Gelatin/Hyaluronic Acid Scaffold Coupled to CpG and MAGE-A5 as a Treatment against Murine Melanoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Ethics Statement
2.3. Reagents
2.4. Tumor Antigen
2.5. Ge and HA Scaffold Construction
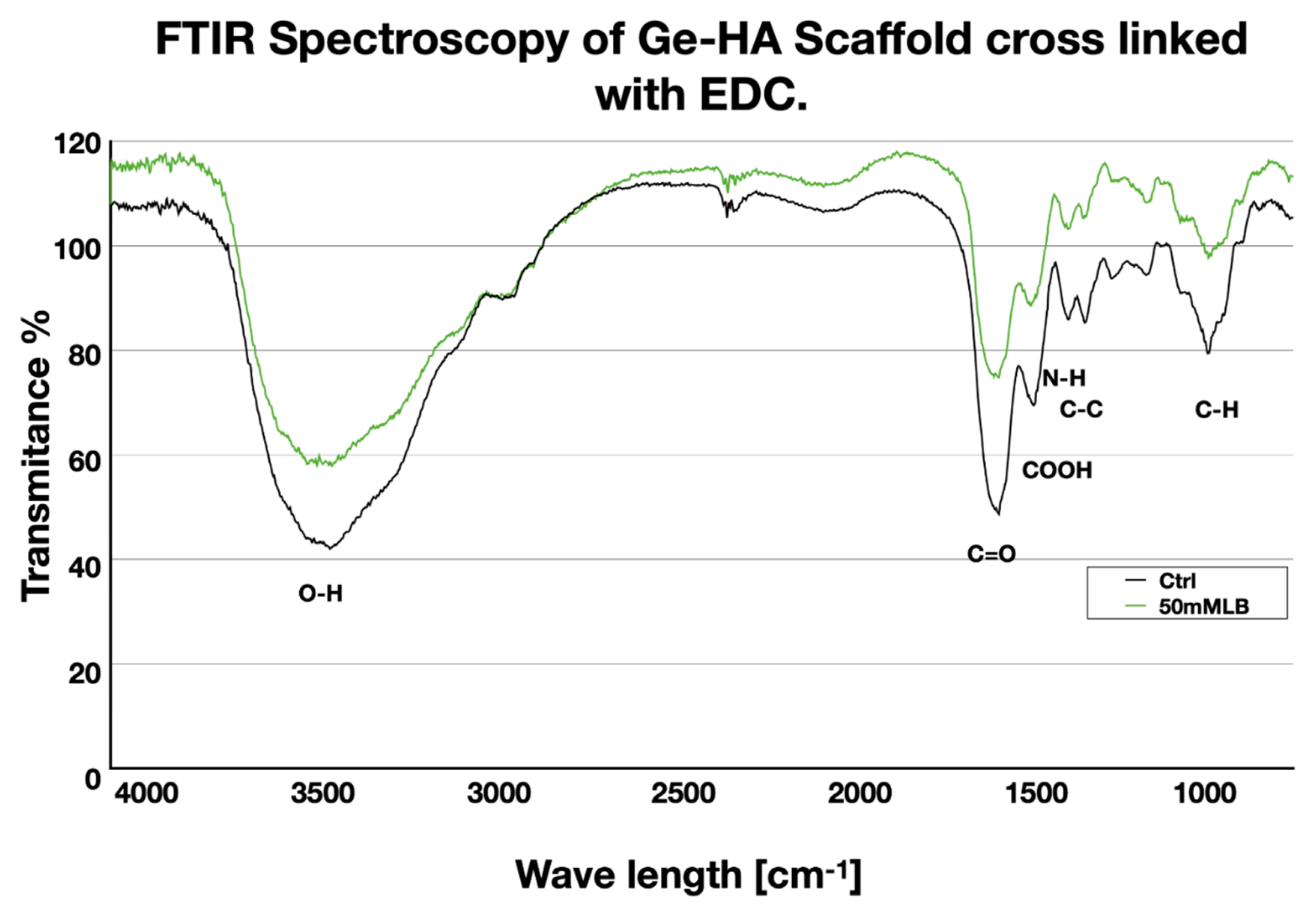
2.6. Fourier Transform Infrared (FT-IR) Characterization
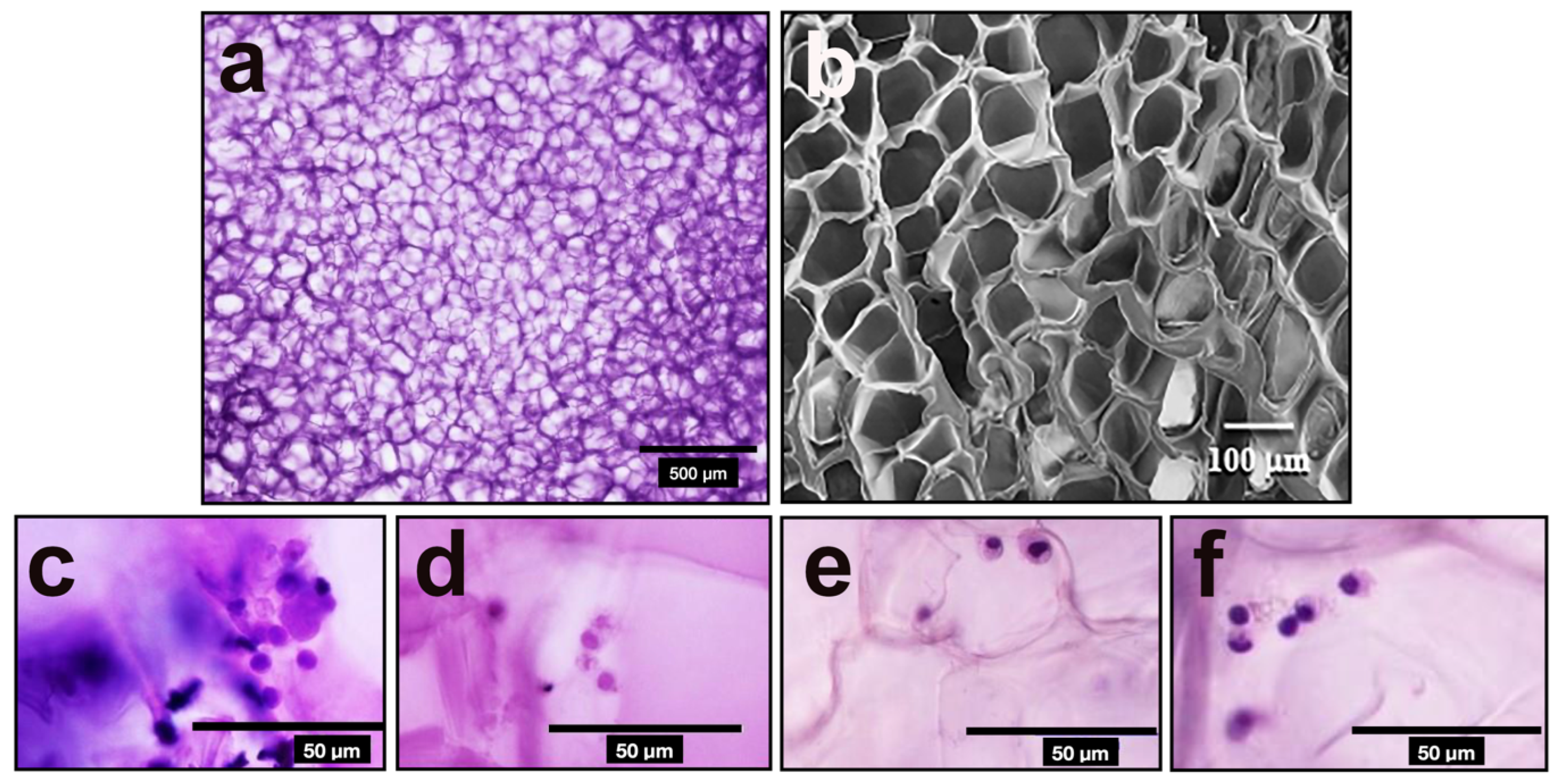
2.7. Scanning Electron Microscopy (SEM)
2.8. Melanoma Induction in B16-F10 Mice
2.9. Effect of the Ge/HA Scaffold Coupled to CpG, MAGE-A5 or Both in Splenocytes In Vitro Tests
2.10. Tumor Growth Rate and Survival
2.11. Histopathological Evaluation of Tumors
2.12. Statistical Analysis
3. Results
3.1. FT-IR Analysis
3.2. Surface Morphology of Ge/HA
3.3. Effect of the Ge/HA Scaffold Coupled to CpG, MAGE-A5 or Both in Splenocytes In Vitro Tests
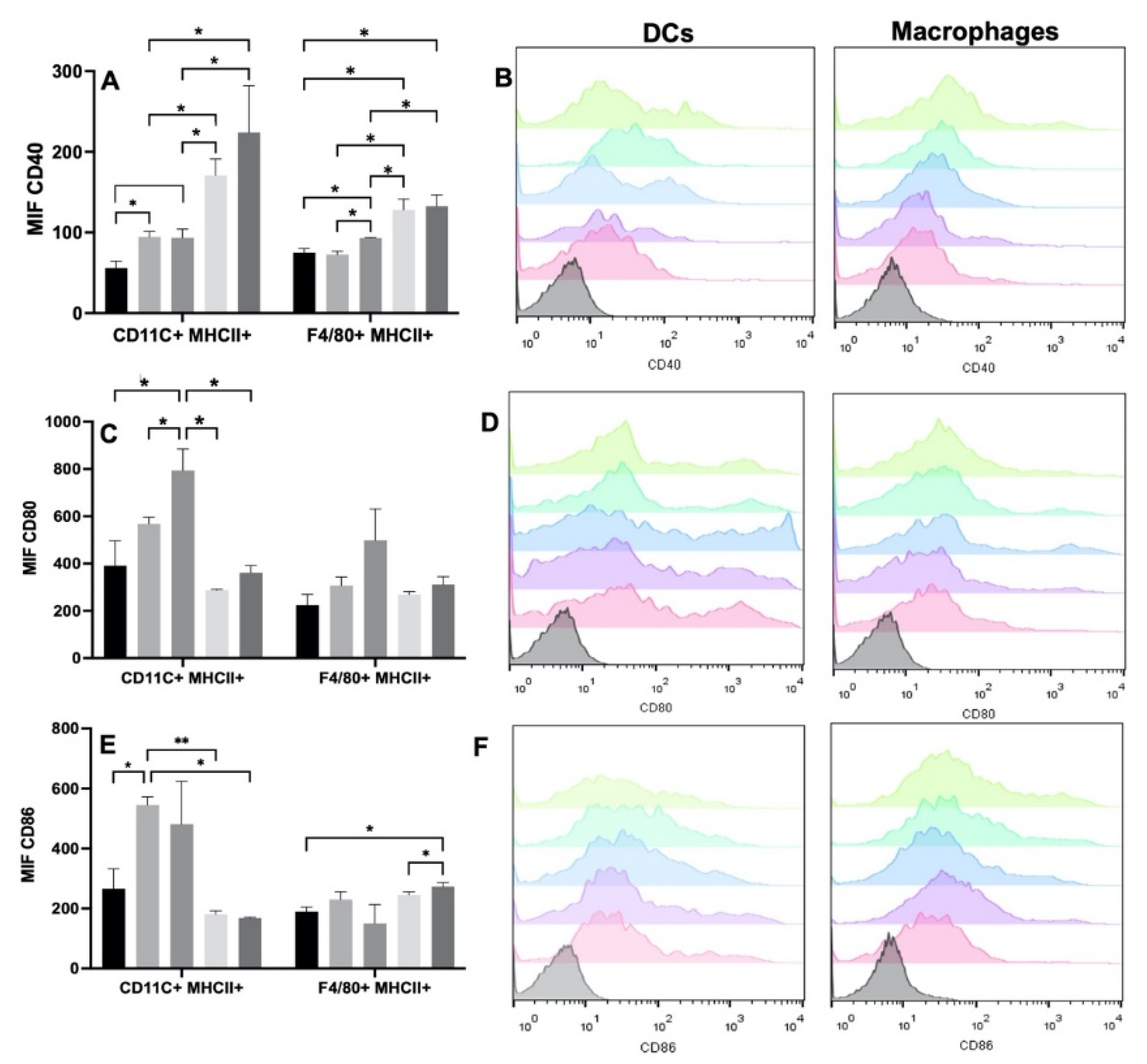
3.3.1. Effect of Scaffolds on Macrophages and Dendritic Cells
3.3.2. Effect of Scaffolds on T Lymphocytes
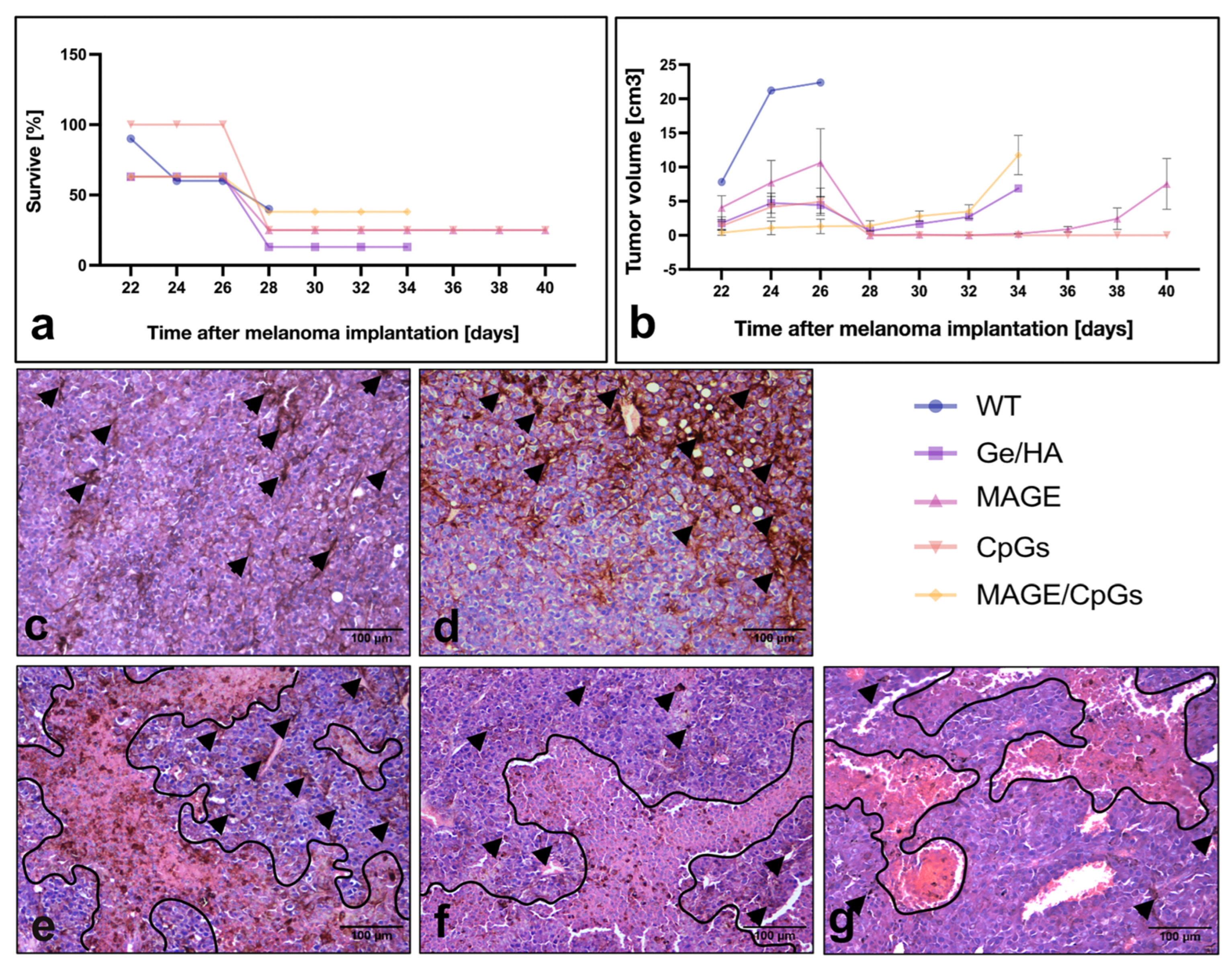
3.4. Survival Analysis and Tumor Growth Rate
3.5. Histopathological Evaluation of Tumor Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moynihan, K.D.; Darrell, J.; Irvine, D.J. Roles for Innate Immunity in Combination Immunotherapies. Cancer Res. 2017, 19, 5215–5221. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Blum, N.T.; Lin, J.; Qu, J.; Huang, P. Biomaterial scaffold-based local drug delivery systems for cancer immunotherapy. Sci. Bull. 2020, 65, 1489–1504. [Google Scholar] [CrossRef]
- Minda, A.G.; Awel, F.S.; Seifudin, K.A.; Gezahegne, M.K. Immunotherapy against cancer: A comprehensive review. J. Cancer Res. Exp. Oncol. 2016, 2, 15–25. [Google Scholar] [CrossRef]
