The Rejuvenating Potential of Plasticizers on Oxidatively Aged Asphalts: Rheological and Molecular Dynamics Perspectives
Abstract
:1. Introduction
2. Materials and Rheological Evaluation Methods
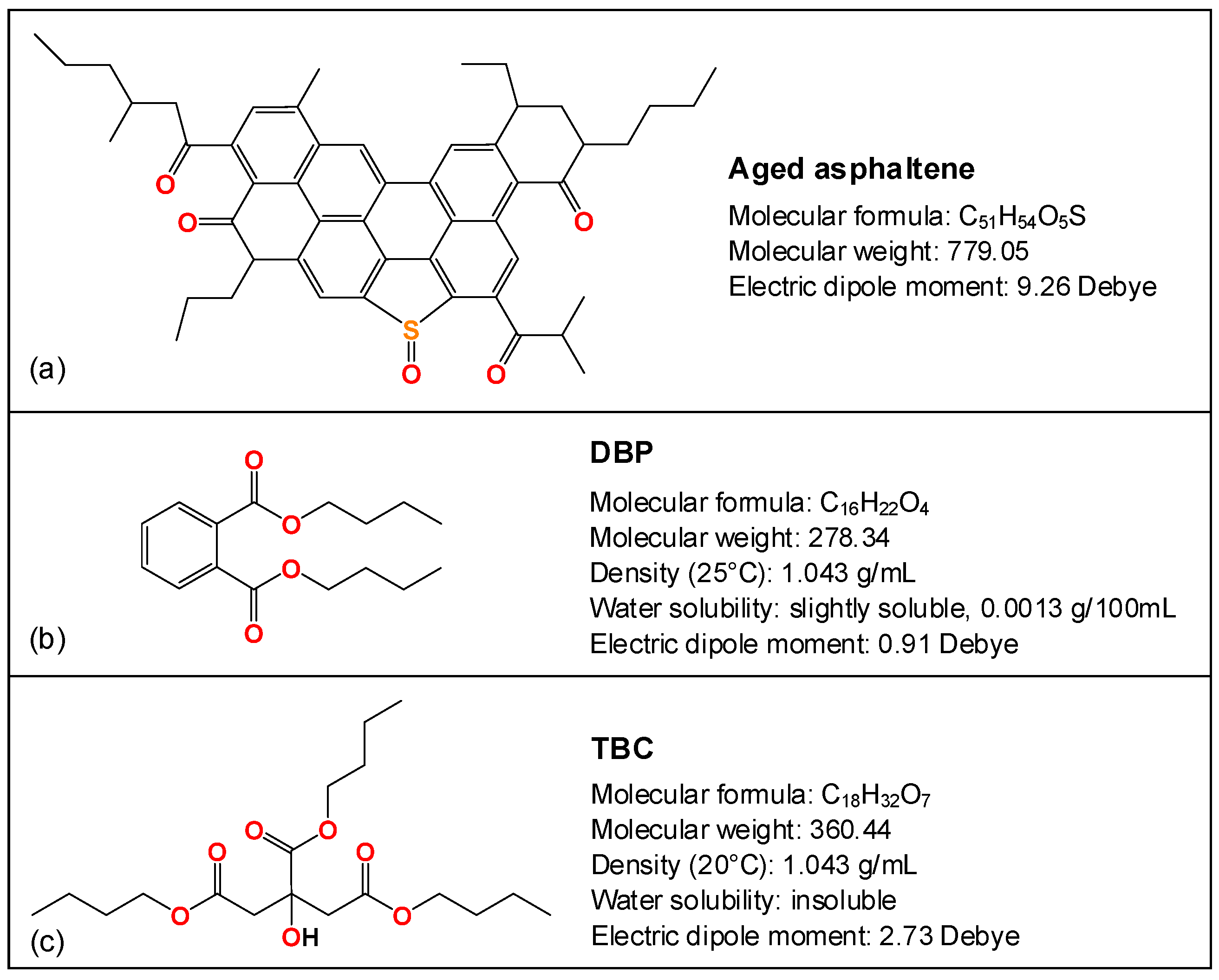
2.1. Materials
2.2. Rheological Characterization
2.2.1. Linear Viscoelastic Properties
2.2.2. Fatigue Performance
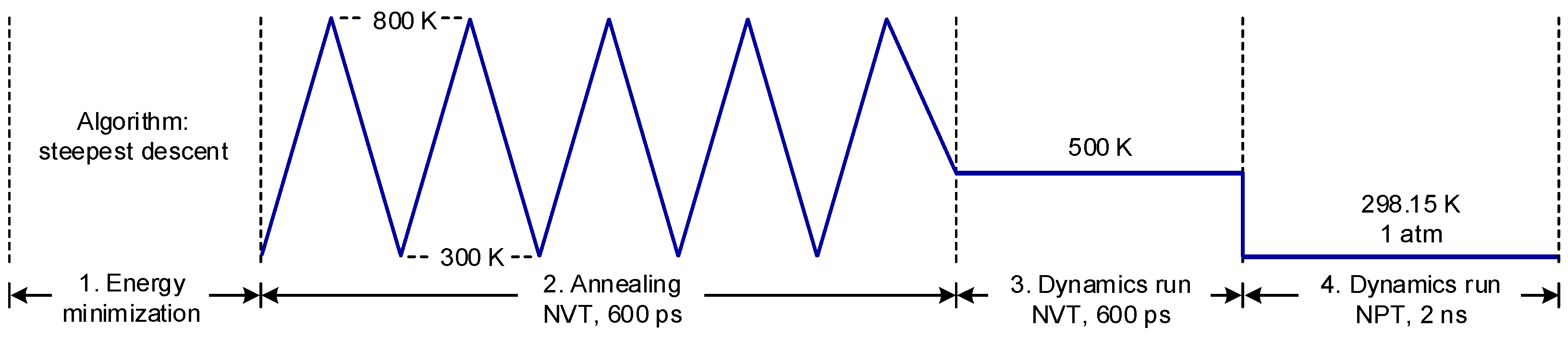
3. Molecular Dynamics Simulation Approach
- Step 1:
- Energy minimization, using the steepest descent algorithm with the maximum number of steps set to 50,000;
- Step 2:
- Annealing, following a triangular temperature profile covering the range from 300 to 800 K. The ending temperature was set at 500 K, the same temperature used for the subsequent dynamics run. The canonical NVT ensemble (constant value for the number of particles, volume, and temperature) was used for this stage;
- Step 3:
- Dynamics run, for a period of 600 ps under 500 K using the NVT ensemble;
- Step 4:
- Dynamics run, for a period of 2 ns to equilibrate the system under the room temperature and atmospheric pressure using the NPT ensemble (constant value for the number of particles, pressure, and temperature). The densities of all the molecular systems evaluated were found to stabilize within 600 ps.
