Development of Azo Dye Immobilized Poly (Glycidyl Methacrylate-Co-Methyl Methacrylate) Polymers Composites as Novel Adsorbents for Water Treatment Applications: Methylene Blue-Polymers Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymerization Process
2.3. Sulphonation Process
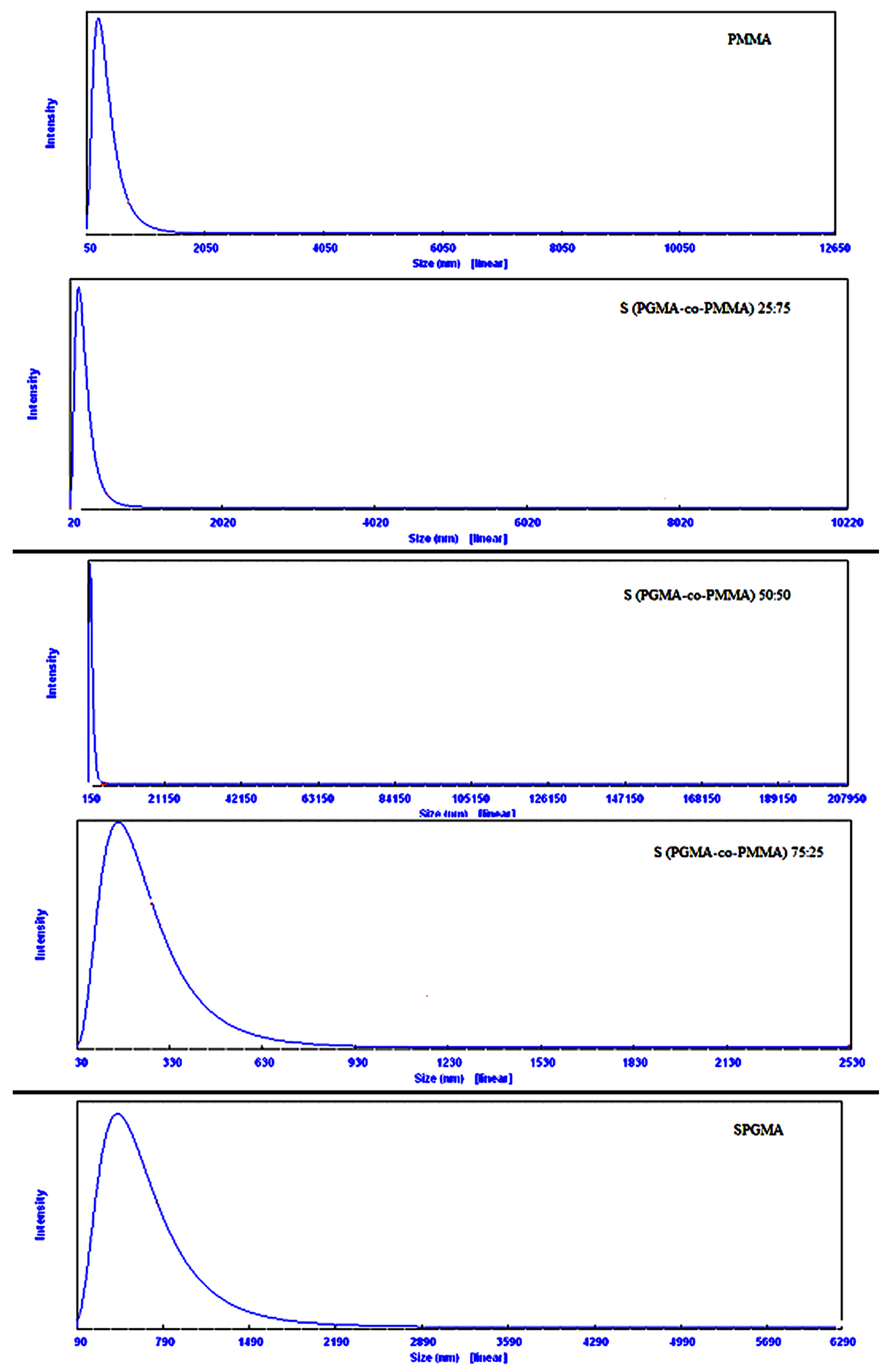
2.4. Particle Size Analysis
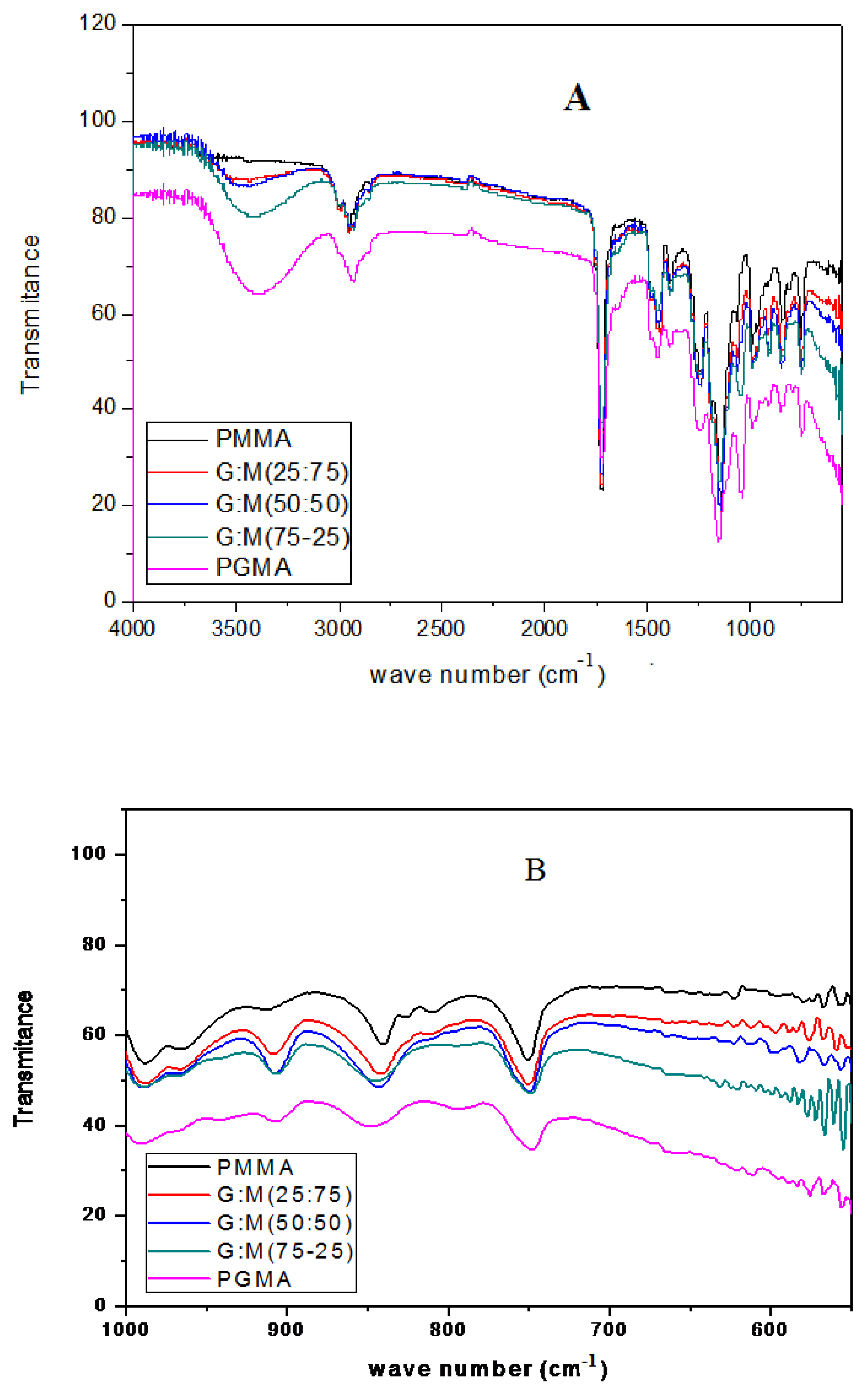
2.5. Fourier Transform Infrared Spectroscopic Analysis
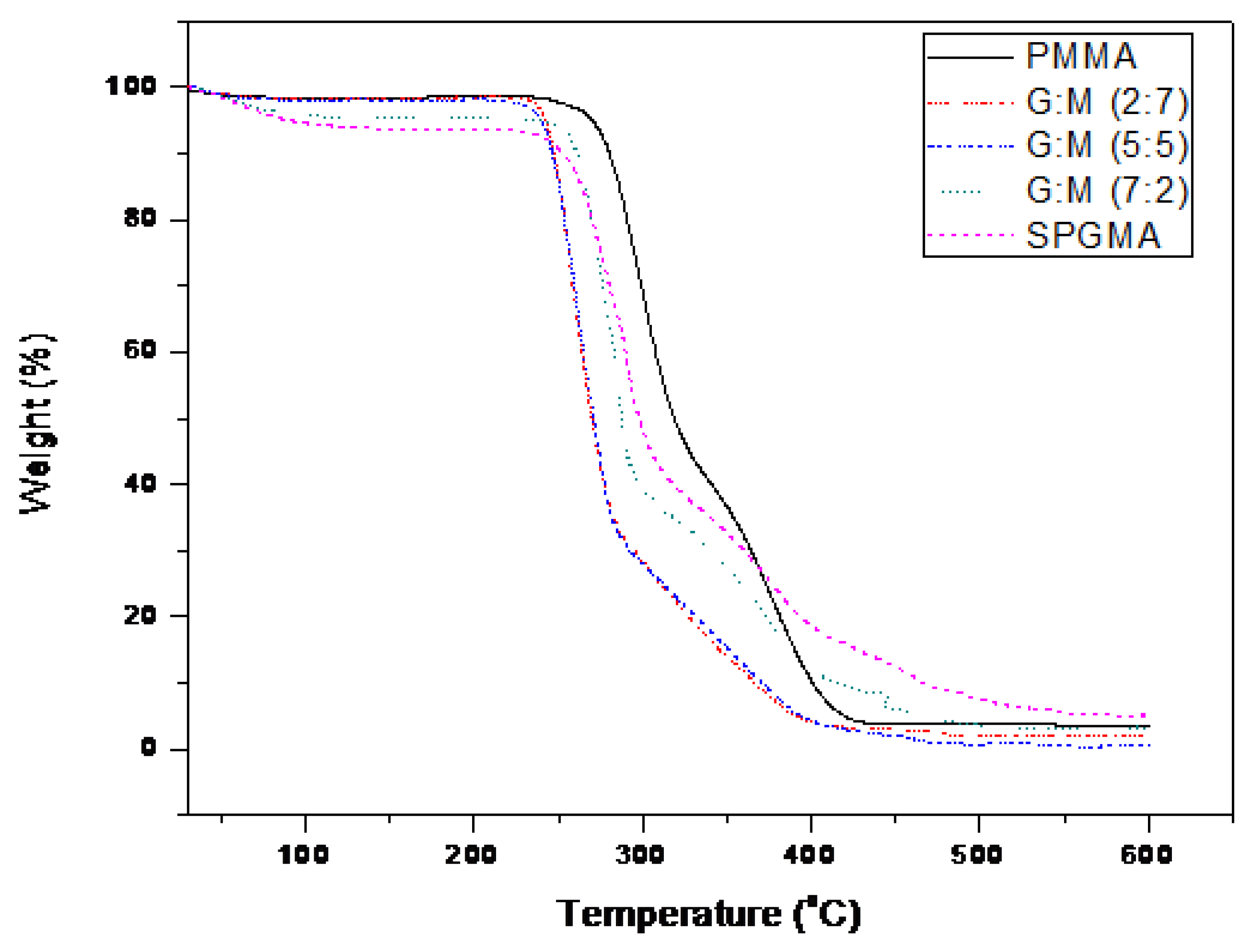
2.6. TGA Analysis
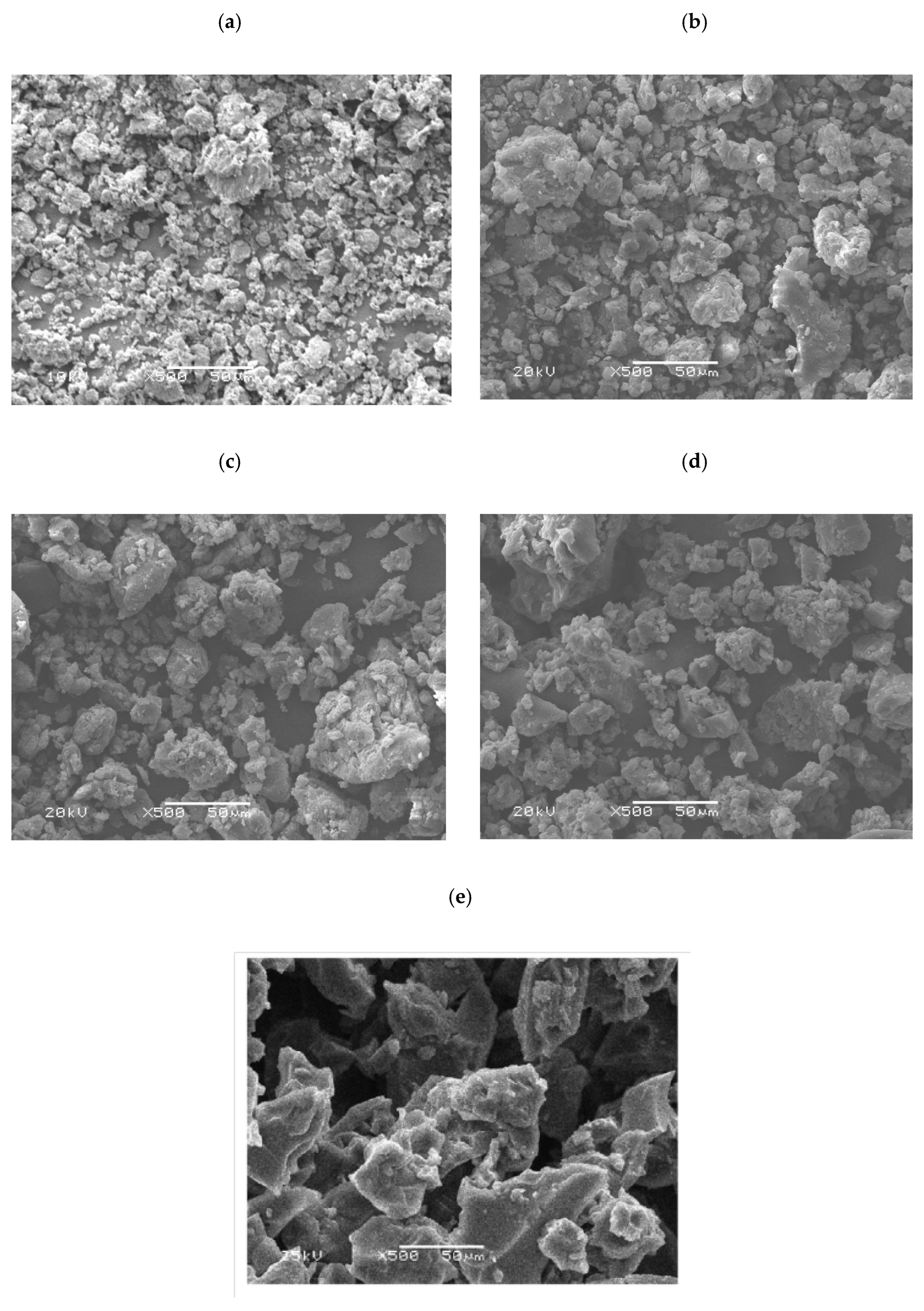
2.7. Scanning Electron Microscopic Analysis
2.8. Preparation of Basic Methylen Blue Solution
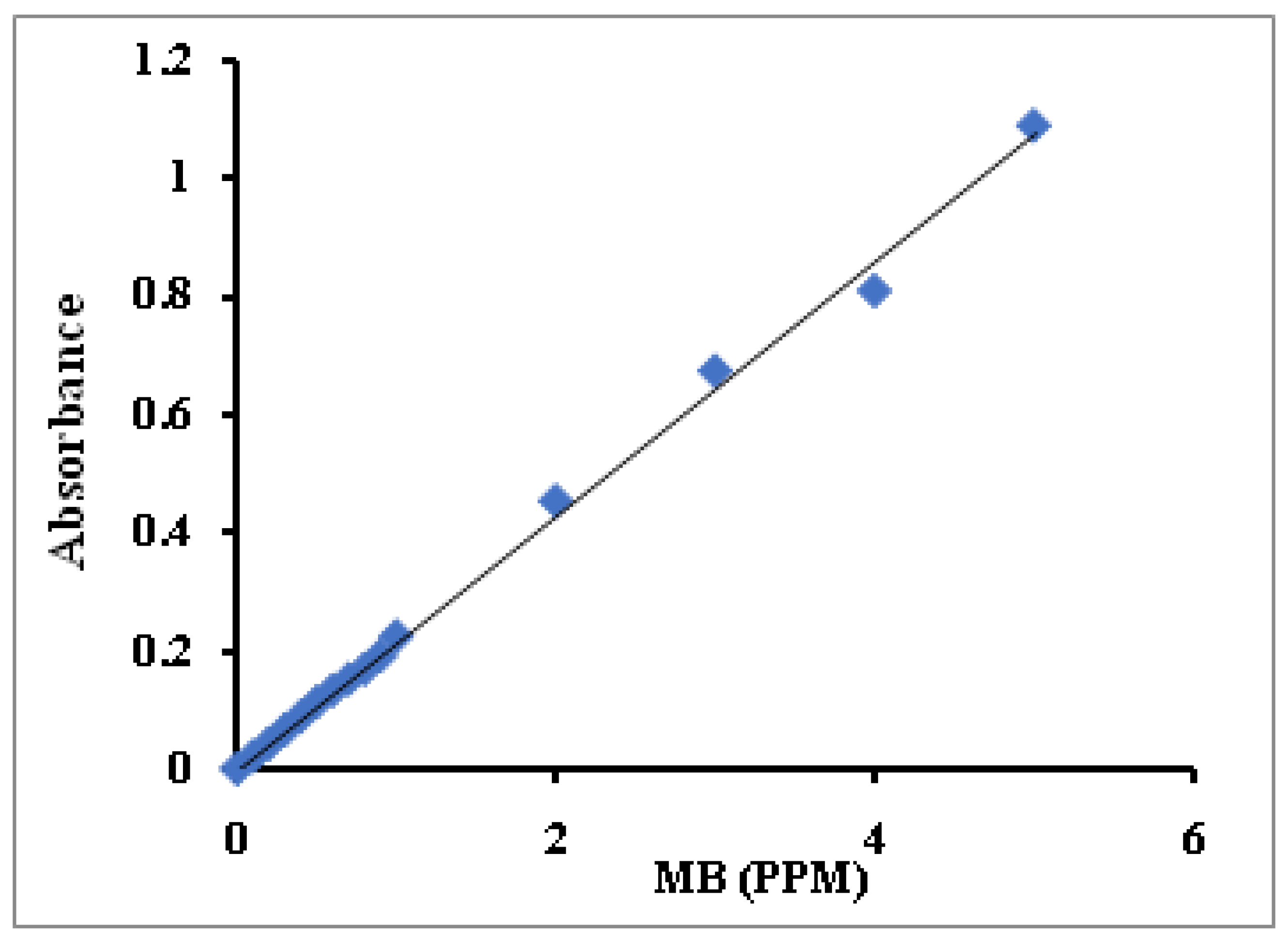
2.9. Standard Curve of MB Concentration
2.10. Methylene Blue-Polymers Composites Formation (Immobilization Process)
2.11. Chromium (VI) and Manganese (VII) Ions Removal
3. Results and Discussion
3.1. Polymerization Process
3.2. Methylene Blue-Polymers Composites Formation (Immobilization Process)
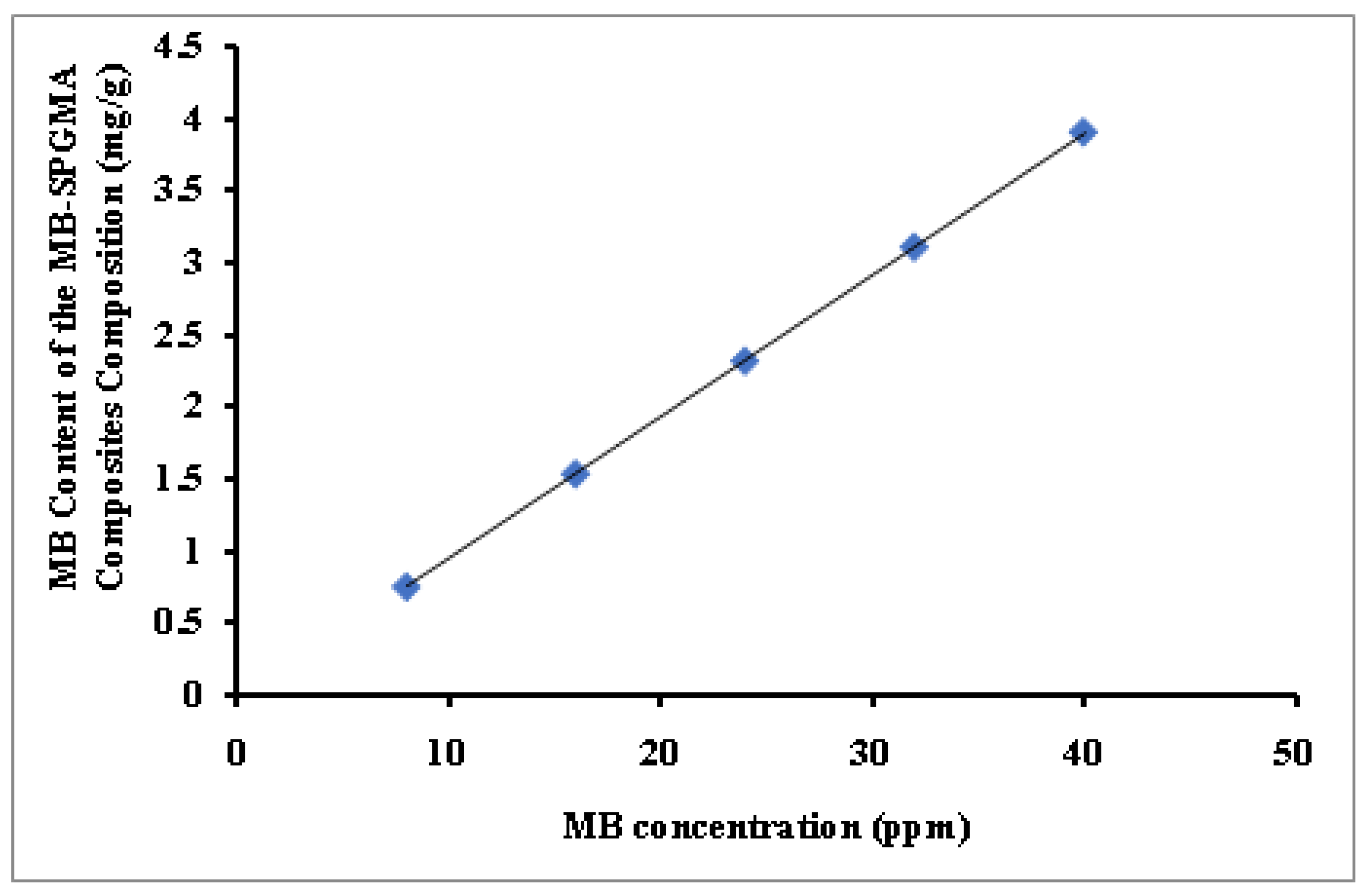
3.2.1. Methylene Blue Concentration
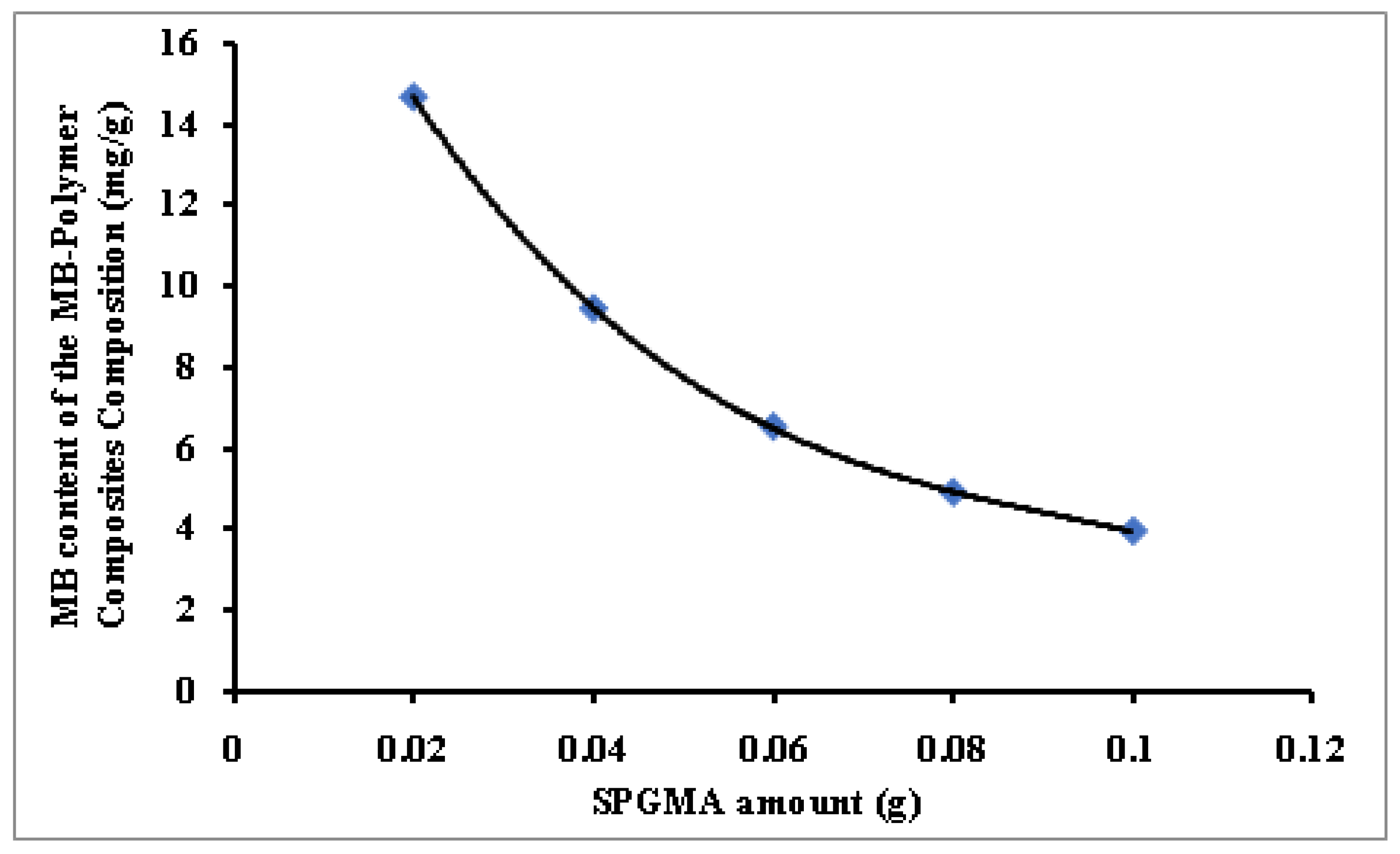
3.2.2. SPGMA Polymer Dose
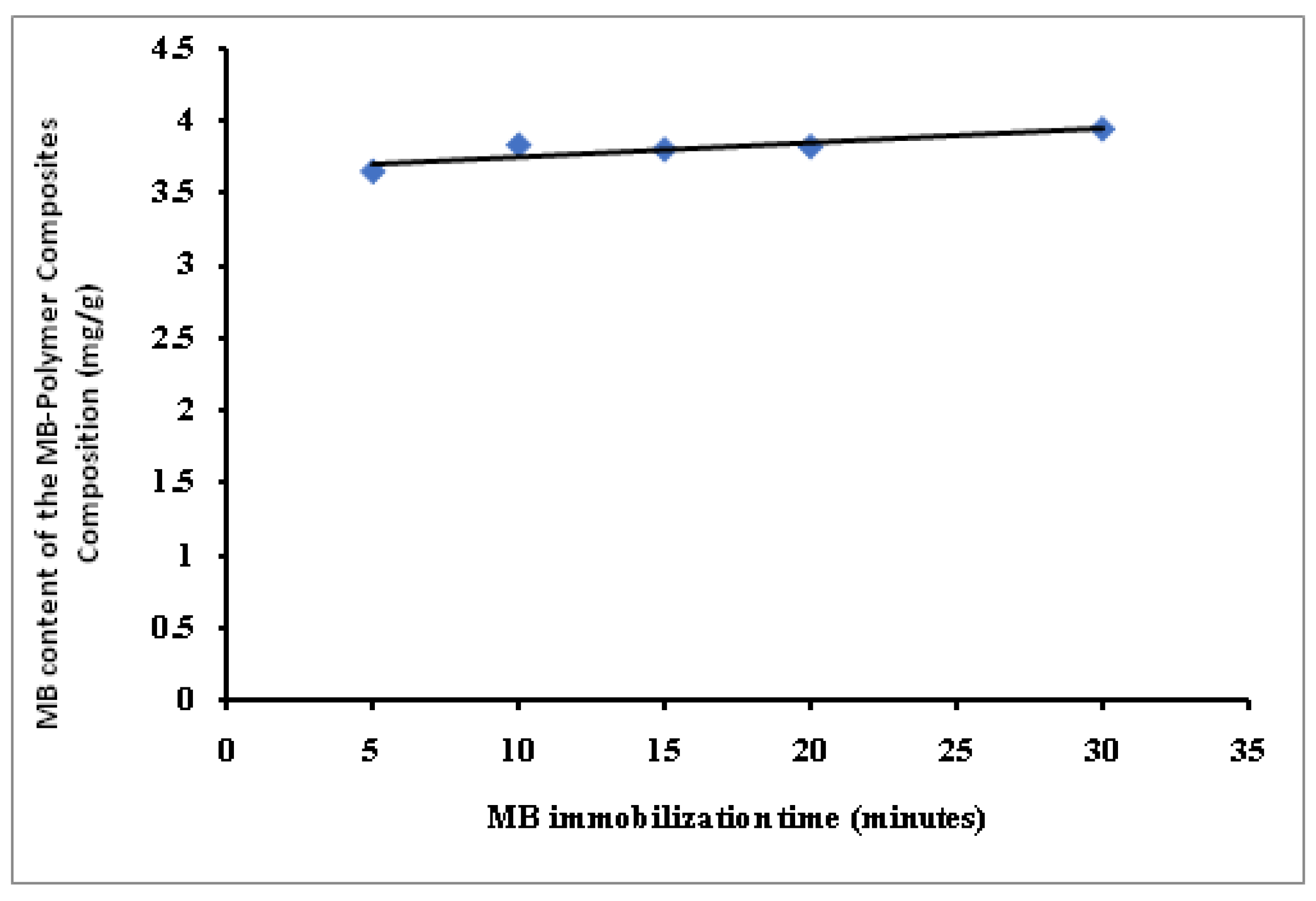
3.2.3. MB Immobilization Time
3.2.4. Agitation Speed
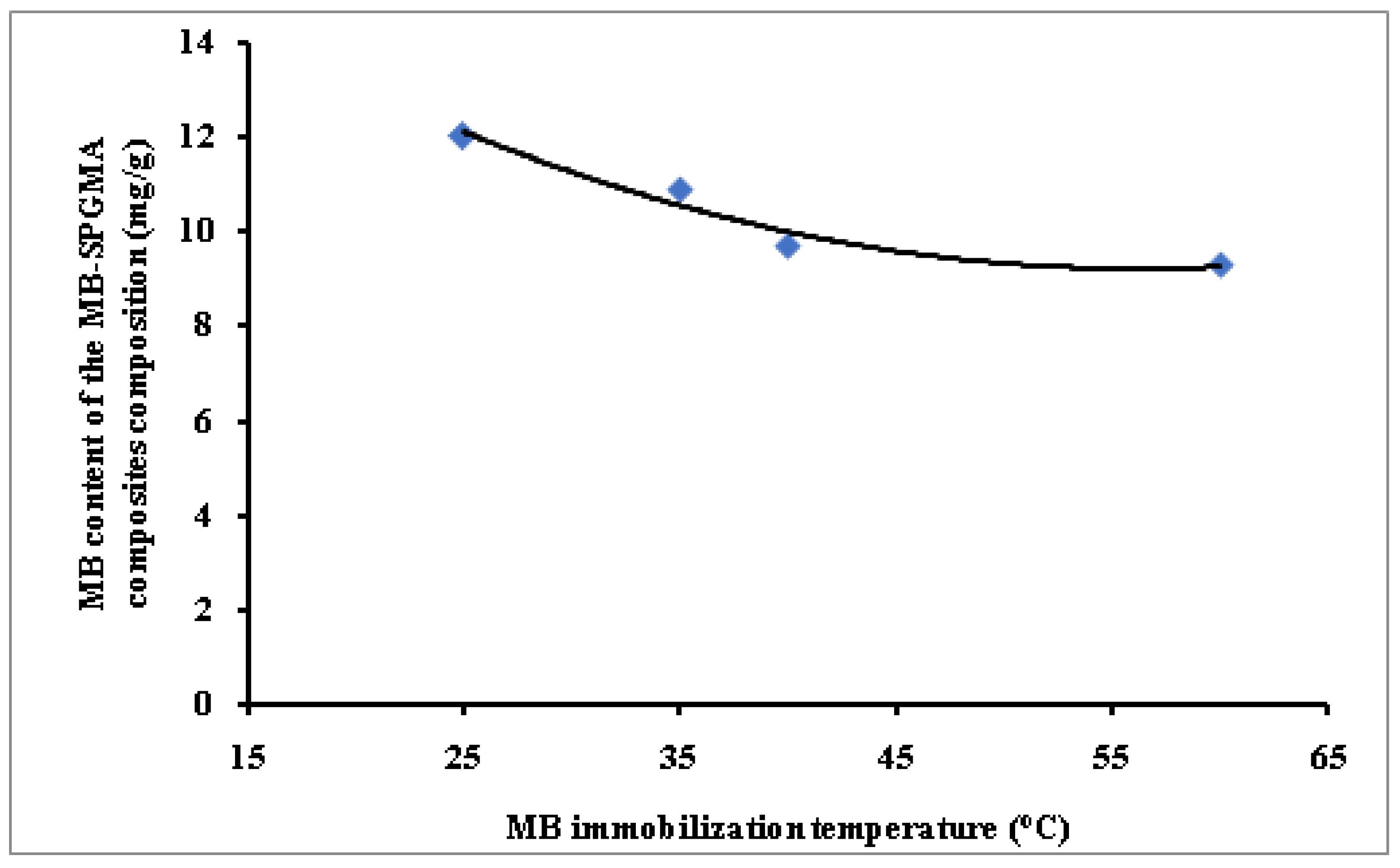
3.2.5. MB Immobilization Temperature
3.3. Matrix Characterization
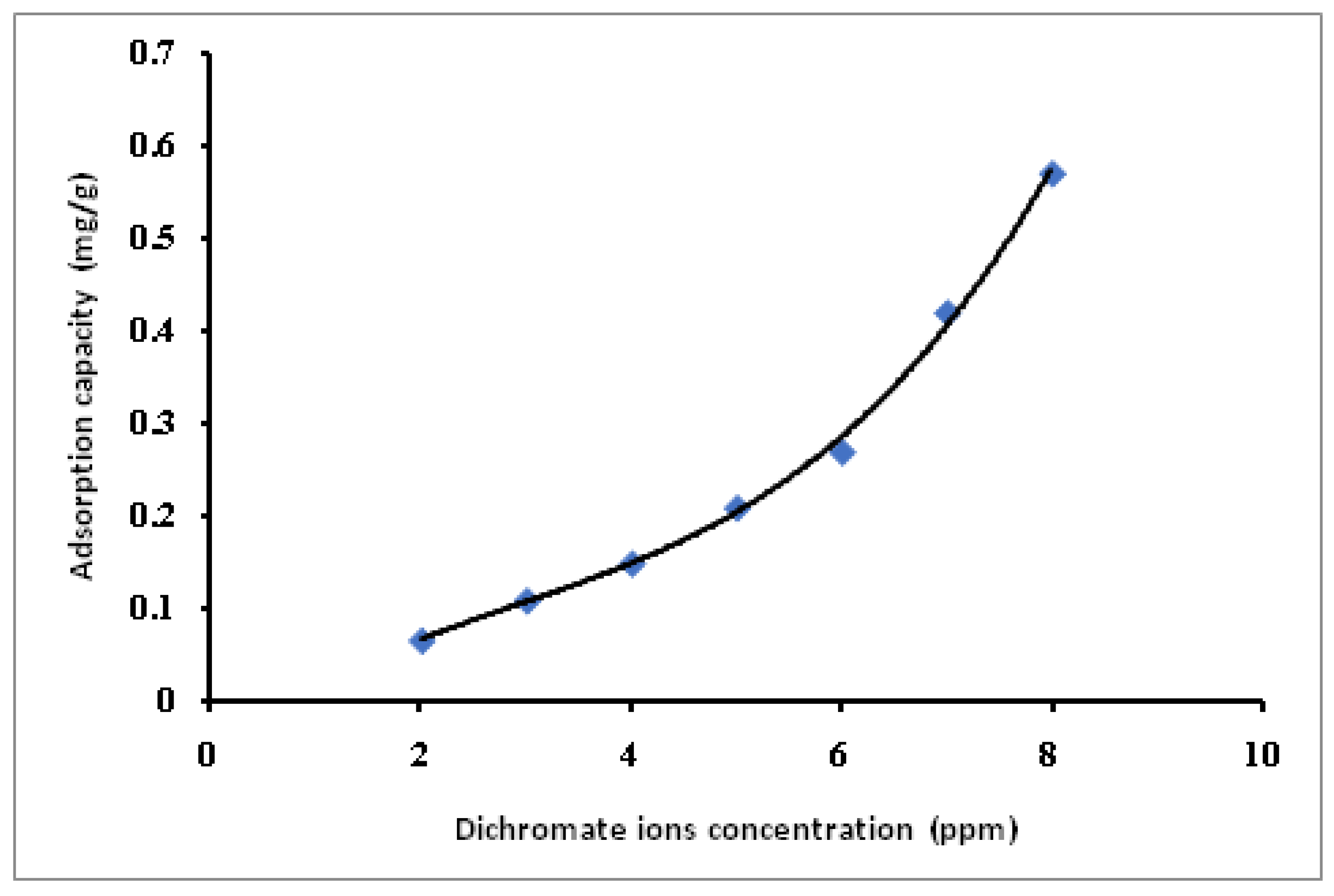
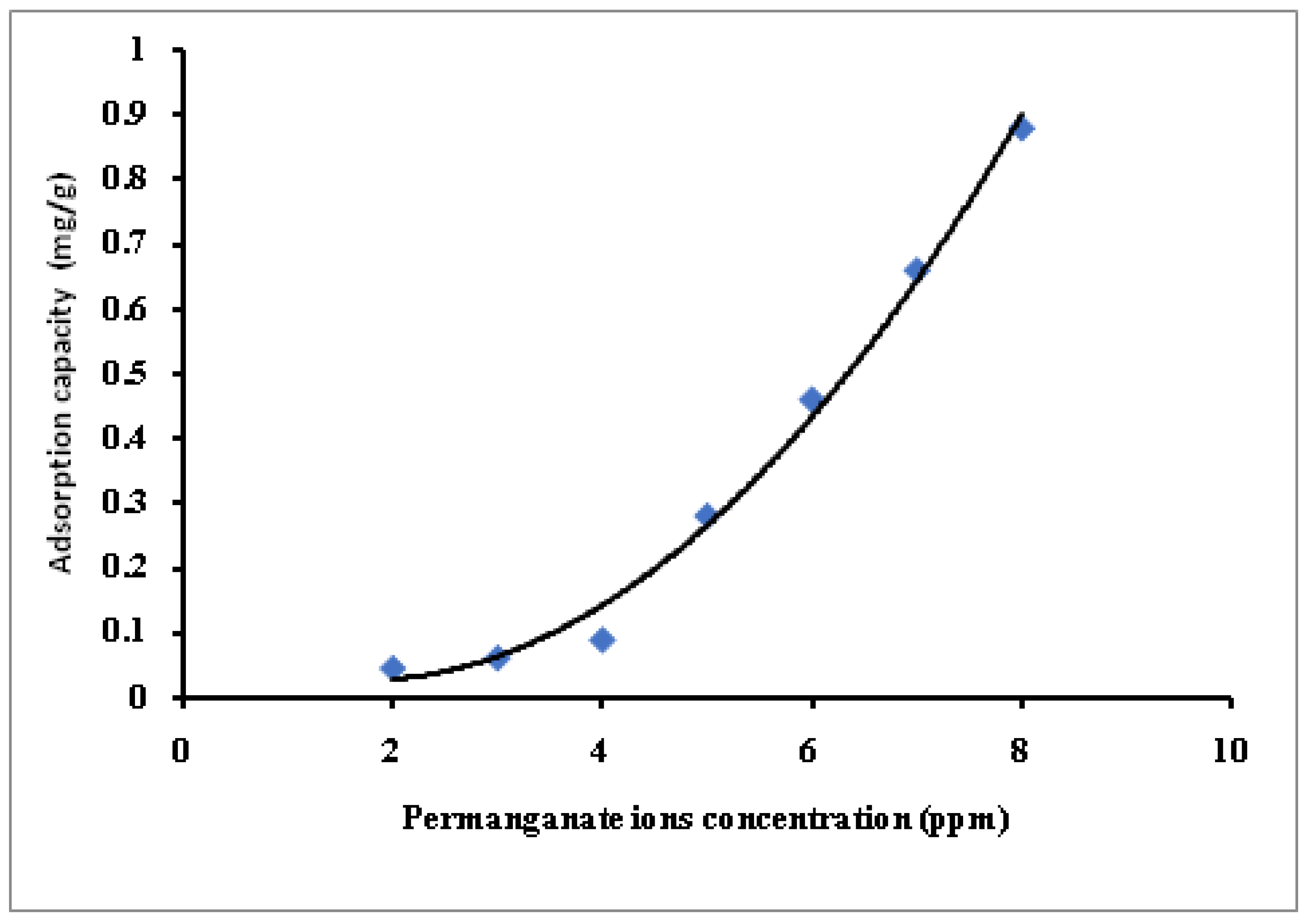
3.4. Dichromate and Permanganate Metal Ions Removal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomez, V.; Larrechi, M.S.; Callao, M.P. Kinetic and adsorption study of acid dye removal using activated carbon. Chemosphere 2007, 69, 1151–1158. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, R.; Nayak, A.; Saleh, T.A.; Barakat, M.A. Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: A review. Adv. Colloid Interface Sci. 2013, 193, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Ayad, M.M.; Abo El-Nasr, A. Adsorption of cationic dye (methylene blue) from water using polyaniline nano-tubes base. J. Phys. Chem. C 2010, 114, 14377–14383. [Google Scholar] [CrossRef]