- Zhao, Z.; Zheng, L.; Chen, W.; Weng, W.; Song, J.; Ji, J. Delivery strategies of cancer immunotherapy: Recent advances and future perspectives. J. Hematol. Oncol. 2019, 1, 126. [Google Scholar] [CrossRef]
- Li, J.; Luo, Y.; Li, B.; Xia, Y.; Wang, H.; Fu, C. Implantable and Injectable Biomaterial Scaffolds for Cancer Immunotherapy. Front. Bioeng. Biotechnol. 2020, 8, 612950. [Google Scholar] [CrossRef]
- Han, S.; Wu, J. Three-dimensional (3D) scaffolds as powerful weapons for tumor immunotherapy. Bioac. Mater. 2022, 17, 300–319. [Google Scholar] [CrossRef]
- Ali, O.A.; Lewin, S.A.; Dranoff, G.; Mooney, D.J. Vaccines Combined with Immune Checkpoint Antibodies Promote Cytotoxic T-cell Activity and Tumor Eradication. Cancer Immunol. Res. 2016, 2, 95–100. [Google Scholar] [CrossRef]
- Verma, V.; Kim, Y.; Lee, M.C.; Lee, J.T.; Cho, S.; Park, I.K.; Min, J.J.; Lee, J.J.; Lee, S.E.; Rhee, J.H. Activated dendritic cells delivered in tissue compatible biomatrices induce in-situ anti-tumor CTL responses leading to tumor regression. Oncotarget 2016, 26, 39894–39906. [Google Scholar] [CrossRef]
- Gariboldi, S.; Palazzo, M.; Zanobbio, L.; Selleri, S.; Sommariva, M.; Sfondrini, L.; Cavicchini, S.; Balsari, A.; Rumio, C. Low molecular weight hyaluronic acid increases the self-defense of skin epithelium by induction of beta-defensin 2 via TLR2 and TLR4. J. Immunol. 2008, 3, 2103–2110. [Google Scholar] [CrossRef]
- Donaldson, A.R.; Tanase, C.E.; Awuah, D.; Vasanthi Bathrinarayanan, P.; Hall, L.; Nikkhah, M.; Khademhosseini, A.; Rose, F.; Alexander, C.; Ghaemmaghami, A.M. Photocrosslinkable Gelatin Hydrogels Modulate the Production of the Major Pro-inflammatory Cytokine, TNF-α, by Human Mononuclear Cells. Front. Bioeng. Biotechnol. 2018, 6, 116. [Google Scholar] [CrossRef]