4. Results and Discussions
4.1. Rheological Performance
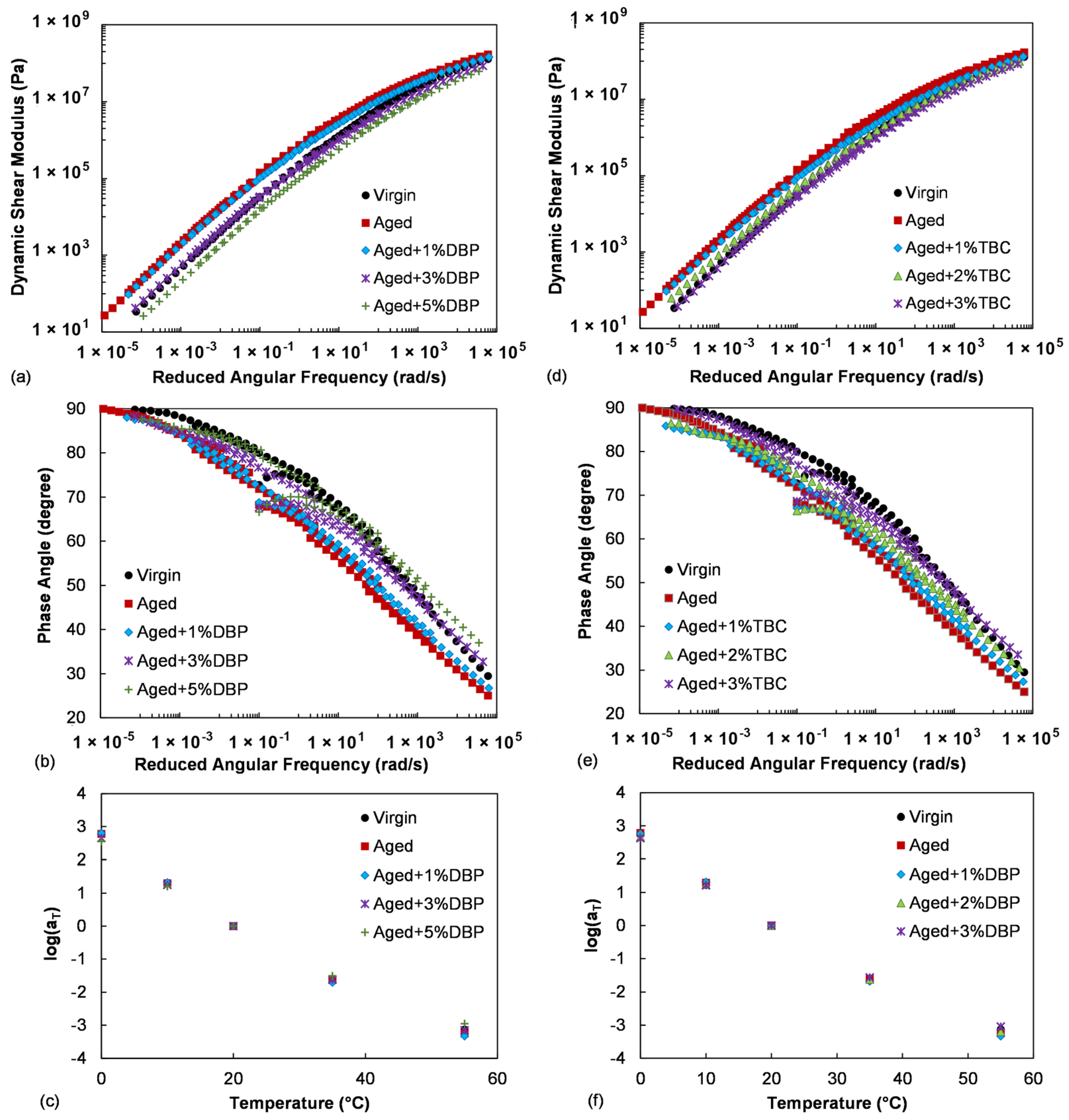
4.1.1. Linear Viscoelastic Characteristics
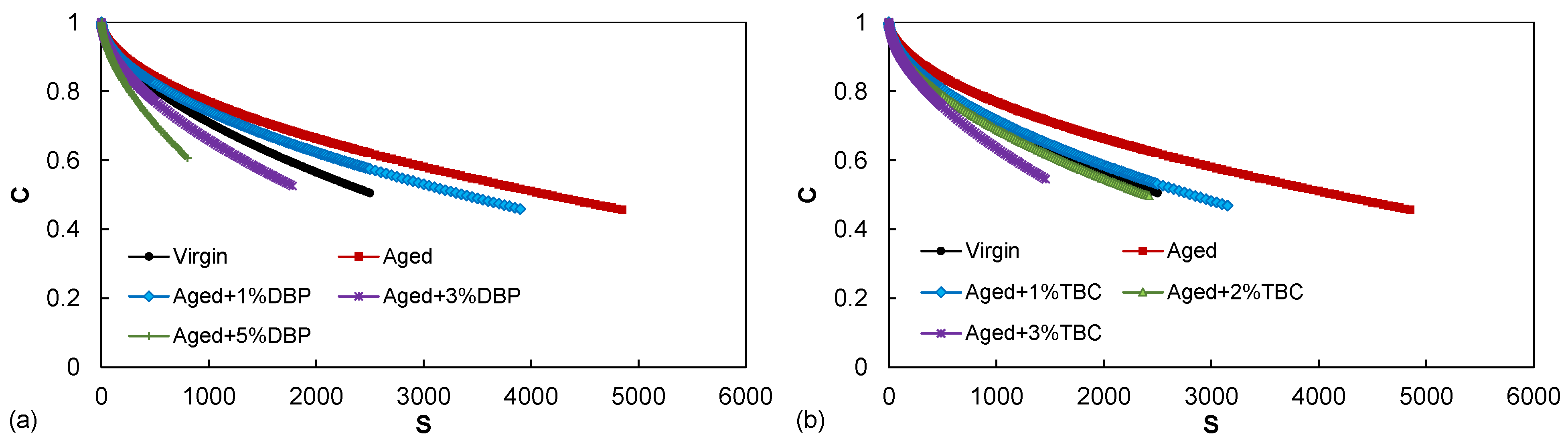
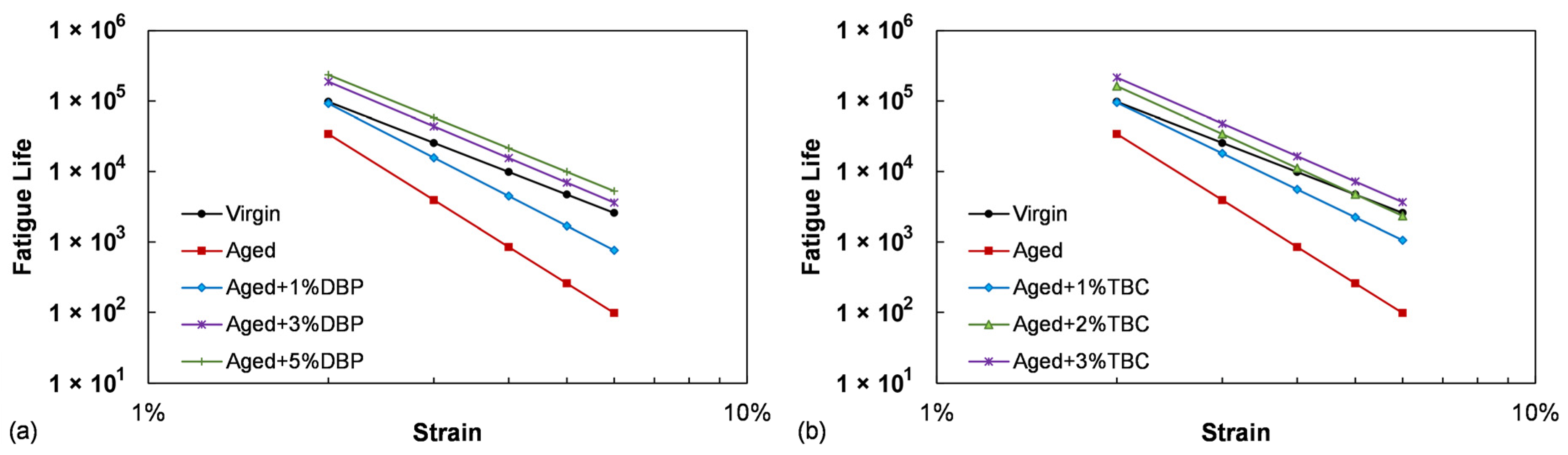
4.1.2. Fatigue Performance
4.2. MD Simulation Results
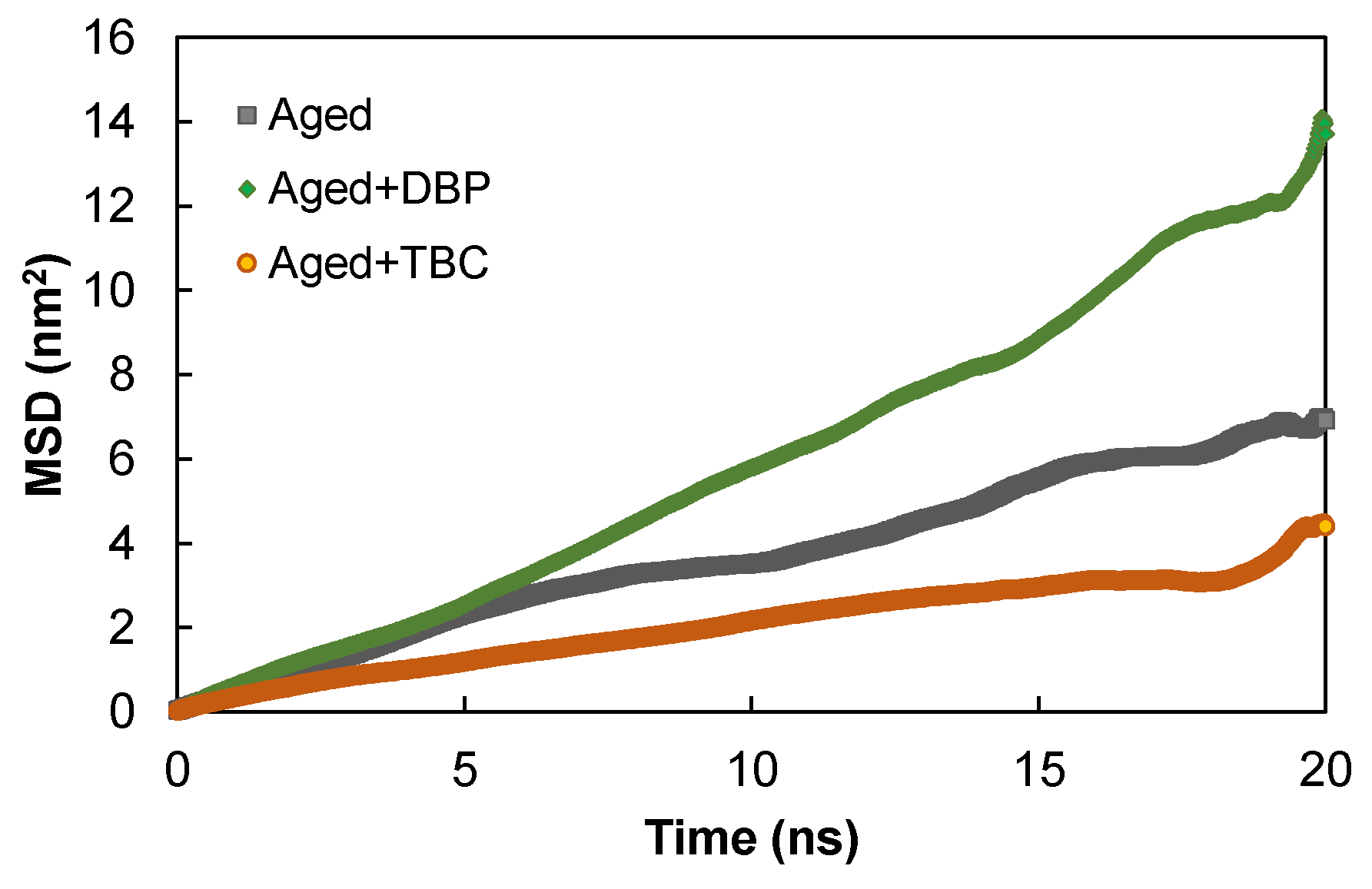
4.2.1. Mean Squared Displacement
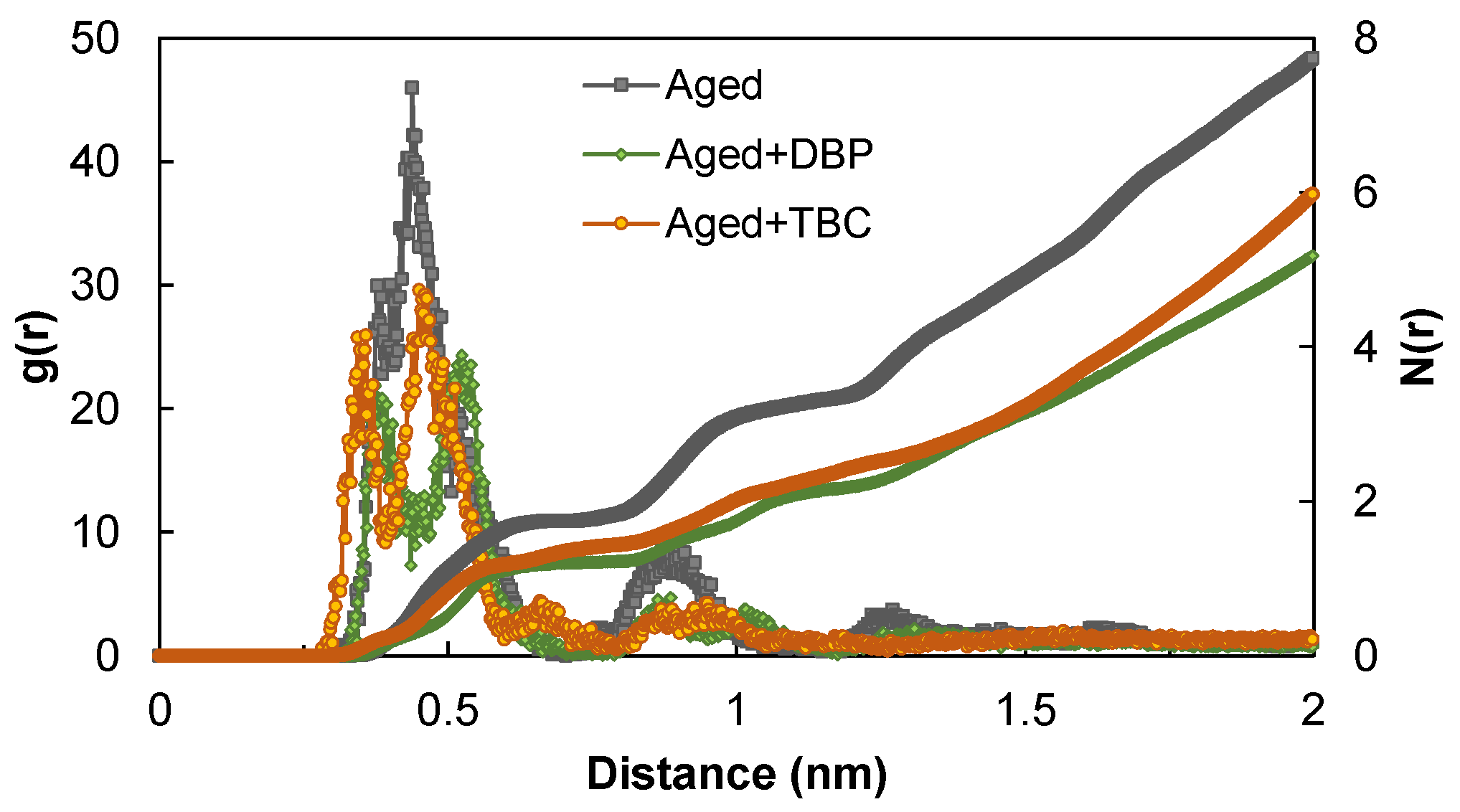
4.2.2. Radial Distribution Function
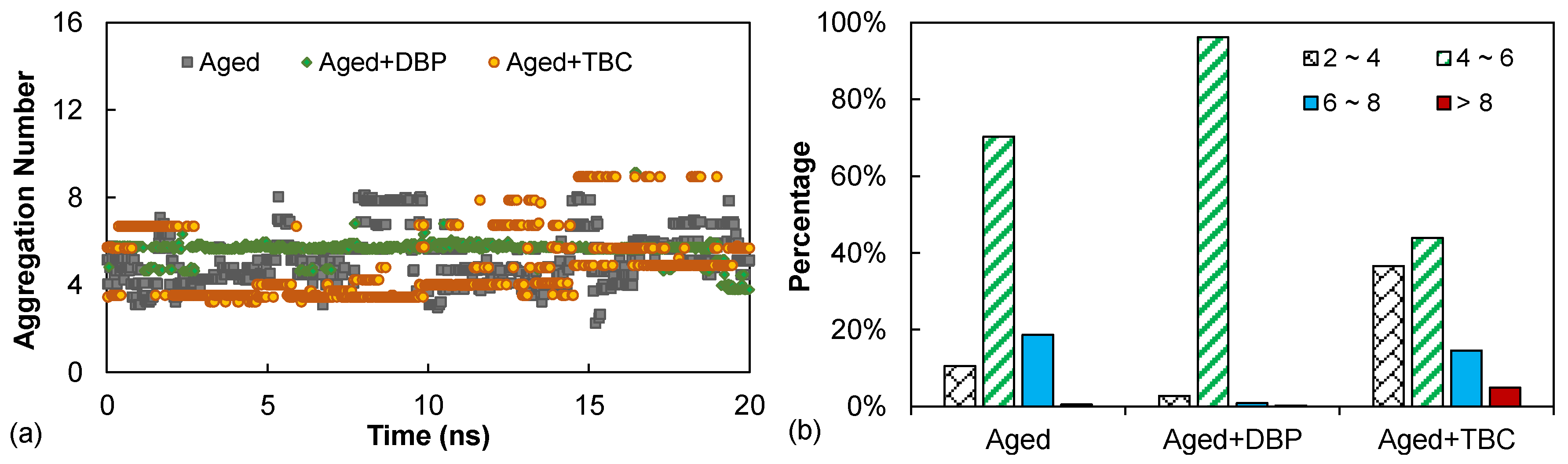
4.2.3. Aggregation Number
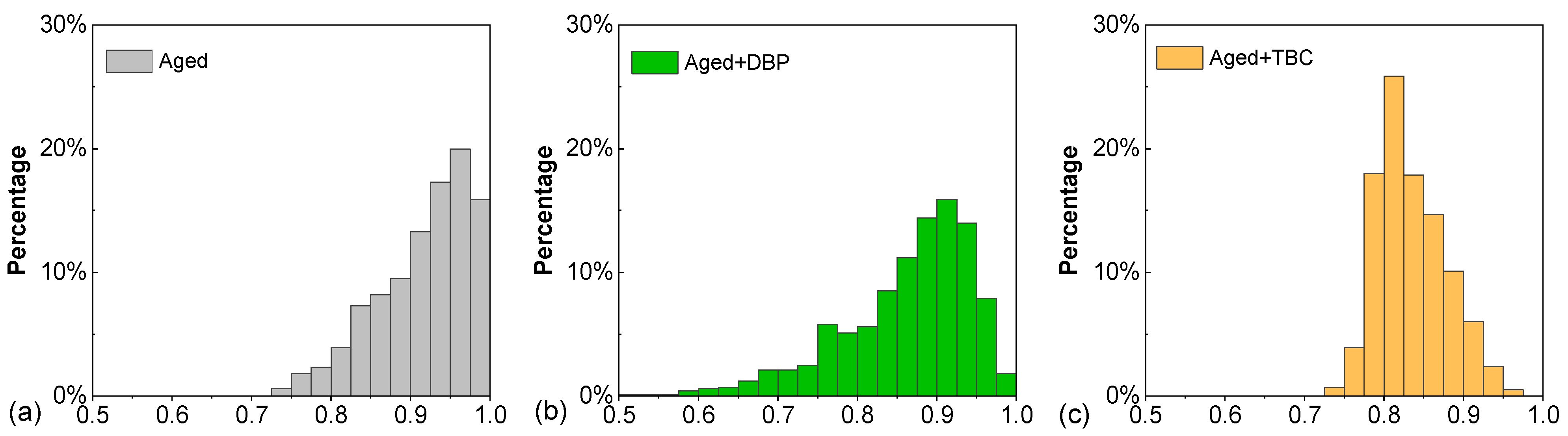
4.2.4. Order Parameter
5. Conclusions
- The two plasticizers were both highly effective in softening the aged asphalt. As interred from their impacts on the dynamic shear modulus and phase angle master curves, incorporation of appropriate dosages of DBP or TBC were able to retore the low-temperature performance to a level comparable to the virgin asphalt without sacrificing the stiffness and elasticity properties required at high temperatures;
