Polyurethane Recycling: Conversion of Carbamates—Catalysis, Side-Reactions and Mole Balance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
2.2. Analytical Method
2.2.1. Sample Preparation
2.2.2. Liquid Chromatography
2.3. Safety Recommendations
3. Results
3.1. Thermal and Catalytic Carbamate Cleavage
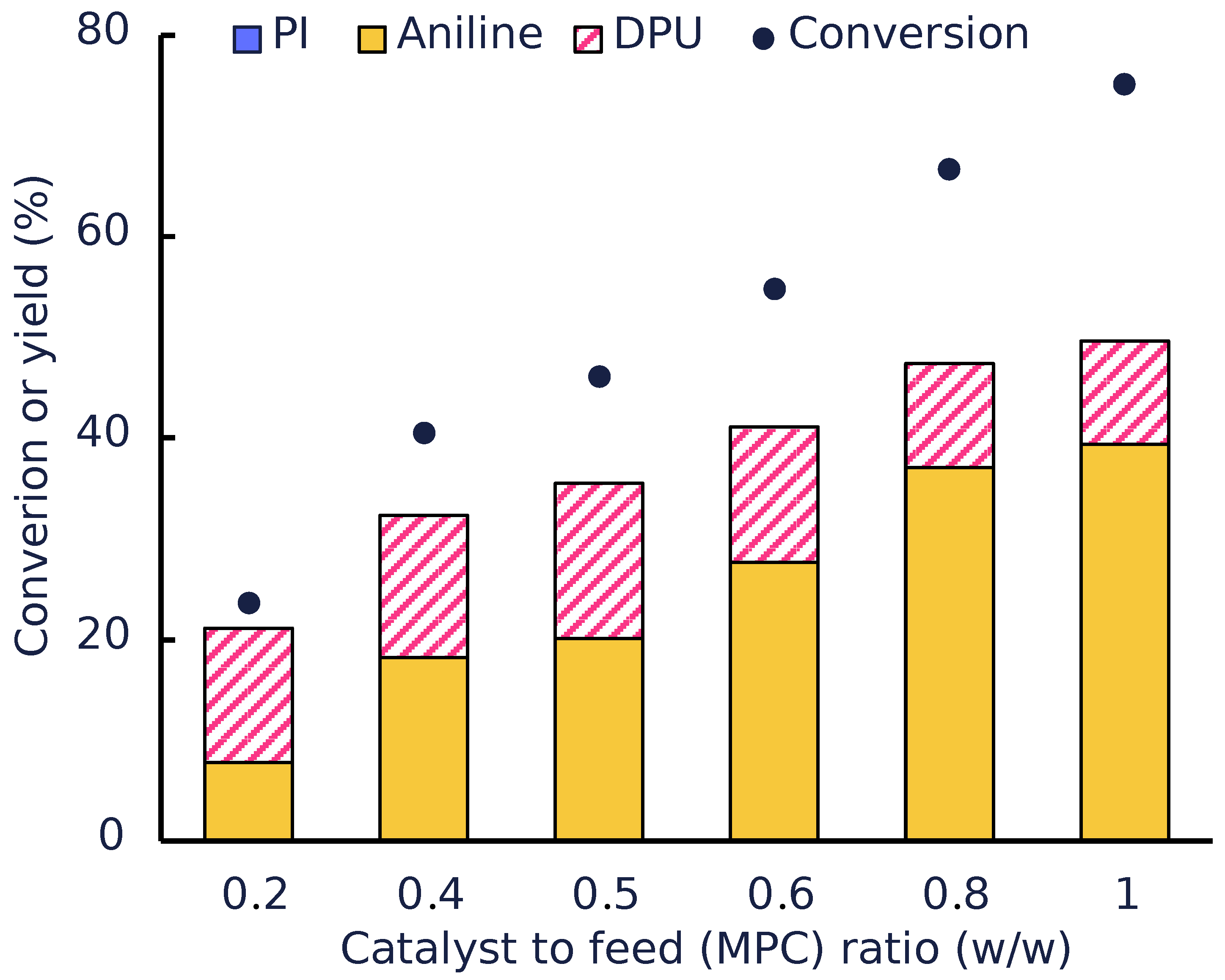
Catalyst to Feed Ratio
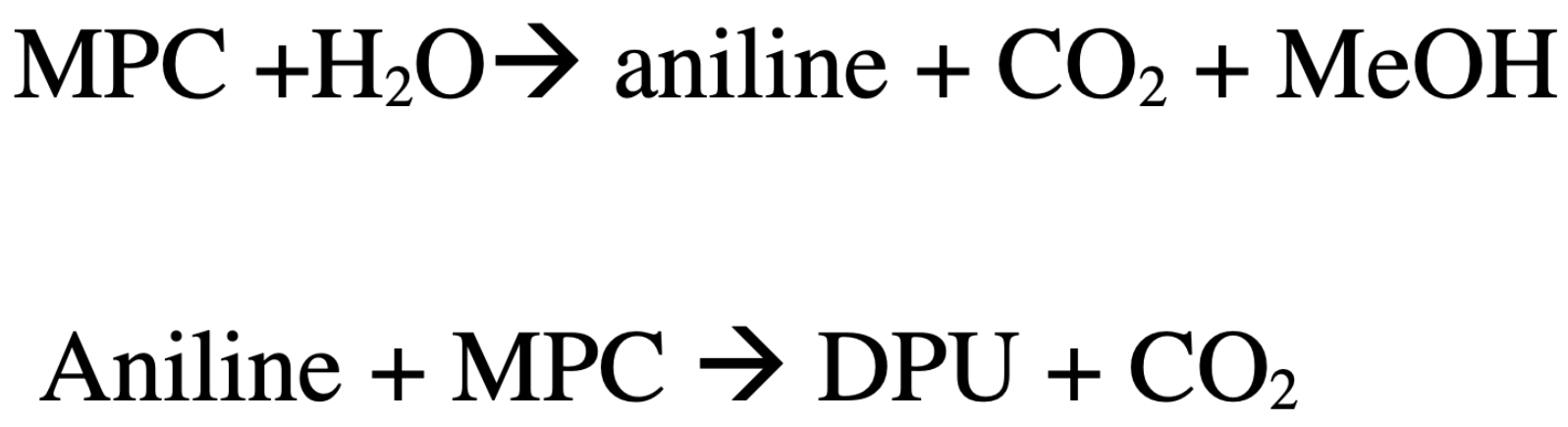
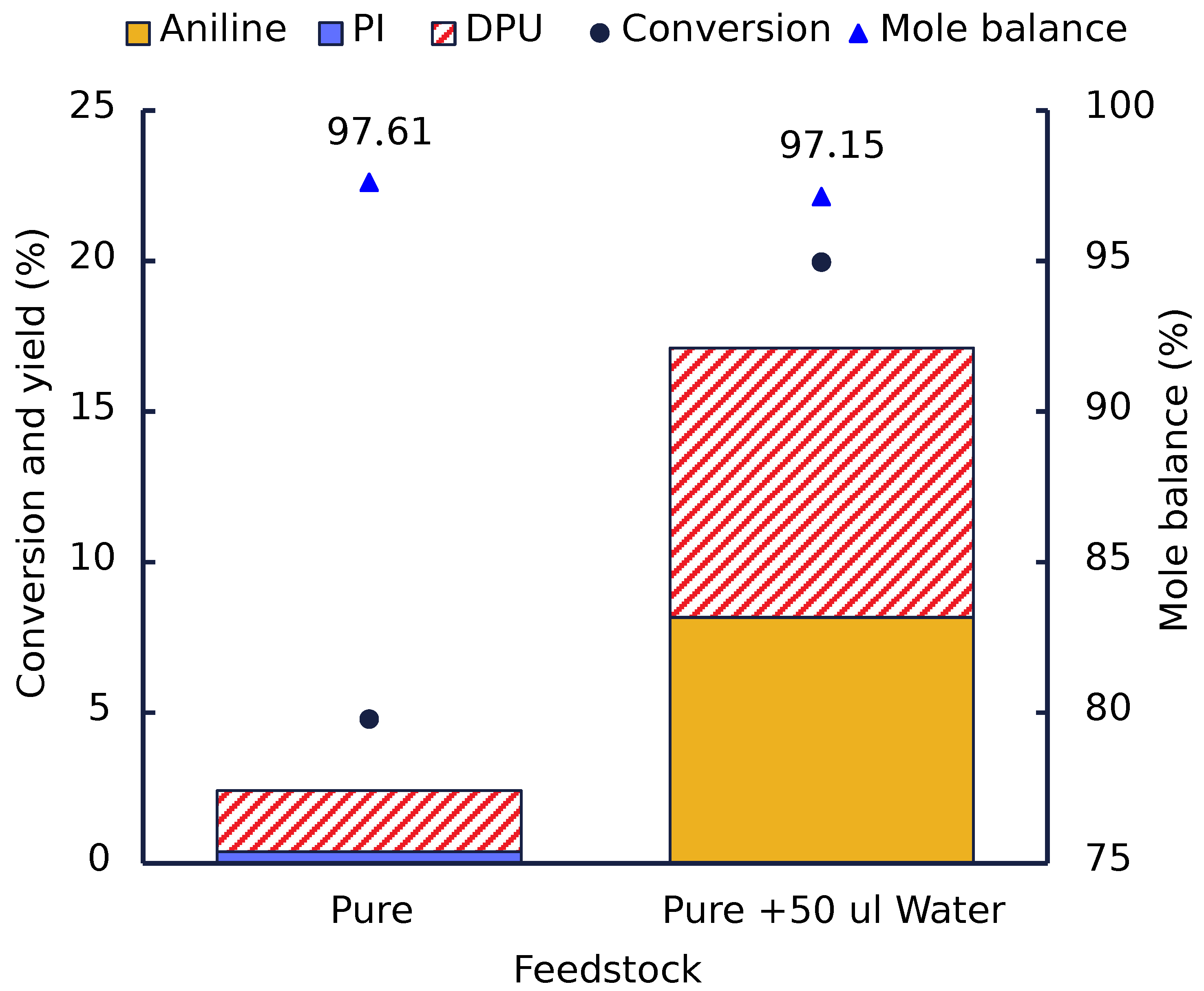
3.2. Effect of Water
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MPC | Methyl N-phenyl carbamate |
| PUR | Polyurethanes |
| TDI | Toluene diisocyanate |