- Vegetti, D.; Karousou, E.; Viola, M.; Deleonibus, S.; De Luca, G.; Passi, A. Hyaluronan: Biosynthesis and signaling. Biochim. Biophys. Acta 2014, 8, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Abdullah, H.Z. A review of gelatin: Properties, sources, process, applications, and commercialization. Mater. Today 2019, 42, 240–250. [Google Scholar] [CrossRef]
- Rowley, A.T.; Nagalla, R.R.; Wang, S.W.; Liu, W.F. Extracellular Matrix-Based Strategies for Immunomodulatory Biomaterials Engineering. Adv. Healthc. Mater. 2019, 8, e1801578. [Google Scholar] [CrossRef]
- Kaul, A.; Short, W.D.; Keswani, S.G.; Wang, X. Immunologic roles of hyaluronan in dermal wound healing. Biomolecules 2021, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Klinman, D.M. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat. Rev. Immunol. 2004, 4, 249–258. [Google Scholar] [CrossRef]
- Hanagata, N. Structure-dependent immunostimulatory effect of CpG oligodeoxynucleotides and their delivery system. Int. J. Nanomed. 2012, 7, 2181–2195. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhao, H.; Li, Y.; Lee, A.L.; Li, Z.; Fu, M.; Li, C.; Yang, Y.Y.; Yuan, P. Synthetic peptide hydrogels as 3D scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2020, 160, 78–104. [Google Scholar] [CrossRef]
- Domingues, B.; Lopes, J.; Soares, P.; Populo, H. Melanoma treatment in review. Immunol. Targets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef]
- Palucka, K.; Banchereau, J. Dendritic-Cell-Based Therapeutic Cancer Vaccines. Immunity 2013, 1, 38–48. [Google Scholar] [CrossRef]
- Piñón-Zárate, G.; Herrera-Enríquez, M.Á.; Hernández-Téllez, B.; Jarquín-Yáñez, K.; Castell-Rodríguez, A.E. GK-1 improves the immune response induced by bone marrow dendritic cells loaded with MAGE-AX in mice with melanoma. J. Immunol. Res. 2014. [Google Scholar] [CrossRef]
