The Development of New Nanocomposite Polytetrafluoroethylene/Fe2O3 NPs to Prevent Bacterial Contamination in Meat Industry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Composite PTFE/Fe2O3 NPs
2.2. Physical Properties Assay
2.3. Measurement of Reactive Oxygen Species Concentration
2.4. Measurement of 8-Oxoguanine and Long-Lived Reactive Protein Species Concentration
2.5. Antimicrobial Avtivity Assay
2.6. Assay of the Influence on Animal Cells Viability
2.7. Statistic
3. Results
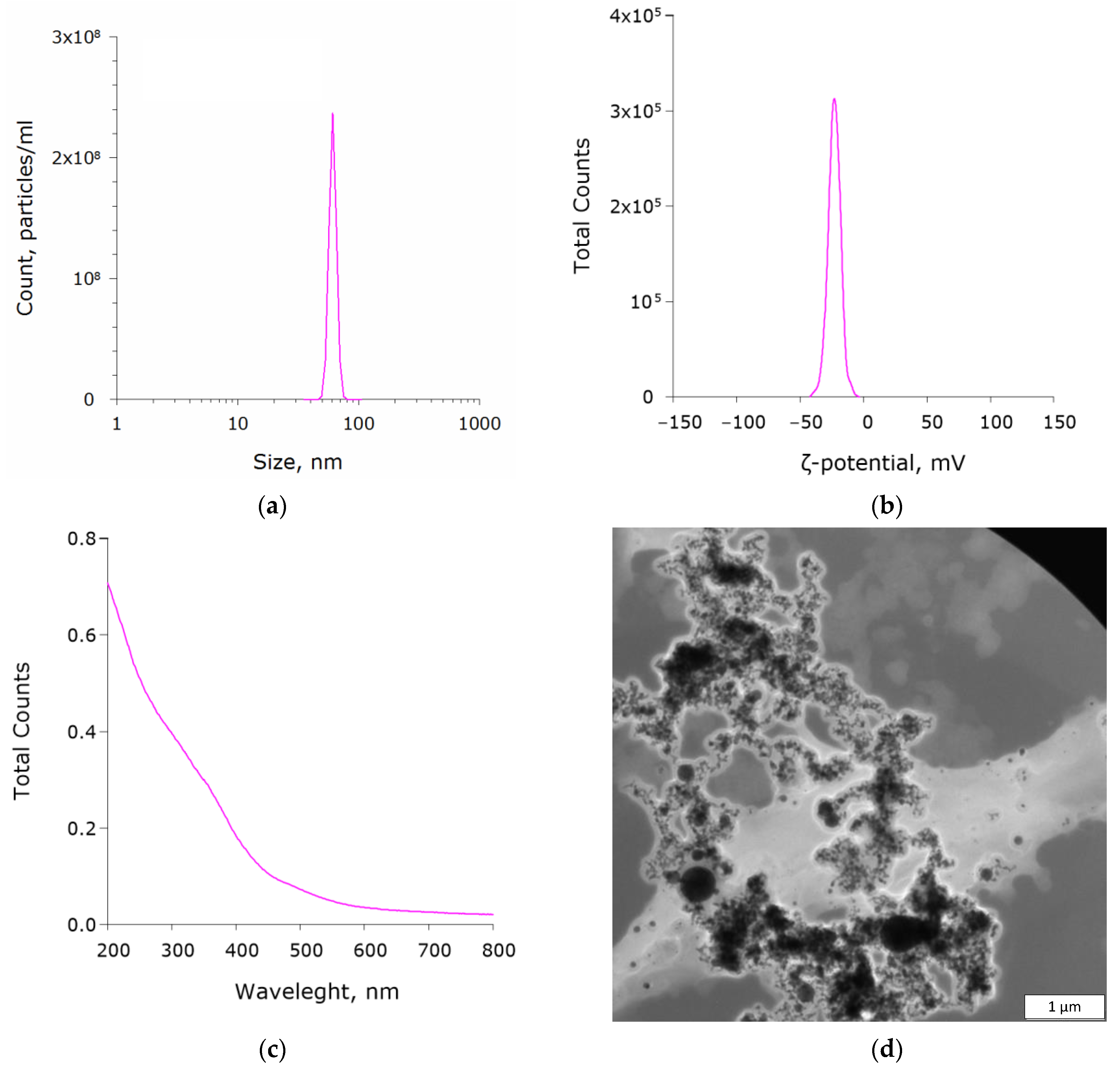
3.1. Physical Properties
3.2. Generation of Reactive Oxigen Species Concentration
3.3. Generation of 8-Oxoguanine and Long-Lived Reactive Protein Species
3.4. Antimicrobial Activity
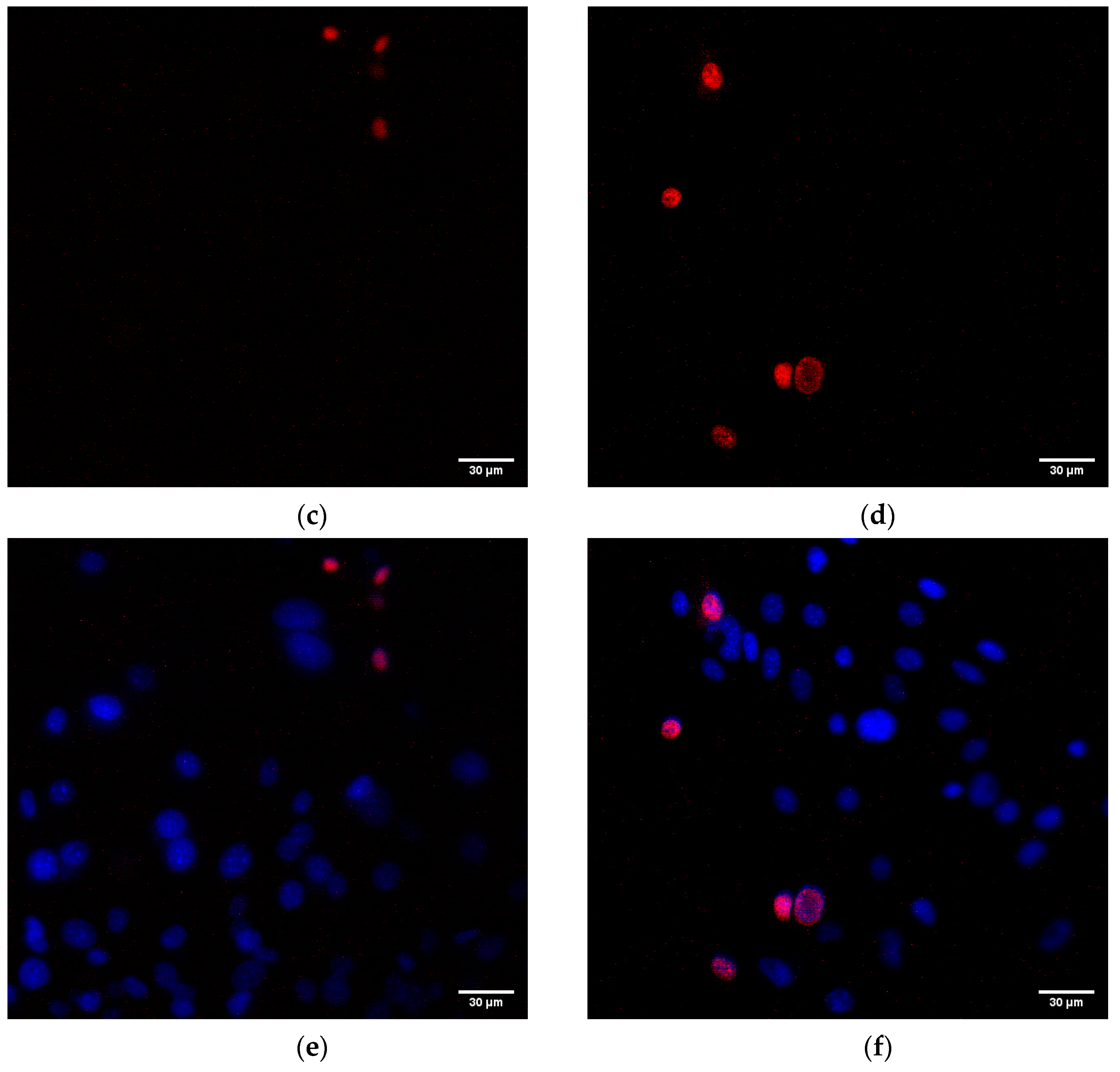
3.5. Influence on the Animal Cells Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sekoai, P.T.; Feng, S.; Zhou, W.; Ngan, W.Y.; Pu, Y.; Yao, Y.; Pan, J.; Habimana, O. Insights into the Microbiological Safety of Wooden Cutting Boards Used for Meat Processing in Hong Kong's Wet Markets: A Focus on Food-Contact Surfaces, Cross-Contamination and the Efficacy of Traditional Hygiene Practices. Microorganisms 2020, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Biofilms and Meat Safety: A Mini-Review. J. Food Prot. 2018, 82, 120–127. [Google Scholar] [CrossRef]
- Lo, M.Y.; Ngan, W.Y.; Tsun, S.M.; Hsing, H.L.; Lau, K.T.; Hung, H.P.; Chan, S.L.; Lai, Y.Y.; Yao, Y.; Pu, Y.; et al. A Field Study Into Hong Kong’s Wet Markets: Raised Questions Into the Hygienic Maintenance of Meat Contact Surfaces and the Dissemination of Microorganisms Associated With Nosocomial Infections. Front. Microbiol. 2019, 10, 2618. [Google Scholar] [CrossRef] [PubMed]
- Dantas, S.T.A.; Rossi, B.F.; Bonsaglia, E.C.R.; Castilho, I.G.; Hernandes, R.T.; Fernandes, A.J.; Rall, V.L.M. Cross-Contamination and Biofilm Formation by Salmonella enterica Serovar Enteritidis on Various Cutting Boards. Foodborne Pathog. Dis. 2018, 15, 81–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srey, S.; Jahid, I.K.; Ha, S.-D. Biofilm formation in food industries: A food safety concern. Food Control. 2013, 31, 572–585. [Google Scholar] [CrossRef]
- Scallan, E.; Griffin, P.M.; Angulo, F.J.; Tauxe, R.V.; Hoekstra, R.M. Foodborne illness acquired in the United States—Unspecified agents. Emerg. Infect. Dis. 2011, 17, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Aspell CPolymer science VRGowariker, N.V. Viswanathan and Jayadev Sreedhar, Halsted Press (John Wiley & Sons), New York, 1986. pp. xv + 505, ISBN 0-470-20322-6. Br. Polym. J. 1988, 20, 88. [Google Scholar] [CrossRef]
- Dhanumalayan, E.; Joshi, G.M. Performance properties and applications of polytetrafluoroethylene (PTFE)—A review. Adv. Compos. Hybrid Mater. 2018, 1, 247–268. [Google Scholar] [CrossRef]
- Burkarter, E.; Saul, C.K.; Thomazi, F.; Cruz, N.C.; Roman, L.S.; Schreiner, W.H. Superhydrophobic electrosprayed PTFE. Surf. Coat. Technol. 2007, 202, 194–198. [Google Scholar] [CrossRef]
- Dearn, K.D.; Hoskins, T.J.; Petrov, D.G.; Reynolds, S.C.; Banks, R. Applications of dry film lubricants for polymer gears. Wear 2013, 298–299, 99–108. [Google Scholar] [CrossRef]
- Mccook, N.L.; Burris, D.L.; Dickrell, P.L.; Sawyer, W.G. Cryogenic Friction Behavior of PTFE based Solid Lubricant Composites. Tribol. Lett. 2005, 20, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Barish, J.A.; Goddard, J.M. Anti-fouling surface modified stainless steel for food processing. Food Bioprod. Processing 2013, 91, 352–361. [Google Scholar] [CrossRef]
