Full Recycling and Re-Use of Bio-Based Epoxy Thermosets: Chemical and Thermomechanical Characterization of the Recycled Matrices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Epoxy Resin Formulation
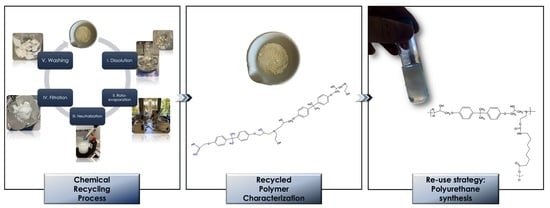
2.3. Chemical Recycling Process
2.4. Re-Use and Up-Scaling Strategy of the Recycled Polymer
2.5. Characterization Techniques
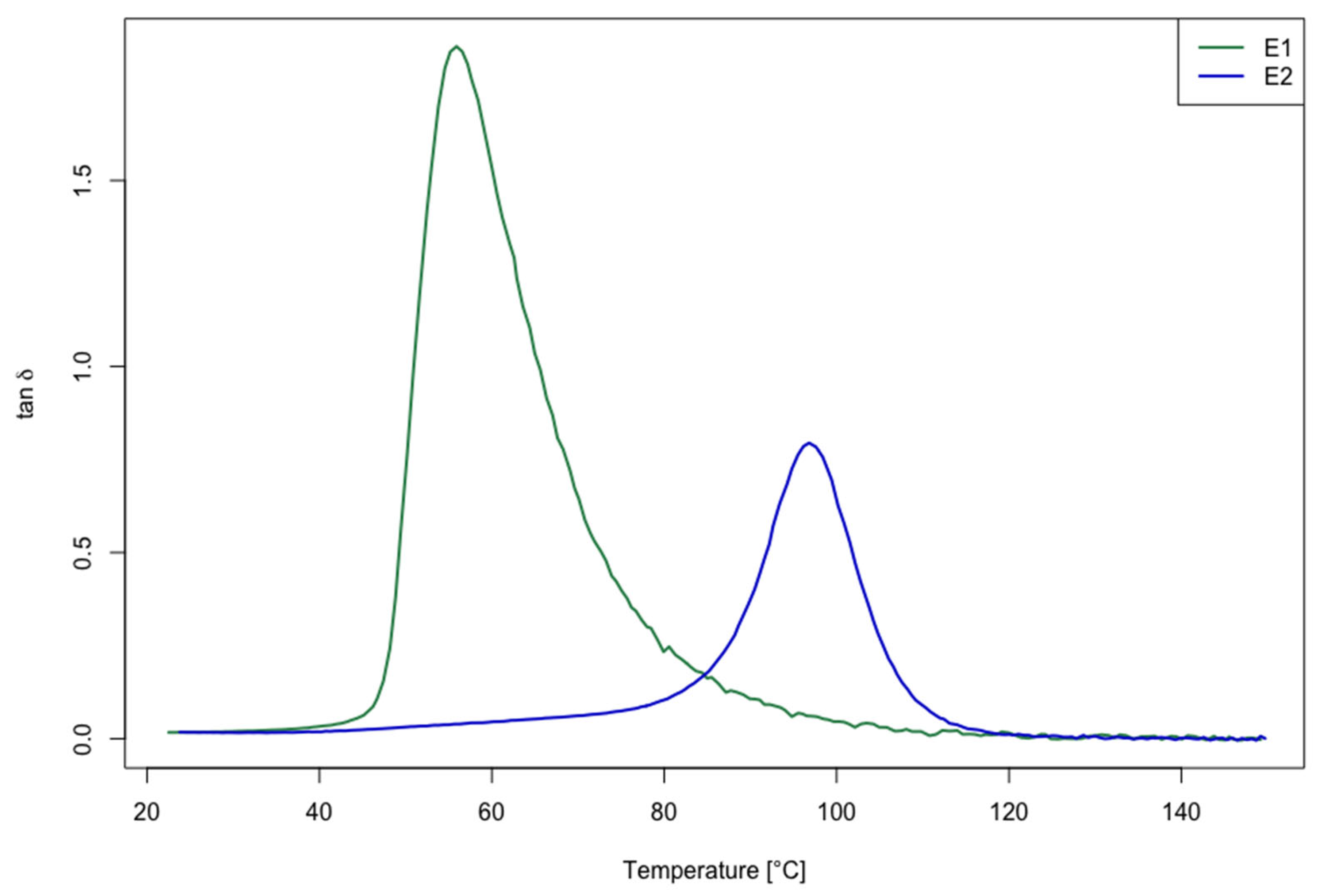
2.5.1. Dynamic Mechanical Analysis (DMA) of the Cured Epoxy Resins
2.5.2. Gel Permeation Chromatography (GPC)
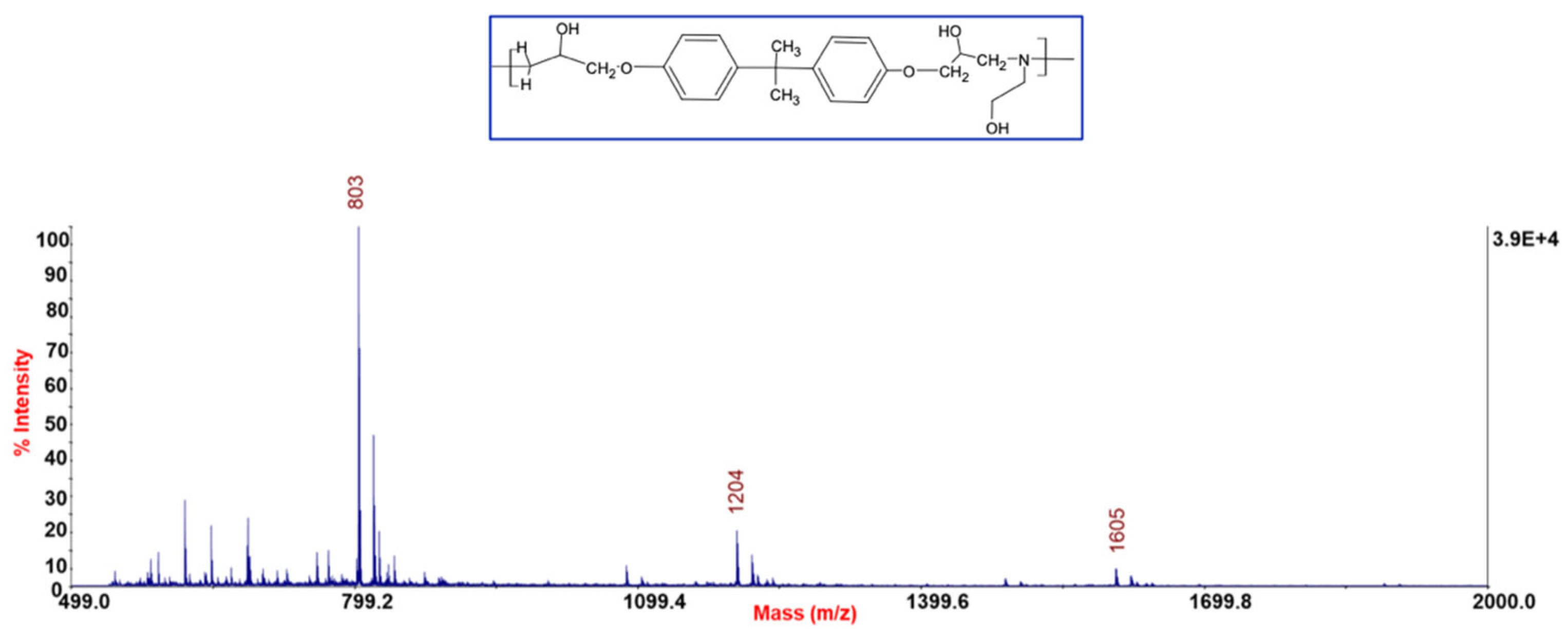
2.5.3. Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF-MS)
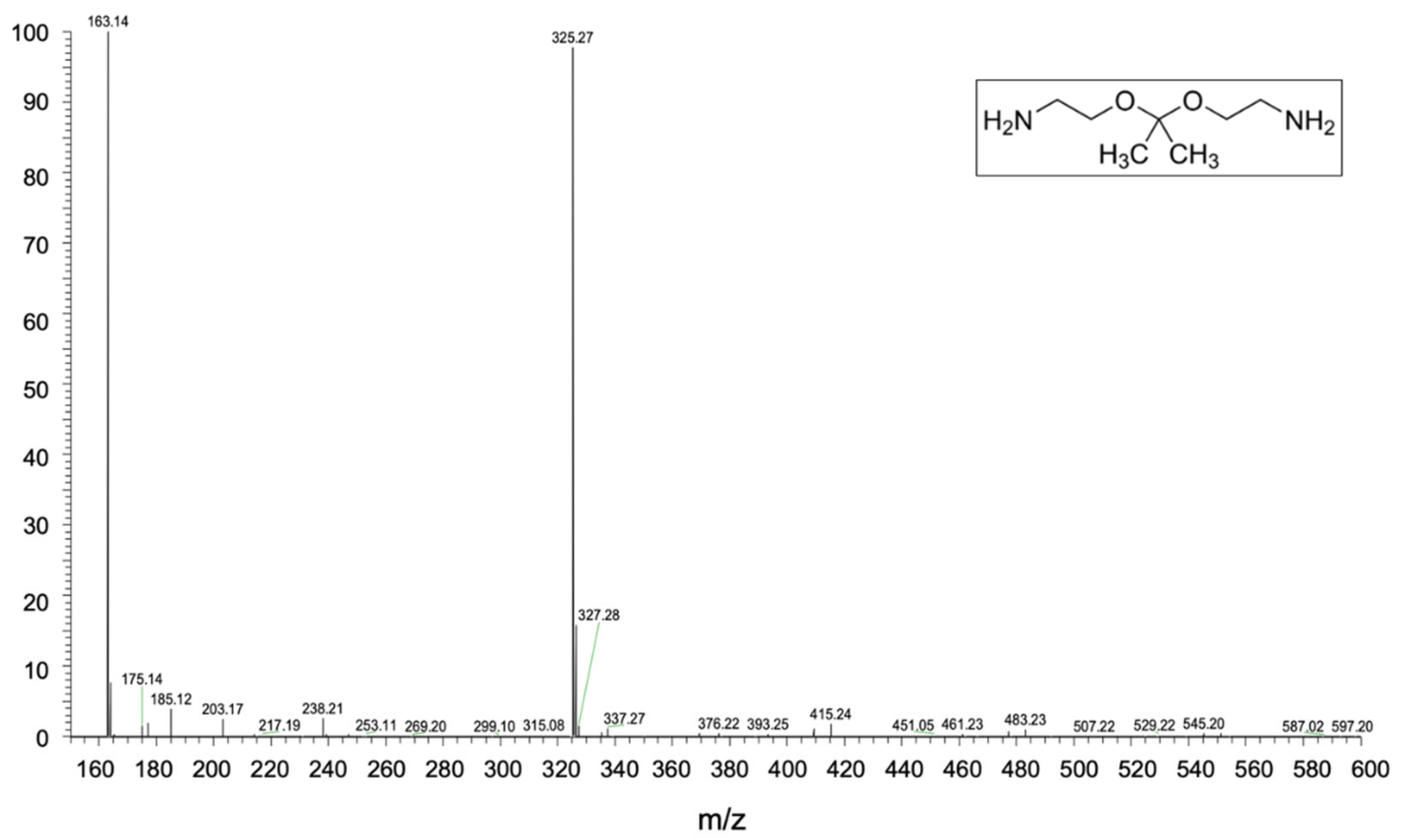
2.5.4. Heated Electrospray Ionization Mass Spectroscopy (HESI-MS)
2.5.5. Proton Nuclear Magnetic Resonance (1H-NMR) Analysis
2.5.6. Thermal Gravimetric Analysis (TGA)
2.5.7. Differential Scanning Calorimetry (DSC)
2.5.8. FT-IR Analysis
3. Results and Discussion
3.1. Thermo-Mechanical Properties of Cured Resins
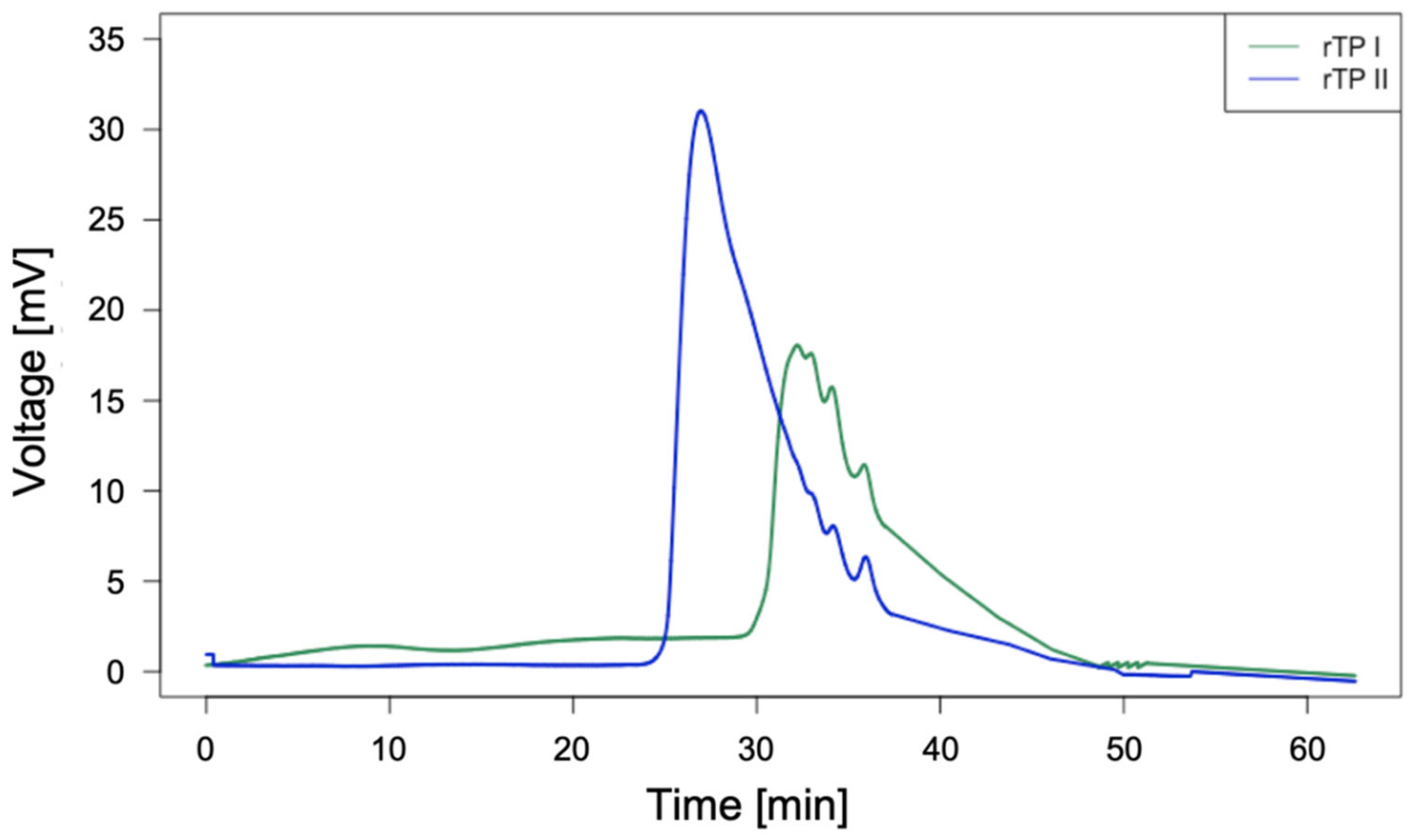
3.2. Chemical Recycling Process Results and Yields
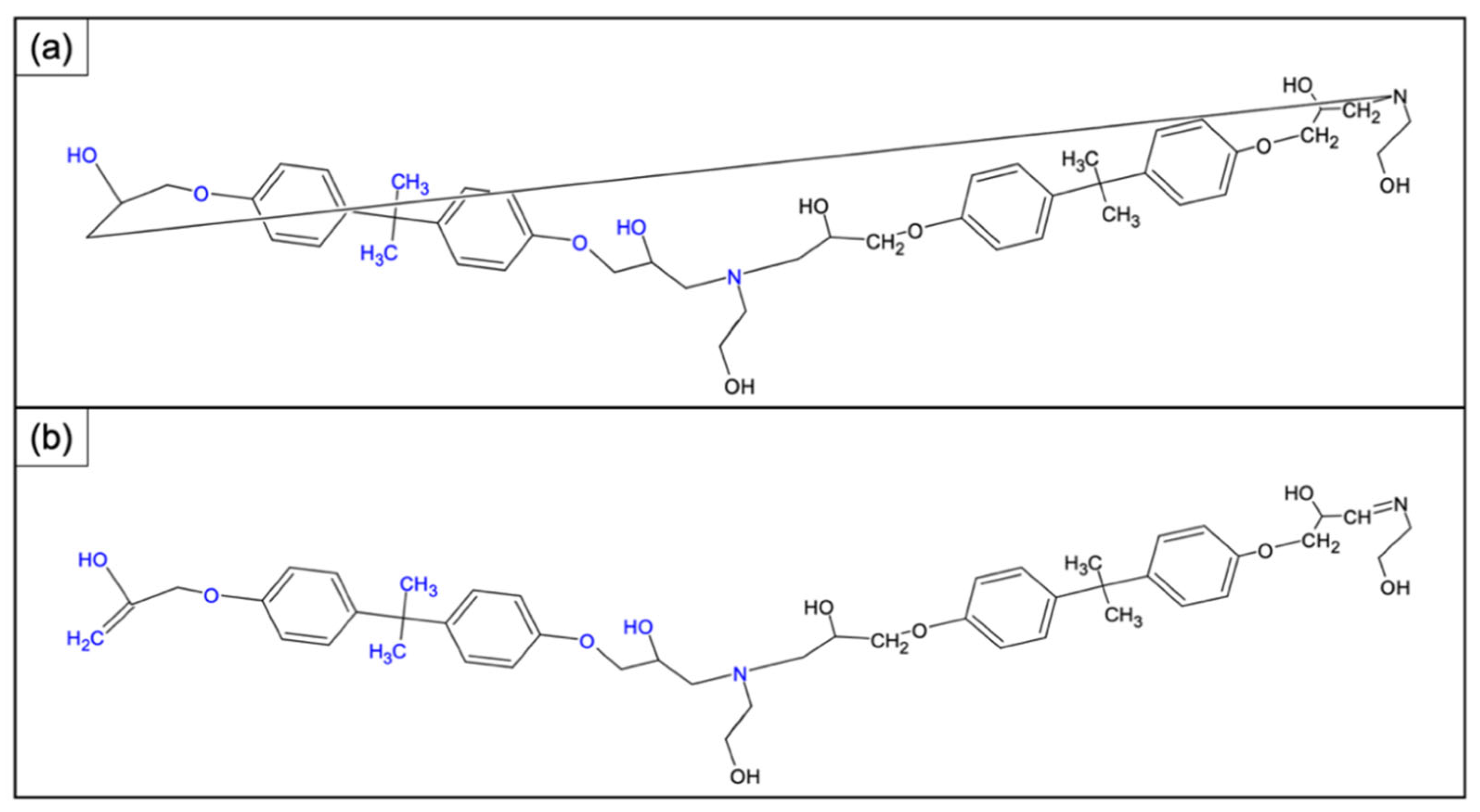
3.3. Chemical Characterization of the Recycled Polymer
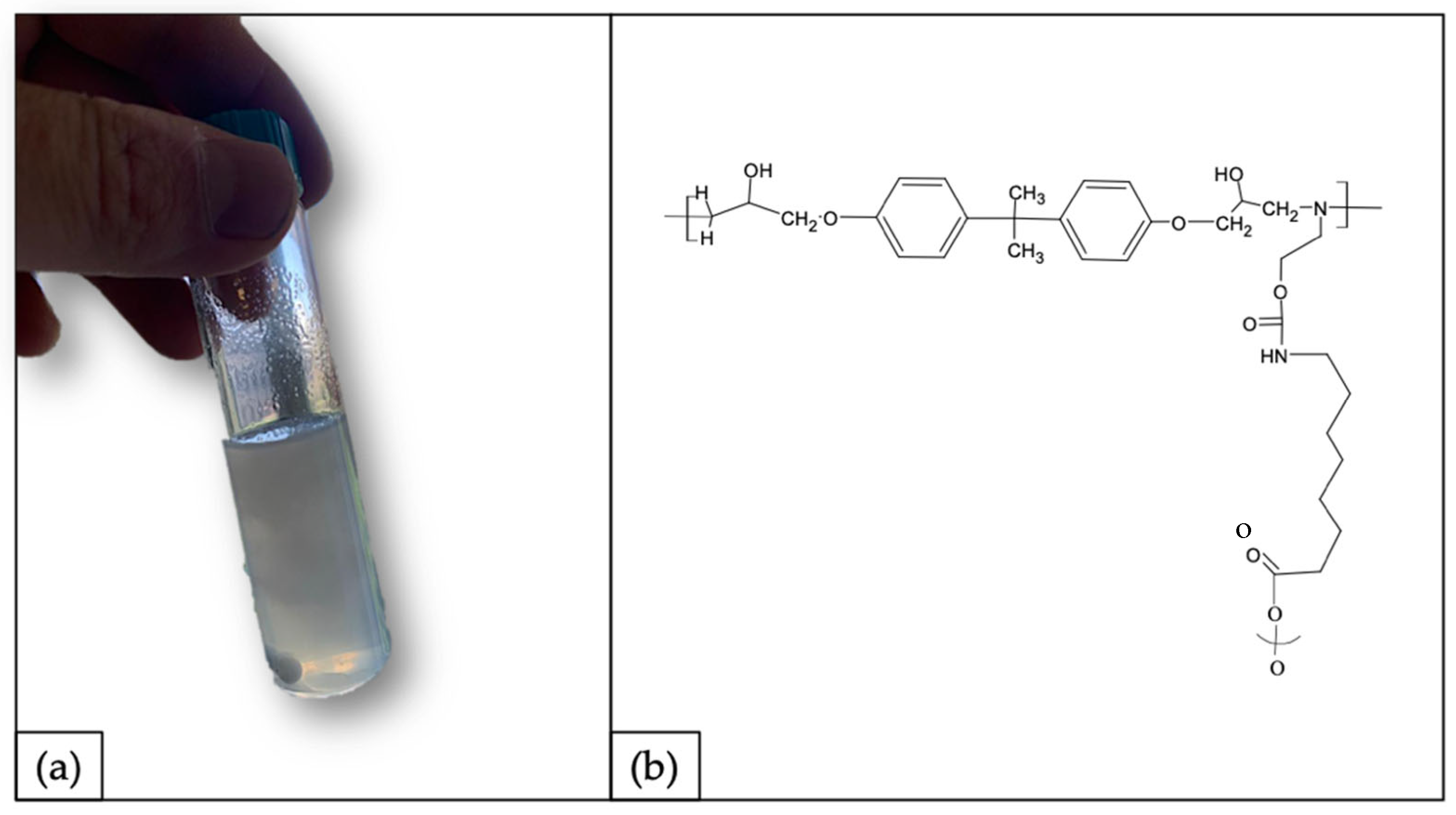
3.4. Re-Use and Up-Scaling Strategy: Polyurethane Synthesis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Krauklis, A.E.; Karl, C.W.; Gagani, A.I.; Jørgensen, J.K. Composite material recycling technology—State-of-the-art and sustainable development for the 2020s. J. Compos. Sci. 2021, 5, 28. [Google Scholar] [CrossRef]
- Post, W.; Susa, A.; Blaauw, R.; Molenveld, K.; Knoop, R.J.I. A Review on the Potential and Limitations of Recyclable Thermosets for Structural Applications. Polym. Rev. 2020, 60, 359–388. [Google Scholar] [CrossRef]
