Development of Polyphenol-Functionalized Gelatin-Poly(vinylpyrrolidone) IPN for Potential Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of Hydrogels
2.2.1. Gamma Radiation Crosslinking of Gelatin Hydrogels
2.2.2. Synthesis of IPN GE/PVP Hydrogels
2.2.3. Coupling of GA
2.3. Physicochemical Characterization
2.3.1. FT-IR Characterization
2.3.2. Thermogravimetric Analysis
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Equilibrium Swelling Time and Water Content Test
2.3.5. Mechanical Testing
2.3.6. Gallic acid Content
2.3.7. Radical Scavenging Activity
3. Results and Discussion
3.1. Crosslinking of GE
3.2. Radiochemical Yield Ratios of Crosslinking and Scission
3.3. FT-IR Analysis
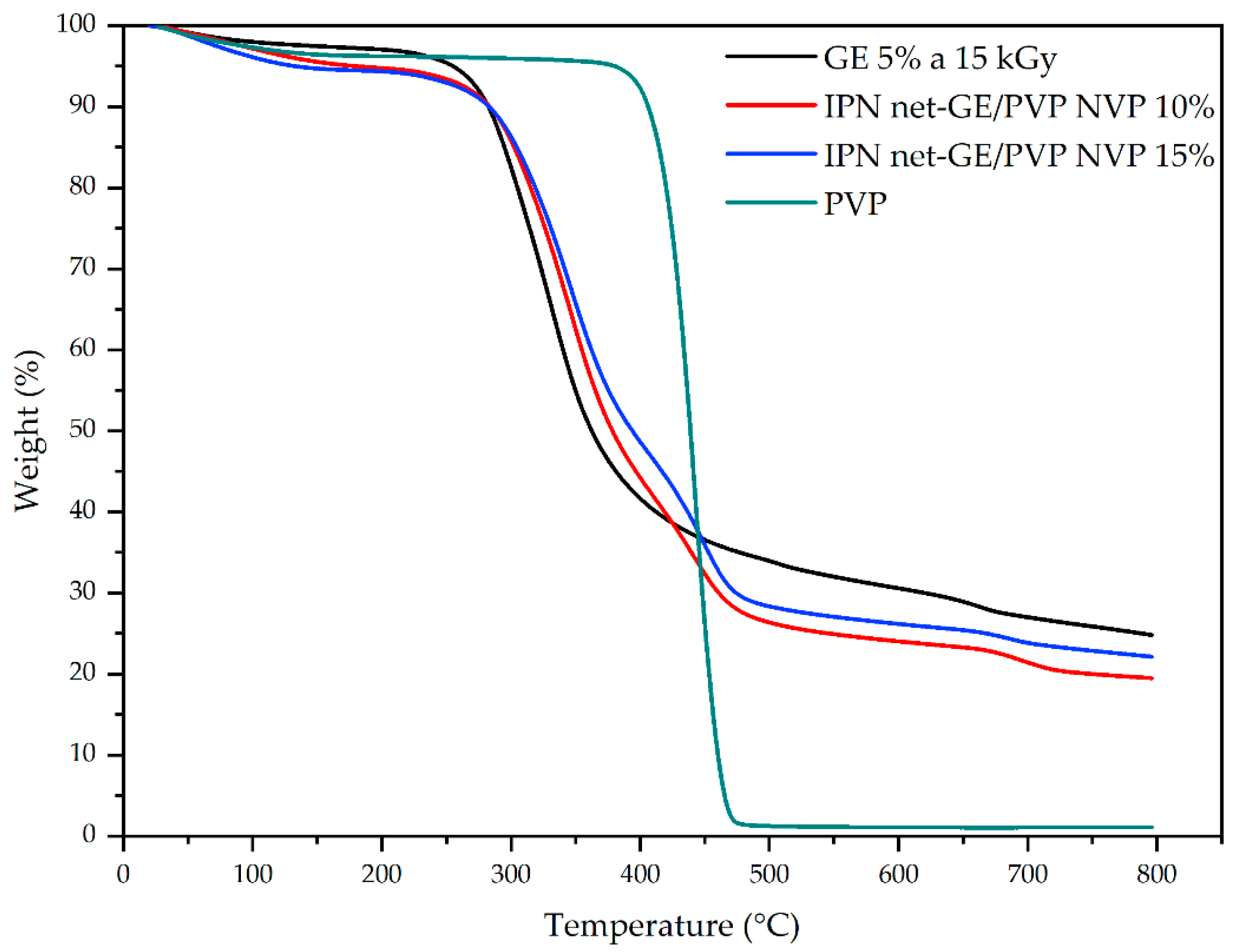
3.4. Thermal Analysis
3.5. Morphology of the Hydrogels
3.6. Swelling Behavior and Water Content
3.7. Mechanical Stability Measurements
3.8. Gallic Acid Content and Radical Scavenging Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, J.; Zhang, H.; Yu, F.; Pei, Y.; Luo, X. Superclear, Porous Cellulose Membranes with Chitosan-Coated Nanofibers for Visualized Cutaneous Wound Healing Dressing. ACS Appl. Mater. Interfaces 2020, 12, 24370–24379. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Critical Review in Oral Biology & Medicine: Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials Based on Chitin and Chitosan in Wound Dressing Applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Baek, S.; Kang, H. Biomaterials to Prevent Post-Operative Adhesion. Materials 2020, 13, 3056. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Publ. Gr. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Dreiss, C.A. Hydrogel Design Strategies for Drug Delivery. Curr. Opin. Colloid Interface Sci. 2020, 48, 1–17. [Google Scholar] [CrossRef]
- Chaudhary, S.; Chakraborty, E. Hydrogel Based Tissue Engineering and Its Future Applications in Personalized Disease Modeling and Regenerative Therapy. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 3. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in Pharmaceutical Formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Sun, X.; Agate, S.; Salem, K.S.; Lucia, L.; Pal, L. Hydrogel-Based Sensor Networks: Compositions, Properties, and Applications—A Review. ACS Appl. Bio Mater. 2021, 4, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Qu, X.; Shi, B.; Zheng, Q.; Lin, X.; Chao, S.; Wang, C.; Zhou, J.; Sun, Y.; et al. Ultra-Stretchable and Fast Self-Healing Ionic Hydrogel in Cryogenic Environments for Artificial Nerve Fiber. Adv. Mater. 2022, 34, 2105416. [Google Scholar] [CrossRef] [PubMed]