- O’brien, M.; Baxendale, I.R.; Ley, S.V. Flow Ozonolysis Using a Semipermeable Teflon AF-2400 Membrane To Effect Gas−Liquid Contact. Org. Lett. 2010, 12, 1596–1598. [Google Scholar] [CrossRef]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.-J.; Park, W.-T.; Yoon, Y.-J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.F.; Preger, Y.; Stahl, S.S.; Root, T.W. PTFE-Membrane Flow Reactor for Aerobic Oxidation Reactions and Its Application to Alcohol Oxidation. Org. Process Res. Dev. 2015, 19, 858–864. [Google Scholar] [CrossRef]
- Rodriguez-Lopez, J.; Alpuche-Aviles, M.A.; Bard, A.J. Selective Insulation with Poly(tetrafluoroethylene) of Substrate Electrodes for Electrochemical Background Reduction in Scanning Electrochemical Microscopy. Anal. Chem. 2008, 80, 1813–1818. [Google Scholar] [CrossRef]
- Feng, S.; Zhong, Z.; Zhang, F.; Wang, Y.; Xing, W. Amphiphobic Polytetrafluoroethylene Membranes for Efficient Organic Aerosol Removal. ACS Appl. Mater. Interfaces 2016, 8, 8773–8781. [Google Scholar] [CrossRef]
- Rondinella, A.; Andreatta, F.; Turrin, D.; Fedrizzi, L. Degradation Mechanisms Occurring in PTFE-Based Coatings Employed in Food-Processing Applications. Coatings 2021, 11, 1419. [Google Scholar] [CrossRef]
- Chao, Q.; Meng, L.; Shuxian, C. Anti-icing characteristics of PTFE super hydrophobic coating on titanium alloy surface. J. Alloys Compd. 2021, 860, 157907. [Google Scholar] [CrossRef]
- Song, W.; Wang, S.; Lu, Y.; Zhang, X.; Xia, Z. Friction behavior of PTFE-coated Si3N4/TiC ceramics fabricated by spray technique under dry friction. Ceram. Int. 2021, 47, 7487–7496. [Google Scholar] [CrossRef]
- Wu, X.; Yang, F.; Gan, J.; Zhao, W.; Wu, Y. A flower-like waterborne coating with self-cleaning, self-repairing properties for superhydrophobic applications. J. Mater. Res. Technol. 2021, 14, 1820–1829. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Smirnova, V.V.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of Al2O3 Nanoparticles. Nanomaterials 2022, 12, 2635. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. Do Iron Oxide Nanoparticles Have Significant Antibacterial Properties? Antibiotics 2021, 10, 884. [Google Scholar] [CrossRef]
- Chausov, D.N.; Burmistrov, D.E.; Kurilov, A.D.; Bunkin, N.F.; Astashev, M.E.; Simakin, A.V.; Vedunova, M.V.; Gudkov, S.V. New Organosilicon Composite Based on Borosiloxane and Zinc Oxide Nanoparticles Inhibits Bacterial Growth, but Does Not Have a Toxic Effect on the Development of Animal Eukaryotic Cells. Materials 2021, 14, 6281. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Simakin, A.V.; Sarimov, R.M.; Kurilov, A.D.; Chausov, D.N. Novel Biocompatible with Animal Cells Composite Material Based on Organosilicon Polymers and Fullerenes with Light-Induced Bacteriostatic Properties. Nanomaterials 2021, 11, 2804. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, V.V.; Chausov, D.N.; Serov, D.A.; Kozlov, V.A.; Ivashkin, P.I.; Pishchalnikov, R.Y.; Uvarov, O.V.; Vedunova, M.V.; Semenova, A.A.; Lisitsyn, A.B.; et al. A Novel Biodegradable Composite Polymer Material Based on PLGA and Silver Oxide Nanoparticles with Unique Physicochemical Properties and Biocompatibility with Mammalian Cells. Materials 2021, 14, 6915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liang, X.; Gadd, G.M.; Zhao, Q. A sol–gel based silver nanoparticle/polytetrafluorethylene (AgNP/PTFE) coating with enhanced antibacterial and anti-corrosive properties. Appl. Surf. Sci. 2021, 535, 147675. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Baimler, I.V.; Uvarov, O.V.; Smirnova, V.V.; Volkov, M.Y.; Semenova, A.A.; Lisitsyn, A.B. Influence of the Concentration of Fe and Cu Nanoparticles on the Dynamics of the Size Distribution of Nanoparticles. Front. Phys. 2020, 8, 578. [Google Scholar] [CrossRef]