- Mishnaevsky, L. Sustainable end-of-life management of wind turbine blades: Overview of current and coming solutions. Materials 2021, 14, 1124. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Boom, R.; Irion, B.; van Heerden, D.-J.; Kuiper, P.; de Wit, H. Recycling of composite materials. Chem. Eng. Process. Process Intensif. 2012, 51, 53–68. [Google Scholar] [CrossRef]
- Oliveux, G.; Dandy, L.O.; Leeke, G.A. Current status of recycling of fibre reinforced polymers: Review of technologies, reuse and resulting properties. Prog. Mater. Sci. 2015, 72, 61–99. [Google Scholar] [CrossRef] [Green Version]
- Hong, M.; Chen, E.Y.X. Chemically recyclable polymers: A circular economy approach to sustainability. Green Chem. 2017, 19, 3692–3706. [Google Scholar] [CrossRef]
- Borjan, D.; Knez, Ž.; Knez, M. Recycling of carbon fiber-reinforced composites—Difficulties and future perspectives. Materials 2021, 14, 4191. [Google Scholar] [CrossRef]
- Pimenta, S.; Pinho, S.T. Recycling carbon fibre reinforced polymers for structural applications: Technology review and market outlook. Waste Manag. 2011, 31, 378–392. [Google Scholar] [CrossRef] [Green Version]
- El Gersifi, K.; Durand, G.; Tersac, G. Solvolysis of bisphenol A diglycidyl ether/anhydride model networks. Polym. Degrad. Stab. 2006, 91, 690–702. [Google Scholar] [CrossRef]
- Oliveux, G.; Dandy, L.O.; Leeke, G.A. Degradation of a model epoxy resin by solvolysis routes. Polym. Degrad. Stab. 2015, 118, 96–103. [Google Scholar] [CrossRef]
- Li, J.; Xu, P.L.; Zhu, Y.K.; Ding, J.-P. A promising strategy for chemical recycling of carbon fiber/thermoset composites: Self-accelerating decomposition in a mild oxidative system. Green Chem. 2012, 14, 3260–3263. [Google Scholar] [CrossRef]