| MDI | Methyl diphenyl diisocyanate |
| HDI | 1,6-hexameylene diisocyanate |
| DPU | 1,3-Diphenylurea |
| MMK | Montmorillonite K-10 |
| HPLC | High performance liquid chromatography |
| LC | Liquid chromatography |
| MS | Mass spectrometer |
| UV | Ultraviolet |
| PP | 1-(2-Pyridyl)piperazine |
| DMSO | Dimethyl sulfoxide |
| PI | Phenyl isocyanate |
| wt% | Weight percent |
| GPC | Gel permeation chromatography |
Appendix A


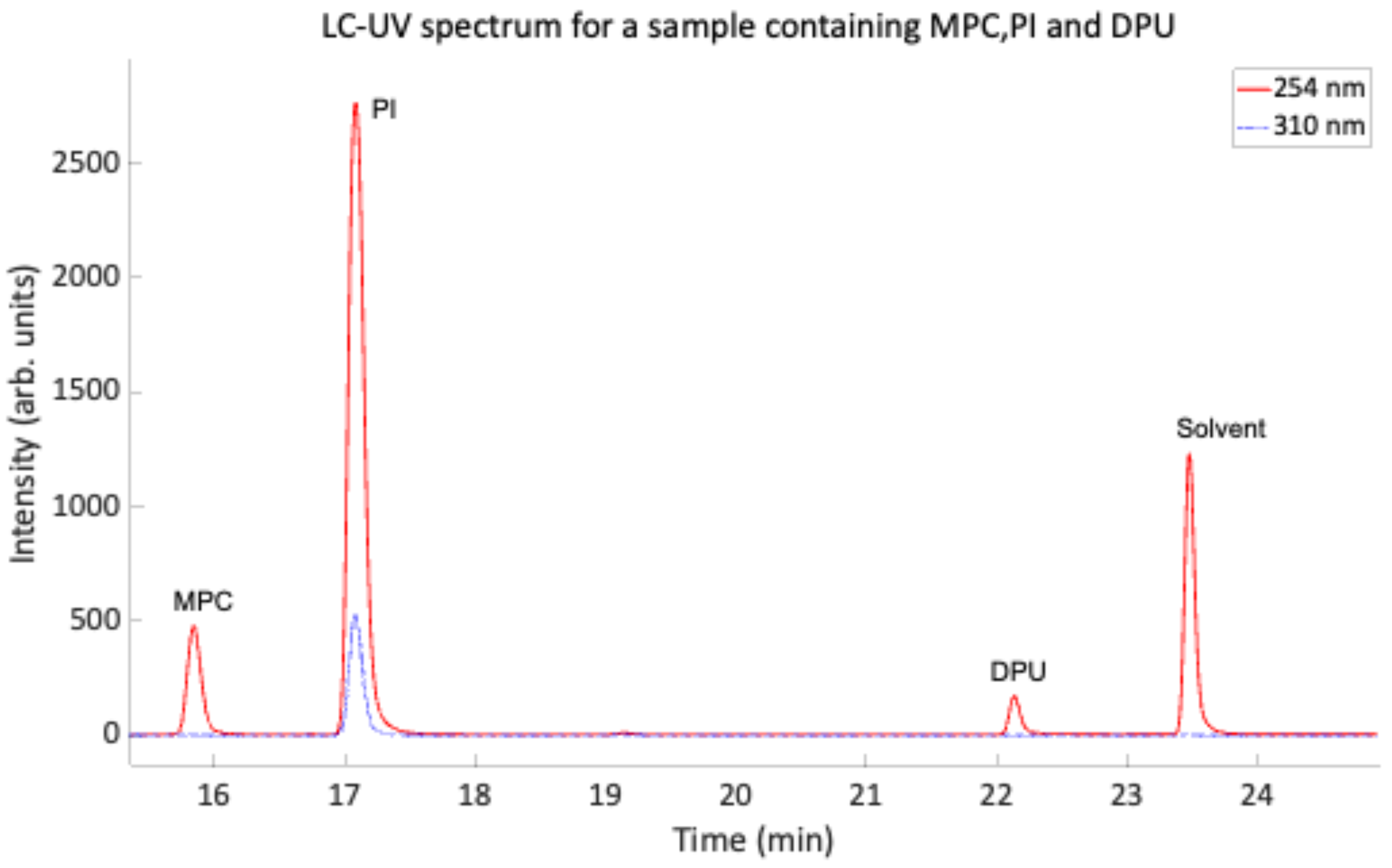

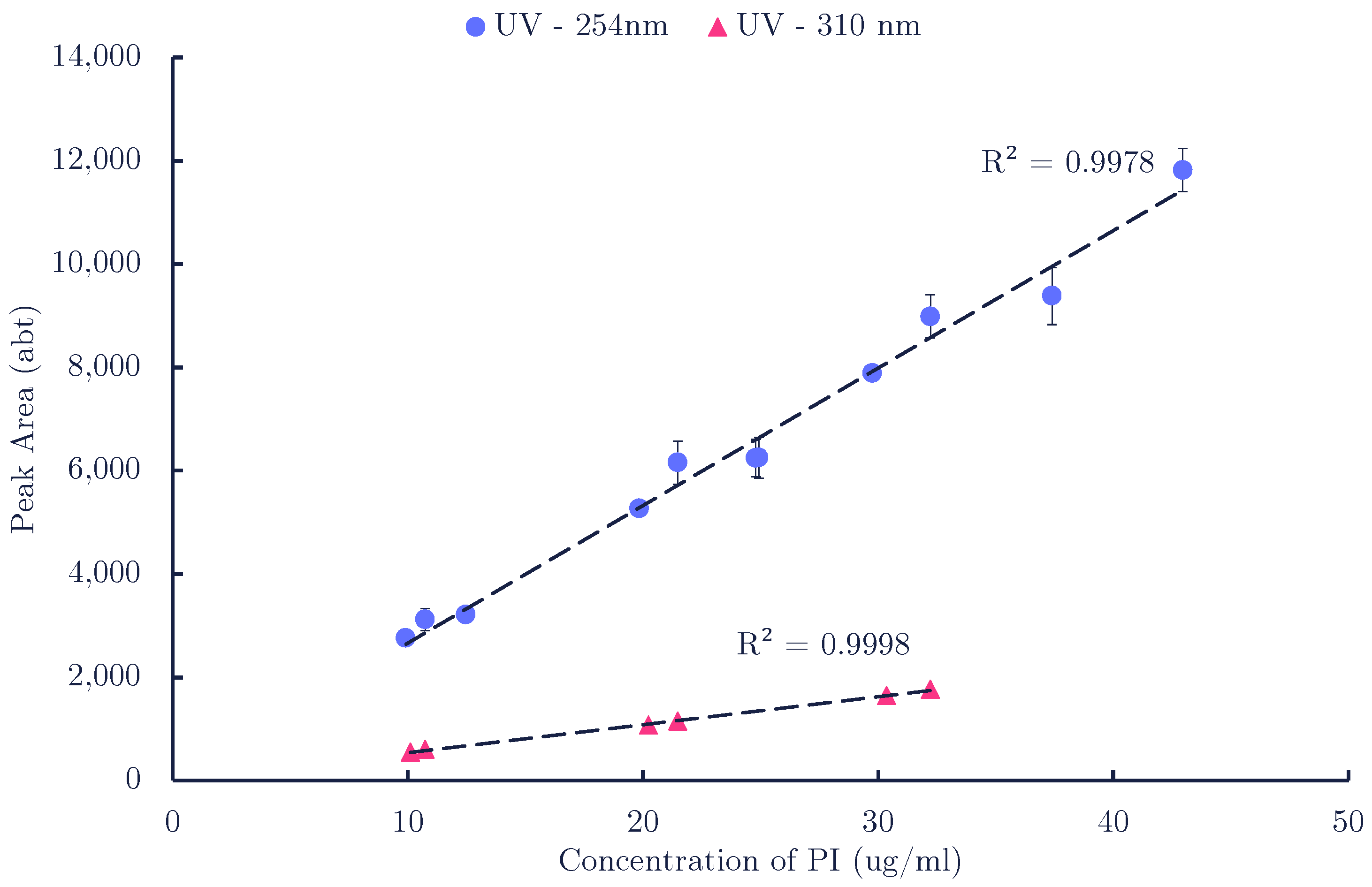
References
- Bayer, O. Das Di-Isocyanat-Polyadditionsverfahren (Polyurethane). Angew. Chem. 1947, 59, 257–272. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, W.C.; Li, Q.; Khan, M.R.; Hu, G.H.; Liu, Y.; Wu, W.; Huang, C.X.; Li, R.K. Recent advances in superhydrophobic polyurethane: Preparations and applications. Adv. Colloid Interface Sci. 2022, 303, 102644. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe. Plastics—The Facts 2021; Plastics Europe: Bruxelles, Belgium, 2021. [Google Scholar]
- Fernández, L. Market Volume of Polyurethane Worldwide from 2015 to 2020, with a Forecast for 2021 to 2026. Available online: https://www.statista.com/statistics/720449/global-polyurethane-market-size-forecast (accessed on 21 November 2021).