- Wong, Y.C.; Szeto, Y.S.; Cheung, W.H.; McKay, G. Equilibrium studies for acid dye adsorption onto chitosan. Langmuir 2003, 19, 7888–7894. [Google Scholar] [CrossRef]
- Baybars, A.F.; Cengiz, Q.; Mustafa, K. Cationic dye (methylene blue) removal from aqueous solution by montmorillonite. Bull. Korean Chem. Soc. 2012, 33, 3184–3190. [Google Scholar]
- Yagub, M.T.; Sen, T.K.; Ang, H.M. Equilibrium, kinetics, and thermodynamics of methylene blue adsorption by pine tree leaves. Water Air Soil Pollut. 2012, 223, 5267–5282. [Google Scholar] [CrossRef]
- Sen, T.K.; Afroze, S.; Ang, H.M. Equilibrium, kinetics and mechanism of removal of methylene blue from aqueous solution by adsorption onto pine cone biomass of Pinus radiate. Water Air Soil Pollut. 2011, 218, 499–515. [Google Scholar] [CrossRef]
- Mohammad, M.; Maitra, S.; Ahmad, N.; Bustam, A.; Sen, T.K. Metal ion removal from aqueous solution using physic seed hull. J. Hazard. Mater. 2010, 179, 363–372. [Google Scholar] [CrossRef]
- Abd EI-Latif, M.M.; Ibrahim, A.M.; EI-Kady, M.F. Adsorption equilibrium, kinetics and thermodynamics of methylene blue from aqueous solutions using biopolymer oak sawdust composite. J. Am. Sci. 2010, 6, 267–283. [Google Scholar]
- Yao, Z.; Wang, L.; Qi, J. Biosorption of methylene blue from aqueous solution using a bioenergy forest waste: Xanthocerassorbifolia seed coat. Clean 2009, 37, 642–648. [Google Scholar]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Srinivasan, A.; Viraraghavan, T. Decolorization of dye wastewaters by biosorbents: A review. J. Environ. Manag. 2010, 91, 1915–1929. [Google Scholar] [CrossRef]
- Leszczyńska, M.; Hubicki, Z. Application of weakly and strongly basic anion exchangers for the removal of brilliant yellow from aqueous solutions. Desalination Water Treat. 2009, 2, 156–161. [Google Scholar] [CrossRef]