- Yue, L.; Amirkhosravi, M.; Gong, X.; Gray, T.; Manas-Zloczower, I. Recycling Epoxy by Vitrimerization: Influence of an Initial Thermoset Chemical Structure. ACS Sustain. Chem. Eng. 2020, 8, 12706–12712. [Google Scholar] [CrossRef]
- Zhao, Q.; Jiang, J.; Li, C.; Li, Y. Efficient recycling of carbon fibers from amine-cured CFRP composites under facile condition. Polym. Degrad. Stab. 2020, 179, 109268. [Google Scholar] [CrossRef]
- Pickering, S.J. Recycling technologies for thermoset composite materials-current status. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1206–1215. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Yildirir, E.; Williams, P.T. Catalytic hydrothermal degradation of carbon reinforced plastic wastes for carbon fibre and chemical feedstock recovery. Waste Biomass Valorization 2013, 4, 87–93. [Google Scholar] [CrossRef]
- Lee, M.; Kim, D.H.; Park, J.J.; You, N.-H.; Goh, M. Fast chemical recycling of carbon fiber reinforced plastic at ambient pressure using an aqueous solvent accelerated by a surfactant. Waste Manag. 2020, 118, 190–196. [Google Scholar] [CrossRef]
- Yuyan, L.; Guohua, S.; Linghui, M. Recycling of carbon fibre reinforced composites using water in subcritical conditions. Mater. Sci. Eng. A 2009, 520, 179–183. [Google Scholar] [CrossRef]
- Shibasaki, Y.; Kamimori, T.; Kadokawa, J.I.; Hatano, B.; Tagaya, H. Decomposition reactions of plastic model compounds in sub- and supercritical water. Polym. Degrad. Stab. 2004, 83, 481–485. [Google Scholar] [CrossRef]
- Okajima, I.; Hiramatsu, M.; Shimamura, Y.; Awaya, T.; Sako, T. Chemical recycling of carbon fiber reinforced plastic using supercritical methanol. J. Supercrit. Fluids 2014, 91, 68–76. [Google Scholar] [CrossRef]
