Nanoparticle-Based Drug Delivery Systems: An Inspiring Therapeutic Strategy for Neurodegenerative Diseases
Abstract
1. Introduction
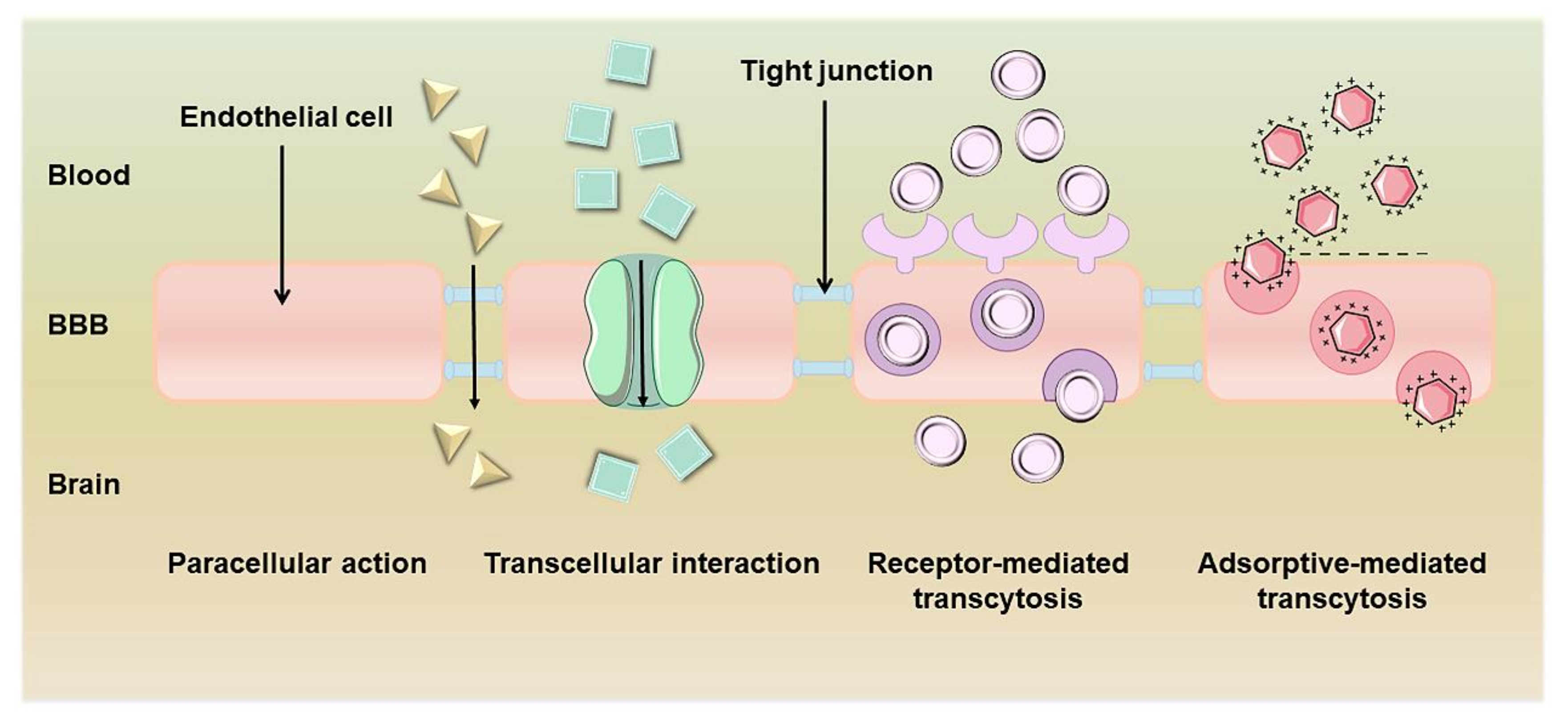
2. The Structure and Role of the BBB
3. Types and Administration Routes of NPs
3.1. Inorganic-Based NPs
3.2. Organic-Based NPs
3.3. Multifunctionally Modified NPs
3.4. Administration Routes of NPs
4. NPs-Based Drug Delivery Systems
4.1. Biological Molecules
4.2. Natural Products
4.3. FDA-Approved Clinical Drugs
5. Application of NPs in Neurodegenerative Diseases
5.1. Alzheimer’s Disease
5.2. Parkinson’s Disease
5.3. Huntington’s Disease
5.4. Amyotrophic Lateral Sclerosis
5.5. Frontotemporal Dementia, Prion Disease, and Glioblastoma
6. Combined Therapeutic Strategies
7. Clinical Trials
8. Prospects and Challenges
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Terreros-Roncal, J.; Moreno-Jimenez, E.P.; Flor-Garcia, M.; Rodriguez-Moreno, C.B.; Trinchero, M.F.; Cafini, F.; Rabano, A.; Llorens-Martin, M. Impact of neurodegenerative diseases on human adult hippocampal neurogenesis. Science 2021, 374, 1106–1113. [Google Scholar] [CrossRef]
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Blennow, K.; Breteler, M.M.B.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, U.; Kayed, R. Amyloid beta, tau, and alpha-synuclein aggregates in the pathogenesis, prognosis, and therapeutics for neurodegenerative diseases. Prog. Neurobiol. 2022, 214, 102270. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative stress and mitochondrial damage in neurodegenerative diseases: From molecular mechanisms to targeted therapies. Oxid. Med. Cell. Longev. 2020, 2020, 1270256. [Google Scholar] [CrossRef] [PubMed]
- Janicki Hsieh, S.; Alexopoulou, Z.; Mehrotra, N.; Struyk, A.; Stoch, S.A. Neurodegenerative diseases: The value of early predictive end points. Clin. Pharmacol. Ther. 2022, 111, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Segarra, M.; Aburto, M.R.; Acker-Palmer, A. Blood-brain barrier dynamics to maintain brain homeostasis. Trends Neurosci. 2021, 44, 393–405. [Google Scholar] [CrossRef]
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W.D. Strategies for delivering therapeutics across the blood-brain barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef]
- Masoudi Asil, S.; Ahlawat, J.; Guillama Barroso, G.; Narayan, M. Nanomaterial based drug delivery systems for the treatment of neurodegenerative diseases. Biomater. Sci. 2020, 8, 4109–4128. [Google Scholar] [CrossRef]
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of nanotechnology in drug delivery to the central nervous system. Biomed. Pharmacother. 2019, 111, 666–675. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Nehra, G.; Bauer, B.; Hartz, A.M.S. Blood-brain barrier leakage in Alzheimer’s disease: From discovery to clinical relevance. Pharmacol. Ther. 2022, 234, 108119. [Google Scholar] [CrossRef]
- Cuny, G.D. Foreword: Neurodegenerative diseases: Challenges and opportunities. Future Med. Chem. 2012, 4, 1647–1649. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Khan, A.I.; Cai, X.; Song, Y.; Lyu, Z.; Du, D.; Dutta, P.; Lin, Y. Overcoming blood-brain barrier transport: Advances in nanoparticle-based drug delivery strategies. Mater. Today. 2020, 37, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Fan, W.; Lau, J.; Deng, L.; Shen, Z.; Chen, X. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem. Soc. Rev. 2019, 48, 2967–3014. [Google Scholar] [CrossRef]
- Han, L.; Jiang, C. Evolution of blood-brain barrier in brain diseases and related systemic nanoscale brain-targeting drug delivery strategies. Acta Pharm. Sin. B 2021, 11, 2306–2325. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Tomita, M.; Awazu, S. Transcellular and paracellular contribution to transport processes in the colorectal route. Adv. Drug Del. Rev. 1997, 28, 191–204. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Anderson, R.G.; Brown, M.S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature 1979, 279, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Lu, W. Adsorptive-mediated brain delivery systems. Curr. Pharm. Biotechnol. 2012, 13, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.J.; Santoso, M.R.; Rezaee, F.; Aghaverdi, H.; Mahmoudi, M.; Perry, G. Advances in Alzheimer’s diagnosis and therapy: The implications of nanotechnology. Trends Biotechnol. 2017, 35, 937–953. [Google Scholar] [CrossRef]
- Krol, S.; Macrez, R.; Docagne, F.; Defer, G.; Laurent, S.; Rahman, M.; Hajipour, M.J.; Kehoe, P.G.; Mahmoudi, M. Therapeutic benefits from nanoparticles: The potential significance of nanoscience in diseases with compromise to the blood brain barrier. Chem. Rev. 2013, 113, 1877–1903. [Google Scholar] [CrossRef]
- Furtado, D.; Bjornmalm, M.; Ayton, S.; Bush, A.I.; Kempe, K.; Caruso, F. Overcoming the blood-brain barrier: The role of nanomaterials in treating neurological diseases. Adv. Mater. 2018, 30, e1801362. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.C.; Chang, Y.M.; Kang, I.K. Immobilization of biomolecules on the surface of inorganic nanoparticles for biomedical applications. Sci. Technol. Adv. Mater. 2010, 11, 014101. [Google Scholar] [CrossRef]