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef] [Green Version]
- Brereton, G.; Emanuel, R.M.; Lomax, R.; Pennington, K.; Ryan, T.; Tebbe, H.; Timm, M.; Ware, P.; Winkler, K.; Yuan, T.; et al. Polyurethanes. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; pp. 1–76. [Google Scholar] [CrossRef]
- Saunders, J.H.; Frisch, K.C. Polyurethanes: Chemistry and Technology; Chemistry, L.K., Ed.; Pt. 1; Interscience Publishers: New York, NY, USA, 1962; Available online: https://ut.on.worldcat.org/oclc/768435228 (accessed on 21 November 2021).
- ISOPA—European Diisocyanite and Polyol Producers Association. TDI Classification and Labelling Requirements Classification; ISOPA: Bruxelles, Belgium, 2012; pp. 1–3. [Google Scholar]
- ISOPA—European Diisocyanite and Polyol Producers Association. Recycling and Recovery of Polyurethanes foam Legislation Recycling & Recovery Technologies Recycling and Recovery of Polyurethanes Eco—Considerations; ISOPA: Bruxelles, Belgium, 2012. [Google Scholar]
- Simón, D.; Rodríguez, J.F.; Carmona, M.; Serrano, A.; Borreguero, A.M. Glycolysis of advanced polyurethanes composites containing thermoregulating microcapsules. Chem. Eng. J. 2018, 350, 300–311. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.; de Lucas, A.; Rodríguez, J. Valorization of crude glycerol as a novel transesterification agent in the glycolysis of polyurethane foam waste. Polym. Degrad. Stab. 2015, 121, 126–136. [Google Scholar] [CrossRef]
- Yang, W.; Dong, Q.; Liu, S.; Xie, H.; Liu, L.; Li, J. Recycling and Disposal Methods for Polyurethane Foam Wastes. Procedia Environ. Sci. 2012, 16, 167–175. [Google Scholar] [CrossRef]
- Deng, Y.; Dewil, R.; Appels, L.; Ansart, R.; Baeyens, J.; Kang, Q. Reviewing the thermo-chemical recycling of waste polyurethane foam. J. Environ. Manag. 2021, 278, 111527. [Google Scholar] [CrossRef]
- Datta, J.; Włoch, M. Recycling of Polyurethanes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 323–358. [Google Scholar] [CrossRef]
- Lange, J.P. Sustainable development: Efficiency and recycling in chemicals manufacturing. Green Chem. 2002, 4, 546–550. [Google Scholar] [CrossRef]
- Zahedifar, P.; Pazdur, L.; Vande Veld, C.M.; Billen, P. Multistage chemical recycling of polyurethanes and dicarbamates: A glycolysis–hydrolysis demonstration. Sustainability 2021, 13, 3583. [Google Scholar] [CrossRef]
- Vanbergen, T.; Verlent, I.; De Geeter, J.; Haelterman, B.; Claes, L.; De Vos, D. Recycling of Flexible Polyurethane Foam by Split-Phase Alcoholysis: Identification of Additives and Alcoholyzing Agents to Reach Higher Efficiencies. ChemSusChem 2020, 13, 3835–3843. [Google Scholar] [CrossRef]
- Herrero, D.S. Glycolysis Process for Polyurethane Waste Recycling. Ph.D. Thesis, Universidad de Castilla-la Mancha, Ciudad Real, Spain, 2017; p. 293. [Google Scholar]
- Alavi Nikje, M.M.; Nikrah, M. Glycerin as a new glycolysing agent for chemical recycling of cold cure polyurethane foam wastes in “split-phase” condition. Polym. Bull. 2007, 58, 411–423. [Google Scholar] [CrossRef]
- Molero, C.; De Lucas, A.; Rodríguez, J.F. Recovery of polyols from flexible polyurethane foam by “split- phase” glycolysis with new catalysts. Polym. Degrad. Stab. 2006, 91, 894–901. [Google Scholar] [CrossRef]
- Dyer, E.; Newborn, G.E. Thermal Degradation of Carbamates of Methylenebis-(4-phenyl Isocyanate) 1. J. Am. Chem. Soc. 1958, 80, 5495–5498. [Google Scholar] [CrossRef]
- Dyer, E.; Wright, G.C. Thermal Degradation of Alkyl N-Phenylcarbamates. J. Am. Chem. Soc. 1959, 81, 2138–2143. [Google Scholar] [CrossRef]
- Daly, N.J.; Ziolkowski, F. The thermal decompositions of carbamates. IV. The ethylN-methyl-carbamate system. Int. J. Chem. Kinet. 1980, 12, 241–252. [Google Scholar] [CrossRef]
- Wang, P.; Liu, S.; Deng, Y. Important Green Chemistry and Catalysis: Non-phosgene Syntheses of Isocyanates—Thermal Cracking Way. Chin. J. Chem. 2017, 35, 821–835. [Google Scholar] [CrossRef] [Green Version]
- Uriz, P.; Serra, M.; Salagre, P.; Castillon, S.; Claver, C.; Fernandez, E. A new and efficient catalytic method for synthesizing isocyanates from carbamates. Tetrahedron Lett. 2002, 43, 1673–1676. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Li, F.; Wang, S.; Zhang, J. Catalytic synthesis of toluene-2,4-diisocyanate from dimethyl carbonate. J. Chem. Technol. Biotechnol. 2001, 76, 857–861. [Google Scholar] [CrossRef]
- Wang, Q.; Kang, W.; Zhang, Y.; Yang, X.; Yao, J.; Chen, T.; Wang, G. Solvent-free thermal decomposition of methylenediphenyl di(phenylcarbamate) catalyzed by nano-Cu2O. Chin. J. Catal. 2013, 34, 548–558. [Google Scholar] [CrossRef]
- Lewandowski, G.; Milchert, E. Thermal decomposition of methylene-4,4′-di(ethylphenyl-carbamate) to methylene-4,4′-di(phenylisocyanate). J. Hazard. Mater. 2005, 119, 19–24. [Google Scholar] [CrossRef]
- Ammar, M.; Cao, Y.; He, P.; Wang, L.; Chen, J.; Li, H. An efficient green route for hexamethylene-1,6-diisocyanate synthesis by thermal decomposition of hexamethylene-1,6-dicarbamate over Co3O4/ZSM-5 catalyst: An indirect utilization of CO2. Chin. J. Chem. Eng. 2017, 25, 1760–1770. [Google Scholar] [CrossRef]
- Cao, Y.; Chi, Y.; Muhammad, A.; He, P.; Wang, L.; Li, H. Non-phosgene synthesis of hexamethylene-1,6-diisocyanate from thermal decomposition of hexamethylene-1,6-dicarbamate over Zn–Co bimetallic supported ZSM-5 catalyst. Chin. J. Chem. Eng. 2019, 27, 549–555. [Google Scholar] [CrossRef]
- Hyun, M.J.; Shin, M.; Kim, Y.J.; Suh, Y.W. Phosgene-free decomposition of dimethylhexane-1,6-dicarbamate over ZnO. Res. Chem. Intermed. 2016, 42, 57–70. [Google Scholar] [CrossRef]
- Jeong, C.; Hyun, M.J.; Suh, Y.W. Activity of coprecipitated CuO/ZnO catalysts in the decomposition of dimethylhexane-1,6-dicarbamate. Catal. Commun. 2015, 70, 34–39. [Google Scholar] [CrossRef]
- Schlecht, P.C.; O’Connor, P.F. NIOSH Manual of Analytical Methods; Isocyanates, Total (Map) Method 5525; US Department of Health and Human Services, Division of Physical Sciences and Engineering: Washington, DC, USA, 2003; pp. 1–18.