- Increase in the blending dosages of both plasticizers consistently improved the fatigue resistance of the aged asphalt. At the same dosages of 1% and 3%, TBC demonstrated a slightly higher effectiveness than DBP in restoring the fatigue performance by providing lower G-R values and higher fatigue lives;
- Compared to DBP, TBC provided stronger interactions with the aged asphaltenes due to the presence of three polar ester groups and a hydroxyl group that formed hydrogen bonds with the latter. The stronger interactions hindered the mobility of asphaltenes at the room temperature condition but temperature rise enabled the asphaltenes to overcome the energy trap. At higher temperatures, TBC promoted the mobility by yielding the highest diffusion coefficients of the asphaltenes;
- Analyses based on RDF and order parameter indicated the deagglomerating effects of both plasticizers. They dispersed and dissociated the asphaltenes by decreasing the number of molecules surrounding a reference and by promoting the orientational randomness of asphaltene molecules so as to weaken the formation and growth of parallel stacking agglomerates;
- The benzene ring structure present in DBP allowed aromatic interactions with the cores of asphaltenes, limiting their growth into larger agglomerates; the resulting aggregation state was dominated by intermediate-sized agglomerates according to the aggregation number results. Owing to its high polarity and hydroxyl group, TBC exhibited a slightly better deagglomerating effect as it yielded a higher percentage of small agglomerates and on average a lower orientational orderliness of the asphaltenes. This observation helped to shed light on the higher rejuvenating effectiveness of TBC as noted in the rheological performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fini, E.H.; Hosseinnezhad, S.; Oldham, D.J.; Chailleux, E.; Gaudefroy, V. Source dependency of rheological and surface characteristics of bio-modified asphalts. Road Mater. Pavement Des. 2017, 18, 408–424. [Google Scholar] [CrossRef]
- Cao, W.; Mohammad, L.N.; Elseifi, M. Assessing the effects of RAP, RAS, and warm-mix technologies on fatigue performance of asphalt mixtures and pavements using viscoelastic continuum damage approach. Road Mater. Pavement Des. 2017, 18, 353–371. [Google Scholar] [CrossRef]
