nanocomposite. | [77] |
| Starch/MWCNT/surfactants such as sodium dodecyl sulfate (SDS), cetyltrimethylammonium bromide (CTAB), and sodium cholate (SC) | Electrically conductive biocomposite film | CTAB reduced the mechanical properties of starch, while SC had no significant effect. SC (18.3–25.3°) and CTAB (20.8–32.3°) reduced the contact angle of starch (42.9–45.2°). CTAB (14.75 S/m) and SC (11.56 S/m) improved the electrical conductivity of starch (2.03 × 10−6 S/m). CTAB (30.2%), SDS (24.4%), and SC (12%) increased the inhibition of free radicals more than starch. | [22]. |
| Maize starch/glycerol (20%)/Na-MMT (10%) nanoclay | Lightweight architectural constructions | Showed intercalated structure and improved tensile properties. | [66] |
| Starch-Based Nanocomposite | Method | Biodegradation | Other Observation | Reference |
|---|---|---|---|---|
| Poly(ethyl methacrylate)-co-starch/graphene oxide/Ag NPs (PEMA-co-starch/GO/Ag NPs) | Active sludge water for 180 days. | 4.5% after 180 days. | GO and Ag NPs (2 wt.%) increased thermal stability, chemical resistance, tensile strength, and oxygen barrier property. Antimicrobial activity against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Bacillus subtilis. | [97] |
| Maize starch/PVA/TiO2 | Soil burial test: buried at 2–3 cm depth in peaty soil with 60% moisture, 98% RH, at 30 °C for 3 months. | Around <20% remaining mass after 80 days. | The addition of fibrous TiO2 (0.01 and 0.05 wt.%) decreased the elongation at break and improved the tensile strength, Young’s modulus UV, and water vapor barrier properties. | [146] |
| Sweet potato starch (SPS)/montmorillonite (MMT)/thyme essential oil (TEO) | Soil burial degradation test. | The addition of MMT hindered the biodegradability (23.25%) of SPS (48.88%). Biodegradability of SPS/MMT increased with the addition of TEO (61–63%) | The addition of MMT and TEO improved water resistance by 50%. The addition of MMT at 3% and TEO at 2% improved the elongation, Young’s modulus, and water vapor barrier properties of SPS. | [100] |
| Corn starch/glycerol/montmorillonite (MMT) nanoclay | Microbiological medium of pure Micrococcus luteus culture incubating at room temperature for 30 days. | Complete decay after 20 days in corn starch and 21–24 days in corn starch filled with nanoclay. | Addition of nanoclay (2–3 wt.%) in corn starch reduced water absorption (by 22%), moisture uptake (40%), oxygen permeation (30%), and swelling thickness (31%). | [15] |
| Cationic starch (CS)/montmorillonite (MMT)/nanocrystalline cellulose (NCC) | Composting conditions at 58 °C for 26 days. | CS/MMT/NCC nanocomposite films showed a higher decomposition rate than pure CS. 90% disintegration after 26 days. | Addition of MMT (5% wt) and NCC (5% wt) increased tensile strength (6.60 MPa) and modulus (2.17 GPa), and decreased elongation at break, water solubility (19.63%), moisture absorption (17.73%), water vapor permeability (4.61 gMm.m−2day.kPa), O2 permeability (28.72 cm3m−1d−1Pa−1). | [127] |
| Cross-linked poly(lactic acid) (PLA)/maleated thermoplastic starch (MTPS)/montmorillonite (MMT) | Samples (1.5 × 1.5 cm) in activated sludge for 3 months. | MTPS and nanoclay improved the biodegradation, while crosslinking of PLA reduced the biodegradation rate. | The addition of MMT improved tensile strength. Increasing MTPS (wt.%) content decreased the tensile strength and increased the elongation at break. | [71] |
| Corn starch-g-poly(AA-co-AAm)/natural char nanoparticles (NCNPs) nanocomposite encapsulated urea. Where: acrylic acid (AA), acrylamide (AAm). | Buried in the soil at pH 7.5 for 30 days. | The degradation rate after 30 days was 23.9%. | The addition of NCNPs decreased the leaching of nitrate and improved soil water-retention capacity. | [84] |
| Thermoplastic corn starch (TPS)/cellulose nanofibrils from pineapple leaf/oxidized sucrose | Sample (40 × 8 × 2 mm) buried at 10 cm depth of a sand and soil mixture (in equal ratio) at ambient temperature for 30 days. | About 30% weight loss in cross-linked films after 30 days, much lower than TPS (80%). | - | [5] |