- Sarimov, R.M.; Nagaev, E.I.; Matveyeva, T.A.; Binhi, V.N.; Burmistrov, D.E.; Serov, D.A.; Astashev, M.E.; Simakin, A.V.; Uvarov, O.V.; Khabatova, V.V.; et al. Investigation of Aggregation and Disaggregation of Self-Assembling Nano-Sized Clusters Consisting of Individual Iron Oxide Nanoparticles upon Interaction with HEWL Protein Molecules. Nanomaterials 2022, 12, 3960. [Google Scholar] [CrossRef]
- Ivanyuk, V.V.; Shkirin, A.V.; Belosludtsev, K.N.; Dubinin, M.V.; Kozlov, V.A.; Bunkin, N.F.; Dorokhov, A.S.; Gudkov, S.V. Influence of Fluoropolymer Film Modified With Nanoscale Photoluminophor on Growth and Development of Plants. Front. Phys. 2020, 8, 616040. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Lyakhov, G.A.; Pustovoy, V.I.; Shcherbakov, I.A. Influence of Mechanical Effects on the Hydrogen Peroxide Concentration in Aqueous Solutions. Phys. Wave Phenom. 2019, 27, 141–144. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Simakin, A.V.; Sevostyanov, M.A.; Konushkin, S.V.; Losertová, M.; Ivannikov, A.Y.; Kolmakov, A.G.; Izmailov, A.Y. Manufacturing and study of mechanical properties, structure and compatibility with biological objects of plates and wire from new Ti-25Nb-13Ta-5Zr alloy. Metals 2020, 10, 1584. [Google Scholar] [CrossRef]
- Gudkov, S.; Garmash, S.; Shtarkman, I.; Chernikov, A.; Karp, O.; Bruskov, V. Long-lived protein radicals induced by X-ray irradiation are the source of reactive oxygen species in aqueous medium. Biochem. Bioph. Rep. 2010, 430, 1. [Google Scholar] [CrossRef]
- Sharapov, M.; Novoselov, V.; Penkov, N.; Fesenko, E.; Vedunova, M.; Bruskov, V.; Gudkov, S. Protective and adaptogenic role of peroxiredoxin 2 (Prx2) in neutralization of oxidative stress induced by ionizing radiation. Free. Radic. Biol. Med. 2019, 134, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Chausov, D.N.; Smirnova, V.V.; Burmistrov, D.E.; Sarimov, R.M.; Kurilov, A.D.; Astashev, M.E.; Uvarov, O.V.; Dubinin, M.V.; Kozlov, V.A.; Vedunova, M.V.; et al. Synthesis of a Novel, Biocompatible and Bacteriostatic Borosiloxane Composition with Silver Oxide Nanoparticles. Materials 2022, 15, 527. [Google Scholar] [CrossRef]
- Astashev, M.E.; Sarimov, R.M.; Serov, D.A.; Matveeva, T.A.; Simakin, A.V.; Ignatenko, D.N.; Burmistrov, D.E.; Smirnova, V.V.; Kurilov, A.D.; Mashchenko, V.I.; et al. Antibacterial behavior of organosilicon composite with nano aluminum oxide without influencing animal cells. React. Funct. Polym. 2022, 170, 105143. [Google Scholar] [CrossRef]
- Seluanov, A.; Vaidya, A.; Gorbunova, V. Establishing primary adult fibroblast cultures from rodents. J. Vis. Exp. 2010, 44, 2033. [Google Scholar] [CrossRef] [Green Version]
- Sevost’yanov, M.A.; Nasakina, E.O.; Baikin, A.S.; Sergienko, K.V.; Konushkin, S.V.; Kaplan, M.A.; Seregin, A.V.; Leonov, A.V.; Kozlov, V.A.; Shkirin, A.V.; et al. Biocompatibility of new materials based on nano-structured nitinol with titanium and tantalum composite surface layers: Experimental analysis in vitro and in vivo. J. Mater. Science. Mater. Med. 2018, 29, 33. [Google Scholar] [CrossRef]
- Kaplan, M.A.; Sergienko, K.V.; Kolmakova, A.A.; Konushkin, S.V.; Baikin, A.S.; Kolmakov, A.G.; Sevostyanov, M.A.; Kulikov, A.V.; Ivanov, V.E.; Belosludtsev, K.N.; et al. Development of a Biocompatible PLGA Polymers Capable to Release Thrombolytic Enzyme Prourokinase. J. Biomater. Sci. Polym. Ed. 2020, 31, 1405–1420. [Google Scholar] [CrossRef]