- Constantino, J.; Gomes, C.; Falcão, A.; Neves, B.M.; Cruz, M.T. Dendritic cell-based immunotherapy: A basic review and recent advances. Immunol. Res. 2017, 4, 798–810. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Schaller, B.; Kobayashi, E.; Hernandez, M.; Zhang, Y.; Miron, R.J. Hyaluronic acid gel-based scaffolds as potential carrier for growth factors: An in vitro bioassay on its osteogenic potential. J. Clin. Med. 2016, 5, 112. [Google Scholar] [CrossRef]
- Kurum, A.; Gao, M.; Tang, L. Synthetic 3D scaffolds for cancer immunotherapy. Curr. Opin. Biotechnol. 2020, 65, 1–8. [Google Scholar] [CrossRef]
- Eggert, A.O.; Andersen, M.H.; Voigt, H.; Schrama, D.; Kämpgen, E.; Straten, P.T.; Becker, J.C. Characterization of mouse MAGE-derived H-2Kb-restricted CTL epitopes. Eur. J. Immunol. 2004, 11, 3285–3290. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.A.; Huebsch, N.; Cao, L.; Dranoff, G.; Mooney, D.J. Infection-mimicking materials to program dendritic cells in situ. Nat. Mater. 2008, 2, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Jarquín-Yañéz, K.; Arenas-Alatorre, J.; Piñón Zárate, G.; Olivares-Arellano, R.M.; Herrera-Enríquez, M.; Hernández-Tellez, B.; Castell-Rodríguez, A.E. Structural Effect of Different EDC Crosslinker Concentration in Gelatin-Hyaluronic Acid Scaffolds. J. Bioeng. Biomed. Sci. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Lewis, J.S.; Roy, K.; Keselowsky, B.G. Materials that harness and modulate the immune system. MRS Bull 2014, 39, 25–34. [Google Scholar] [CrossRef][Green Version]
- Silva, A.L.; Rosalia, R.A.; Varypataki, E.; Sibuea, S.; Ossendorp, F.; Jiskoot, W. Poly-(lactic-co-glycolic-acid)-based particulate vaccines: Particle uptake by dendritic cells is a key parameter for immune activation. Vaccine 2015, 33, 847–854. [Google Scholar] [CrossRef]