- Cao, W.; Wang, C. A new comprehensive analysis framework for fatigue characterization of asphalt binder using the Linear Amplitude Sweep test. Constr. Build. Mater. 2018, 171, 1–12. [Google Scholar] [CrossRef]
- Molenaar, A.; Hagos, E.; van de Vev, M. Effects of aging on the mechanical characteristics of bituminous binders in PAC. J. Mater. Civil. Eng. 2010, 22, 779–787. [Google Scholar] [CrossRef]
- Petersen, J.C. A dual, sequential mechanism for the oxidation of petroleum asphalts. Pet. Sci. Technol. 1998, 16, 1023–1051. [Google Scholar] [CrossRef]
- Petersen, J.C.; Harnsberger, P.M. Asphalt aging: A dual oxidation mechanism and its interrelationships with asphalt composition and oxidative age hardening. Transp. Res. Rec. 1998, 1638, 47–55. [Google Scholar] [CrossRef]
- Petersen, J.C.; Glaser, R. Asphalt oxidation mechanisms and the role of oxidation products on age hardening revisited. Road Mater. Pavement Des. 2011, 12, 795–819. [Google Scholar] [CrossRef]
- Pan, T.; Lu, Y.; Lloyd, S. Quantum-chemistry study of asphalt oxidative aging: An XPS-aided analysis. Ind. Eng. Chem. Res. 2012, 51, 7957–7966. [Google Scholar] [CrossRef]
- Sylvester, T.; Tabatabaee, H. Rejuvenation vs. Softening of recycled binders. In Proceedings of the IAPA 80th Annual Conference, Champaign, IL, USA, 11–12 December 2017; Available online: https://il-asphalt.org/files/8714/8969/2569/2017Sylvester.pdf (accessed on 16 September 2022).
- Haghshenas, H.F.; Rea, R.; Reinke, G.; Zaumanis, M.; Fini, E. Relationship between colloidal index and chemo-rheological properties of asphalt binders modified by various recycling agents. Constr. Build. Mater. 2022, 318, 126161. [Google Scholar] [CrossRef]
- Ma, T.; Huang, X.; Zhao, Y.; Zhang, Y. Evaluation of diffusion and distribution of the rejuvenator for hot asphalt recycling. Constr. Build. Mater. 2015, 98, 530–536. [Google Scholar] [CrossRef]
- NCAT. NCAT Researchers Explore Multiple Uses of Rejuvenators. Asph. Technol. News 2014, 26, 1–16. Available online: https://eng.auburn.edu/research/centers/ncat/research/newsletters/atnspring2014.pdf (accessed on 16 September 2022).