- Suyama, K.; Kubota, M.; Shirai, M.; Yoshida, H. Effect of alcohols on the degradation of crosslinked unsaturated polyester in sub-critical water. Polym. Degrad. Stab. 2006, 91, 983–986. [Google Scholar] [CrossRef]
- Hyde, J.R.; Lester, E.; Kingman, S.; Pickering, S.; Wong, K.-H. Supercritical propanol, a possible route to composite carbon fibre recovery: A viability study. Compos. Part A Appl. Sci. Manuf. 2006, 37, 2171–2175. [Google Scholar] [CrossRef]
- Jiang, G.; Pickering, S.J.; Lester, E.H.; Turner, T.A.; Wong, K.-H.; Warrior, N.A. Characterisation of carbon fibres recycled from carbon fibre/epoxy resin composites using supercritical n-propanol. Compos. Sci. Technol. 2009, 69, 192–198. [Google Scholar] [CrossRef]
- Piñero-Hernanz, R.; García-Serna, J.; Dodds, C.; Hyde, J.; Poliakoff, M.; Cocero, M.J.; Kingman, S.; Pickering, S.; Lester, E. Chemical recycling of carbon fibre composites using alcohols under subcritical and supercritical conditions. J. Supercrit. Fluids 2008, 46, 83–92. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Yang, Q.; Deng, T.; Wang, Y.; Yang, Y.; Jia, S.; Qin, Z.; Hou, X. Chemical recycling of unsaturated polyester resin and its composites via selective cleavage of the ester bond. Green Chem. 2015, 17, 4527–4532. [Google Scholar] [CrossRef]
- Gao, Y.; Romero, P.; Zhang, H.; Huang, M.; Lai, F. Unsaturated polyester resin concrete: A review. Constr. Build. Mater. 2019, 228, 116709. [Google Scholar] [CrossRef]
- Arturi, K.R.; Sokoli, H.U.; Søgaard, E.G.; Vogel, F.; Bjelić, S. Recovery of value-added chemicals by solvolysis of unsaturated polyester resin. J. Clean Prod. 2018, 170, 131–136. [Google Scholar] [CrossRef]
- Kamimura, A.; Yamamoto, S.; Yamada, K. Depolymerization of unsaturated polyesters and waste fiber-reinforced plastics by using ionic liquids: The use of microwaves to accelerate the reaction rate. ChemSusChem 2011, 4, 644–649. [Google Scholar] [CrossRef]