- Torres, M.P.; Wilson-Welder, J.H.; Lopac, S.K.; Phanse, Y.; Carrillo-Conde, B.; Ramer-Tait, A.E.; Bellaire, B.H.; Wannemuehler, M.J.; Narasimhan, B. Polyanhydride microparticles enhance dendritic cell antigen presentation and activation. Acta Biomater. 2011, 7, 2857–2864. [Google Scholar] [CrossRef]
- Sinha, A.; Choi, Y.; Nguyen, M.H.; Nguyen, T.L.; Choi, S.W.; Kim, J. A 3D Macroporous Alginate Graphene Scaffold with an Extremely Slow Release of a Loaded Cargo for In Situ Long-Term Activation of Dendritic Cells. Adv. Heal. Mater. 2019, 5, e1800571. [Google Scholar] [CrossRef]
- Stephan, S.B.; Taber, A.M.; Jileaeva, I.; Pegues, E.P.; Sentman, C.L.; Stephan, M.T. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat. Biotechnol. 2015, 1, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.T.; Moffett, H.F.; Stephan, S.B.; Opel, C.F.; Dumigan, A.G.; Jiang, X.; Pillarisetty, V.G.; Pillai, S.P.S.; Wittrup, K.D.; Stephan, M.T. Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. J. Clin. Investig. 2017, 6, 2176–2191. [Google Scholar] [CrossRef] [PubMed]
- Jarquín-Yáñez, K.; Herrera-Enriquez, M.Á.; Lemini, C.; Melendez-Moreno, E.; Villena-López, P.; Ávila, M.E.; Hernández-Téllez, B.; Piñón-Zárate, G.; Sampedro-Carrillo, E.A.; Castell-Rodríguez, A.E. Epicutaneous Administration of 17β-Estradiol Induces Langerhans Cells Depletion. Immunol. Investig. 2021, 2, 1561–1581. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 5, 646–674. [Google Scholar] [CrossRef]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double- stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Z.; Cui, B.; Liu, H.Z.; Mi, S.; Yan, J.; Yan, H.M. Blocking TLR2 activity attenuates pulmonary metastases of tumor. PLoS ONE 2009, 4, e6520. [Google Scholar] [CrossRef]
