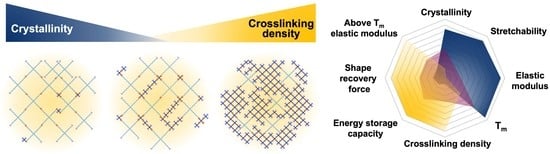
Influences of Crystallinity and Crosslinking Density on the Shape Recovery Force in Poly(ε-Caprolactone)-Based Shape-Memory Polymer Blends
Abstract
:1. Introduction
2. Materials and Methods
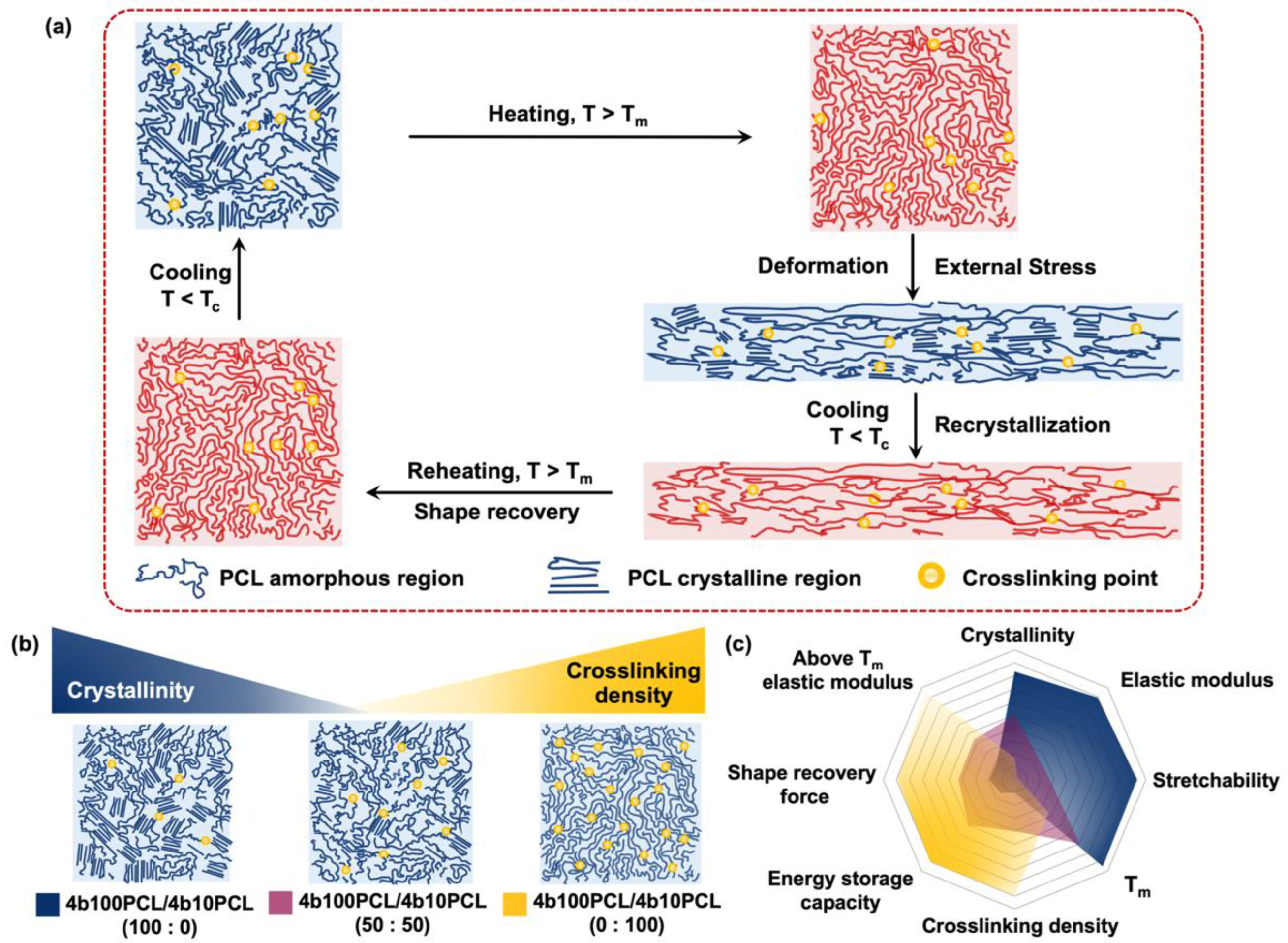
3. Results and Discussions
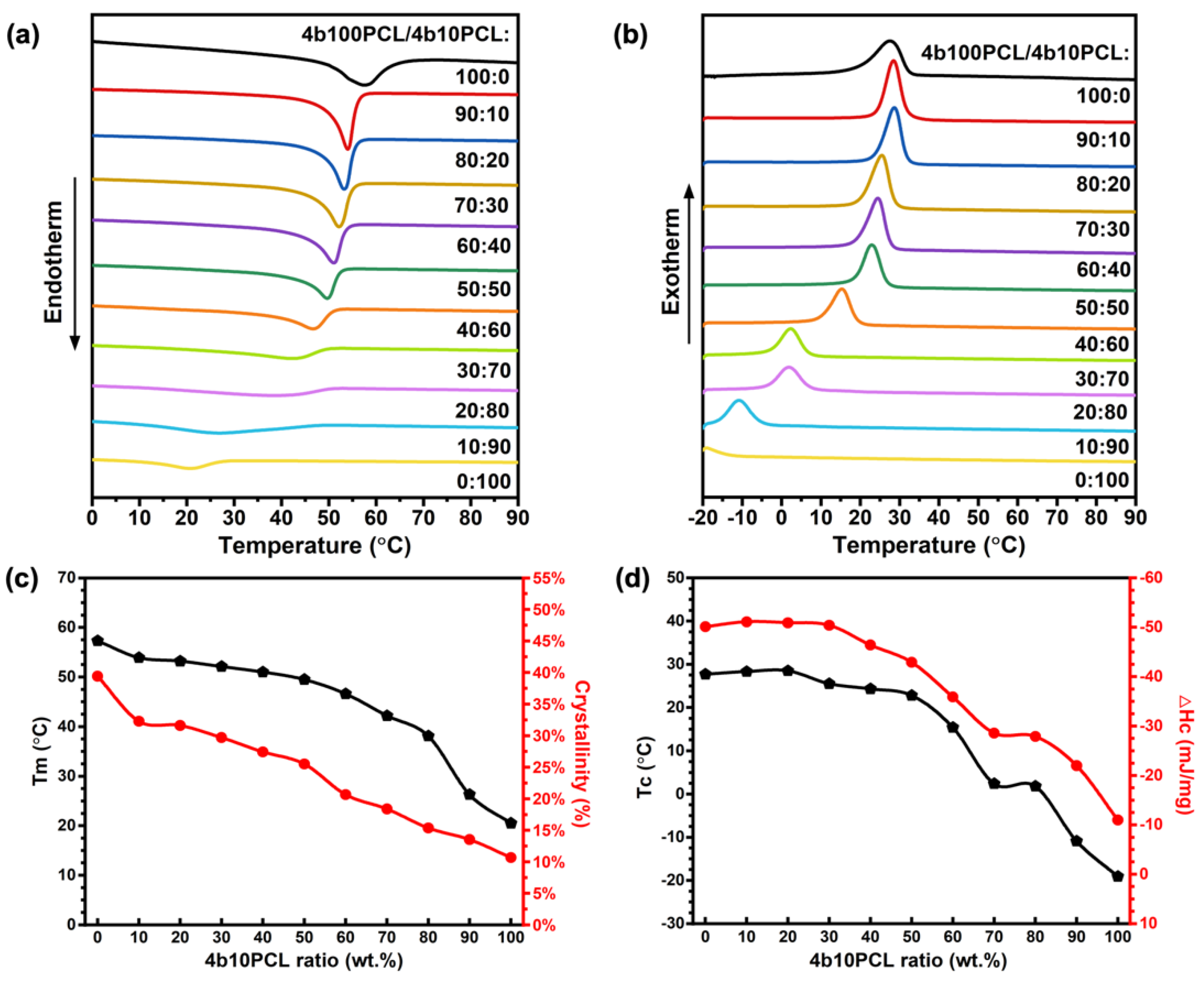
3.1. Thermal Properties
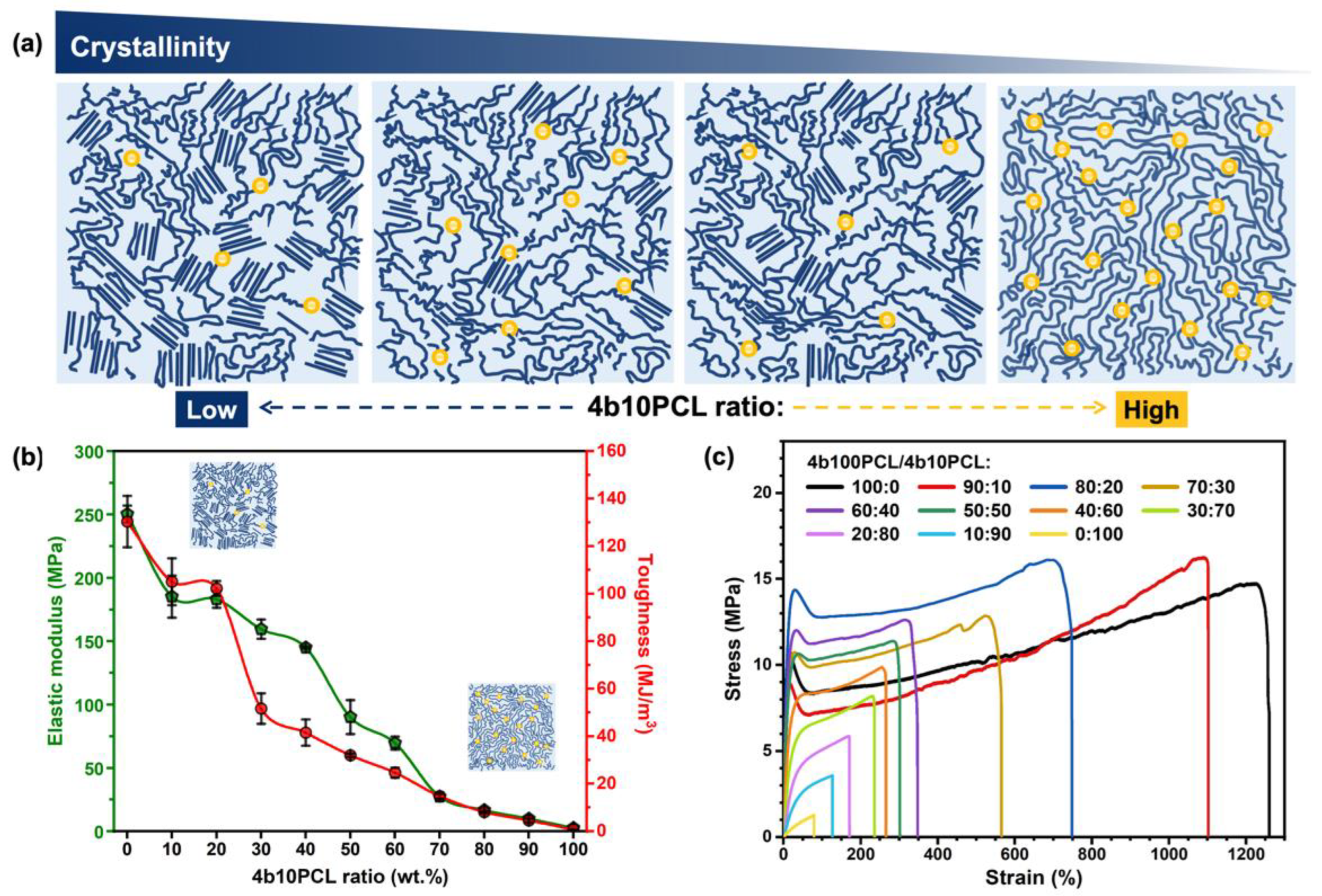
3.2. Mechanical Properties
3.3. Shape-Memory Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lendlein, A.; Langer, R. Biodegradable, Elastic Shape-Memory Polymers for Potential Biomedical Applications. Science 2002, 296, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Ebara, M.; Uto, K.; Idota, N.; Hoffman, J.M.; Aoyagi, T. Shape-memory surface with dynamically tunable nano-geometry activated by body heat. Adv. Mater. 2012, 24, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, S.; Niiyama, E.; Sugo, K.; Uto, K.; Takenaka, S.; Kikuchi, A.; Ebara, M. Shape-memory balloon offering simultaneous thermo/chemotherapies to improve anti-osteosarcoma efficacy. Biomater. Sci. 2021, 9, 6957–6965. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Uto, K.; Niiyama, E.; Ebara, M.; Nagao, T. Hybridizing Poly(ε-caprolactone) and Plasmonic Titanium Nitride Nanoparticles for Broadband Photoresponsive Shape Memory Films. ACS Appl. Mater. Interfaces 2016, 8, 5634–5640. [Google Scholar] [CrossRef]
- Shou, Q.; Uto, K.; Iwanaga, M.; Ebara, M.; Aoyagi, T. Near-infrared light-responsive shape-memory poly(ε-caprolactone) films that actuate in physiological temperature range. Polym. J. 2014, 46, 492–498. [Google Scholar] [CrossRef]