- Das, M.; Chacko, R.; Varughese, S. An Efficient Method of Recycling of CFRP Waste Using Peracetic Acid. ACS Sustain. Chem. Eng. 2018, 6, 1564–1571. [Google Scholar] [CrossRef]
- Xu, P.; Li, J.; Ding, J. Chemical recycling of carbon fibre/epoxy composites in a mixed solution of peroxide hydrogen and N,N-dimethylformamide. Compos. Sci. Technol. 2013, 82, 54–59. [Google Scholar] [CrossRef]
- Ma, Y.; Nutt, S. Chemical treatment for recycling of amine/epoxy composites at atmospheric pressure. Polym. Degrad. Stab. 2018, 153, 307–317. [Google Scholar] [CrossRef]
- Dang, W.; Kubouchi, M.; Yamamoto, S.; Sembokuya, H.; Tsuda, K. An approach to chemical recycling of epoxy resin cured with amine using nitric acid. Polymer 2002, 43, 2953–2958. [Google Scholar] [CrossRef]
- Prinçaud, M.; Aymonier, C.; Loppinet-Serani, A.; Perry, N.; Sonnemann, G. Environmental feasibility of the recycling of carbon fibers from CFRPs by solvolysis using supercritical water. ACS Sustain. Chem. Eng. 2014, 2, 1498–1502. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Wang, X.L.; Tian, F.; An, W.-L.; Xu, S.; Wang, Y.-Z. A fast and mild closed-loop recycling of anhydride-cured epoxy through microwave-assisted catalytic degradation by trifunctional amine and subsequent reuse without separation. Green Chem. 2019, 21, 2487–2493. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Z.; Wang, B.; Li, P.; Zhu, J.; Ma, S. Closed-loop chemical recycling of thermosetting polymers and their applications: A review. Green Chem. 2022, 24, 5691–5708. [Google Scholar] [CrossRef]