- Deng, J.J.; Yu, P.; Wang, Y.X.; Yang, L.F.; Mao, L.Q. Visualization and quantification of neurochemicals with gold nanoparticles: Opportunities and challenges. Adv. Mater. 2014, 26, 6933–6943. [Google Scholar] [CrossRef]
- Meola, A.; Rao, J.; Chaudhary, N.; Sharma, M.; Chang, S.D. Gold nanoparticles for brain tumor imaging: A systematic review. Front. Neurol. 2018, 9, 328. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; Karuppusamy, I.; Saravanan, M.; Muthukumar, H.; Ponnuchamy, K.; Ramkumar, V.S.; Pugazhendhi, A. Synthesis of silver nanoparticles and their biomedical applications—A comprehensive review. Curr. Pharm. Des. 2019, 25, 2650–2660. [Google Scholar] [CrossRef]
- Zuberek, M.; Stepkowski, T.M.; Kruszewski, M.; Grzelak, A. Exposure of human neurons to silver nanoparticles induces similar pattern of ABC transporters gene expression as differentiation: Study on proliferating and post-mitotic LUHMES cells. Mech. Ageing Dev. 2018, 171, 7–14. [Google Scholar] [CrossRef]
- Huang, C.L.; Hsiao, I.L.; Lin, H.C.; Wang, C.F.; Huang, Y.J.; Chuang, C.Y. Silver nanoparticles affect on gene expression of inflammatory and neurodegenerative responses in mouse brain neural cells. Environ. Res. 2015, 136, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Baldrighi, M.; Trusel, M.; Tonini, R.; Giordani, S. Carbon nanomaterials interfacing with neurons: An in vivo perspective. Front. Neurosci. 2016, 10, 250. [Google Scholar] [CrossRef]
- Paviolo, C.; Cognet, L. Near-infrared nanoscopy with carbon-based nanoparticles for the exploration of the brain extracellular space. Neurobiol. Dis. 2021, 153, 105328. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Mohammadinejad, R.; Kailasa, S.K.; Ahmadi, Z.; Afshar, E.G.; Pardakhty, A. Carbon dots as versatile nanoarchitectures for the treatment of neurological disorders and their theranostic applications: A review. Adv. Colloid Interface Sci. 2020, 278, 102123. [Google Scholar] [CrossRef]
- Zhang, W.; Sigdel, G.; Mintz, K.J.; Seven, E.S.; Zhou, Y.Q.; Wang, C.Y.; Leblanc, R.M. Carbon dots: A future blood-brain barrier penetrating nanomedicine and drug nanocarrier. Int. J. Nanomed. 2021, 16, 5003–5016. [Google Scholar] [CrossRef]
- Mintz, K.J.; Mercado, G.; Zhou, Y.Q.; Ji, Y.W.; Hettiarachchi, S.D.; Liyanagea, P.Y.; Pandey, R.R.; Chusuei, C.C.; Dallman, J.; Leblanc, R.M. Tryptophan carbon dots and their ability to cross the blood-brain barrier. Colloids Surf. B Biointerfaces 2019, 176, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Q.; Liyanage, P.Y.; Devadoss, D.; Guevara, L.R.R.; Cheng, L.; Graham, R.M.; Chand, H.S.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; et al. Nontoxic amphiphilic carbon dots as promising drug nanocarriers across the blood-brain barrier and inhibitors of beta-amyloid. Nanoscale 2019, 11, 22387–22397. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q.; Shang, T.; Zhang, X.D.; Ye, T.; Wang, D.J.; Rei, L. Passage of magnetic Tat-conjugated Fe3O4@SiO2 nanoparticles across in vitro blood-brain barrier. Nanoscale Res. Lett. 2016, 11, 451. [Google Scholar] [CrossRef]
- Kim, D.; Kwon, H.J.; Hyeon, T. Magnetite/ceria nanoparticle assemblies for extracorporeal cleansing of amyloid-beta in Alzheimer’s disease. Adv. Mater. 2019, 31, e1807965. [Google Scholar] [CrossRef]
- Abbas, H.; Refai, H.; El Sayed, N.; Rashed, L.A.; Mousa, M.R.; Zewail, M. Superparamagnetic iron oxide loaded chitosan coated bilosomes for magnetic nose to brain targeting of resveratrol. Int. J. Pharm. 2021, 610, 121244. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.Y.; Chen, D.X.; Wang, Y.; Li, H.F.; Zhang, Y.B.; Chen, H.Y.; Li, X.; Huo, M.F. Nanozymes-recent development and biomedical applications. J. Nanobiotechnol. 2022, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Camarena, Á.; Merino, M.; Sánchez-Sánchez, A.V.; Blasco, S.; Llinares, J.M.; Mullor, J.L.; García-España, E. An antioxidant boehmite amino-nanozyme able to disaggregate Huntington’s inclusion bodies. Chem. Commun. 2022, 58, 5021–5024. [Google Scholar] [CrossRef]
- Bukhari, S.N.A. Nanotherapeutics for Alzheimer’s disease with preclinical evaluation and clinical trials: Challenges, promises and limitations. Curr. Drug Deliv. 2022, 19, 17–31. [Google Scholar] [CrossRef]
- Utekhina, A.Y.; Sergeev, G.B. Organic nanoparticles. Russ. Chem. Rev. 2011, 80, 219–233. [Google Scholar] [CrossRef]
- Tsou, Y.H.; Zhang, X.Q.; Zhu, H.; Syed, S.; Xu, X.Y. Drug delivery to the brain across the blood-brain barrier using nanomaterials. Small 2017, 13, 1701921. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Impact of nanoparticles on brain health: An up to date overview. J. Clin. Med. 2018, 7, 490. [Google Scholar] [CrossRef]
- Ramos-Zaldivar, H.M.; Polakovicova, I.; Salas-Huenuleo, E.; Corvalan, A.H.; Kogan, M.J.; Yefi, C.P.; Andia, M.E. Extracellular vesicles through the blood-brain barrier: A review. Fluids Barriers CNS 2022, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Ordonez-Gutierrez, L.; Re, F.; Bereczki, E.; Ioja, E.; Gregori, M.; Andersen, A.J.; Anton, M.; Moghimi, S.M.; Pei, J.J.; Masserini, M.; et al. Repeated intraperitoneal injections of liposomes containing phosphatidic acid and cardiolipin reduce amyloid-beta levels in APP/PS1 transgenic mice. Nanomedicine 2015, 11, 421–430. [Google Scholar] [CrossRef]