- Kim, S.; Takahashi, H.; Lin, W.W.; Descargues, P.; Grivennikov, S.; Kim, Y. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 2009, 457, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.M.; Marchicio, J.; López, M.; Ziblat, A.; Elias, F.; Fló, J. PyNTTTTGT and CpG Immunostimulatory Oligonucleotides: Effect on Granulocyte/Monocyte Colony-Stimulating Factor (GM-CSF) Secretion by Human CD56+ (NK and NKT) Cells. PLoS ONE 2015, 2, e0117484. [Google Scholar] [CrossRef]
 Without treatment,
Without treatment,  Ge/Ha,
Ge/Ha,  CpG,
CpG,  MAGE-A5,
MAGE-A5,  MAGE-A5/CpG.
MAGE-A5/CpG.
 Without treatment,
Without treatment,  Ge/Ha,
Ge/Ha,  CpG,
CpG,  MAGE-A5,
MAGE-A5,  MAGE-A5/CpG.
MAGE-A5/CpG.
 Without treatment,
Without treatment,  Ge/Ha,
Ge/Ha,  CpG,
CpG,  MAGE-A5,
MAGE-A5,  MAGE-A5/CpG. (B) Expression of CD137 in CD4 and CD8 T lymphocytes. No significant changes were observed in the percentage of CD4 T lymphocytes. CD8 T lymphocytes only showed an increase in the Ge/HA group compared to the Ge/HA/MAGE-A5 and Ge/HA/CpG/MAGE-A5 groups (* p = 0.01: Ge/HA vs. Ge/HA/MAGE-A5; ** p = 0.003: Ge/HA vs. Ge/HA/MAGE-A5/CpG). CD137 expression was enhanced in both CD4+ and CD8+ T lymphocytes. CD4 T lymphocytes showed higher levels of CD137 in the groups treated with Ge/HA, Ge/HA/CpG, and Ge/HA/CpG/MAGE-A5 than in the WT group (* p = 0.03: WT vs. Ge/HA, Ge/HA/CpG, Ge/HA/MAGE-A5/CpG). CD8+ lymphocytes showed the highest CD137 expression in the Ge/HA/MAGE-A5- and Ge/HA/CpG/MAGE-A5-treated groups compared to the WT groups (* p = 0.04 WT vs. Ge/HA/MAGE-A5; * p = 0.02: Ge/HA vs. Ge/HA/MAGE-A5/CpG; * p = 0.01: Ge/HA/CpG vs. Ge/HA/MAGE-A5/CpG; ** p = 0.005: Ge/HA/CpG vs. Ge/HA/MAGE-A5; ** p = Ge/HA vs. Ge/HA/MAGE-A5/CpG; **** p < 0.0001: Ge/HA vs. Ge/HA/MAGE-A5).