- Starinets, V.S.; Serov, D.A.; Penkov, N.V.; Belosludtseva, N.V.; Dubinin, M.V.; Belosludtsev, K.N. Alisporivir Normalizes Mitochondrial Function of Primary Mouse Lung Endothelial Cells Under Conditions of Hyperglycemia. Biochem. Mosc. 2022, 87, 605–616. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative Stress and Oxidative Damage in Carcinogenesis. Toxicol. Pathol. 2009, 38, 96–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burmistrov, D.E.; Simakin, A.V.; Smirnova, V.V.; Uvarov, O.V.; Ivashkin, P.I.; Kucherov, R.N.; Ivanov, V.E.; Bruskov, V.I.; Sevostyanov, M.A.; Baikin, A.S.; et al. Bacteriostatic and Cytotoxic Properties of Composite Material Based on ZnO Nanoparticles in PLGA Obtained by Low Temperature Method. Polymers 2022, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Amutha, S.; Sridhar, S. Green synthesis of magnetic iron oxide nanoparticle using leaves of Glycosmis mauritiana and their antibacterial activity against human pathogens. J. Innov. Pharm. Biol. Sci. 2018, 5, 22–26. [Google Scholar]
- Gabrielyan, L.; Hakobyan, L.; Hovhannisyan, A.; Trchounian, A. Effects of iron oxide (Fe3O4) nanoparticles on Escherichia coli antibiotic-resistant strains. J. Appl. Microbiol. 2019, 126, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dixit, C.K. 3—Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Woodhead Publishing: Sawston, UK, 2017; pp. 43–58. Available online: https://www.sciencedirect.com/science/article/pii/B9780081005576000031 (accessed on 22 September 2022). [CrossRef]
- Martins, S.A.; Dias, F.W.; Nunes, L.S.; Borges, L.A.; D’almeida, J.R. Mechanical Characterization of Silica Reinforced-PTFE Matrix Composites. Procedia Eng. 2011, 10, 2651–2656. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.S.K.; Ching, Y.C.; Liu, S.; Ching, K.Y.; Razali, S.; Gan, S.N. Effects of PTFE Micro-Particles on the Fiber-Matrix Interface of Polyoxymethylene/Glass Fiber/Polytetrafluoroethylene Composites. Materials 2018, 11, 2164. [Google Scholar] [CrossRef] [Green Version]
- Khayet, M.; Wang, R. Mixed Matrix Polytetrafluoroethylene/Polysulfone Electrospun Nanofibrous Membranes for Water Desalination by Membrane Distillation. ACS Appl. Mater. Interfaces 2018, 10, 24275–24287. [Google Scholar] [CrossRef]
- Xu, H.X.; Yuan, X.H.; Zhu, E.B.; Li, S.L.; Chen, L.; Han, Z.R. Comparison of Mechanical and Frictional Properties of PTFE Matrix Composites Reinforced by Different Fibers and Graphite. Key Eng. Mater. 2014, 575, 203–208. [Google Scholar]
- Ibrahim, N.H.; Romli, A.Z.; Ibrahim, N.N.I.N. Hardness optimization of epoxy filled PTFE with/without TiO2 filler. IOP Conf. Ser. Mater. Sci. Eng. 2020, 839, 012002. [Google Scholar] [CrossRef]
- Wegner, A.M.; Haudenschild, D.R. NADPH oxidases in bone and cartilage homeostasis and disease: A promising therapeutic target. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2020, 38, 2104–2112. [Google Scholar] [CrossRef]
- Shcherbakov, I.A.; Baimler, I.V.; Gudkov, S.V.; Lyakhov, G.A.; Mikhailova, G.N.; Pustovoy, V.I.; Sarimov, R.M.; Simakin, A.V.; Troitsky, A.V. Influence of a Constant Magnetic Field on Some Properties of Water Solutions. Dokl. Phys. 2020, 65, 273–275. [Google Scholar] [CrossRef]
- Menshchikova, E.B.; Kozhin, P.M.; Chechushkov, A.V.; Khrapova, M.V.; Zenkov, N.K. The Oral Delivery of Water-Soluble Phenol TS-13 Ameliorates Granuloma Formation in an In Vivo Model of Tuberculous Granulomatous Inflammation. Oxidative Med. Cell. Longev. 2021, 2021, 6652775. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Douki, T.; Gasparutto, D.; Ravanat, J.-L. Oxidative damage to DNA: Formation, measurement and biochemical features. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2003, 531, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, M.G.; Gordeeva, A.E.; Goncharov, R.G.; Tikhonova, I.V.; Ravin, V.K.; Temnov, A.A.; Fesenko, E.E.; Novoselov, V.I. The Effect of Exogenous Peroxiredoxin 6 on the State of Mesenteric Vessels and the Small Intestine in Ischemia–Reperfusion Injury. Biophysics 2017, 62, 998–1008. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995, 82–83, 969–974. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Yanykin, D.V.; Paskhin, M.O.; Nagaev, E.V.; Efimov, A.D.; Kaziev, A.V.; Ageychenkov, D.G.; Gudkov, S.V. Additive Production of a Material Based on an Acrylic Polymer with a Nanoscale Layer of Zno Nanorods Deposited Using a Direct Current Magnetron Discharge: Morphology, Photoconversion Properties, and Biosafety. Materials 2021, 14, 6586. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Reviews. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Gabrielyan, L.; Badalyan, H.; Gevorgyan, V.; Trchounian, A. Comparable antibacterial effects and action mechanisms of silver and iron oxide nanoparticles on Escherichia coli and Salmonella typhimurium. Sci. Rep. 2020, 10, 13145. [Google Scholar] [CrossRef] [PubMed]
- Gabrielyan, L.; Hovhannisyan, A.; Gevorgyan, V.; Ananyan, M.; Trchounian, A. Antibacterial effects of iron oxide (Fe3O4) nanoparticles: Distinguishing concentration-dependent effects with different bacterial cells growth and membrane-associated mechanisms. Appl. Microbiol. Biotechnol. 2019, 103, 2773–2782. [Google Scholar] [CrossRef]
- Sousa, C.; Sequeira, D.; Kolen’ko, Y.V.; Pinto, I.M.; Petrovykh, D.Y. Analytical Protocols for Separation and Electron Microscopy of Nanoparticles Interacting with Bacterial Cells. Anal. Chem. 2015, 87, 4641–4648. [Google Scholar] [CrossRef] [Green Version]
- Arakha, M.; Pal, S.; Samantarrai, D.; Panigrahi, T.K.; Mallick, B.C.; Pramanik, K.; Mallick, B.; Jha, S. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015, 5, 14813. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Serov, D.A.; Astashev, M.E.; Semenova, A.A.; Lisitsyn, A.B. Ag2O Nanoparticles as a Candidate for Antimicrobial Compounds of the New Generation. Pharmaceuticals 2022, 15, 968. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z.Q.; Lin, M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J. Appl. Microbiol. 2009, 107, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.K.; Debanath, M.K.; Paul, B.; Medhi, S.; Saikia, E. Antibacterial and nonlinear dynamical analysis of flower and hexagon-shaped ZnO microstructures. Sci. Rep. 2020, 10, 2598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sihem, L.; Hanine, D.; Faiza, B. Antibacterial Activity of α-Fe2O3 and α-Fe2O3@Ag Nanoparticles Prepared by Urtica Leaf Extract. Nanotechnologies Russ. 2020, 15, 198–203. [Google Scholar] [CrossRef]
- Shkodenko, L.; Kassirov, I.; Koshel, E. Metal Oxide Nanoparticles Against Bacterial Biofilms: Perspectives and Limitations. Microorganisms 2020, 8, 1545. [Google Scholar] [CrossRef]
- Kudrinskiy, A.A.; Ivanov, A.Y.; Kulakovskaya, E.V.; Klimov, A.I.; Zherebin, P.M.; Khodarev, D.V.; Le, A.-T.; Tam, L.T.; Lisichkin, G.V.; Krutyakov, Y.A. The Mode of Action of Silver and Silver Halides Nanoparticles against Saccharomyces cerevisiae Cells. J. Nanoparticles 2014, 2014, 568635. [Google Scholar] [CrossRef] [Green Version]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [Green Version]
- Fenton, H.J.H. Oxidation of Tartaric Acid in Presence of Iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef] [Green Version]
- Abou-Gamra, Z.M. Kinetic and Thermodynamic Study for Fenton-Like Oxidation of Amaranth Red Dye. Adv. Chem. Eng. Sci. 2014, 4, 285–291. [Google Scholar] [CrossRef]
- Maji, S.K.; Mukherjee, N.; Mondal, A.; Adhikary, B. Synthesis, characterization and photocatalytic activity of α-Fe2O3 nanoparticles. Polyhedron 2012, 33, 145–149. [Google Scholar] [CrossRef]