- Purkait, M.K.; Maiti, A.; Das Gupta, S. Removal of congo red using activated carbon and its regeneration. J. Hazard. Mater. 2007, 145, 287–295. [Google Scholar] [CrossRef]
- Hernandez-Montoya, V.; Perez-Cruz, M.A.; Mendoza-Castillo, D.I.; Moreno-Virgen, M.R.; Bonilla-Petriciolet, A. Competitive adsorption of dyes and heavy metals on zeolitic structures. J. Environ. Manag. 2013, 116, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Errais, E.; Duplay, J.; Elhabiri, M.; Khodja, M.; Ocampo, R. Anionic RR120 dye adsorption onto raw clay: Surface properties and adsorption mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2012, 403, 69–78. [Google Scholar] [CrossRef]
- Ofomaja, A.E. Equilibrium sorption of methylene blue using mansonia wood sawdust as biosorbent. Desalination Water Treat. 2009, 3, 1–10. [Google Scholar] [CrossRef]
- Reddy, M.C.; Sivaramakrishna, L.; Reddy, A.V. The use of an agricultural waste material, Jujuba seeds for the removal of anionic dye (Congo red) from aqueous medium. J. Hazard. Mater. 2012, 203, 118–127. [Google Scholar] [CrossRef]
- Sarioglu, M.; Atay, U.A. Removal of Methylene blue by using biosolid. Glob. Nest J. 2006, 8, 113–120. [Google Scholar]
- Deniz, F.; Karaman, S. Removal of Basic Red 46 dye from aqueous solution by pine tree leaves. Chem. Eng. J. 2011, 170, 67–74. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res. 2012, 46, 1933–1946. [Google Scholar] [CrossRef] [PubMed]
- Auta, M.; Hameed, B.H. Coalesced chitosan activated carbon composite for batch and fixed-bed adsorption of cationic and anionic dyes. Colloids Surf. B Biointerfaces 2013, 105, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Poinern, G.E.J.; Senanayake, G.; Shah, N.; Thi-Le, X.N.; Parkinson, G.M. Adsorption of the aurocyanide, View the MathML source complex on granular activated carbons derived from macadamia nut shells—A preliminary study. Miner. Eng. 2011, 24, 1694–1702. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K.; Phan, C. Synthesis and characterization of novel-activated carbon from waste biomass pine cone and its application in the removal of congo red dye from aqueous solution by adsorption. Water Air Soil Pollut. 2014, 225, 1–16. [Google Scholar] [CrossRef]