- Cai, X.; Zhang, J.; Xu, G.; Gong, M.; Chen, X.; Yang, J. Internal aging indexes to characterize the aging behavior of two bio-rejuvenated asphalts. J. Clean. Prod. 2019, 220, 1231–1238. [Google Scholar] [CrossRef]
- Zhang, R.; You, Z.; Wang, H.; Ye, M.; Yap, Y.K.; Si, C. The impact of bio-oil as rejuvenator for aged asphalt binder. Constr. Build. Mater. 2019, 196, 134–143. [Google Scholar] [CrossRef]
- Chen, M.; Leng, B.; Wu, S.; Sang, Y. Physical, chemical and rheological properties of waste edible vegetable oil rejuvenated asphalt binders. Constr. Build. Mater. 2014, 66, 286–298. [Google Scholar] [CrossRef]
- Fini, E.H.; Kalberer, E.W.; Shahbazi, A.; Basti, M.; You, Z.; Ozer, H.; Aurangzeb, Q. Chemical characterization of biobinder from swine manure: Sustainable modifier for asphalt binder. J. Mater. Civil. Eng. 2011, 23, 1506–1513. [Google Scholar] [CrossRef]
- Zaumanis, M.; Mallick, R.; Frank, R. Evaluation of different recycling agents for restoring aged asphalt binder and performance of 100% recycled asphalt. Mater. Struct. 2014, 48, 2475–2488. [Google Scholar] [CrossRef]
- Bajaj, A.; Martin, A.E.; King, G.; Glover, C.; Kaseer, F.; Arámbula-Mercado, E. Evaluation and classification of recycling agents for asphalt binders. Constr. Build. Mater. 2020, 260, 119864. [Google Scholar] [CrossRef]
- Menapace, I.; Cucalon, L.G.; Kaseer, F.; Arámbula-Mercado, E.; Martin, A.E.; Masad, E.; King, G. Effect of recycling agents in recycled asphalt binders observed with microstructural and rheological tests. Constr. Build. Mater. 2018, 158, 61–74. [Google Scholar] [CrossRef]
- Oldham, D.J.; Fini, E.H.; Chailleux, E. Application of a bio-binder as a rejuvenator for wet processed asphalt shingles in pavement construction. Constr. Build. Mater. 2015, 86, 75–84. [Google Scholar] [CrossRef]
- Cavalli, M.C.; Zaumanis, M.; Mazza, E.; Partl, M.N.; Poulikakos, L.D. Effect of ageing on the mechanical and chemical properties of binder from RAP treated with bio-based rejuvenators. Compos. B Eng. 2018, 141, 174–181. [Google Scholar] [CrossRef]
- Reedijk, J. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Oxford, UK, 2014. [Google Scholar]
- Zhang, F.; Hu, C. The research for high-elastic modified asphalt. J. Appl. Polym. Sci. 2015, 132, 42132. [Google Scholar] [CrossRef]
- Fu, Z.; Shi, K.; Ma, F.; Song, R.; Chen, L.; Dai, J.; Shen, W. Rheological properties of dioctyl adipate-modified asphalt binder. Int. J. Pavement Eng. 2021, 23, 2644–2653. [Google Scholar] [CrossRef]
- Song, R.; Sha, A.; Shi, K.; Li, J.; Li, X.; Zhang, F. Polyphosphoric acid and plasticizer modified asphalt: Rheological properties and modification mechanism. Constr. Build. Mater. 2021, 309, 125158. [Google Scholar] [CrossRef]
- Jamal, I.H.; Yaseen, G.; Aziz, A. Influence of Cereclor on the performance of aged asphalt binder. Int. J. Pavement Eng. 2020, 21, 1309–1320. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, G.; Gong, M.; Yang, J. Recycling long-term-aged asphalts using bio-binder/plasticizer-based rejuvenator. Constr. Build. Mater. 2017, 147, 117–129. [Google Scholar] [CrossRef]
- Rajib, A.I.; Samieadel, A.; Zalghout, A.; Kaloush, K.E.; Sharma, B.K.; Fini, E.H. Do all rejuvenators improve asphalt performance? Road Mater. Pavement Des. 2022, 23, 358–376. [Google Scholar] [CrossRef]
- Kaseer, F.; Martin, A.E.; Arámbula-Mercado, E. Use of recycling agents in asphalt mixtures with high recycled materials contents in the United States: A literature review. Constr. Build. Mater. 2019, 211, 974–987. [Google Scholar] [CrossRef]
- Cucalon, L.G.; King, G.; Kaseer, F.; Arambula-Mercado, E.; Martin, A.E.; Turner, T.F.; Glover, C.J. Compatibility of recycled binder blends with recycling agents: Rheological and physicochemical evaluation of rejuvenation and aging processes. Ind. Eng. Chem. Res. 2017, 56, 8375–8384. [Google Scholar] [CrossRef]
- AASHTO T240; Standard Method of Test for Effect of Heat and Air on a Moving Film of Asphalt Binder (Rolling Thin-Film Oven Test). AASHTO: Washington, DC, USA, 2013.
- AASHTO R28; Standard Practice for Accelerated Aging of Asphalt Binder Using a Pressurized Aging Vessel (PAV). AASHTO: Washington, DC, USA, 2012.