- Baer, G.M.; Small, W.; Wilson, T.S.; Benett, W.J.; Matthews, D.L.; Hartman, J.; Maitland, D.J. Fabrication and in vitro deployment of a laser-activated shape memory polymer vascular stent. Biomed. Eng. Online 2007, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- Small, W., IV; Wilson, T.S.; Benett, W.J.; Loge, J.M.; Maitland, D.J. Laser-activated shape memory polymer intravascular thrombectomy device. Opt. Express 2005, 13, 8204. [Google Scholar] [CrossRef]
- Uto, K.; Ebara, M. Magnetic-Responsive Microparticles that Switch Shape at 37 °C. Appl. Sci. 2017, 7, 1203. [Google Scholar] [CrossRef] [Green Version]
- Mohr, R.; Kratz, K.; Weigel, T.; Lucka-Gabor, M.; Moneke, M.; Lendlein, A. Initiation of shape-memory effect by inductive heating of magnetic nanoparticles in thermoplastic polymers. Proc. Natl. Acad. Sci. USA 2006, 103, 3540–3545. [Google Scholar] [CrossRef] [Green Version]
- Yungerman, I.; Starodumov, I.; Fulati, A.; Uto, K.; Ebara, M.; Moskovitz, Y. Full-Atomistic Optimized Potentials for Liquid Simulations and Polymer Consistent Force Field Models for Biocompatible Shape-Memory Poly(ε-caprolactone). J. Phys. Chem. B 2022, 126, 3961–3972. [Google Scholar] [CrossRef]
- Schönfeld, D.; Chalissery, D.; Wenz, F.; Specht, M.; Eberl, C.; Pretsch, T. Actuating shape memory polymer for thermoresponsive soft robotic gripper and programmable materials. Molecules 2021, 26, 522. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Chang, H.H.; Naguib, H.E. Biocompatible shape memory polymer actuators with high force capabilities. Eur. Polym. J. 2015, 67, 186–198. [Google Scholar] [CrossRef]
- Chan, B.Q.Y.; Low, Z.W.K.; Heng, S.J.W.; Chan, S.Y.; Owh, C.; Loh, X.J. Recent Advances in Shape Memory Soft Materials for Biomedical Applications. ACS Appl. Mater. Interfaces 2016, 8, 10070–10087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, N.; Hingorani, H.; Ding, N.; Wang, D.; Yuan, C.; Zhang, B.; Gu, G.; Ge, Q. Fast-Response, Stiffness-Tunable Soft Actuator by Hybrid Multimaterial 3D Printing. Adv. Funct. Mater. 2019, 29, 1806698. [Google Scholar] [CrossRef]