- Thöle, M.; Nobmann, S.; Huwyler, J.; Bartmann, A.; Fricker, G. Uptake of cationzied albumin coupled liposomes by cultured porcine brain microvessel endothelial cells and intact brain capillaries. J. Drug Target. 2002, 10, 337–344. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Amiji, M.M. A review of nanocarrier-based CNS delivery systems. Curr. Drug Del. 2006, 3, 219–232. [Google Scholar] [CrossRef]
- Cormode, D.P.; Jarzyna, P.A.; Mulder, W.J.M.; Fayad, Z.A. Modified natural nanoparticles as contrast agents for medical imaging. Adv. Drug Del. Rev. 2010, 62, 329–338. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y.; Sun, J.; Han, Y.; Gong, W.; Li, Y.; Feng, Y.; Wang, H.; Yang, M.; Li, Z.; et al. Neuronal mitochondria-targeted delivery of curcumin by biomimetic engineered nanosystems in Alzheimer’s disease mice. Acta Biomater. 2020, 108, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; El Andaloussi, S.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Del. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Morad, G.; Carman, C.V.; Hagedorn, E.J.; Perlin, J.R.; Zon, L.I.; Mustafaoglu, N.; Park, T.E.; Ingber, D.E.; Daisy, C.C.; Moses, M.A. Tumor-derived extracellular vesicles breach the Intact blood-brain barrier via transcytosis. ACS Nano. 2019, 13, 13853–13865. [Google Scholar] [CrossRef] [PubMed]
- Lorca, C.; Laparra, M.; Cespedes, M.V.; Casani, L.; Florit, S.; Jove, M.; Mota-Martorell, N.; Vilella, E.; Gallart-Palau, X.; Serra, A. Industrial by-products as a novel circular source of biocompatible extracellular vesicles. Adv. Funct. Mater. 2022, 32, 2202700. [Google Scholar] [CrossRef]
- Ruoslahti, E. Peptides as targeting elements and tissue penetration devices for nanoparticles. Adv. Mater. 2012, 24, 3747–3756. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.Y.; Liu, R.; Zhang, J.J. Progress of stimuli-responsive nanomaterials in tumor analysis. Chin. J. Anal. Chem. 2021, 49, 1133–1141. [Google Scholar] [CrossRef]
- Kardani, K.; Milani, A.; Shabani, S.H.; Bolhassani, A. Cell penetrating peptides: The potent multi-cargo intracellular carriers. Expert Opin. Drug Deliv. 2019, 16, 1227–1258. [Google Scholar] [CrossRef]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-penetrating peptides: From basic research to clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Ryu, J.; Kim, K.A.; Lee, H.J.; Bahn, J.H.; Han, K.; Choi, E.Y.; Lee, K.S.; Kwon, H.Y.; Choi, S.Y. Mutational analysis of a human immunodeficiency virus type 1 Tat protein transduction domain which is required for delivery of an exogenous protein into mammalian cells. J. Gen. Virol. 2002, 83, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The third helix of the antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994, 269, 10444–10450. [Google Scholar] [CrossRef]
- Tuennemann, G.; Ter-Avetisyan, G.; Martin, R.M.; Stoeckl, M.; Herrmann, A.; Cardoso, C. Live-cell analysis of cell penetration ability and toxicity of oligo-arginines. J. Pept. Sci. 2008, 14, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Crook, Z.R.; Girard, E.; Sevilla, G.P.; Merrill, M.; Friend, D.; Rupert, P.B.; Pakiam, F.; Nguyen, E.; Yin, C.; Ruff, R.O.; et al. A TfR-binding cystine-dense peptide promotes blood-brain barrier penetration of bioactive molecules. J. Mol. Biol. 2020, 432, 3989–4009. [Google Scholar] [CrossRef]
- Arora, S.; Sharma, D.; Singh, J. GLUT-1: An effective target to deliver brain-derived neurotrophic factor gene across the blood brain barrier. ACS Chem. Neurosci. 2020, 11, 1620–1633. [Google Scholar] [CrossRef]
- Dedrick, R.L. Arterial drug infusion: Pharmacokinetic problems and pitfalls. J. Natl. Cancer Inst. 1988, 80, 84–89. [Google Scholar] [CrossRef]
- Cooke, J.N.R.; Ellis, J.A.; Hossain, S.; Nguyen, J.; Bruce, J.N.; Joshi, S. Computational pharmacokinetic rationale for intra-arterial delivery to the brain. Drug Del. Transl. Res. 2016, 6, 622–629. [Google Scholar] [CrossRef]
- Ferreira, N.N.; de Oliveira, E.; Granja, S.; Boni, F.I.; Ferreira, L.M.B.; Cury, B.S.F.; Santos, L.C.R.; Reis, R.M.; Lima, E.M.; Baltazar, F.; et al. Nose-to-brain co-delivery of drugs for glioblastoma treatment using nanostructured system. Int. J. Pharm. 2021, 603, 120714. [Google Scholar] [CrossRef]
- Moradi, F.; Dashti, N. Targeting neuroinflammation by intranasal delivery of nanoparticles in neurological diseases: A comprehensive review. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 133–148. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Holzwarth, U.; Schleh, C.; Kozempel, J.; Wenk, A.; Haberl, N.; Hirn, S.; Schaffler, M.; Lipka, J.; Semmler-Behnke, M.; et al. Quantitative biokinetics of titanium dioxide nanoparticles after oral application in rats: Part 2. Nanotoxicology 2017, 11, 443–453. [Google Scholar] [CrossRef]
- Lim, S.; Park, J.; Shim, M.K.; Um, W.; Yoon, H.Y.; Ryu, J.H.; Lim, D.-K.; Kim, K. Recent advances and challenges of repurposing nanoparticle-based drug delivery systems to enhance cancer immunotherapy. Theranostics 2019, 9, 7906–7923. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.; Andrabi, S.S.; Mansoor, S.; Kuang, Y.; Kwon, B.K.; Labhasetwar, V. Antioxidant therapy in oxidative stress-induced neurodegenerative diseases: Role of nanoparticle-based drug delivery systems in clinical translation. Antioxidants 2022, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.d.S.; Kanekiyo, T.; Singh, J. Nerve growth factor gene delivery across the blood-brain barrier to reduce beta amyloid accumulation in AD mice. Mol. Pharm. 2020, 17, 2054–2063. [Google Scholar] [CrossRef]