- Karlsson, D.; Dahlin, J.; Skarping, G.; Dalene, M. Determination of isocyanates, aminoisocyanates and amines in air formed during the thermal degradation of polyurethane. J. Environ. Monit. 2002, 4, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.S.; Outterside, S.M. MDI and TDI: Safety, Health and the Environment; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Schulz, M.; Salthammer, T. Sensitive determination of airborne diisocyanates by HPLC: 4,4′-Diphenylmethane-diisocyanate (MDI). Fresenius J. Anal. Chem. 1998, 362, 289–293. [Google Scholar] [CrossRef]
- Hardy, H.L.; Walker, R.F. Novel reagent for the determination of atmospheric isocyanate monomer concentrations. Analyst 1979, 104, 890. [Google Scholar] [CrossRef]
- Health Council of the Netherlands. Di- and Triisocyanates. Health-Based Recommendation on Occupational Exposure Limits; No. 2018/20; The Hague, Health Council of the Netherlands: Hague, The Netherlands, 2018. [Google Scholar]
- Roberge, B.; Aubin, S.; Ostiguy, C.; Lesage, J. Guide for Safe Use of Isocyanates. Available online: www.irsst.qc.ca (accessed on 21 November 2021).
- ECHA. Phenyl Isocyanate infocard. Eur. Chem. Agency 2006, 2006, pdb.caut643. [Google Scholar] [CrossRef]
- ISOPA—European Diisocyanite and Polyol Producers Association. Walk the Talk MDI Users; The Economist: London, UK, 2014; Volume 410. [Google Scholar]
- ISOPA—European Diisocyanite and Polyol Producers Association. Walk the Talk TDI Users; The Economist: London, UK, 2014; Volume 410, pp. 1–28. [Google Scholar]
- Center for the Polyurethanes Industry. Guidance for Working with TDI: Things You Should Know Purpose; Center for the Polyurethanes Industry: Arlington, VA, USA, 2012. [Google Scholar]
- Safe Work Australia. Guide to Handling Isocyanates; Safe Work Australia: Canberra, Australia, 2015; pp. 1–14.
- Center for the Polyurethanes Industry. Occupational Hygiene Air Monitoring for MDI & TDI Guidance; Center for the Polyurethanes Industry: Arlington, VA, USA, 2012. [Google Scholar]