- Ansari, M.O.; Parveen, N.; Ahmad, M.F.; Wani, A.L.; Afrin, S.; Rahman, Y.; Jameel, S.; Khan, Y.A.; Siddique, H.R.; Tabish, M.; et al. Evaluation of DNA interaction, genotoxicity and oxidative stress induced by iron oxide nanoparticles both in vitro and in vivo: Attenuation by thymoquinone. Sci. Rep. 2019, 9, 6912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolen’ko, Y.V.; Bañobre-López, M.; Rodríguez-Abreu, C.; Carbó-Argibay, E.; Deepak, F.L.; Petrovykh, D.Y.; Cerqueira, M.F.; Kamali, S.; Kovnir, K.; Shtansky, D.V.; et al. High-Temperature Magnetism as a Probe for Structural and Compositional Uniformity in Ligand-Capped Magnetite Nanoparticles. J. Phys. Chem. C 2014, 118, 28322–28329. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wei, W.; Wu, X.; Zhao, Y.; Dai, H. The antibacterial and antibiofilm activities of mesoporous hollow Fe3O4 nanoparticles in an alternating magnetic field. Biomater. Sci. 2020, 8, 4492–4507. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakellis, P.; Kaprou, G.D.; Papavieros, G.; Mastellos, D.C.; Constantoudis, V.; Tserepi, A.; Gogolides, E. Enhanced antibacterial activity of ZnO-PMMA nanocomposites by selective plasma etching in atmospheric pressure. Micro Nano Eng. 2021, 13, 100098. [Google Scholar] [CrossRef]
- Ismail, R.A.; Sulaiman, G.M.; Abdulrahman, S.A.; Marzoog, T.R. Antibacterial activity of magnetic iron oxide nanoparticles synthesized by laser ablation in liquid. Mater. Sci. Eng. C 2015, 53, 286–297. [Google Scholar] [CrossRef]
- Saqib, S.; Munis, M.F.H.; Zaman, W.; Ullah, F.; Shah, S.N.; Ayaz, A.; Farooq, M.; Bahadur, S. Synthesis, characterization and use of iron oxide nano particles for antibacterial activity. Microsc. Res. Tech. 2019, 82, 415–420. [Google Scholar] [CrossRef]
- Bashir, M.; Ali, S.; Farrukh, M.A. Green synthesis of Fe2O3 nanoparticles from orange peel extract and a study of its antibacterial activity. J. Korean Phys. Soc. 2020, 76, 848–854. [Google Scholar] [CrossRef]
- Prabhu, Y.; Rao, K.V.; Kumari, B.S.; Kumar, V.S.S.; Pavani, T. Synthesis of Fe3O4 nanoparticles and its antibacterial application. Int. Nano Lett. 2015, 5, 85–92. [Google Scholar] [CrossRef]
- Al-Kattan, A.; Grojo, D.; Drouet, C.; Mouskeftaras, A.; Delaporte, P.; Casanova, A.; Robin, J.D.; Magdinier, F.; Alloncle, P.; Constantinescu, C. Short-Pulse Lasers: A Versatile Tool in Creating Novel Nano-/Micro-Structures and Compositional Analysis for Healthcare and Wellbeing Challenges. Nanomaterials 2021, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Burmistrov, D.E.; Lednev, V.N.; Simakin, A.V.; Uvarov, O.V.; Kucherov, R.N.; Ivashkin, P.I.; Dorokhov, A.S.; Izmailov, A.Y. Biosafety Construction Composite Based on Iron Oxide Nanoparticles and PLGA. Inventions 2022, 7, 61. [Google Scholar] [CrossRef]
- Bhushan, M.; Kumar, Y.; Periyasamy, L.; Viswanath, A.K. Antibacterial applications of α-Fe2O3/Co3O4 nanocomposites and study of their structural, optical, magnetic and cytotoxic characteristics. Appl. Nanosci. 2018, 8, 137–153. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, P.; Neogi, S. Gentamicin coated iron oxide nanoparticles as novel antibacterial agents. Mater. Res. Express 2017, 4, 095005. [Google Scholar] [CrossRef]
- Nehra, P.; Chauhan, R.; Garg, N.; Verma, K. Antibacterial and antifungal activity of chitosan coated iron oxide nanoparticles. Br. J. Biomed. Sci. 2018, 75, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Khashan, K.S.; Sulaiman, G.M.; Mahdi, R. Preparation of iron oxide nanoparticles-decorated carbon nanotube using laser ablation in liquid and their antimicrobial activity. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1699–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Wang, L.; Liang, X.; Vorstius, J.; Keatch, R.; Corner, G.; Nabi, G.; Davidson, F.; Gadd, G.M.; Zhao, Q. Enhanced Antibacterial and Antiadhesive Activities of Silver-PTFE Nanocomposite Coating for Urinary Catheters. ACS Biomater. Sci. Eng. 2019, 5, 2804–2814. [Google Scholar] [CrossRef]