- Arora, S.; Layek, B.; Singh, J. Design and validation of liposomal ApoE2 gene delivery system to evade blood-brain barrier for effective treatment of Alzheimer’s disease. Mol. Pharm. 2021, 18, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lindholm, K.; Yang, L.B.; Yue, X.; Citron, M.; Yao, R.Q.; Beach, T.; Sue, L.; Sabbagh, M.; Cai, H.B.; et al. Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer’s disease patients. Proc. Natl. Acad. Sci. USA 2004, 101, 3632–3637. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, F.; Liu, Y.; Zheng, M.; Wang, Y.; Zhang, D.; Anraku, Y.; Zou, Y.; Li, J.; Wu, H.; et al. Blood-brain barrier-penetrating siRNA nanomedicine for Alzheimer’s disease therapy. Sci. Adv. 2020, 6, eabc7031. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, T.; Chen, Q.; Li, C.; Chu, Y.; Guo, Q.; Zhang, Y.; Zhou, W.; Chen, H.; Zhou, Z.; et al. Biomimetic dendrimer-peptide conjugates for early multi-target therapy of Alzheimer’s disease by inflammatory microenvironment modulation. Adv. Mater. 2021, 33, e2100746. [Google Scholar] [CrossRef]
- Blanco, S.; Peralta, S.; Morales, M.E.; Martinez-Lara, E.; Pedrajas, J.R.; Castan, H.; Peinado, M.A.; Ruiz, M.A. Hyaluronate nanoparticles as a delivery system to carry neuroglobin to the brain after stroke. Pharmaceutics 2020, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Khadija, J.F.; Harun-Or-Rashid, M.; Rahaman, M.S.; Nafady, M.H.; Islam, M.R.; Akter, A.; Emran, T.B.; Wilairatana, P.; Mubarak, M.S. Bioactive compounds and their derivatives: An insight into prospective phytotherapeutic approach against Alzheimer’s disease. Oxid. Med. Cell. Longev. 2022, 2022, 5100904. [Google Scholar] [CrossRef]
- Soares, T.B.; Loureiro, L.; Carvalho, A.; Real Oliveira, M.E.C.D.; Dias, A.; Sarmento, B.; Lucio, M. Lipid nanocarriers loaded with natural compounds: Potential new therapies for age related neurodegenerative diseases? Prog. Neurobiol. 2018, 168, 21–41. [Google Scholar] [CrossRef]
- Liu, H.; Han, Y.; Wang, T.; Zhang, H.; Xu, Q.; Yuan, J.; Li, Z. Targeting microglia for therapy of Parkinson’s disease by using biomimetic ultrasmall nanoparticles. J. Am. Chem. Soc. 2020, 142, 21730–21742. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Yin, T.; Gong, Y.; Yuan, G.; Chen, L.; Liu, J. Quercetin-modified gold-palladium nanoparticles as a potential autophagy inducer for the treatment of Alzheimer’s disease. J. Colloid Interface Sci. 2019, 552, 388–400. [Google Scholar] [CrossRef]
- Yang, R.; Zheng, Y.; Wang, Q.J.; Zhao, L. Curcumin-loaded chitosan-bovine serum albumin nanoparticles potentially enhanced A beta 42 phagocytosis and modulated macrophage polarization in Alzheimer’s disease. Nanoscale Res. Lett. 2018, 13, 330. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, N.; Zheng, G.; Yang, L. Oral administration of resveratrol-selenium-peptide nanocomposites alleviates Alzheimer’s disease-like pathogenesis by Inhibiting Abeta aggregation and regulating gut microbiota. ACS Appl. Mater. Interfaces 2021, 13, 46406–46420. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.A.; Andrade, S.; Duarte, A.; Neves, A.R.; Queiroz, J.F.; Nunes, C.; Sevin, E.; Fenart, L.; Gosselet, F.; Coelho, M.A.; et al. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules 2017, 22, 277. [Google Scholar] [CrossRef] [PubMed]
- Fornaguera, C.; Feiner-Gracia, N.; Caldero, G.; Garcia-Celma, M.J.; Solans, C. Galantamine-loaded PLGA nanoparticles, from nano-emulsion templating, as novel advanced drug delivery systems to treat neurodegenerative diseases. Nanoscale 2015, 7, 12076–12084. [Google Scholar] [CrossRef]
- Carbone, M.; Duty, S.; Rattray, M. Riluzole neuroprotection in a Parkinson’s disease model involves suppression of reactive astrocytosis but not GLT-1 regulation. BMC Neurosci. 2012, 13, 38. [Google Scholar] [CrossRef]
- Fazil, M.; Shadab; Baboota, S.; Sahni, J.K.; Ali, J. Nanotherapeutics for Alzheimer’s disease (AD): Past, present and future. J. Drug Target. 2012, 20, 97–113. [Google Scholar] [CrossRef]
- Benjamin, B.; Burns, A. Donepezil for Alzheimer’s disease. Expert Rev. Neurother. 2007, 7, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.J.; Mao, S.Y.; Zhang, Q.F.; Yu, H.Y.; Li, Y.; He, X.L.; Yang, S.Y.; Zhang, Z.R.; Yi, Z.Q.; Song, Y.J.; et al. Brain-targeted polysorbate 80-emulsified donepezil drug-loaded nanoparticles for neuroprotection. Nanoscale Res. Lett. 2021, 16, 132. [Google Scholar] [CrossRef] [PubMed]
- Baysal, I.; Ucar, G.; Gultekinoglu, M.; Ulubayram, K.; Yabanoglu-Ciftci, S. Donepezil loaded PLGA-b-PEG nanoparticles: Their ability to induce destabilization of amyloid fibrils and to cross blood brain barrier in vitro. J. Neural. Transm. 2017, 124, 33–45. [Google Scholar] [CrossRef]
