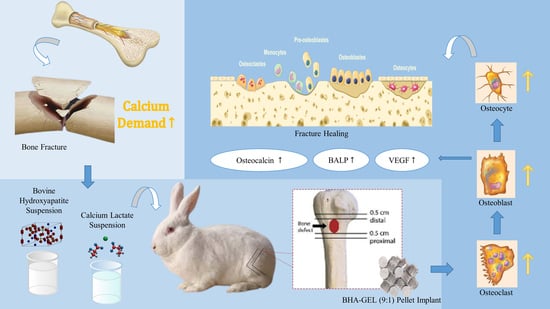
Acceleration of Bone Fracture Healing through the Use of Bovine Hydroxyapatite or Calcium Lactate Oral and Implant Bovine Hydroxyapatite–Gelatin on Bone Defect Animal Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Materials
2.3. BHA–GEL Pellet Preparation
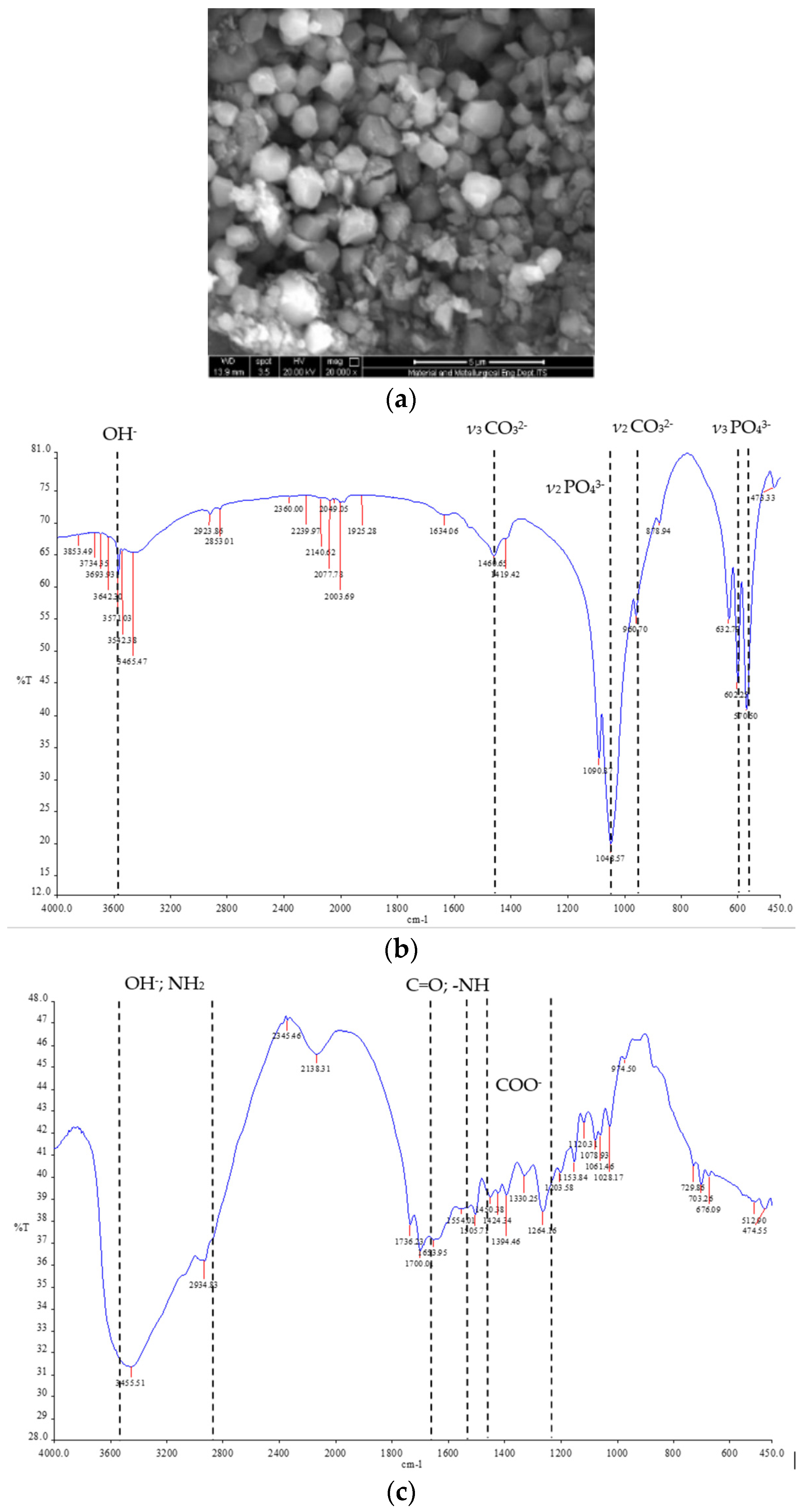
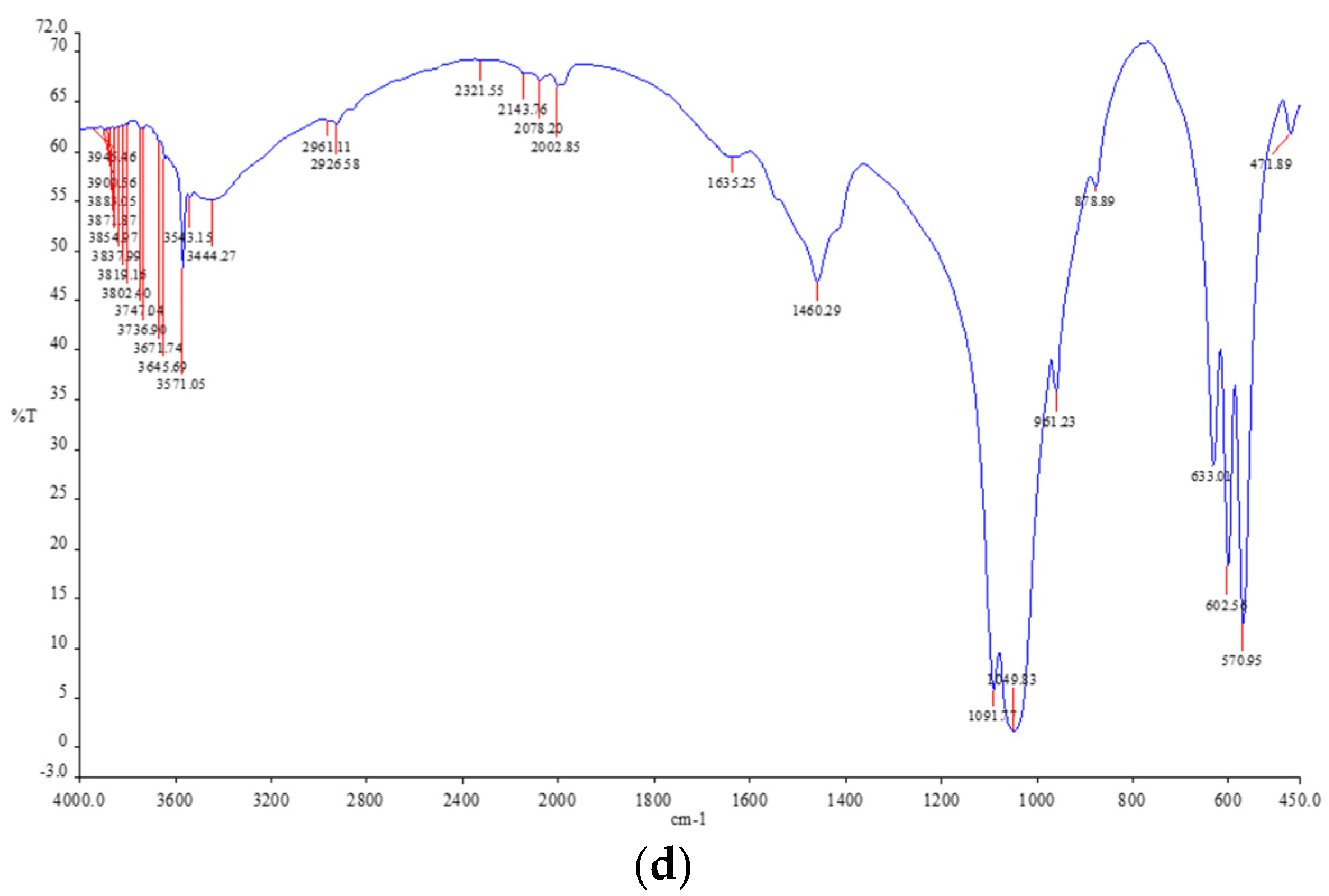
2.4. Characterizations of Pellet
2.5. Blood Sampling Technique
2.6. Bone Sampling Technique
2.7. Radiology Examination
2.8. Haematoxylin and Eosin Staining
2.9. Immunohistochemistry
2.10. Bone Alkaline Phosphatase
2.11. Calcium Concentration
2.12. Statistical Analysis
3. Results
3.1. Characterizations of Pellet
3.2. Radiology Examination
3.3. Examination of the number of Osteoblasts, Osteoclasts and Osteocytes through Hematoxylin-Eosin Staining
3.4. Examination of the Amount and Distribution of VEGF through Immunohistochemistry
3.5. Examination of the Amount and Distribution of Osteocalcin through Immunohistochemistry
3.6. Examination of BALP Levels through ELISA
3.7. Examination of Calcium Levels in the Blood through the Calcium Colorimetric Assay Kit
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrett, K.E.; Barman, S.M.; Boitano, S.; Brooks, H. Ganong’s Review of Medical Physiology; McGraw-Hill Education: New York City, NY, USA, 2012; 768p. [Google Scholar]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture Healing: Mechanisms and Interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noorisa, R.; Apriliwati, D.; Aziz, A.; Bayusentono, S. The Characteristic of Patients With Femoral Fracture In Department Of Orthopaedic And Traumatology RSUD Dr. Soetomo Surabaya 2013–2016. J. Orthop. Traumatol. Surabaya 2017, 6, 1–11. [Google Scholar] [CrossRef]
- Ferdiansyah Mahyudin, N. Graft Tulang & Material Pengganti Tulang (Karaktersitik Dan Strategi Aplikasi Klinis); Airlangga University Press: Surabaya, Indonesia, 2018. [Google Scholar]
- Budiatin, A.S.; Zainuddin, M.; Khotib, J.; Ferdiansyah. Pelepasan Gentamisin Dari Pelet Bovine-Hydroxyapatite-Gelatin Sebagai Ssitem Penghantaran Obat Dan Pengisi Tulang. J. Farm. Dan Ilmu Kefarmasian Indones. 2014, 1, 10–15. [Google Scholar]