- La Rosa, A.D.; Banatao, D.R.; Pastine, S.J.; Latteri, A.; Cicala, G. Recycling treatment of carbon fibre/epoxy composites: Materials recovery and characterization and environmental impacts through life cycle assessment. Compos. Part B Eng. 2016, 104, 17–25. [Google Scholar] [CrossRef]
- La Rosa, A.D.; Blanco, I.; Banatao, D.R.; Pastine, S.J.; Bjorklung, A.; Cicala, G. Innovative chemical process for recycling thermosets cured with recyclamines® by converting bio-epoxy composites in reusable thermoplastic-an LCA study. Materials 2018, 11, 353. [Google Scholar] [CrossRef] [Green Version]
- Saitta, L.; Prasad, V.; Tosto, C.; Murphy, N.; Ivankovic, A.; Cicala, G.; Scarselli, G. Characterization of biobased epoxy resins to manufacture eco-composites showing recycling properties. Polym. Compos. 2022, 1–14. [Google Scholar] [CrossRef]
- Desroches, M.; Escouvois, M.; Auvergne, R.; Cailol, S.; Boutevin, B. From Vegetable Oils to Polyurethanes: Synthetic routes to polyols and Main Industrial Products. Polym. Rev. 2012, 52, 38–79. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Zhang, C.; Kessler, M.R. Polyols and polyurethanes prepared from epoxidized soybean oil ring-opened by polyhydroxy fatty acids with varying oh numbers. J. Appl. Polym. Sci. 2015, 132, 41213. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, J.; Hse, C.-J.; Shupe, T.F. Preparation of polyurethane foams using fractionated products in liquefied wood. J. Appl. Polym. Sci. 2014, 131, 40096. [Google Scholar] [CrossRef]
- Cinelli, P.; Anguillesi, I.; Lazzeri, A. Green synthesis of flexible polyurethane foams from liquefied lignin. Eur. Polym. J. 2013, 49, 1174–1184. [Google Scholar] [CrossRef]
- Cicala, G.; Pergolizzi, E.; Piscopo, F.; Carbone, D.; Recca, G. Hybrid composites manufactured by resin infusion with a fully recyclable bioepoxy resin. Compos. Part B Eng. 2018, 132, 69–76. [Google Scholar] [CrossRef]