- Matsunaga, S.; Kishi, T.; Iwata, N. Memantine monotherapy for Alzheimer’s disease: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0123289. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Cano, A.; Calpena, A.C.; Camins, A.; Carmona, N.; Silva, A.M.; Souto, E.B.; et al. Memantine loaded PLGA PEGylated nanoparticles for Alzheimer’s disease: In vitro and in vivo characterization. J. Nanobiotechnol. 2018, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, V.; Gaud, R.; Bajaj, A.; Wairkar, S. Pharmacokinetics and pharmacodynamics of intranasally administered selegiline nanoparticles with improved brain delivery in Parkinson’s disease. Nanomedicine. 2018, 14, 2609–2618. [Google Scholar] [CrossRef]
- Verma, S.K.; Arora, I.; Javed, K.; Akhtar, M.; Samim, M. Enhancement in the neuroprotective power of riluzole against cerebral ischemia using a brain targeted drug delivery vehicle. ACS Appl. Mater. Interfaces 2016, 8, 19716–19723. [Google Scholar] [CrossRef]
- Sharma, M.; Tiwari, V.; Chaturvedi, S.; Wahajuddin, M.; Shukla, S.; Panda, J.J. Self-fluorescent lone tryptophan nanoparticles as theranostic agents against Alzheimer’s disease. ACS Appl. Mater. Interfaces 2022, 14, 13079–13093. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, R.; Zhang, D.; Zhang, Z.; Zhu, J.; Xu, L.; Guo, Y. Neuroprotective effects of maize tetrapeptide-anchored gold nanoparticles in Alzheimer’s disease. Colloids Surf. B Biointerfaces 2021, 200, 111584. [Google Scholar] [CrossRef]
- Zhang, X.J.; Li, Y.Y.; Hu, Y.N. Green synthesis of silver nanoparticles and their preventive effect in deficits in recognition and spatial memory in sporadic Alzheimer’s rat model. Colloids Surf A 2020, 605, 125288. [Google Scholar] [CrossRef]
- Guo, X.; Lie, Q.; Liu, Y.; Jia, Z.; Gong, Y.; Yuan, X.; Liu, J. Multifunctional selenium quantum dots for the treatment of Alzheimer’s disease by reducing Abeta-neurotoxicity and oxidative stress and alleviate neuroinflammation. ACS Appl. Mater. Interfaces 2021, 13, 30261–30273. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Jin, L.; Wu, Z.; Xie, Y.; Zhang, P.; Wang, Q.; Yan, S.; Chen, B.; Liang, H.; Naman, C.B.; et al. PLGA-PEG nanoparticles facilitate in vivo anti-Alzheimer’s effects of fucoxanthin, a marine carotenoid derived from edible brown algae. J. Agric. Food Chem. 2021, 69, 9764–9777. [Google Scholar] [CrossRef] [PubMed]
- Corneo, E.D.; Silveira, G.D.; Scussel, R.; Correa, M.; Abel, J.D.; Luiz, G.P.; Feuser, P.E.; Silveira, P.C.L.; Machado-de-Avila, R.A. Effects of gold nanoparticles administration through behavioral and oxidative parameters in animal model of Parkinson’s disease. Colloids Surf. B Biointerfaces 2020, 196, 111302. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Qu, A.; Wang, W.; Lu, M.; Shi, B.; Chen, C.; Hao, C.; Xu, L.; Sun, M.; Xu, C.; et al. Facet-dependent biodegradable Mn3O4 nanoparticles for ameliorating Parkinson’s disease. Adv. Healthc. Mater. 2021, 10, 2101316. [Google Scholar] [CrossRef]
- Chen, T.; Liu, W.; Xiong, S.; Li, D.; Fang, S.; Wu, Z.; Wang, Q.; Chen, X. Nanoparticles mediating the sustained puerarin release facilitate improved brain delivery to treat Parkinson’s disease. ACS Appl. Mater. Interfaces 2019, 11, 45276–45289. [Google Scholar] [CrossRef] [PubMed]
- Rusiecka, I.; Ruczynski, J.; Kozlowska, A.; Backtrog, E.; Mucha, P.; Kocic, I.; Rekowski, P. TP10-dopamine conjugate as a potential therapeutic agent in the treatment of Parkinson’s disease. Bioconjug. Chem. 2019, 30, 760–774. [Google Scholar] [CrossRef]
- Wahyuningtyas, D.; Chen, W.-H.; He, R.-Y.; Huang, Y.-A.; Tsao, C.-K.; He, Y.-J.; Yu, C.-Y.; Lu, P.-C.; Chen, Y.-C.; Wang, S.-H.; et al. Polyglutamine-specific gold nanoparticle complex alleviates mutant huntingtin-induced toxicity. ACS Appl. Mater. Interfaces 2021, 13, 60894–60906. [Google Scholar] [CrossRef]
- Cong, W.; Bai, R.; Li, Y.F.; Wang, L.; Chen, C. Selenium nanoparticles as an efficient nanomedicine for the therapy of Huntington’s disease. ACS Appl. Mater. Interfaces 2019, 11, 34725–34735. [Google Scholar] [CrossRef]
- Birolini, G.; Valenza, M.; Ottonelli, I.; Passoni, A.; Favagrossa, M.; Duskey, J.T.; Bombaci, M.; Vandelli, M.A.; Colombo, L.; Bagnati, R.; et al. Insights into kinetics, release, and behavioral effects of brain-targeted hybrid nanoparticles for cholesterol delivery in Huntington’s disease. J. Control. Release 2021, 330, 587–598. [Google Scholar] [CrossRef]
- Marcuzzo, S.; Isaia, D.; Bonanno, S.; Malacarne, C.; Cavalcante, P.; Zacheo, A.; Laquintana, V.; Denora, N.; Sanavio, B.; Salvati, E.; et al. FM19G11-Loaded gold nanoparticles enhance the proliferation and self-renewal of ependymal stem progenitor cells derived from ALS mice. Cells 2019, 8, 279. [Google Scholar] [CrossRef]
- Leyton-Jaimes, M.F.; Ivert, P.; Hoeber, J.; Han, Y.; Feiler, A.; Zhou, C.; Pankratova, S.; Shoshan-Barmatz, V.; Israelson, A.; Kozlova, E.N. Empty mesoporous silica particles significantly delay disease progression and extend survival in a mouse model of ALS. Sci. Rep. 2020, 10, 20675. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.X.; Chung, E.P.; Teague, C.D.; Bowser, R.; Sirianni, R.W. Intravenously administered, retinoid activating nanoparticles increase lifespan and reduce neurodegeneration in the SOD1(G93A) mouse model of ALS. Front. Bioeng. Biotechnol. 2020, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Bertoni, H.; Kozielski, K.L.; Rui, Y.; Lal, B.; Vaughan, H.; Wilson, D.R.; Mihelson, N.; Eberhart, C.G.; Laterra, J.; Green, J.J. Bioreducible polymeric nanoparticles containing multiplexed cancer stem cell regulating miRNAs inhibit glioblastoma growth and prolong survival. Nano Lett. 2018, 18, 4086–4094. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chetelat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Briggs, R.; Kennelly, S.P.; O’Neill, D. Drug treatments in Alzheimer’s disease. Clin. Med. 2016, 16, 247–253. [Google Scholar] [CrossRef]