- Dai, Y.; Wang, Y.; Yao, J.; Wang, Q.; Liu, L.; Chu, W.; Wang, G. Phosgene-Free Synthesis of Phenyl Isocyanate by Catalytic Decomposition of Methyl N-Phenyl Carbamate over Bi2O3 Catalyst. Catal. Lett. 2008, 123, 307–316. [Google Scholar] [CrossRef]

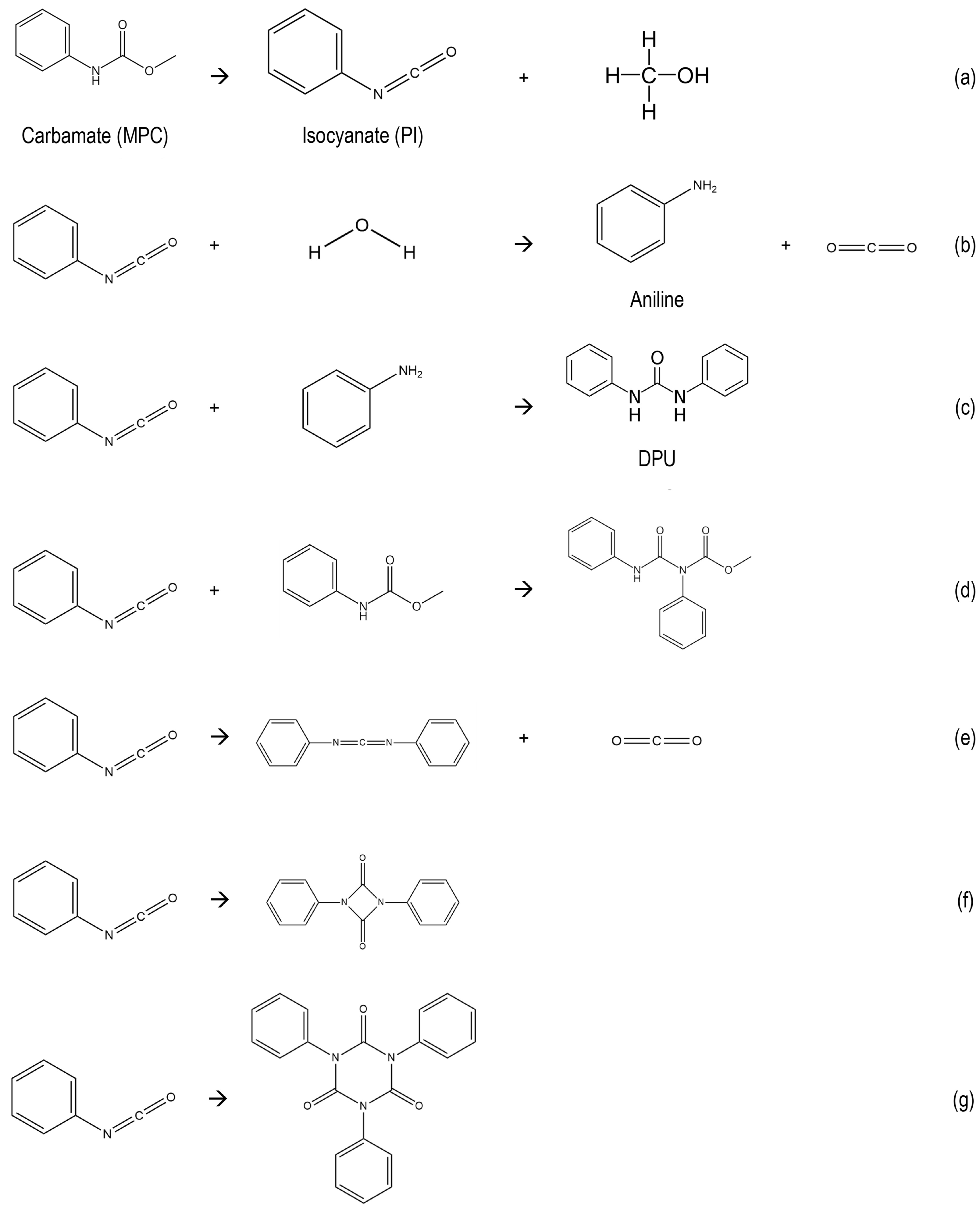

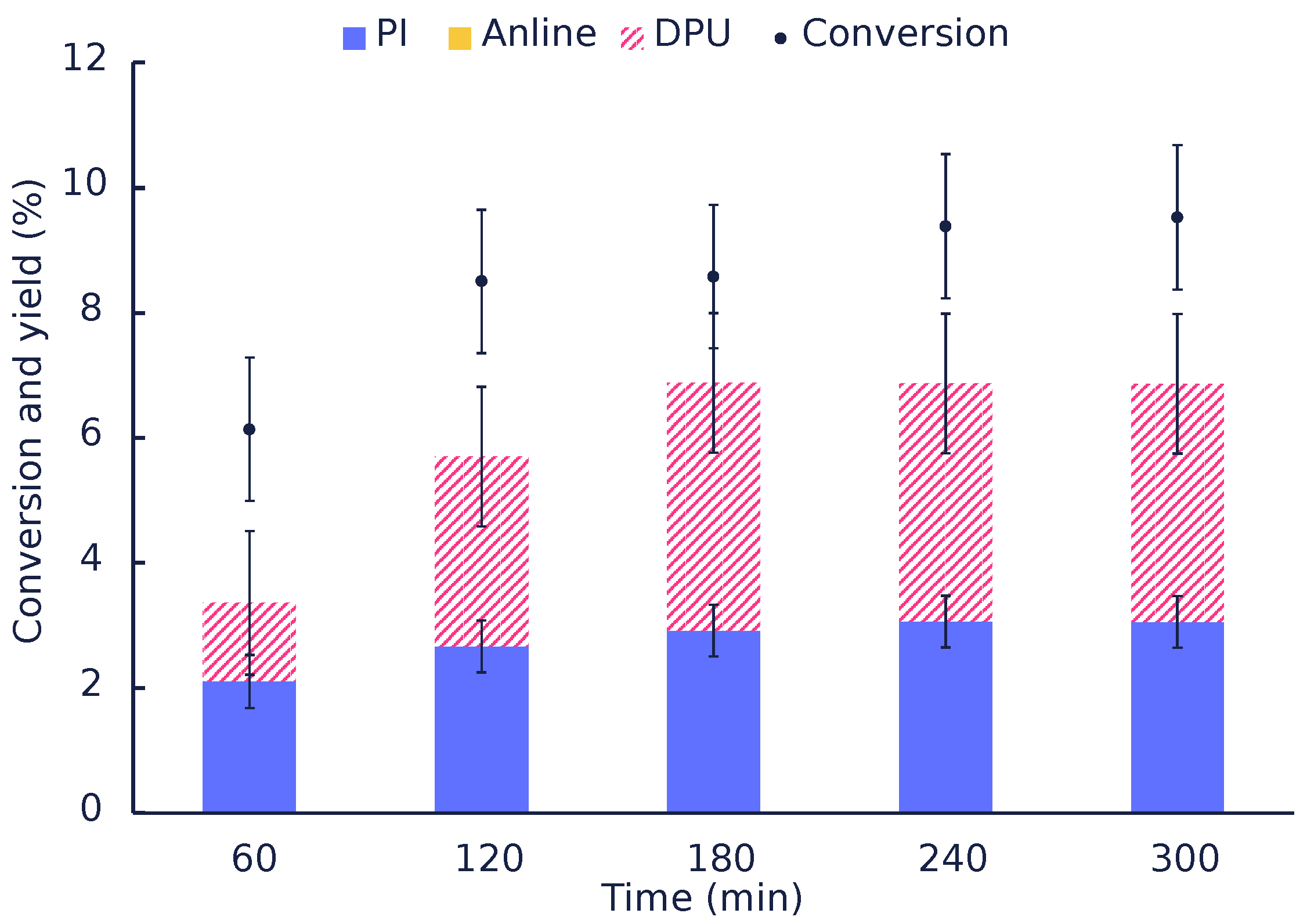
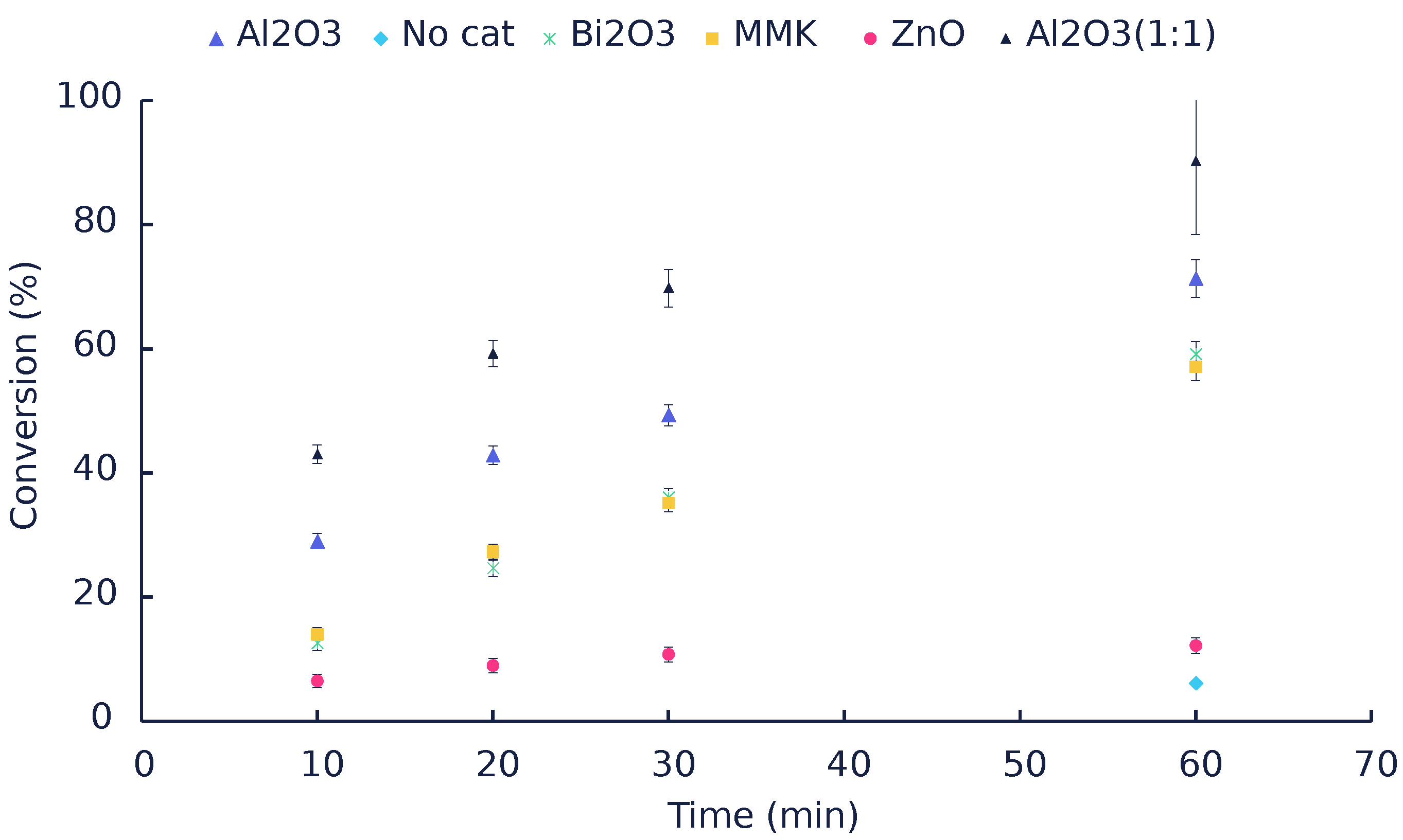
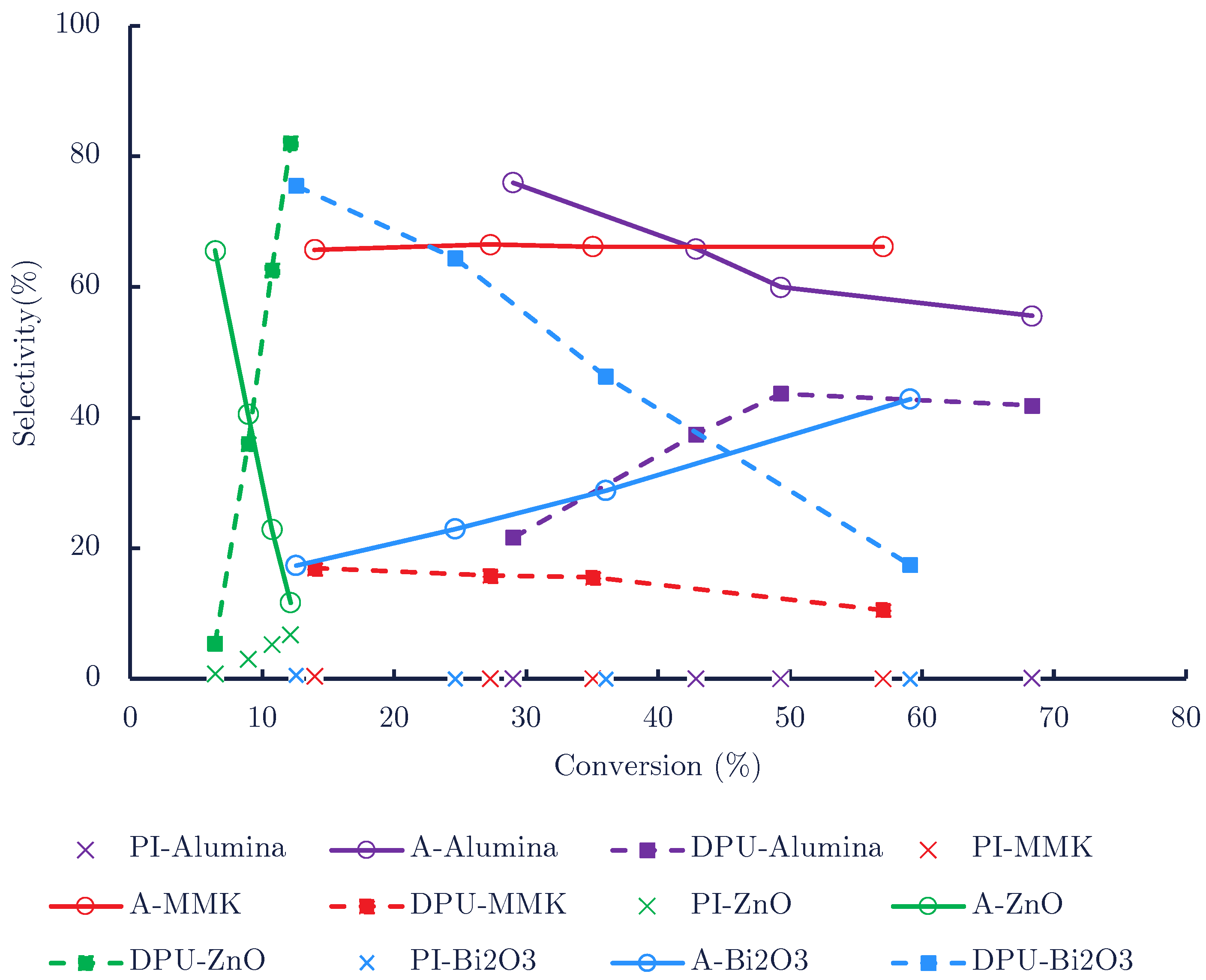
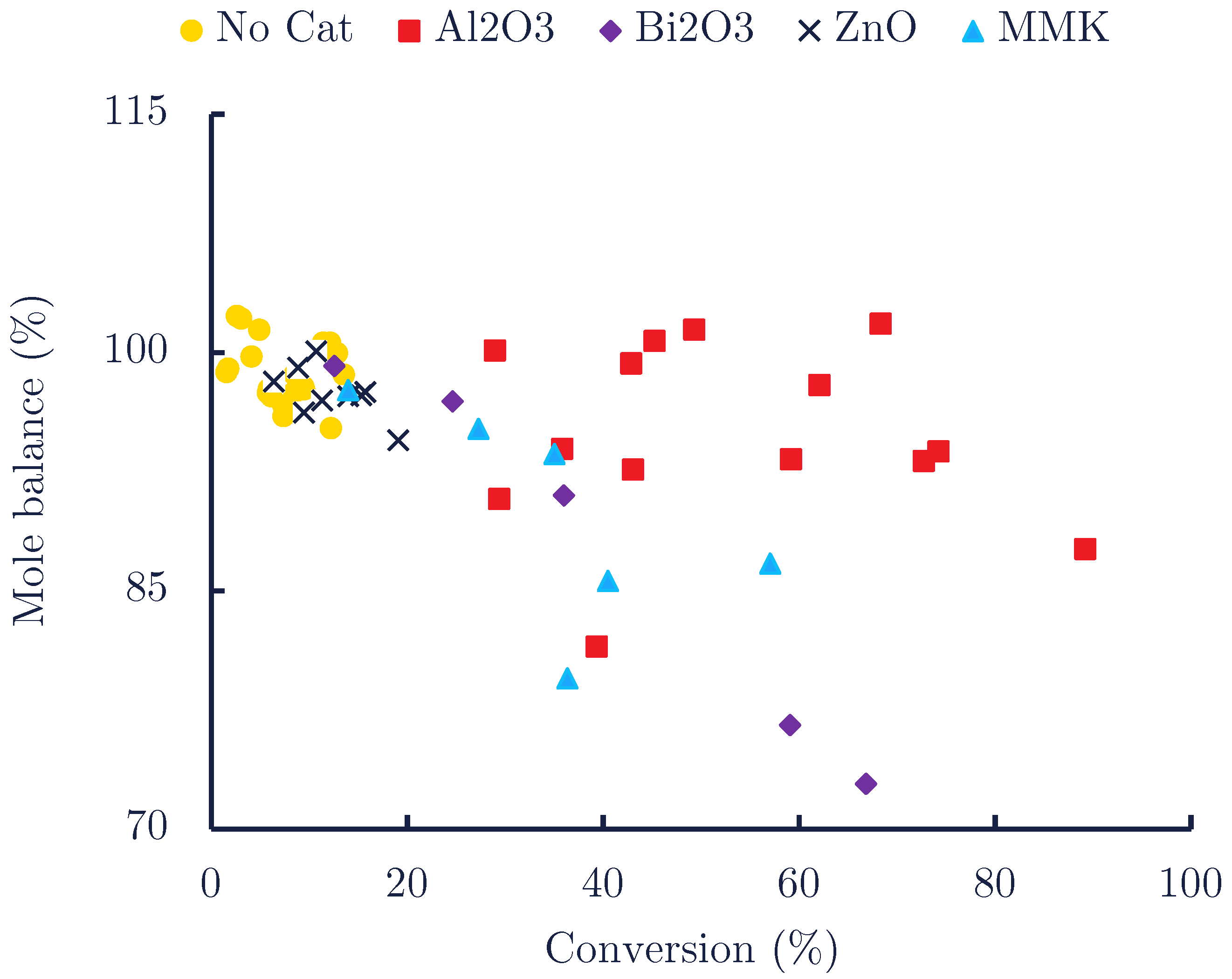
| Catalyst | Time (min) | Conversion (mol%) | Mole Balance (%) | |||
|---|---|---|---|---|---|---|
| ZnO | 10 | 6.5 | 4.2 | 0.1 | 0.3 | 98.2 |
| 20 | 8.9 | 3.6 | 0.3 | 3.2 | 98.2 | |
| 30 | 10.7 | 2.5 | 0.6 | 6.7 | 99.0 | |
| 60 | 12.2 | 1.4 | 0.8 | 10.0 | 100.1 | |
| MMK | 10 | 14.0 | 9.2 | 0.1 | 2.4 | 97.6 |
| 20 | 27.3 | 18.1 | 0.0 | 4.3 | 95.2 | |
| 30 | 35.1 | 23.2 | 0.0 | 5.4 | 93.6 | |
| 60 | 57.0 | 37.7 | 0.0 | 6.0 | 86.7 | |
| Bi2O3 | 10 | 12.6 | 2.2 | 0.1 | 9.5 | 99.2 |
| 20 | 24.6 | 5.7 | 0.0 | 15.9 | 96.9 | |
| 30 | 36.1 | 10.4 | 0.0 | 16.7 | 91.0 | |
| 60 | 59.1 | 25.3 | 0.0 | 10.3 | 76.5 | |
| Al2O3 | 10 | 29.0 | 22.0 | 0.0 | 6.3 | 99.3 |
| 20 | 42.9 | 28.2 | 0.0 | 16.1 | 101.4 | |