- Khan, S.A.; Abbasi, N.; Hussain, D.; Khan, T.A. Sustainable Mitigation of Paracetamol with a Novel Dual-Functionalized Pullulan/Kaolin Hydrogel Nanocomposite from Simulated Wastewater. Langmuir 2022, 38, 8280–8295. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Gouda, M.H.; Abu-Saied, M.A.; El-Shazly, Y.M.S.; Farag, H.A. Development of Grafted Cotton Fabrics Ions Exchanger for Dye Removal Applications: Methylene Blue Model. Desalination Water Treat. 2016, 57, 22049–22060. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Elkady, M.F.; Abdel Rahman, A.M.; Soliman, E.A.; Elzatahry, A.A.; Youssef, M.E.; Eweida, B.Y. Preparation and characterization of iminodiacetic acid functionalized alginate beads for removal of contaminates from waste water: I. methylene blue cationic dye model. Desalination Water Treat. 2012, 40, 15–23. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Aly, K.M.; Khan, Z.A.; Meky, A.E.; Saleh, T.S.; Elbogamy, A.S. Development of Novel Acid-Base Ions Exchanger for Basic Dye Removal: Phosphoric Acid Doped Pyrazole-g-Polyglycidyl Methacrylate. Desalination Water Treat. 2016, 57, 24047–24055. [Google Scholar] [CrossRef]
- Aksu, Z. Biosorption of heavy metals by microalgae in batch and continuous systems. In Wastewater Treatment with Algae; Wong, Y.-S., Tam, N.F.Y., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 37–53. [Google Scholar]
- Dönmez, G.; Aksu, Z. The effect of copper (II) ions on growth and bioaccumulation properties of some yeasts. Process Biochem. 1999, 35, 135–142. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Adsorption of phenolic compounds on low-cost adsorbents: A review. Adv. Colloid Interface Sci. 2008, 143, 48–67. [Google Scholar] [CrossRef]