- Ardhiyanto, H. Peran Hidroksiapatit Sebagai Material Bone Graft Dalam Menstimulasi Kepadatan Kolagen Tipe L Pada Proses Penyembuhan Tulang. Stomatognatic-J. Kedokt. Gigi 2015, 9, 16–18. [Google Scholar]
- Marfil, P.H.M.; Anhê, A.C.B.M.; Telis, V. Texture and Microstructure of Gelatin/Corn Starch-Based Gummy Confections. Food Biophys. 2012, 7, 236–243. [Google Scholar] [CrossRef]
- Budiatin, A.S.; ZAINUDDIN, M.; KHOTIB, J. Biocompatible Composite as Gentamicin Delivery System for Osteomyelitis and Bone Regeneration. Int. J. Pharm. Pharm. Sci. 2014, 6, 4. [Google Scholar]
- Chao, W.W.; Lin, B.F. Isolation and Identification of Bioactive Compounds in Andrographis Paniculata (Chuanxinlian). Chin. Med. 2010, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Gani, M.A. Studi Komparasi Osteokonduktivitas Natural Dan Synthetic Hydroxyapatite Serta Keterlibatan Polaritas Makrofag Pada Defek Tulang; Universitas Airlangga: Surabaya, Indonesia, 2021. [Google Scholar]
- Khotib, J.; Lasandara, C.S.; Samirah, S.; Budiatin, A.S. Acceleration of Bone Fracture Healing through the Use of Natural Bovine Hydroxyapatite Implant on Bone Defect Animal Model. Folia Med. Indones. 2019, 55, 176. [Google Scholar] [CrossRef] [Green Version]
- Mandagi, C.A.F.; Bidjuni, H.; Hamel, R.S. Karakteristik Yang Berhubungan Dengan Tingkat Nyeri Pada Pasien Fraktur Di Ruang Bedah Rumah Sakit Umum Gmim Bethesda Tomohon. J. Keperawatan. 2017, 5, 1–7. [Google Scholar]
- Schmidt, K.H.; Wörner, U.M.; Buck, H.J. Examination of New Bone Growth on Aluminium Oxide Implant Contact Surfaces after Oral Administration of Ossein-Hydroxyapatite Compound to Rats. Curr. Med. Res. Opin. 1988, 11, 107–115. [Google Scholar] [CrossRef]
- Straub, D.A. Calcium Supplementation in Clinical Practice: A Review of Forms, Doses, and Indications. Nutr. Clin. Pract. 2007, 22, 286–296. [Google Scholar] [CrossRef]
- Wang, Y.; Von Euw, S.; Fernandes, F.M.; Cassaignon, S.; Selmane, M.; Laurent, G.; Pehau-Arnaudet, G.; Coelho, C.; Bonhomme-Coury, L.; Giraud-Guille, M.M.; et al. Water-Mediated Structuring of Bone Apatite. Nat. Mater. 2013, 12, 1144–1153. [Google Scholar] [CrossRef] [Green Version]