| 30 | 49.3 | 29.5 | 0.0 | 21.5 | 101.8 | |
| 60 | 68.3 | 38.0 | 0.1 | 28.6 | 98.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamani, S.; Lange, J.-P.; Kersten, S.R.A.; Ruiz, M.P. Polyurethane Recycling: Conversion of Carbamates—Catalysis, Side-Reactions and Mole Balance. Polymers 2022, 14, 4869. https://doi.org/10.3390/polym14224869
Zamani S, Lange J-P, Kersten SRA, Ruiz MP. Polyurethane Recycling: Conversion of Carbamates—Catalysis, Side-Reactions and Mole Balance. Polymers. 2022; 14(22):4869. https://doi.org/10.3390/polym14224869
Chicago/Turabian StyleZamani, Shahab, Jean-Paul Lange, Sascha R. A. Kersten, and M. Pilar Ruiz. 2022. "Polyurethane Recycling: Conversion of Carbamates—Catalysis, Side-Reactions and Mole Balance" Polymers 14, no. 22: 4869. https://doi.org/10.3390/polym14224869
APA StyleZamani, S., Lange, J.-P., Kersten, S. R. A., & Ruiz, M. P. (2022). Polyurethane Recycling: Conversion of Carbamates—Catalysis, Side-Reactions and Mole Balance. Polymers, 14(22), 4869. https://doi.org/10.3390/polym14224869