- Kamrin, M.A. Phthalate risks, phthalate regulation, and public health: A review. J. Toxicol. Environ. Health B 2009, 12, 157–174. [Google Scholar] [CrossRef]
- Cao, W.; Wang, C. Fatigue performance characterization and prediction of asphalt binders using the linear amplitude sweep based viscoelastic continuum damage approach. Int. J. Fatigue 2019, 119, 112–125. [Google Scholar] [CrossRef]
- Christensen, D.W.; Anderson, D.A. Interpretation of dynamic mechanical test data for paving grade asphalt cements. J. Assoc. Asph. Paving Technol. 1992, 61, 67–116. [Google Scholar]
- Glover, J.C.; Davison, R.R.; Domke, C.H.; Ruan, Y.; Juristyarini, P.; Knorr, D.B.; Jung, S.H. Development of a New Method for Assessing Asphalt Binder Durability with Field Evaluation; Report No. FHWA/TX/05-1872-2; Texas Transportation Institute: College Station, TX, USA, 2005. [Google Scholar]
- Rowe, G.M. Prepared discussion following the paper by Anderson et al. titled Evaluation of the relationship between asphalt binder properties and non-load related cracking. J. Assoc. Asph. Paving Technol. 2011, 80, 649–662. [Google Scholar]
- Pournoman, S.; Hajj, E.Y.; Morian, N.; Martin, A.E. Impact of recycled materials and recycling agents on asphalt binder oxidative aging predictions. Transp. Res. Rec. 2018, 2672, 277–289. [Google Scholar] [CrossRef]
- Sedghi, M.; Goual, L.; Welch, W.; Kubelka, J. Effect of asphaltene structure on association and aggregation using molecular dynamics. J. Phys. Chem. B 2013, 117, 5765–5776. [Google Scholar] [CrossRef]
- Goual, L.; Sedghi, M.; Wang, X.; Zhu, Z. Asphaltene aggregation and impact of alkylphenols. Langmuir 2014, 30, 5394–5403. [Google Scholar] [CrossRef]
- Li, D.D.; Greenfield, M.L. Chemical compositions of improved model asphalt systems for molecular simulations. Fuel 2014, 115, 347–356. [Google Scholar] [CrossRef]
- Mullins, O.C. The modified Yen model. Energy Fuels 2010, 24, 2179–2207. [Google Scholar] [CrossRef]
- Li, C.; Ma, F.; Fu, Z.; Dai, J.; Wen, Y.; Shi, K. Using Cereclor plasticizer to modify the virgin asphalt binder: A case of rheological properties improvement. Constr. Build. Mater. 2022, 318, 126039. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Yao, H.; Dai, Q.; You, Z. Molecular dynamics simulation of physicochemical properties of the asphalt model. Fuel 2016, 164, 83–93. [Google Scholar] [CrossRef]
- Wypych, A. Databook of Plasticizers, 2nd ed.; ChemTec Publishing: Toronto, ON, USA, 2017. [Google Scholar]
- Haghshenas, H.F.; Kim, Y.-R.; Kommidi, S.R.; Nguyen, D.; Haghshenas, D.F.; Morton, M.D. Evaluation of long-term effects of rejuvenation on reclaimed binder properties based on chemical-rheological tests and analyses. Mater. Struct. 2018, 51, 134. [Google Scholar] [CrossRef]
- Nsengiyumva, G.; Haghshenas, H.F.; Kim, Y.-R.; Kommidi, S.R. Mechanical-chemical characterization of the effects of type, dosage, and treatment methods of rejuvenators in aged bituminous materials. Transp. Res. Rec. 2020, 2674, 126–138. [Google Scholar] [CrossRef]
- Haghshenas, H.F.; Fini, E.; Rea, R.; Khodaii, A. Increasing the efficacy of recycling agents with simultaneous addition of zinc diethyldithiocarbamate as an antioxidant. Constr. Build. Mater. 2021, 271, 121892. [Google Scholar] [CrossRef]
- Peskir, G. On the diffusion coefficient: The Einstein relation and beyond. Stoch. Models 2003, 19, 383–405. [Google Scholar] [CrossRef]
- Li, D.D.; Greenfield, M.L. Viscosity, relaxation time, and dynamics within a model asphalt of larger molecules. J. Chem. Phys. 2014, 140, 034507. [Google Scholar] [CrossRef]
- Headen, T.F.; Boek, E.S.; Skipper, N.T. Evidence for asphaltene nanoaggregation in toluene and heptane from molecular dynamics simulations. Energy Fuels 2009, 23, 1220–1229. [Google Scholar] [CrossRef]
- Samieadel, A.; Rajib, A.I.; Dandamudi, K.P.R.; Deng, S.; Fini, E.H. Improving recycled asphalt using sustainable hybrid rejuvenators with enhanced intercalation into oxidized asphaltenes nanoaggregates. Constr. Build. Mater. 2020, 262, 120090. [Google Scholar] [CrossRef]
- Murata, K.; Tanaka, H. Microscopic identification of the order parameter governing liquid-liquid transition in a molecular liquid. Proc. Natl. Acad. Sci. USA 2015, 112, 5956–5961. [Google Scholar] [CrossRef] [Green Version]
- Paoletti, A.; Ricci, F.P.; Passari, L. Magnetization and state of order in MnNi3. J. Appl. Phys. 1966, 37, 3236. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, W.; Li, X. The Rejuvenating Potential of Plasticizers on Oxidatively Aged Asphalts: Rheological and Molecular Dynamics Perspectives. Polymers 2022, 14, 4624. https://doi.org/10.3390/polym14214624
Cao W, Li X. The Rejuvenating Potential of Plasticizers on Oxidatively Aged Asphalts: Rheological and Molecular Dynamics Perspectives. Polymers. 2022; 14(21):4624. https://doi.org/10.3390/polym14214624
Chicago/Turabian StyleCao, Wei, and Xinyan Li. 2022. "The Rejuvenating Potential of Plasticizers on Oxidatively Aged Asphalts: Rheological and Molecular Dynamics Perspectives" Polymers 14, no. 21: 4624. https://doi.org/10.3390/polym14214624