MAGE-A5/CpG. (B) Expression of CD137 in CD4 and CD8 T lymphocytes. No significant changes were observed in the percentage of CD4 T lymphocytes. CD8 T lymphocytes only showed an increase in the Ge/HA group compared to the Ge/HA/MAGE-A5 and Ge/HA/CpG/MAGE-A5 groups (* p = 0.01: Ge/HA vs. Ge/HA/MAGE-A5; ** p = 0.003: Ge/HA vs. Ge/HA/MAGE-A5/CpG). CD137 expression was enhanced in both CD4+ and CD8+ T lymphocytes. CD4 T lymphocytes showed higher levels of CD137 in the groups treated with Ge/HA, Ge/HA/CpG, and Ge/HA/CpG/MAGE-A5 than in the WT group (* p = 0.03: WT vs. Ge/HA, Ge/HA/CpG, Ge/HA/MAGE-A5/CpG). CD8+ lymphocytes showed the highest CD137 expression in the Ge/HA/MAGE-A5- and Ge/HA/CpG/MAGE-A5-treated groups compared to the WT groups (* p = 0.04 WT vs. Ge/HA/MAGE-A5; * p = 0.02: Ge/HA vs. Ge/HA/MAGE-A5/CpG; * p = 0.01: Ge/HA/CpG vs. Ge/HA/MAGE-A5/CpG; ** p = 0.005: Ge/HA/CpG vs. Ge/HA/MAGE-A5; ** p = Ge/HA vs. Ge/HA/MAGE-A5/CpG; **** p < 0.0001: Ge/HA vs. Ge/HA/MAGE-A5).
 Without treatment,
Without treatment,  Ge/Ha,
Ge/Ha,  CpG,
CpG,  MAGE-A5,
MAGE-A5,  MAGE-A5/CpG. (B) Expression of CD137 in CD4 and CD8 T lymphocytes. No significant changes were observed in the percentage of CD4 T lymphocytes. CD8 T lymphocytes only showed an increase in the Ge/HA group compared to the Ge/HA/MAGE-A5 and Ge/HA/CpG/MAGE-A5 groups (* p = 0.01: Ge/HA vs. Ge/HA/MAGE-A5; ** p = 0.003: Ge/HA vs. Ge/HA/MAGE-A5/CpG). CD137 expression was enhanced in both CD4+ and CD8+ T lymphocytes. CD4 T lymphocytes showed higher levels of CD137 in the groups treated with Ge/HA, Ge/HA/CpG, and Ge/HA/CpG/MAGE-A5 than in the WT group (* p = 0.03: WT vs. Ge/HA, Ge/HA/CpG, Ge/HA/MAGE-A5/CpG). CD8+ lymphocytes showed the highest CD137 expression in the Ge/HA/MAGE-A5- and Ge/HA/CpG/MAGE-A5-treated groups compared to the WT groups (* p = 0.04 WT vs. Ge/HA/MAGE-A5; * p = 0.02: Ge/HA vs. Ge/HA/MAGE-A5/CpG; * p = 0.01: Ge/HA/CpG vs. Ge/HA/MAGE-A5/CpG; ** p = 0.005: Ge/HA/CpG vs. Ge/HA/MAGE-A5; ** p = Ge/HA vs. Ge/HA/MAGE-A5/CpG; **** p < 0.0001: Ge/HA vs. Ge/HA/MAGE-A5).
MAGE-A5/CpG. (B) Expression of CD137 in CD4 and CD8 T lymphocytes. No significant changes were observed in the percentage of CD4 T lymphocytes. CD8 T lymphocytes only showed an increase in the Ge/HA group compared to the Ge/HA/MAGE-A5 and Ge/HA/CpG/MAGE-A5 groups (* p = 0.01: Ge/HA vs. Ge/HA/MAGE-A5; ** p = 0.003: Ge/HA vs. Ge/HA/MAGE-A5/CpG). CD137 expression was enhanced in both CD4+ and CD8+ T lymphocytes. CD4 T lymphocytes showed higher levels of CD137 in the groups treated with Ge/HA, Ge/HA/CpG, and Ge/HA/CpG/MAGE-A5 than in the WT group (* p = 0.03: WT vs. Ge/HA, Ge/HA/CpG, Ge/HA/MAGE-A5/CpG). CD8+ lymphocytes showed the highest CD137 expression in the Ge/HA/MAGE-A5- and Ge/HA/CpG/MAGE-A5-treated groups compared to the WT groups (* p = 0.04 WT vs. Ge/HA/MAGE-A5; * p = 0.02: Ge/HA vs. Ge/HA/MAGE-A5/CpG; * p = 0.01: Ge/HA/CpG vs. Ge/HA/MAGE-A5/CpG; ** p = 0.005: Ge/HA/CpG vs. Ge/HA/MAGE-A5; ** p = Ge/HA vs. Ge/HA/MAGE-A5/CpG; **** p < 0.0001: Ge/HA vs. Ge/HA/MAGE-A5).