- Waldemer, R.H.; Tratnyek, P.G. Kinetics of contaminant degradation by permanganate. Environ. Sci. Technol. 2006, 40, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Kumari, S.; Virvadiya, C. Adsorption Analysis of Mn(VII) from Aqueous Medium by Activated Orange Peels Powder. Int. Res. J. Pure Appl. Chem. 2015, 9, 1–8. [Google Scholar] [CrossRef]
- Zhang, K.; Li, C.; He, J.; Liu, R. Adsorption of permanganate onto activated carbon particles. Hua Xi Yi Ke Da Xue Xue Bao 1997, 28, 344–346. [Google Scholar] [PubMed]
- Mahmoud, M.E.; Yakout, A.A.; Saad, S.R.; Osman, M.M. Removal of potassium permanganate from water by modified carbonaceous materials. Desalination Water Treat. 2016, 57, 15559–15569. [Google Scholar] [CrossRef]
- Virvadiya, C.; Kumari, S.; Choudhary, V.; Gupta, V. Combined bio- and chemosorption of Mn(VII) from aqueous solution by Prosopis cineraria leaf powder. Eur. Chem. Bull. 2014, 3, 315–318. [Google Scholar]
- Chaudhary, M. Use of Millet Husk as a Biosorbent for the Removal of chromium and Manganese Ions from the Aqueous Solutions. Int. J. Chem. Environ. Pharm. Res. 2011, 2, 30–33. [Google Scholar]
- Wan Ngah, W.S.; Hanafiah, M.A.K.M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresour. Technol. 2007, 99, 3935–3948. [Google Scholar] [CrossRef]
- Waranusantigula, P.; Pokethitiyook, P.; Kruatrachue, M.; Upatham, E.S. Kinetics of cbasic dye (methylene blue) biosorption by giant duckweed (Spirodelapolyrrhiza). Environ. Pollut. 2003, 125, 385–392. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J. Hazard. Mater. 2006, 137, 762–811. [Google Scholar] [CrossRef]
- Varga, M.; Takács, M.; Záray, G.; Varga, I. Comparative study of sorption kinetics and equilibrium of chromium (VI) on charcoals prepared from different low-cost materials. Microchem. J. 2013, 107, 25–30. [Google Scholar] [CrossRef]
- Srinivasan, K. Evaluation of Rice Husk Carbon for the Removal of Trace Inorganic Form Water. Master’s Thesis, Indian Institute of Technology Madras, Chennai, India, 1986. [Google Scholar]