- Firlar, I.; Altunbek, M.; Mccarthy, C.; Ramalingam, M. Functional Hydrogels for Treatment of Chronic Wounds. Gels 2022, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Puertas-Bartolomé, M.; Mora-Boza, A.; García-Fernández, L. Emerging Biofabrication Techniques: A Review on Natural Polymers for Biomedical Applications. Polymers 2021, 13, 1209. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rujitanaroj, P.; Pimpha, N.; Supaphol, P. Wound-Dressing Materials with Antibacterial Activity from Electrospun Gelatin Fiber Mats Containing Silver Nanoparticles. Polymer 2008, 49, 4723–4732. [Google Scholar] [CrossRef]
- Wisotzki, E.I.; Hennes, M.; Schuldt, C.; Engert, F.; Knolle, W.; Decker, U.; Käs, J.A.; Zink, M.; Mayr, S.G. Tailoring the Material Properties of Gelatin Hydrogels by High Energy Electron Irradiation. J. Mater. Chem. B 2014, 2, 4297–4309. [Google Scholar] [CrossRef]
- Biswal, D.; Anupriya, B.; Uvanesh, K.; Anis, A.; Banerjee, I.; Pal, K. Effect of Mechanical and Electrical Behavior of Gelatin Hydrogels on Drug Release and Cell Proliferation. J. Mech. Behav. Biomed. Mater. 2016, 53, 174–186. [Google Scholar] [CrossRef]
- Mondragon, G.; Peña-Rodriguez, C.; González, A.; Eceiza, A.; Arbelaiz, A. Bionanocomposites Based on Gelatin Matrix and Nanocellulose. Eur. Polym. J. 2015, 62, 1–9. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Z. Effects of Gelatin-Polyphenol and Gelatin–Genipin Cross-Linking on the Structure of Gelatin Hydrogels. Int. J. Food Prop. 2018, 20, S2822–S2832. [Google Scholar] [CrossRef]
- Ishak, W.H.W.; Ahmad, I.; Ramli, S.; Amin, M.C.I.M. Gamma Irradiation-Assisted Synthesis of Cellulose Nanocrystal-Reinforced Gelatin Hydrogels. Nanomaterials 2018, 8, 749. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, S.; Islam, A.; Gull, N.; Ali, A.; Khan, S.M.; Zia, S.; Anwar, K.; Khan, S.U.; Jamil, T. γ-Irradiated Chitosan Based Injectable Hydrogels for Controlled Release of Drug (Montelukast Sodium). Int. J. Biol. Macromol. 2018, 114, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Fazolin, G.N.; Varca, G.H.C.; de Freitas, L.F.; Rokita, B.; Kadlubowski, S.; Lugão, A.B. Simultaneous Intramolecular Crosslinking and Sterilization of Papain Nanoparticles by Gamma Radiation. Radiat. Phys. Chem. 2020, 171, 108697. [Google Scholar] [CrossRef]
- Kaur, H.; Chatterji, P.R. Interpenetrating Hydrogel Networks. Swelling and Mechanical Properties of the Gelatin-Polyacrylamide Interpenetrating Networks. Macromolecules 1990, 40, 401–410. [Google Scholar] [CrossRef]
- Ajji, Z.; Othman, I.; Rosiak, J.M. Production of Hydrogel Wound Dressings Using Gamma Radiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2005, 229, 375–380. [Google Scholar] [CrossRef]