- Olivier, F.; Rochet, N.; Delpeux-Ouldriane, S.; Chancolon, J.; Sarou-Kanian, V.; Fayon, F.; Bonnamy, S. Strontium Incorporation into Biomimetic Carbonated Calcium-Deficient Hydroxyapatite Coated Carbon Cloth: Biocompatibility with Human Primary Osteoblasts. Mater. Sci. Eng. C 2020, 116, 111192. [Google Scholar] [CrossRef] [PubMed]
- Germaini, M.M.; Detsch, R.; Grünewald, A.; Magnaudeix, A.; Lalloue, F.; Boccaccini, A.R.; Champion, E. Osteoblast and Osteoclast Responses to Porous A/B Carbonate-Substituted Hydroxyapatite Ceramics for Bone Regeneration. Biomed. Mater. 2017, 12, 035008. [Google Scholar] [CrossRef] [PubMed]
- Bristow, S.M.; Gamble, G.D.; Stewart, A.; Horne, L.; House, M.E.; Aati, O.; Mihov, B.; Horne, A.M.; Reid, I.R. Acute and 3-Month Effects of Microcrystalline Hydroxyapatite, Calcium Citrate and Calcium Carbonate on Serum Calcium and Markers of Bone Turnover: A Randomised Controlled Trial in Postmenopausal Women. Br. J. Nutr. 2014, 112, 1611–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stellon, A.; Davies, A.; Webb, A.; Williams, R. Microcrystalline Hydroxyapatite Compound in Prevention of Bone Loss in Corticosteroid-Treated Patients with Chronic Active Hepatitis. Postgrad. Med. J. 1985, 61, 791–796. [Google Scholar] [CrossRef] [Green Version]
- Adrianto, H.B. Peran Hidroksiapatit Sebagai Bone Graft Dalam Proses Penyembuhan Tulang. Stomatognatic-J. Kedokt. Gigi 2011, 8, 118–121. [Google Scholar]
- Sweetman, S.C. Martindale; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Muliani, I.; Nyoman, M.K.; Ketut, T. Pemberian Kalsium Laktat Dan Berenang Meningkatkan Osteoblast Pada Epiphysis Tulang Radius Mencit Perimenopause. J. Vet. 2014, 15, 39–45. [Google Scholar]
- Wagner, G.; Kindrick, S.; Hertzler, S.; DiSilvestro, R.A. Effects of Various Forms of Calcium on Body Weight and Bone Turnover Markers in Women Participating in a Weight Loss Program. J. Am. Coll. Nutr. 2007, 26, 456–461. [Google Scholar] [CrossRef]
- Szterner, P.; Biernat, M. The Synthesis of Hydroxyapatite by Hydrothermal Process with Calcium Lactate Pentahydrate: The Effect of Reagent Concentrations, PH, Temperature, and Pressure. Bioinorg. Chem. Appl. 2022, 2022, 3481677. [Google Scholar] [CrossRef]
- Gaby, K. Bioavailability and Solubility of Different Calcium-Salts as a Basis for Calcium Enrichment of Beverages. Food Nutr. Sci. 2010, 2010, 3035. [Google Scholar]