| Starch/polyethylhexylacrylate (PEHA)/polyvinylalcohol (PVA)/nano CaCO3 nanocomposite | Activated sludge water for 90 days. | Starch/PEHA/PVA/CaCO3 (8 wt.%) degraded by 65% after 15 days | CaCO3 increased the tensile strength, thermal conductivity, thermal stability, and chemical resistance. Antimicrobial activity against Candida albicans, Escherichia coli, Pseudomonas aeruginosa. | [149] |
| Poly(lactic acid) (PLA)/thermoplastic cassava starch (TPCS)/graphene nanoplatelets (GRH) | Samples (1 cm2) buried in inoculated vermiculite and compost under aerobic controlled conditions: at 58 ± 2 °C, RH 50 ± 5%, and airflow rate 40 ± 2 cm3min−1). | The addition of GRH decreased the biodegradation rate from 0.11 to 0.06 d−1 in vermiculite and 0.09 to 0.08 d−1 in compost media. | In PLA, adding TPCS and GRH reduced the crystallinity (34.5 to 4.5%). | [148] |
| Polylactic acid (PLA)/starch (S)/poly-ε-caprolactone (PCL)/nano hydroxyapatite (nHAp)/ | In-vitro hydrolytic degradation test, 0.15 g samples (1 × 1 × 0.15 cm) was hot pressed and incubated in 50 mL phosphate buffer with pH 7.4 at 37 °C. | The increase in nHAp content (1–7%), faster the degradation (13–10 months). | Incorporating nHA (3%) improved the hydrophilicity and antibacterial activity (against Escherichia coli and Staphylococcus aureus). | [110] |
| Starch-graft-poly(acrylamide) (PAM)/graphene oxide (GO)/hydroxyapatite NPs (nHAp) nanocomposite | Soaked in PBS buffer solutions (pH 7.4) containing lysozyme (5000 U/mL) at 37 °C for 15 days. | Biodegradation decreased with increasing nHAp content. Degradability was 41–11% lower than that of PAM/GO (55%) after 15 days. | With increasing nHAp content, porosity, water content, and water uptake were decreased. | [126] |
| Thermoplastic starch (TPS)/beta-tricalcium phosphate (β-TCP) NPs | In vitro degradation tests were performed in a simulated body fluid (SBF) for 28 days. | Degraded 51% after 28 days, higher than TPS (47%). | Adding β-TCP at 10% improved the mechanical properties of TPS. | [105] |
| Starch/PVA/Ag NPs | Under controlled aerobic composting conditions at 58 ± 2 °C for 45 days (based on EN ISO 14855-1: 2012 standard). Disintegration test under composting conditions: 5 g of film samples (25 × 25 mm) at 58 ± 2 °C for 73 days (ISO 20200: 2004). | Biodegradation is 58% after 45 days, which is higher than that of PVA (54%) and lower than starch (134%). Poor disintegration behavior in comparison to starch. | - | [14] |
| Polyvinyl alcohol (PVA)/corn starch (CS)/linseed polyol (LP)/Ag NPs | Soil burial of samples (2 × 2 cm) at a depth of 10 cm. | Biodegradability after 4 weeks PVA < PVA/CS < PVA/CS/LP < PVA/CS/LP/Ag NPs | Improved contact angle (53°), water absorption capacity (equilibrium swelling percentage 129%), thermal stability (10% weight loss at 308 °C), and biodegradation than PVA/CS film. Ag NPs improved antimicrobial behavior against Proteus mirabilis, Candida albicans, Escherichia coli, Enterococcus faecalis, Staphylococcus aureus, Klebsiella pneumoniae, among others. | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamage, A.; Thiviya, P.; Mani, S.; Ponnusamy, P.G.; Manamperi, A.; Evon, P.; Merah, O.; Madhujith, T. Environmental Properties and Applications of Biodegradable Starch-Based Nanocomposites. Polymers 2022, 14, 4578. https://doi.org/10.3390/polym14214578
Gamage A, Thiviya P, Mani S, Ponnusamy PG, Manamperi A, Evon P, Merah O, Madhujith T. Environmental Properties and Applications of Biodegradable Starch-Based Nanocomposites. Polymers. 2022; 14(21):4578. https://doi.org/10.3390/polym14214578
Chicago/Turabian StyleGamage, Ashoka, Punniamoorthy Thiviya, Sudhagar Mani, Prabaharan Graceraj Ponnusamy, Asanga Manamperi, Philippe Evon, Othmane Merah, and Terrence Madhujith. 2022. "Environmental Properties and Applications of Biodegradable Starch-Based Nanocomposites" Polymers 14, no. 21: 4578. https://doi.org/10.3390/polym14214578
APA StyleGamage, A., Thiviya, P., Mani, S., Ponnusamy, P. G., Manamperi, A., Evon, P., Merah, O., & Madhujith, T. (2022). Environmental Properties and Applications of Biodegradable Starch-Based Nanocomposites. Polymers, 14(21), 4578. https://doi.org/10.3390/polym14214578