- Joe, E.; Ringman, J.M. Cognitive symptoms of Alzheimer’s disease: Clinical management and prevention. BMJ 2019, 367, l6217. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Grundkeiqbal, I.; Iqbal, K.; Tung, Y.C.; Quinlan, M.; Wisniewski, H.M.; Binder, L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA 1986, 83, 4913–4917. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Fleming, A.; Bourdenx, M.; Fujimaki, M.; Karabiyik, C.; Krause, G.J.; Lopez, A.; Puri, C.; Scrivo, A.; Skidmore, J.; Son, S.M.; et al. The different autophagy degradation pathways and neurodegeneration. Neuron 2022, 110, 935–966. [Google Scholar] [CrossRef]
- Fang, E.F.; Hou, Y.J.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.M.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X.L.; et al. Mitophagy inhibits amyloid-beta and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 2019, 22, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.P.; Ferreira, G.K.; Pires, A.J.; de Bem Silveira, G.; de Souza, D.L.; Brandolfi, J.A.; de Souza, C.T.; Paula, M.M.S.; Silveira, P.C.L. Gold nanoparticles prevent cognitive deficits, oxidative stress and inflammation in a rat model of sporadic dementia of Alzheimer’s type. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, D.; Aarsland, D.; Chaudhuri, K.R.; Dobkin, R.D.; Leentjens, A.F.G.; Rodriguez-Violante, M.; Schrag, A. The neuropsychiatry of Parkinson’s disease: Advances and challenges. Lancet Neurol. 2022, 21, 89–102. [Google Scholar] [CrossRef]
- Elkouzi, A.; Vedam-Mai, V.; Eisinger, R.S.; Okun, M.S. Emerging therapies in Parkinson disease—Repurposed drugs and new approaches. Nat. Rev. Neurol. 2019, 15, 204–223. [Google Scholar] [CrossRef]
- Charvin, D.; Medori, R.; Hauser, R.A.; Rascol, O. Therapeutic strategies for Parkinson disease: Beyond dopaminergic drugs. Nat. Rev. Drug Discov. 2018, 17, 804–822. [Google Scholar] [CrossRef] [PubMed]
- Zand, Z.; Khaki, P.A.; Salihi, A.; Sharifi, M.; Nanakali, N.M.Q.; Alasady, A.A.B.; Aziz, F.M.; Shahpasand, K.; Hasan, A.; Falahati, M. Cerium oxide NPs mitigate the amyloid formation of alpha-synuclein and associated cytotoxicity. Int. J. Nanomed. 2019, 14, 6989–7000. [Google Scholar] [CrossRef] [PubMed]
- Ruotolo, R.; De Giorgio, G.; Minato, I.; Bianchi, M.G.; Bussolati, O.; Marmiroli, N. Cerium oxide nanoparticles rescue alpha-synuclein-induced toxicity in a yeast model of Parkinson’s disease. Nanomaterials 2020, 10, 235. [Google Scholar] [CrossRef]
- Ahlawat, J.; Neupane, R.; Deemer, E.; Sreenivasan, S.T.; Narayan, M. Chitosan-ellagic acid nanohybrid for mitigating rotenone-induced oxidative stress. ACS Appl. Mater. Interfaces 2020, 12, 18964–18977. [Google Scholar] [CrossRef]
- Vonsattel, J.P.G.; Keller, C.; Cortes Ramirez, E.P. Huntington’s disease—Neuropathology. Handb. Clin. Neurol. 2011, 100, 83–100. [Google Scholar] [CrossRef]
- Claassen, D.O.; Carroll, B.; De Boer, L.M.; Wu, E.; Ayyagari, R.; Gandhi, S.; Stamler, D. Indirect tolerability comparison of deutetrabenazine and tetrabenazine for Huntington disease. J. Clin. Mov. Disord. 2017, 4, 3. [Google Scholar] [CrossRef]
- Debnath, K.; Pradhan, N.; Singh, B.K.; Jana, N.R.; Jana, N.R. Poly(trehalose) nanoparticles prevent amyloid aggregation and suppress polyglutamine aggregation in a Huntington’s disease model mouse. ACS Appl. Mater. Interfaces 2017, 9, 24126–24139. [Google Scholar] [CrossRef] [PubMed]
- Debnath, K.; Shekhar, S.; Kumar, V.; Jana, N.R.; Jana, N.R. Efficient Inhibition of protein aggregation, disintegration of aggregates, and lowering of cytotoxicity by green tea polyphenol-based self-assembled polymer nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 20309–20318. [Google Scholar] [CrossRef] [PubMed]
- van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; Van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef] [PubMed]
- Goutman, S.A.; Hardiman, O.; Al-Chalabi, A.; Chio, A.; Savelieff, M.G.; Kiernan, M.C.; Feldman, E.L. Emerging insights into the complex genetics and pathophysiology of amyotrophic lateral sclerosis. Lancet Neurol. 2022, 21, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, M.C.; Vucic, S.; Talbot, K.; McDermott, C.J.; Hardiman, O.; Shefner, J.M.; Al-Chalabi, A.; Huynh, W.; Cudkowicz, M.; Talman, P.; et al. Improving clinical trial outcomes in amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2021, 17, 104–118. [Google Scholar] [CrossRef]
- DeCoteau, W.; Heckman, K.L.; Estevez, A.Y.; Reed, K.J.; Costanzo, W.; Sandford, D.; Studlack, P.; Clauss, J.; Nichols, E.; Lipps, J.; et al. Cerium oxide nanoparticles with antioxidant properties ameliorate strength and prolong life in mouse model of amyotrophic lateral sclerosis. Nanomedicine 2016, 12, 2311–2320. [Google Scholar] [CrossRef]
- Maden, M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat. Rev. Neurosci. 2007, 8, 755–765. [Google Scholar] [CrossRef]