- Khotib, J.; Gani, M.A.; Budiatin, A.S.; Lestari, M.L.A.D.; Rahadiansyah, E.; Ardianto, C. Signaling Pathway and Transcriptional Regulation in Osteoblasts during Bone Healing: Direct Involvement of Hydroxyapatite as a Biomaterial. Pharmaceuticals 2021, 14, 615. [Google Scholar] [CrossRef] [PubMed]
- Wölfl, C.; Schweppenhäuser, D.; Gühring, T.; Takur, C.; Höner, B.; Kneser, U.; Grützner, P.A.; Kolios, L. Characteristics of Bone Turnover in the Long Bone Metaphysis Fractured Patients with Normal or Low Bone Mineral Density (BMD). PLoS ONE 2014, 9, e96058. [Google Scholar] [CrossRef] [Green Version]
- Castelo-Branco, C.; Cancelo Hidalgo, M.J.; Palacios, S.; Ciria-Recasens, M.; Fernández-Pareja, A.; Carbonell-Abella, C.; Manasanch, J.; Haya-Palazuelos, J. Efficacy and Safety of Ossein-Hydroxyapatite Complex versus Calcium Carbonate to Prevent Bone Loss. Climacteric 2020, 23, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, C.; Ciria-Recasens, M.; Cancelo-Hidalgo, M.J.; Palacios, S.; Haya-Palazuelos, J.; Carbonell-Abelló, J.; Blanch-Rubió, J.; Martinez-Zapata, M.J.; Manasanch, J.; Pérez-Edo, L. Efficacy of Ossein-Hydroxyapatite Complex Compared with Calcium Carbonate to Prevent Bone Loss: A Meta-Analysis. Menopause 2009, 16, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, C.; Dávila Guardia, J. Use of Ossein—Hydroxyapatite Complex in the Prevention of Bone Loss: A Review. Climacteric 2015, 18, 29–37. [Google Scholar] [CrossRef]
- Costescu, A.; Pasuk, I.; Ungureanu, F.; Dinischiotu, A.; Costache, M.; Huneau, F.; Galaup, S.; le Coustumer, P.; Predoi, D. Physico-Chemical Properties Of Nano-Sized Hexagonal Hydroxyapatite Powder Synthesized By Sol-Gel. Dig. J. Nanomater. Biostructures. 2010, 5, 989–1000. [Google Scholar]
- Budiatin, A.S.; Samirah; Gani, M.A.; Nilamsari, W.P.; Ardianto, C. The Characterization of Bovine Bone-Derived Hydroxyapatite Isolated Using Novel Non-Hazardous Method. J. Biomim. Biomater. Biomed. Eng. 2020, 45, 49–56. [Google Scholar] [CrossRef]