- Wang, J.; Tu, Z.; Zhang, H.; Wang, M.-M.; Liu, W.; Qu, J.-P. Actuation Mechanisms of a Semicrystalline Elastomer-Based Polymer Artificial Muscle with High Actuation Strain. Macromolecules 2022, 55, 3986–3999. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, X.X. Two-Way Reversible Shape Memory Polymers Containing Polydopamine Nanospheres: Light Actuation, Robotic Locomotion, and Artificial Muscles. ACS Biomater. Sci. Eng. 2018, 4, 3099–3106. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Wen, J.; Liu, J.; Zhang, D.; Li, J.; Chu, H. Ultra-Stretchable, Variable Modulus, Shape Memory Multi-Purpose Low Hysteresis Hydrogel Derived from Solvent-Induced Dynamic Micelle Sea-Island Structure. Adv. Funct. Mater. 2021, 31, 2011259. [Google Scholar] [CrossRef]
- Kumar, B.; Hu, J.; Pan, N. Smart medical stocking using memory polymer for chronic venous disorders. Biomaterials 2016, 75, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Fulati, A.; Uto, K.; Iwanaga, M.; Watanabe, M.; Ebara, M. Smart Shape-Memory Polymeric String for the Contraction of Blood Vessels in Fetal Surgery of Sacrococcygeal Teratoma. Adv. Healthc. Mater. 2022, 11, 2200050. [Google Scholar] [CrossRef]
- Zhao, Q.; Qi, H.J.; Xie, T. Recent progress in shape memory polymer: New behavior, enabling materials, and mechanistic understanding. Prog. Polym. Sci. 2015, 49–50, 79–120. [Google Scholar] [CrossRef]
- Xia, Y.; He, Y.; Zhang, F.; Liu, Y.; Leng, J. A Review of Shape Memory Polymers and Composites: Mechanisms, Materials, and Applications. Adv. Mater. 2021, 33, 2000713. [Google Scholar] [CrossRef] [PubMed]