- Razzak, M.T.; Darwis, D.; Zainuddin; Sukirno. Irradiation of Polyvinyl Alcohol and Polyvinyl Pyrrolidone Blended Hydrogel for Wound Dressing. Radiat. Phys. Chem. 2001, 62, 107–113. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Olejniczak, J. Medical Applications of Radiation Formed Hydrogels. Radiat. Phys. Chem. 1993, 42, 903–906. [Google Scholar] [CrossRef]
- Ng, T.B.; He, J.S.; Niu, S.M.; Pi, Z.F.; Shao, W.; Liu, F.; Zhao, L. A Gallic Acid Derivative and Polysaccharides with Antioxidative Activity from Rose (Rosa Rugosa) Flowers. J. Pharm. Pharmacol. 2010, 56, 537–545. [Google Scholar] [CrossRef]
- Ma, J.; Luo, X.D.; Protiva, P.; Yang, H.; Ma, C.; Basile, M.J.; Weinstein, I.B.; Kennelly, E.J. Bioactive Novel Polyphenols from the Fruit of Manilkara Zapota (Sapodilla). J. Nat. Prod. 2003, 66, 983–986. [Google Scholar] [CrossRef]
- Mansouri, M.T.; Soltani, M.; Naghizadeh, B.; Farbood, Y.; Mashak, A.; Sarkaki, A. A Possible Mechanism for the Anxiolytic-like Effect of Gallic Acid in the Rat Elevated plus Maze. Pharmacol. Biochem. Behav. 2014, 117, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Charlesby, A. Atomic Radiation and Polymers; Pergamon Press Ltd.: Oxford, UK; London, UK, 1960; ISBN 0471359246. [Google Scholar]
- Xu, Y.; Wei, Z.; Xue, C.; Huang, Q. Assembly of Zein–Polyphenol Conjugates via Carbodiimide Method: Evaluation of Physicochemical and Functional Properties. Lwt 2022, 154, 112708. [Google Scholar] [CrossRef]
- Pérez-Calixto, D.; Amat-Shapiro, S.; Zamarrón-Hernández, D.; Vázquez-Victorio, G.; Puech, P.H.; Hautefeuille, M. Determination by Relaxation Tests of the Mechanical Properties of Soft Polyacrylamide Gels Made for Mechanobiology Studies. Polymers 2021, 13, 629. [Google Scholar] [CrossRef] [PubMed]
- van Otterloo, J.; Cruden, A.R. Rheology of Pig Skin Gelatine: Defining the Elastic Domain and Its Thermal and Mechanical Properties for Geological Analogue Experiment Applications. Tectonophysics 2016, 683, 86–97. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Analytical Methods The Folin–Ciocalteu Assay Revisited: Improvement of Its Speci Fi City for Total Phenolic Content Determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Ikawa, M.; Shaper, T.D.; Dollard, C.A.; Sasner, J.J. Utilization of Folin-Ciocalteau Phenol Reagent for the Detection of Certain Nitrogen Compounds. J. Agric. Food Chem. 2003, 51, 1811–1815. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Lee, D.H.; Arisaka, Y.; Tonegawa, A.; Kang, T.W.; Tamura, A.; Yui, N. Cellular Orientation on Repeatedly Stretching Gelatin Hydrogels with Supramolecular Crosslinking. Polymers 2019, 11, 2095. [Google Scholar] [CrossRef]