- Khoo, W.; Nor, F.M.; Ardhyananta, H.; Kurniawan, D. Preparation of Natural Hydroxyapatite from Bovine Femur Bones Using Calcination at Various Temperatures. Procedia Manuf. 2015, 2, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Brahimi, S.; Ressler, A.; Boumchedda, K.; Hamidouche, M.; Kenzour, A.; Djafar, R.; Antunović, M.; Bauer, L.; Hvizdoš, P.; Ivanković, H. Preparation and Characterization of Biocomposites Based on Chitosan and Biomimetic Hydroxyapatite Derived from Natural Phosphate Rocks. Mater. Chem. Phys. 2022, 276, 125421. [Google Scholar] [CrossRef]
- Abdelmagid, S.; Barbe, M.; Hadjiargyrou, M.; Owen, T.; Razmpour, R.; Rehman, S.; Popoff, S.; Safadi, F. Temporal and Spatial Expression of Osteoactivin During Fracture Repair. J. Cell. Biochem. 2010, 111, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Budiatin, A.S.; Gani, M.A.; Samirah; Ardianto, C.; Raharjanti, A.M.; Septiani, I.; Putri, N.P.K.P.; Khotib, J. Bovine Hydroxyapatite-Based Bone Scaffold with Gentamicin Accelerates Vascularization and Remodeling of Bone Defect. Int. J. Biomater. 2021, 2021, 5560891. [Google Scholar] [CrossRef]
- Shi, P.; Liu, M.; Fan, F.; Yu, C.; Lu, W.; Du, M. Characterization of Natural Hydroxyapatite Originated from Fish Bone and Its Biocompatibility with Osteoblasts. Mater. Sci. Eng. C. 2018, 90, 706–712. [Google Scholar] [CrossRef]
- He, L.H.; Liu, M.; He, Y.; Xiao, E.; Zhao, L.; Zhang, T.; Yang, H.Q.; Zhang, Y. TRPV1 Deletion Impaired Fracture Healing and Inhibited Osteoclast and Osteoblast Differentiation. Sci. Rep. 2017, 7, 42385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, K.; Olsen, B.R. The Roles of Vascular Endothelial Growth Factor in Bone Repair and Regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture Healing under Healthy and Inflammatory Conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef]
- Li, B.; Wang, H.; Qiu, G.; Su, X.; Wu, Z. Synergistic Effects of Vascular Endothelial Growth Factor on Bone Morphogenetic Proteins Induced Bone Formation in Vivo: Influencing Factors and Future Research Directions. BioMed Res. Int. 2016, 2016, 2869572. [Google Scholar] [CrossRef] [Green Version]
- Nofikasari, I.; Rufaida, A.; Aqmarina, C.D.; Failasofia, F.; Fauzia, A.R.; Handajani, J. Efek Aplikasi Topikal Gel Ekstrak Pandan Wangi Terhadap Penyembuhan Luka Gingiva. Maj. Kedokt. Gigi Indones. 2016, 2, 53–59. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Yang, C.; Li, Y.; Dai, Z. An Overview of Osteocalcin Progress. J. Bone Miner. Metab. 2016, 34, 367–379. [Google Scholar] [CrossRef]