- Iqbal, M.; Saeed, A.; Zafars, S.I. Hybrid biosorbent: An innovative matrix to enhance the biosorption of Cd(II) from aqueous solution. J. Hazard. Mater. 2007, 148, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Malathi, S.; Srinivasan, K.; Gomathi, M. Studies on the removal of Cr (VI) from aqueous solution by activated carbon developed from Cottonseed activated withsulphuric acid. Int. J. ChemTech Res. 2015, 8, 795–802. [Google Scholar]
- Ansari, R. Application of Polyaniline and its Composites for adsorption/Recovery of Chromium (VI) from Aqueous Solutions. Acta Chim. Slov. 2006, 53, 88–94. [Google Scholar]
- Jassal, P.S.; Raut, V.P.; Anand, N. Removal of Chromium (VI) ions from Aqueous solution onto Chitosan and Cross-linked Chitosan Beads. Proc. Indian Natl. Sci. Acad. 2010, 76, 1–6. [Google Scholar]
- Marin, N.M. Natural and Synthetic Polymers Modified with Acid Blue 113 for Removal of Cr3+, Zn2+ and Mn2+. Polymers 2022, 14, 2139. [Google Scholar] [CrossRef] [PubMed]
- Mohy-Eldin, M.S.; Elkady, M.F.; Abu-Saied, M.A.; Rahman, A.A.; Soliman, E.A.; Elzatahry, A.A.; Youssef, M.E. Removal of cadmium ions from synthetic aqueous solutions using a novel nano-sulphonated poly glycidylmethacrylate cation exchanger: Kinetic and equilibrium studies. J. Appl. Polym. Sci. 2010, 118, 3111–3122. [Google Scholar] [CrossRef]
- Elkady, M.F.; Abu-Saied, M.A.; Abdel Rahman, A.M.; Soliman, E.A.; Elzatahry, A.A.; Youssef, M.E.; Mohy Eldin, M.S. Novel nano-sulphonated polyglycidyl methacrylate cation exchanger for removal of heavy metals: Optimization of the operational conditions. Desalination 2011, 279, 152–162. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Aggour, Y.A.; Elaassar, M.R.; Beghet, G.E.; Atta, R.R. Development of nano-crosslinked polyacrylonitrile ions exchanger particles for dyes removal. Desalination Water Treat. 2016, 57, 4255–4266. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Al-Bogami, A.S.; Aly, K.M.; Khan, Z.A.; Mekky, A.E.M.; Saleh, T.S.; Hakamy, A.A.-W. Removal of Chromium (VI) Metal Ions Using Amberlite IRA-420 Anions Exchanger. Desalination Water Treat. 2017, 60, 335–342. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Alamry, K.A.; Al-Malki, M.A. Kinetic and Isothermal Studies of Manganese (VII) Ions Removal using Amberlite IRA-420 Anions Exchanger. Desalination Water Treat. 2017, 72, 30–40. [Google Scholar] [CrossRef]
- Eren, Z.; Acar, F.N. Adsorption of reactive black 5 from an aqueous solution: Equilibrium and kinetic studies. Desalination 2006, 194, 1–10. [Google Scholar] [CrossRef]
- Kannan, N.; Sundaram, M.M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons: A comparative study. Dye. Pigment. 2001, 51, 25–40. [Google Scholar] [CrossRef]
- Bulut, Y.; Aydin, A. A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 2006, 194, 259–267. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; El-Sakka, S.A.; El-Masry, M.M.; Abdel-Gawad, I.I.; Garybe, S.S. Removal of methylene blue dye from aqueous medium by nano polyacrylonitrile particles. Desalination Water Treat. 2012, 44, 151–160. [Google Scholar] [CrossRef]
- Kakurai, T.; Yoshida, E.; Noguch, T. The Reaction of Glycidylmethacrylate Copolymers with Amines. Kobunshi Kagaku 1968, 25, 413–418. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, W.; Lu, Z.; Ming, C. Photografted poly (methyl methacrylate)-based high performance protein microarray for hepatitis B virus biomarker detection in human serum. MedChemComm 2010, 1, 132–135. [Google Scholar] [CrossRef]
| GMA (%) | MMA (%) | Polymerization Yield (%) |
|---|---|---|
| 100 | 0 | 100 |
| 0 | 100 | 89 |
| 75 | 25 | 85.6 |
| 50 | 50 | 92.3 |
| 25 | 75 | 93.5 |
| Comonomers Composition (GMA:MMA) (v/v) | MB-PGMA-Co-PMMA (mg/g) | MB-SPGMA-Co-PMMA (mg/g) |
|---|---|---|
| 100:0 | 0.9738 | 0.974 |
| 0:100 | 0.0935 | 0.258 |
| 75:25 | 0.255 | 0.978 |
| 50:50 | 0.4700 | 0.974 |
| 25:75 | 0.1676 | 0.853 |