- Teixeira, M.I.; Lopes, C.M.; Goncalves, H.; Catita, J.; Silva, A.M.; Rodrigues, F.; Amaral, M.H.; Costa, P.C. Formulation, characterization, and cytotoxicity evaluation of lactoferrin functionalized lipid nanoparticles for riluzole delivery to the brain. Pharmaceutics 2022, 14, 185. [Google Scholar] [CrossRef]
- Boeve, B.F.; Boxer, A.L.; Kumfor, F.; Pijnenburg, Y.; Rohrer, J.D. Advances and controversies in frontotemporal dementia: Diagnosis, biomarkers, and therapeutic considerations. Lancet Neurol. 2022, 21, 258–272. [Google Scholar] [CrossRef]
- Waris, A.; Ali, A.; Khan, A.U.; Asim, M.; Zamel, D.; Fatima, K.; Raziq, A.; Khan, M.A.; Akbar, N.; Baset, A.; et al. Applications of various types of nanomaterials for the treatment of neurological disorders. Nanomaterials 2022, 12, 2140. [Google Scholar] [CrossRef]
- McKinnon, C.; Nandhabalan, M.; Murray, S.A.; Plaha, P. Glioblastoma: Clinical presentation, diagnosis, and management. BMJ 2021, 374, n1560. [Google Scholar] [CrossRef] [PubMed]
- Binyamin, O.; Larush, L.; Frid, K.; Keller, G.; Friedman-Levi, Y.; Ovadia, H.; Abramsky, O.; Magdassi, S.; Gabizon, R. Treatment of a multiple sclerosis animal model by a novel nanodrop formulation of a natural antioxidant. Int. J. Nanomed. 2015, 10, 7165–7174. [Google Scholar] [CrossRef] [PubMed]
- El Moustaine, D.; Perrier, V.; Smeller, L.; Lange, R.; Torrent, J. Full-length prion protein aggregates to amyloid fibrils and spherical particles by distinct pathways. FEBS J. 2008, 275, 2021–2031. [Google Scholar] [CrossRef]
- Lin, T.T.; Zhao, P.F.; Jiang, Y.F.; Tang, Y.S.; Jin, H.Y.; Pan, Z.Z.; He, H.N.; Yang, V.C.; Huang, Y.Z. Blood-brain-barrier-penetrating albumin nanoparticles for biomimetic drug delivery via albumin-binding protein pathways for antiglioma therapy. ACS Nano 2016, 10, 9999–10012. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Shi, Z.Y.; Liu, R.Y.; Wu, Y.Y.; Zhang, X. Combined-therapeutic strategies synergistically potentiate glioblastoma multiforme treatment via nanotechnology. Theranostics 2020, 10, 3223–3239. [Google Scholar] [CrossRef]
- Kuang, J.; Song, W.; Yin, J.; Zeng, X.; Han, S.; Zhao, Y.P.; Tao, J.; Liu, C.J.; He, X.H.; Zhang, X.Z. iRGD modified chemo-immunotherapeutic nanoparticles for enhanced immunotherapy against glioblastoma. Adv. Funct. Mater. 2018, 28, 1800025. [Google Scholar] [CrossRef]
- Yu, G.C.; Yu, S.; Saha, M.L.; Zhou, J.; Cook, T.R.; Yung, B.C.; Chen, J.; Mao, Z.W.; Zhang, F.W.; Zhou, Z.J.; et al. A discrete organoplatinum(II) metallacage as a multimodality theranostic platform for cancer photochemotherapy. Nat. Commun. 2018, 9, 4335. [Google Scholar] [CrossRef] [PubMed]
- Poon, C.; McMahon, D.; Hynynen, K. Noninvasive and targeted delivery of therapeutics to the brain using focused ultrasound. Neuropharmacology 2017, 120, 20–37. [Google Scholar] [CrossRef]
- Karakatsani, M.E.; Blesa, J.; Konofagou, E.E. Blood-brain barrier opening with focused ultrasound in experimental models of Parkinson’s disease. Mov. Disord. 2019, 34, 1252–1261. [Google Scholar] [CrossRef]
- Mead, B.P.; Kim, N.; Miller, G.W.; Hodges, D.; Mastorakos, P.; Klibanov, A.L.; Mandell, J.W.; Hirsh, J.; Suk, J.S.; Hanes, J.; et al. Novel focused ultrasound gene therapy approach noninvasively restores dopaminergic neuron function in a rat Parkinson’s disease model. Nano Lett. 2017, 17, 3533–3542. [Google Scholar] [CrossRef]
- Vucic, S.; Kiernan, M.C.; Menon, P.; Huynh, W.; Rynders, A.; Ho, K.S.; Glanzman, R.; Hotchkin, M.T. Study protocol of RESCUE-ALS: A Phase 2, randomised, double-blind, placebo-controlled study in early symptomatic amyotrophic lateral sclerosis patients to assess bioenergetic catalysis with CNM-Au8 as a mechanism to slow disease progression. BMJ Open 2021, 11, e041479. [Google Scholar] [CrossRef] [PubMed]
- Thivat, E.; Casile, M.; Moreau, J.; Molnar, I.; Dufort, S.; Seddik, K.; Le Duc, G.; De Beaumont, O.; Loeffler, M.; Durando, X.; et al. Phase I/II study testing the combination of AGuIX nanoparticles with radiochemotherapy and concomitant temozolomide in patients with newly diagnosed glioblastoma (NANO-GBM trial protocol). BMC Cancer 2023, 23, 344. [Google Scholar] [CrossRef] [PubMed]
- Olaru, D.G.; Olaru, A.; Kassem, G.H.; Popescu-Driga, M.V.; Pinosano, L.R.; Dumitrascu, D.I.; Popescu, E.L.; Hermann, D.M.; Popa-Wagner, A. Toxicity and health impact of nanoparticles. Basic biology and clinical perspective. Rom. J. Morphol. Embryol. 2019, 60, 787–792. [Google Scholar] [PubMed]
- Bencsik, A.; Lestaevel, P.; Canu, I.G. Nano- and neurotoxicology: An emerging discipline. Prog. Neurobiol. 2018, 160, 45–63. [Google Scholar] [CrossRef]
- Wen, M.M.; El-Salamouni, N.S.; El-Refaie, W.M.; Hazzah, H.A.; Ali, M.M.; Tosi, G.; Farid, R.M.; Blanco-Prieto, M.J.; Billa, N.; Hanafy, A.S. Nanotechnology-based drug delivery systems for Alzheimer’s disease management: Technical, industrial, and clinical challenges. J. Control. Release 2017, 245, 95–107. [Google Scholar] [CrossRef]


| Nanomaterials | Administration Route | Animal Models | Effectiveness | Ref. |
|---|---|---|---|---|
| Self-fluorescent solo tryptophan nanoparticles | Intraventricular administration | Streptozotocin induced AD model rat | Attenuates cognitive deficits and inhibits Aβ42 oligomerization | [98] |