- Hesaraki, S.; Nazarian, H.; Pourbaghi-Masouleh, M.; Borhan, S. Comparative Study of Mesenchymal Stem Cells Osteogenic Differentiation on Low-Temperature Biomineralized Nanocrystalline Carbonated Hydroxyapatite and Sintered Hydroxyapatite. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2014, 102, 108–118. [Google Scholar] [CrossRef]
- Jafary, F.; Hanachi, P.; Gorjipour, K. Osteoblast Differentiation on Collagen Scaffold with Immobilized Alkaline Phosphatase. Int. J. Organ. Transplant. Med. 2017, 8, 195. [Google Scholar] [PubMed]
- Golub, E.E.; Boesze-Battaglia, K. The Role of Alkaline Phosphatase in Mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Greenblatt, M.B.; Tsai, J.N.; Wein, M.N. Bone Turnover Markers in the Diagnosis and Monitoring of Metabolic Bone Disease. Clin. Chem. 2017, 63, 464–474. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, J.; Horowitz, M.; Choi, Y.; Takayanagi, H.; Schett, G. Osteoimmunology: Interactions of the Immune and Skeletal Systems; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Yudaniayanti, I.S. Aktifitas Alkaline Phosphatase Pada Proses Kesembuhan Patah Tulang Femur Dengan Terapi CaCO3 Dosis Tinggi Pada Tikus Jantan (Sprague Dawley). Media Kedokt. Hewan 2005, 21, 15–18. [Google Scholar]
- Birmingham, E.; Niebur, G.L.; McHugh, P.E. Osteogenic Differentiation of Mesenchymal Stem Cells Is Regulated by Osteocyte and Osteoblast Cells in a Simplified Bone Niche. Eur. Cells Mater. 2012, 23, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Recker, R.R.; Weaver, C.M. Absorbability of Calcium Sources: The Limited Role of Solubility. Calcif. Tissue Int. 1990, 46, 300–304. [Google Scholar] [CrossRef]
- Ueda, Y.; Taira, Z. Effect of Anions or Foods on Absolute Bioavailability of Calcium from Calcium Salts in Mice by Pharmacokinetics. J. Exp. Pharmacol. 2013, 5, 65. [Google Scholar]
- Buclin, T.; Jacquet, A.F.; Burckhardt, P. Intestinal Absorption of Calcium Gluconate and Oseine-Mineral Complex: An Evaluation by Conventional Analyses. Schweiz. Med. Wochenschr. 1986, 116, 1780–1783. [Google Scholar]