- Gabbot, P. Principles and Applications of Thermal Analysis; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Gupta, V.B.; Drzal, L.T.; Lee, C.Y.-C.; Rich, M.J. The temperature-dependence of some mechanical properties of a cured epoxy resin system. Polym. Eng. Sci. 1985, 25, 812–823. [Google Scholar] [CrossRef]
- Yang, G.; Xie, W.; Huang, M.; Champagne, V.K.; Lee, J.-H.; Klier, J.; Schiffman, J.D. Polymer Particles with a Low Glass Transition Temperature Containing Thermoset Resin Enable Powder Coatings at Room Temperature. Ind. Eng. Chem. Res. 2019, 58, 908–916. [Google Scholar] [CrossRef]
- Ferrari, F.; Corcione, C.E.; Striani, R.; Saitta, L.; Cicala, G.; Greco, A. Fully recyclable bio-based epoxy formulations using epoxidized precursors fromwaste flour: Thermal and mechanical characterization. Polymers 2021, 13, 2768. [Google Scholar] [CrossRef]
- Fox, T.G.; Flory, P.J. Second-order transition temperatures and related properties of polystyrene. I. Influence of molecular weight. J. Appl. Phys. 1950, 21, 581–591. [Google Scholar] [CrossRef]
- Barral, L.; Cano, J.; López, J.; Lopez-Bueno, I.; Nogueira, P.; Abad, M.J.; Ramirez, C. Decomposition behavior of epoxy-resin systems cured by diamines. Eur. Polym. J. 2000, 36, 1231–1240. [Google Scholar] [CrossRef]
- Ismoilov, K.; Akram, W.; Chauhan, S.; Ergasheva, K.; Artikboeva, R.; Islomova, Z.; Quan, H. Synthesis and Evaluation of Properties of a Novel Cationic Waterborne Polyurethane Finishing Agent. J. Chem. Eng. Process Technol. 2019, 10, 398. [Google Scholar] [CrossRef]
- La Rosa, A.D.; Greco, S.; Tosto, C.; Cicala, G. LCA and LCC of a chemical recycling process of waste CF-thermoset composites for the production of novel CF-thermoplastic composites. Open loop and closed loop scanarios. J. Clean Prod. 2021, 304, 127158. [Google Scholar] [CrossRef]