- Maccallum, N.; Howell, C.; Kim, P.; Sun, D.; Friedlander, R.; Ranisau, J.; Ahanotu, O.; Lin, J.J.; Vena, A.; Hatton, B.; et al. Liquid-Infused Silicone As a Biofouling-Free Medical Material. ACS Biomater. Sci. Eng. 2015, 1, 43–51. [Google Scholar] [CrossRef]
- Awad, T.S.; Asker, D.; Hatton, B.D. Food-Safe Modification of Stainless Steel Food-Processing Surfaces to Reduce Bacterial Biofilms. ACS Appl. Mater. Interfaces 2018, 10, 22902–22912. [Google Scholar] [CrossRef]
- Huang, K.; Goddard, J.M. Influence of fluid milk product composition on fouling and cleaning of Ni–PTFE modified stainless steel heat exchanger surfaces. J. Food Eng. 2015, 158, 22–29. [Google Scholar] [CrossRef]
- Zore, A.; Bezek, K.; Jevšnik, M.; Abram, A.; Runko, V.; Slišković, I.; Raspor, P.; Kovačević, D.; Bohinc, K. Bacterial adhesion rate on food grade ceramics and Teflon as kitchen worktop surfaces. Int. J. Food Microbiol. 2020, 332, 108764. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shen, M.; Xia, J.; Shi, X. Recent developments of cancer nanomedicines based on ultrasmall iron oxide nanoparticles and nanoclusters. Nanomedicine 2021, 16, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and Detrimental Effects of Reactive Oxygen Species on Lifespan: A Comprehensive Review of Comparative and Experimental Studies. Front. Cell Dev. Biol. 2021, 9, 628157. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.; Ríos-Momberg, M.; Hewitt, D.; Hansberg, W. Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol. 2005, 13, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Branzei, D.; Foiani, M. Regulation of DNA repair throughout the cell cycle. Nat. Rev. Mol. Cell Biol. 2008, 9, 297–308. [Google Scholar] [CrossRef]
- Eidet, J.R.; Pasovic, L.; Maria, R.; Jackson, C.J.; Utheim, T.P. Objective assessment of changes in nuclear morphology and cell distribution following induction of apoptosis. Diagn. Pathol. 2014, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippi-Chiela, E.C.; Oliveira, M.M.; Jurkovski, B.; Callegari-Jacques, S.M.; Da Silva, V.D.; Lenz, G. Nuclear morphometric analysis (NMA): Screening of senescence, apoptosis and nuclear irregularities. PLoS ONE 2012, 7, e0042522. [Google Scholar] [CrossRef] [Green Version]
- Saliani, M.; Jalal, R.; Goharshadi, E.K. Effects of pH and temperature on antibacterial activity of zinc oxide nanofluid against Escherichia coli O157: H7 and Staphylococcus aureus. Jundishapur J. Microbiol. 2015, 8, 17115. [Google Scholar] [CrossRef] [Green Version]
- Hanini, A.; Schmitt, A.; Kacem, K.; Chau, F.; Ammar, S.; Gavard, J. Evaluation of iron oxide nanoparticle biocompatibility. Int. J. Nanomed. 2011, 6, 787–794. [Google Scholar] [CrossRef] [Green Version]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [Green Version]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Rahgozar, S.; Zarrabi, A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neurosci. 2017, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Sil, P.C. Iron oxide nanoparticles mediated cytotoxicity via PI3K/AKT pathway: Role of quercetin. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2014, 71, 106–115. [Google Scholar] [CrossRef]
- Lai, X.; Wei, Y.; Zhao, H.; Chen, S.; Bu, X.; Lu, F.; Qu, D.; Yao, L.; Zheng, J.; Zhang, J. The effect of Fe2O3 and ZnO nanoparticles on cytotoxicity and glucose metabolism in lung epithelial cells. J. Appl. Toxicol. JAT 2015, 35, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; Del Pino, P.; Maffre, P.; Hartmann, R.; Gallego, M.; Rivera-Fernández, S.; De La Fuente, J.M.; Nienhaus, G.U.; Parak, W.J. Surface Functionalization of Nanoparticles with Polyethylene Glycol: Effects on Protein Adsorption and Cellular Uptake. ACS Nano 2015, 9, 6996–7008. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Perálvarez-Marín, A.; Minelli, C.; Faraudo, J.; Roig, A.; Laromaine, A. Albumin-coated SPIONs: An experimental and theoretical evaluation of protein conformation, binding affinity and competition with serum proteins. Nanoscale 2016, 8, 14393–14405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Yang, X.L.; Hu, Q.; Liu, M.S.; Peng, T.; Xu, W.F.; Huang, Q.H.; Li, B.; Liao, X.L. Arg-Gly-Asp Peptide-Functionalized Superparamagnetic γ-Fe2O3 Nanoparticles Enhance Osteoblast Migration In Vitro. J. Nanosci. Nanotechnol. 2020, 20, 6173–6179. [Google Scholar] [CrossRef]








| № | Synthesis Method | Composition | Size | MIC | Microorganism | Effect | Ref |
|---|---|---|---|---|---|---|---|
| 1 | Laser ablation in dimethyl formamide or sodium dodecyl sulphate | α-Fe2O3 | 50–110 | 4.25 mg/mL | E. coli, P. aeruginosa, S. aureus, S. marcescens | BS 1 | [78] |
| 2 | Co-precipitation method | Fe2O3 | 25–40 | 10–50 µg/mL | E. coli, S. aureus, S. dysentery | BS | [79] |
| 3 | Co-precipitation method | Fe2O3 | ~50 | 0.5 mg/mL | B. subtilis, E. coli, P. aeruginosa, S. aureus | BS | [80] |
| 4 | Wet chemical method | Fe3O4 | 33–40 | 25–100 µg/mL | E. coli, P. vulgaris, S. aureus, Xanthomonas sp. | BS | [81] |
| 5 | Co-precipitation method | Fe3O4 | 6–9 | 32–128 μg/mL | E. coli, L. monocytogenes, P. aeruginosa, S. marcescens | BS | [82] |
| 6 | Co-precipitation method | Fe3O4 | 10.64 ± 4.73 | 50–500 µg/mL | E. coli, E. hirae | BS | [60] |
| 7 | Co-precipitation method | α-Fe2O3, ZnO/α-Fe2O3 | ~30 | 400–800 µg/mL | B. subtilis, E. coli, S. aureus, S. typhimurium | BS | [84] |
| 8 | Co-precipitation method | Fe2O3, FeO, coated with gentamicin | 10–15 | 200 µg/mL | B. subtilis, E. coli, P. aeruginosa, S. aureus | BC 2 | [85] |
| 10 | Co-precipitation method | Fe3O4 coated with chitozan | ~11 | 30–40 μg/mL | A. niger, B. subtilis, C. albicans, E. coli, F. solani | BS | [86] |
| 11 | Laser ablation in water | Fe2O3 NPs/carbon nanotubes | 6–7 | 400–800 μg/mL | E. coli, K. pneumonia, S. aureus | BS | [87] |
| 12 | Electrolysis | Ag NPs/PTFE | 150 | ~5 mg/mL | E. coli, S. aureus | BC | [88] |
| 13 | Electrolysis + sol–gel method | Ag NPs/PTFE | 500 | ~50 mg/mL | E. coli | BC | [27] |
| 14 | Laser ablation in water | Fe2O3/PLGA | 50 nm | 10 μg/mL | E. coli | BS | [83] |
| 15 | Laser ablation in water | Fe2O3/PTFE | 60 nm | 10 μg/mL | E. coli | BS | Current study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serov, D.A.; Baimler, I.V.; Burmistrov, D.E.; Baryshev, A.S.; Yanykin, D.V.; Astashev, M.E.; Simakin, A.V.; Gudkov, S.V. The Development of New Nanocomposite Polytetrafluoroethylene/Fe2O3 NPs to Prevent Bacterial Contamination in Meat Industry. Polymers 2022, 14, 4880. https://doi.org/10.3390/polym14224880
Serov DA, Baimler IV, Burmistrov DE, Baryshev AS, Yanykin DV, Astashev ME, Simakin AV, Gudkov SV. The Development of New Nanocomposite Polytetrafluoroethylene/Fe2O3 NPs to Prevent Bacterial Contamination in Meat Industry. Polymers. 2022; 14(22):4880. https://doi.org/10.3390/polym14224880
Chicago/Turabian StyleSerov, Dmitriy A., Ilya V. Baimler, Dmitriy E. Burmistrov, Alexey S. Baryshev, Denis V. Yanykin, Maxim E. Astashev, Alexander V. Simakin, and Sergey V. Gudkov. 2022. "The Development of New Nanocomposite Polytetrafluoroethylene/Fe2O3 NPs to Prevent Bacterial Contamination in Meat Industry" Polymers 14, no. 22: 4880. https://doi.org/10.3390/polym14224880
APA StyleSerov, D. A., Baimler, I. V., Burmistrov, D. E., Baryshev, A. S., Yanykin, D. V., Astashev, M. E., Simakin, A. V., & Gudkov, S. V. (2022). The Development of New Nanocomposite Polytetrafluoroethylene/Fe2O3 NPs to Prevent Bacterial Contamination in Meat Industry. Polymers, 14(22), 4880. https://doi.org/10.3390/polym14224880