| Criteria | Score | Characteristics |
|---|---|---|
| No callus | 0 | no callus tissue, fracture line clear |
| Minimal callus | 1 | 25% callus tissue, fracture line still clearly visible |
| Callus evident but healing incomplete | 2 | 50% callus tissue, fracture line blurred |
| Callus evident with stability expected | 3 | 75% callus tissue, fracture line barely visible |
| Complete healing with bone remodeling | 4 | 100% callus tissue, no remaining fracture line visible |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budiatin, A.S.; Khotib, J.; Samirah, S.; Ardianto, C.; Gani, M.A.; Putri, B.R.K.H.; Arofik, H.; Sadiwa, R.N.; Lestari, I.; Pratama, Y.A.; et al. Acceleration of Bone Fracture Healing through the Use of Bovine Hydroxyapatite or Calcium Lactate Oral and Implant Bovine Hydroxyapatite–Gelatin on Bone Defect Animal Model. Polymers 2022, 14, 4812. https://doi.org/10.3390/polym14224812
Budiatin AS, Khotib J, Samirah S, Ardianto C, Gani MA, Putri BRKH, Arofik H, Sadiwa RN, Lestari I, Pratama YA, et al. Acceleration of Bone Fracture Healing through the Use of Bovine Hydroxyapatite or Calcium Lactate Oral and Implant Bovine Hydroxyapatite–Gelatin on Bone Defect Animal Model. Polymers. 2022; 14(22):4812. https://doi.org/10.3390/polym14224812
Chicago/Turabian StyleBudiatin, Aniek Setiya, Junaidi Khotib, Samirah Samirah, Chrismawan Ardianto, Maria Apriliani Gani, Bulan Rhea Kaulika Hadinar Putri, Huzaifah Arofik, Rizka Nanda Sadiwa, Indri Lestari, Yusuf Alif Pratama, and et al. 2022. "Acceleration of Bone Fracture Healing through the Use of Bovine Hydroxyapatite or Calcium Lactate Oral and Implant Bovine Hydroxyapatite–Gelatin on Bone Defect Animal Model" Polymers 14, no. 22: 4812. https://doi.org/10.3390/polym14224812
APA StyleBudiatin, A. S., Khotib, J., Samirah, S., Ardianto, C., Gani, M. A., Putri, B. R. K. H., Arofik, H., Sadiwa, R. N., Lestari, I., Pratama, Y. A., Rahadiansyah, E., & Susilo, I. (2022). Acceleration of Bone Fracture Healing through the Use of Bovine Hydroxyapatite or Calcium Lactate Oral and Implant Bovine Hydroxyapatite–Gelatin on Bone Defect Animal Model. Polymers, 14(22), 4812. https://doi.org/10.3390/polym14224812