- Miyasako, H.; Yamamoto, K.; Nakao, A.; Aoyagi, T. Preparation of cross-linked poly[(ε-caprolactone)-co-lactide] and biocompatibility studies for tissue engineering materials. Macromol. Biosci. 2007, 7, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-Y.; Li, L.; Shi, L.-Y.; Yang, K.-K.; Wang, Y.-Z. Effect of Self-Nucleation and Stress-Induced Crystallization on the Tunable Two-Way Shape-Memory Effect of a Semicrystalline Network. Macromolecules 2022, 55, 5104–5114. [Google Scholar] [CrossRef]
- Chen, J.; Deng, C.; Hong, R.; Fu, Q.; Zhang, J. Effect of thermal annealing on crystal structure and properties of PLLA/PCL blend. J. Polym. Res. 2020, 27, 221. [Google Scholar] [CrossRef]
- Uto, K.; Yamamoto, K.; Hirase, S.; Aoyagi, T. Temperature-responsive cross-linked poly(ε-caprolactone) membrane that functions near body temperature. J. Control Release 2006, 110, 408–413. [Google Scholar] [CrossRef]
- Sedov, I.; Magsumov, T.; Abdullin, A.; Yarko, E.; Mukhametzyanov, T.; Klimovitsky, A.; Schick, C. Influence of the Cross-Link Density on the Rate of Crystallization of Poly(ε-Caprolactone). Polymers 2018, 10, 902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lepoittevin, B.; Devalckenaere, M.; Pantoustier, N.; Alexandre, M.; Kubies, D.; Calberg, C.; Jérôme, R.; Dubois, P. Poly(ε-caprolactone)/clay nanocomposites prepared by melt intercalation: Mechanical, thermal and rheological properties. Polymer 2002, 43, 4017–4023. [Google Scholar] [CrossRef]
- Miao, W.; Zou, W.; Luo, Y.; Zheng, N.; Zhao, Q.; Xie, T. Structural tuning of polycaprolactone based thermadapt shape memory polymer. Polym. Chem. 2020, 11, 1369–1374. [Google Scholar] [CrossRef]