| Agitation Speed (rpm) | 150 | 200 | 250 | 300 |
|---|---|---|---|---|
| MB content of the MB-SPGMA composites composition (mg/g) | 3.91 | 3.88 | 3.89 | 3.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Aassar, M.R.; Tamer, T.M.; El-Sayed, M.Y.; Omer, A.M.; Althobaiti, I.O.; Youssef, M.E.; Alolaimi, R.F.; El-Agammy, E.F.; Alruwaili, M.S.; Mohy-Eldin, M.S. Development of Azo Dye Immobilized Poly (Glycidyl Methacrylate-Co-Methyl Methacrylate) Polymers Composites as Novel Adsorbents for Water Treatment Applications: Methylene Blue-Polymers Composites. Polymers 2022, 14, 4672. https://doi.org/10.3390/polym14214672
El-Aassar MR, Tamer TM, El-Sayed MY, Omer AM, Althobaiti IO, Youssef ME, Alolaimi RF, El-Agammy EF, Alruwaili MS, Mohy-Eldin MS. Development of Azo Dye Immobilized Poly (Glycidyl Methacrylate-Co-Methyl Methacrylate) Polymers Composites as Novel Adsorbents for Water Treatment Applications: Methylene Blue-Polymers Composites. Polymers. 2022; 14(21):4672. https://doi.org/10.3390/polym14214672
Chicago/Turabian StyleEl-Aassar, Mohamed R., Tamer M. Tamer, Mohamed Y. El-Sayed, Ahmed M. Omer, Ibrahim O. Althobaiti, Mohamed E. Youssef, Rawan F. Alolaimi, Emam F. El-Agammy, Manar S. Alruwaili, and Mohamed S. Mohy-Eldin. 2022. "Development of Azo Dye Immobilized Poly (Glycidyl Methacrylate-Co-Methyl Methacrylate) Polymers Composites as Novel Adsorbents for Water Treatment Applications: Methylene Blue-Polymers Composites" Polymers 14, no. 21: 4672. https://doi.org/10.3390/polym14214672
APA StyleEl-Aassar, M. R., Tamer, T. M., El-Sayed, M. Y., Omer, A. M., Althobaiti, I. O., Youssef, M. E., Alolaimi, R. F., El-Agammy, E. F., Alruwaili, M. S., & Mohy-Eldin, M. S. (2022). Development of Azo Dye Immobilized Poly (Glycidyl Methacrylate-Co-Methyl Methacrylate) Polymers Composites as Novel Adsorbents for Water Treatment Applications: Methylene Blue-Polymers Composites. Polymers, 14(21), 4672. https://doi.org/10.3390/polym14214672