- Rahma, A.; Munir, M.M.; Khairurrijal; Prasetyo, A.; Suendo, V.; Rachmawati, H. Intermolecular Interactions and the Release Pattern of Electrospun Curcumin-Polyvinyl(Pyrrolidone) Fiber. Biol. Pharm. Bull. 2016, 39, 163–173. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, X.; Tse, H.-F.; Rong, J. Gallic Acid-L-Leucine Conjugate Protects Mice against LPS-Induced Inflammation and Sepsis via Correcting Proinflammatory Lipid Mediator Profiles and Oxidative Stress. Oxid. Med. Cell. Longev. 2018, 2018, 1081287. [Google Scholar] [CrossRef]
- Albouchi, F.; Avola, R.; Maria Lo Dico, G.; Calabrese, V.; Graziano, A.C.E.; Abderrabba, M.; Cardile, V. Melaleuca Styphelioides Sm. Polyphenols Modulate Interferon Gamma/Histamine-Induced Inflammation in Human NCTC 2544 Keratinocytes. Molecules 2018, 23, 2526. [Google Scholar] [CrossRef] [PubMed]







| Concentration | 5% | 10% |
| Gd/Gc | −0.0436 | 0.1115 |
| Dg (kGy) | 0.2829 | 0.3978 |
| GE Concentration | Total Absorbed Dose (kGy) | Water Content (%) a | Equilibrium Swelling (%) b |
|---|---|---|---|
| 5% | 5 | 92.22 ± 1.64 | 1220 ± 252.43 |
| 10 | 88.43 ± 0.25 | 764.81 ± 18.28 | |
| 15 | 91.01 ± 0.54 | 1014.79 ± 68.98 | |
| 30 | 83.49 ± 1.19 | 507 ± 42.36 | |
| 10% | 5 | 89.76 ± 2.59 | 923.86 ± 283.16 |
| 10 | 85.71 ± 1.62 | 606.12 ± 82.48 | |
| 15 | 86.09 ± 1.22 | 622.69 ± 61.08 | |
| 30 | 82.01 ± 0.47 | 485.40 ± 16.00 |
| E (kPa) | |
|---|---|
| net-GE | 15.82 ± 2.59 |
| net-GE/IPN-PVP 10% | 25.71 ± 4.75 |
| net-GE/IPN-PVP 15% | 44.85 ± 7.55 |
| Sample | Gallic Acid Content (mg GA/g Sample) a | Antioxidant Activity b |
|---|---|---|
| net-GE (pristine) | 0.20 ± 0.21 | 13.085 ± 1.22 |
| net-GE + GA | 1.39 ± 0.19 | 83.281± 5.85 |
| IPN net-GE/PVP | 1.64 ± 0.34 | 91.61 ± 1.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escutia-Guadarrama, L.; Morales, D.; Pérez-Calixto, D.; Burillo, G. Development of Polyphenol-Functionalized Gelatin-Poly(vinylpyrrolidone) IPN for Potential Biomedical Applications. Polymers 2022, 14, 4705. https://doi.org/10.3390/polym14214705
Escutia-Guadarrama L, Morales D, Pérez-Calixto D, Burillo G. Development of Polyphenol-Functionalized Gelatin-Poly(vinylpyrrolidone) IPN for Potential Biomedical Applications. Polymers. 2022; 14(21):4705. https://doi.org/10.3390/polym14214705
Chicago/Turabian StyleEscutia-Guadarrama, Lidia, David Morales, Daniel Pérez-Calixto, and Guillermina Burillo. 2022. "Development of Polyphenol-Functionalized Gelatin-Poly(vinylpyrrolidone) IPN for Potential Biomedical Applications" Polymers 14, no. 21: 4705. https://doi.org/10.3390/polym14214705
APA StyleEscutia-Guadarrama, L., Morales, D., Pérez-Calixto, D., & Burillo, G. (2022). Development of Polyphenol-Functionalized Gelatin-Poly(vinylpyrrolidone) IPN for Potential Biomedical Applications. Polymers, 14(21), 4705. https://doi.org/10.3390/polym14214705