| Experimental Group | CD40 | CD80 | CD86 | p |
|---|---|---|---|---|
| Without treatment (WT) | DC: 56 ± 8.4 | DC: 391 ± 105.65 | DC: 266.5 ± 65.9 | dna |
| MO: 74.9 ± 5.1 | MO: 224 ± 45.6 | MO: 189.5 ± 15.3 | dna | |
| Ge/HA | DC: 94.6 ± 6.58 | DC: 567 ± 28.57 | DC:245.5 ± 26.9 | CD40: p = 0.012. Ge/HA vs. WT, MAGE-A5 and MAGE-A5/CpG CD86: p =0.006. Ge/HA vs. MAGE-A5. p = 0.012. Ge/HA vs. WT and MAGE-A5/CpG |
| MO:72.75 ± 4.07 | MO:307.5 ± 35.5 | MO: 230 ± 25.9 | ns | |
| MAGE-A5 | DC: 170 ± 20.5 | DC: 287.5 ± 3.7 | DC: 181 ± 10.9 | ns |
| MO: 128 ± 13.3 | MO: 269 ± 12.17 | MO: 246 ± 9.8 | CD40: p = 0.0125. MAGE-A5 vs. WT, Ge/HA, CpG and MAGE-A5/CpG | |
| CpG | DC: 93.3 ± 10.8 | DC: 795 ± 91.2 | DC: 480.5 ± 142.9 | CD40: p = 0.012. CpG vs. MAGE-A5 and MAGE-A5/CpG CD80: p = 0.013. CpG vs. Ge/HA and MAGE-A5 |
| MO:3.05 ± 0.7 | MO: 499 ± 132.2 | MO: 150 ± 62.2 | CD40: p = 0.0125. CpG vs. WT, Ge/HA, MAGE-A5, MAGE-A5/CpG. | |
| MAGE-A5/CpG | DC: 224 ± 57.7 | MO: 361 ± 30.6 | DC: 168 ± 288 | ns |
| MO: 132 ± 14.1 | MO: 311.5 ± 33.19 | MO: 273 ± 13.2 | CD40: p = 0.0125. CpG/MAGE-A5 vs. WT CD86: p = 0.04. MAGE-A5/CpG vs. WT and MAGE-A5. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piñón-Zárate, G.; Hernández-Téllez, B.; Jarquín-Yáñez, K.; Herrera-Enríquez, M.Á.; Fuerte-Pérez, A.E.; Valencia-Escamilla, E.A.; Castell-Rodríguez, A.E. Gelatin/Hyaluronic Acid Scaffold Coupled to CpG and MAGE-A5 as a Treatment against Murine Melanoma. Polymers 2022, 14, 4608. https://doi.org/10.3390/polym14214608
Piñón-Zárate G, Hernández-Téllez B, Jarquín-Yáñez K, Herrera-Enríquez MÁ, Fuerte-Pérez AE, Valencia-Escamilla EA, Castell-Rodríguez AE. Gelatin/Hyaluronic Acid Scaffold Coupled to CpG and MAGE-A5 as a Treatment against Murine Melanoma. Polymers. 2022; 14(21):4608. https://doi.org/10.3390/polym14214608
Chicago/Turabian StylePiñón-Zárate, Gabriela, Beatriz Hernández-Téllez, Katia Jarquín-Yáñez, Miguel Ángel Herrera-Enríquez, América Eréndira Fuerte-Pérez, Esther Alejandra Valencia-Escamilla, and Andrés Eliú Castell-Rodríguez. 2022. "Gelatin/Hyaluronic Acid Scaffold Coupled to CpG and MAGE-A5 as a Treatment against Murine Melanoma" Polymers 14, no. 21: 4608. https://doi.org/10.3390/polym14214608
APA StylePiñón-Zárate, G., Hernández-Téllez, B., Jarquín-Yáñez, K., Herrera-Enríquez, M. Á., Fuerte-Pérez, A. E., Valencia-Escamilla, E. A., & Castell-Rodríguez, A. E. (2022). Gelatin/Hyaluronic Acid Scaffold Coupled to CpG and MAGE-A5 as a Treatment against Murine Melanoma. Polymers, 14(21), 4608. https://doi.org/10.3390/polym14214608