| Gold nanoparticles | Subcutaneous administration | D-galactose and aluminum chloride induced AD model mice | Modulate animal behavior, oxidative stress, neurotransmitter levels, and cholinergic system | [99] |
| Silver nanoparticles | Intraventricular administration | Streptozotocin induced AD model rat | Prevent recognition and spatial memory impairment | [100] |
| Selenium quantum dots | Intravenous administration | Aβ1–42 induced AD model mice | Inhibits Aβ aggregation, and reduces oxidative stress | [101] |
| Magnetite/ceria nanoparticles | Intravenous administration | 5×FAD transgenic AD model mice | Reduce Aβ levels, and prevent memory deficits | [40] |
| PLGA-PEG nanoparticles | Intravenous administration | Aβ oligomers induced AD model mice | Prevention of cognitive impairment induced by Aβ | [102] |
| Gold nanoparticles | Intraperitoneal administration | Alkaline reserpine induced PD model mice | Reduce secondary neurodegenerative processes and neuronal cell death | [103] |
| Mn3O4 nanoparticles | Striatum administration | MPTP-induced PD model mice | Decrease the content of α-syn in cerebrospinal fluid | [104] |
| Six-armed star-shaped PLGA nanoparticles | Oral administration | MPTP-induced PD model mice | Reduce dopamine depletion in MPTP-mediated neurotoxicity in mice | [105] |
| TP10-dopamine nanoparticles | Intravenous administration | MPTP-induced PD model mice | High affinity for dopamine D1 and D2 receptors and obvious resistance to PD activity | [106] |
| Gold nanoparticles | - | HD fruit fly larva model | Improved motor performance and longevity | [107] |
| Selenium nanoparticles | - | HD Caenorhabditis elegans model | Reduces neuron death, and alleviates oxidative stress | [108] |
| PLGA/cholesterol nanoparticles | Intraperitoneal administration | R6/2 transgenic HD model mice | Enhance biosynthesis of endogenous cholesterol, prevented cognitive decline | [109] |
| Gold nanoparticles | - | SOD1-G93A transgenic ALS model mice | Promote the self-renewal and proliferation of epSPC | [110] |
| Silica nanoparticles | Intrathecal administration | SOD1-G93A transgenic ALS model mice | Delay disease progression and increased survival in mice | [111] |
| PLA-PEG nanoparticles | Intravenous administration | SOD1-G93A transgenic ALS model mice | Improve motor performance and longevity | [112] |
| Poly(beta-amino ester) nanoparticles | Intratumoral administration | Mice with GBM1A glioma xenograft | Inhibit glioblastoma growth and prolong survival | [113] |
| Status | Study Title | Conditions | Interventions | Phase | Identifier |
|---|---|---|---|---|---|
| Not yet recruiting | Study of APH-1105 in patients with mild to moderate Alzheimer’s disease | Dementia Alzheimer Disease 1 Alzheimer Disease 2 Alzheimer Disease 3 | Drug: APH-1105 Other: Placebo | Phase 2 | NCT03806478 |
| Completed | 31P-MRS imaging to assess the effects of CNM-Au8 on impaired neuronal redox state in Parkinson’s disease (REPAIR-PD) | Parkinson’s Disease | Drug: Gold Nanocrystals | Phase 2 | NCT03815916 |
| Completed | Exploratory study using nanotechnology to Detect Biomarkers of Parkinson’s Disease From Exhaled Breath | Parkinson’s Disease Parkinsonism | Other: collection of exhaled breath | - | NCT01246336 |
| Completed | Therapeutic nanocatalysis to slow disease progression of Amyotrophic Lateral Sclerosis (ALS) (RESCUE-ALS) | Amyotrophic Lateral Sclerosis | Drug: CNM-Au8 Drug: Placebo | Phase 2 | NCT04098406 [151] |
| Withdrawn (study execution discontinued at this time) | 31P-MRS imaging to assess the effects of CNM-Au8 on impaired neuronal redox state in Amyotrophic Lateral Sclerosis (REPAIR-ALS) (REPAIR-ALS) | Amyotrophic Lateral Sclerosis | Drug: Gold Nanocrystals | Phase 2 | NCT03843710 |
| Completed | A Phase I trial of nanoliposomal CPT-11 (NL CPT-11) in patients with recurrent high-grade gliomas | Glioblastoma Gliosarcoma Anaplastic Astrocytoma Anaplastic Oligodendroglioma | Drug: Nanoliposomal CPT-11 | Phase 1 | NCT00734682 |
| Recruiting | AGuIX nanoparticles with radiotherapy plus concomitant Temozolomide in the treatment of newly diagnosed glioblastoma (NANO-GBM) | Glioblastoma | Drug: Polysiloxane Gd-Chelates based nanoparticles (AGuIX) Radiation: radiotherapy Drug: Temozolomide | Phase 1 Phase 2 | NCT04881032 [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, L.; Li, X.; Ji, R.; Hao, Z.; Kong, M.; Wen, X.; Guan, F.; Ma, S. Nanoparticle-Based Drug Delivery Systems: An Inspiring Therapeutic Strategy for Neurodegenerative Diseases. Polymers 2023, 15, 2196. https://doi.org/10.3390/polym15092196
Duan L, Li X, Ji R, Hao Z, Kong M, Wen X, Guan F, Ma S. Nanoparticle-Based Drug Delivery Systems: An Inspiring Therapeutic Strategy for Neurodegenerative Diseases. Polymers. 2023; 15(9):2196. https://doi.org/10.3390/polym15092196
Chicago/Turabian StyleDuan, Linyan, Xingfan Li, Rong Ji, Zhizhong Hao, Mingyue Kong, Xuejun Wen, Fangxia Guan, and Shanshan Ma. 2023. "Nanoparticle-Based Drug Delivery Systems: An Inspiring Therapeutic Strategy for Neurodegenerative Diseases" Polymers 15, no. 9: 2196. https://doi.org/10.3390/polym15092196
APA StyleDuan, L., Li, X., Ji, R., Hao, Z., Kong, M., Wen, X., Guan, F., & Ma, S. (2023). Nanoparticle-Based Drug Delivery Systems: An Inspiring Therapeutic Strategy for Neurodegenerative Diseases. Polymers, 15(9), 2196. https://doi.org/10.3390/polym15092196