- Rodriguez, E.D.; Luo, X.; Mather, P.T. Linear/Network Poly(ε-caprolactone) Blends Exhibiting Shape Memory Assisted Self-Healing (SMASH). ACS Appl. Mater. Interfaces 2011, 3, 152–161. [Google Scholar] [CrossRef]
- Babaie, A.; Rezaei, M.; Sofla, R.L.M. Investigation of the effects of polycaprolactone molecular weight and graphene content on crystallinity, mechanical properties and shape memory behavior of polyurethane/graphene nanocomposites. J. Mech. Behav. Biomed. Mater. 2019, 96, 53–68. [Google Scholar] [CrossRef]
- Baniasadi, M.; Maleki-Bigdeli, M.-A.; Baghani, M. Force and multiple-shape-recovery in shape-memory-polymers under finite deformation torsion-extension. Smart Mater. Struct. 2020, 29, 055011. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fulati, A.; Uto, K.; Ebara, M. Influences of Crystallinity and Crosslinking Density on the Shape Recovery Force in Poly(ε-Caprolactone)-Based Shape-Memory Polymer Blends. Polymers 2022, 14, 4740. https://doi.org/10.3390/polym14214740
Fulati A, Uto K, Ebara M. Influences of Crystallinity and Crosslinking Density on the Shape Recovery Force in Poly(ε-Caprolactone)-Based Shape-Memory Polymer Blends. Polymers. 2022; 14(21):4740. https://doi.org/10.3390/polym14214740
Chicago/Turabian StyleFulati, Ailifeire, Koichiro Uto, and Mitsuhiro Ebara. 2022. "Influences of Crystallinity and Crosslinking Density on the Shape Recovery Force in Poly(ε-Caprolactone)-Based Shape-Memory Polymer Blends" Polymers 14, no. 21: 4740. https://doi.org/10.3390/polym14214740
APA StyleFulati, A., Uto, K., & Ebara, M. (2022). Influences of Crystallinity and Crosslinking Density on the Shape Recovery Force in Poly(ε-Caprolactone)-Based Shape-Memory Polymer Blends. Polymers, 14(21), 4740. https://doi.org/10.3390/polym14214740