- White, B.J.E.; Silvis, H.C.; Winkler, M.S.; Glass, T.W.; Kirkpatrick, D.E. Poly(hydroxyaminoethers): A New Family of Epoxy-Based Thermoplastics. Adv. Mater. 2000, 12, 1791–1800. [Google Scholar] [CrossRef]
- European Council. Directive 2000/53/EC of the European Parliament and of the Council on End-of-Life Vehicles. Off. J. Eur. Union L 2000, 269, 34–269. Available online: https://eur-lex.europa.eu/eli/dir/2000/53/oj (accessed on 31 October 2022).






| Sample ID | Formulation | Mixing Ratio by Weight | Curing Cycle |
|---|---|---|---|
| E1 | Polar Bear + RecyclamineTM R*101 | 100:22 | at 25 °C for 24 h |
| E2 | Polar Bear + RecyclamineTM R*101 | 100:22 | at 25 °C for 24 h + 100 °C for 3 h |
| Sample ID | Tg [°C] |
|---|---|
| E1 | 60.30 ± 0.26 |
| E2 | 103.40 ± 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dattilo, S.; Cicala, G.; Riccobene, P.M.; Puglisi, C.; Saitta, L. Full Recycling and Re-Use of Bio-Based Epoxy Thermosets: Chemical and Thermomechanical Characterization of the Recycled Matrices. Polymers 2022, 14, 4828. https://doi.org/10.3390/polym14224828
Dattilo S, Cicala G, Riccobene PM, Puglisi C, Saitta L. Full Recycling and Re-Use of Bio-Based Epoxy Thermosets: Chemical and Thermomechanical Characterization of the Recycled Matrices. Polymers. 2022; 14(22):4828. https://doi.org/10.3390/polym14224828
Chicago/Turabian StyleDattilo, Sandro, Gianluca Cicala, Paolo Maria Riccobene, Concetto Puglisi, and Lorena Saitta. 2022. "Full Recycling and Re-Use of Bio-Based Epoxy Thermosets: Chemical and Thermomechanical Characterization of the Recycled Matrices" Polymers 14, no. 22: 4828. https://doi.org/10.3390/polym14224828