Molecular Dynamics Studies of the Mechanical Behaviors and Thermal Conductivity of Polyisoprene with Different Degrees of Polymerization
Abstract
:1. Introduction
2. Materials and Methods
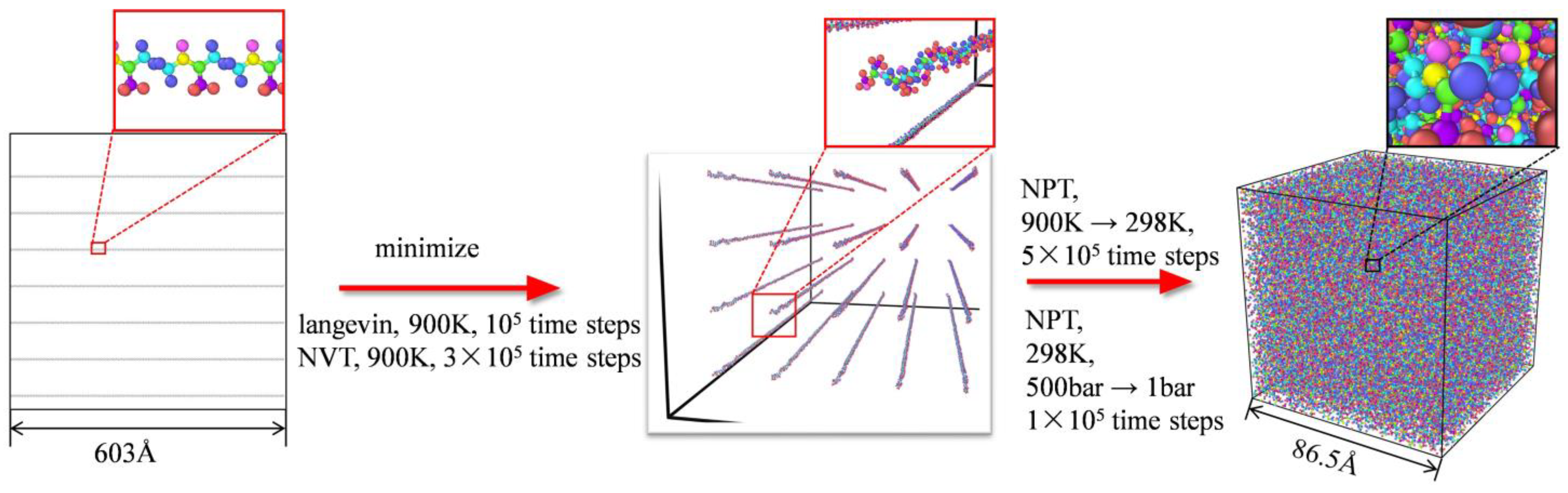
2.1. Construction of the Simulation Model
2.2. Interatomic Potential
2.3. Uniaxial Tensile Simulation
2.4. Thermal Conductivity Calculation
3. Results and Analysis of Tensile Simulation
3.1. Stress–Strain Behavior
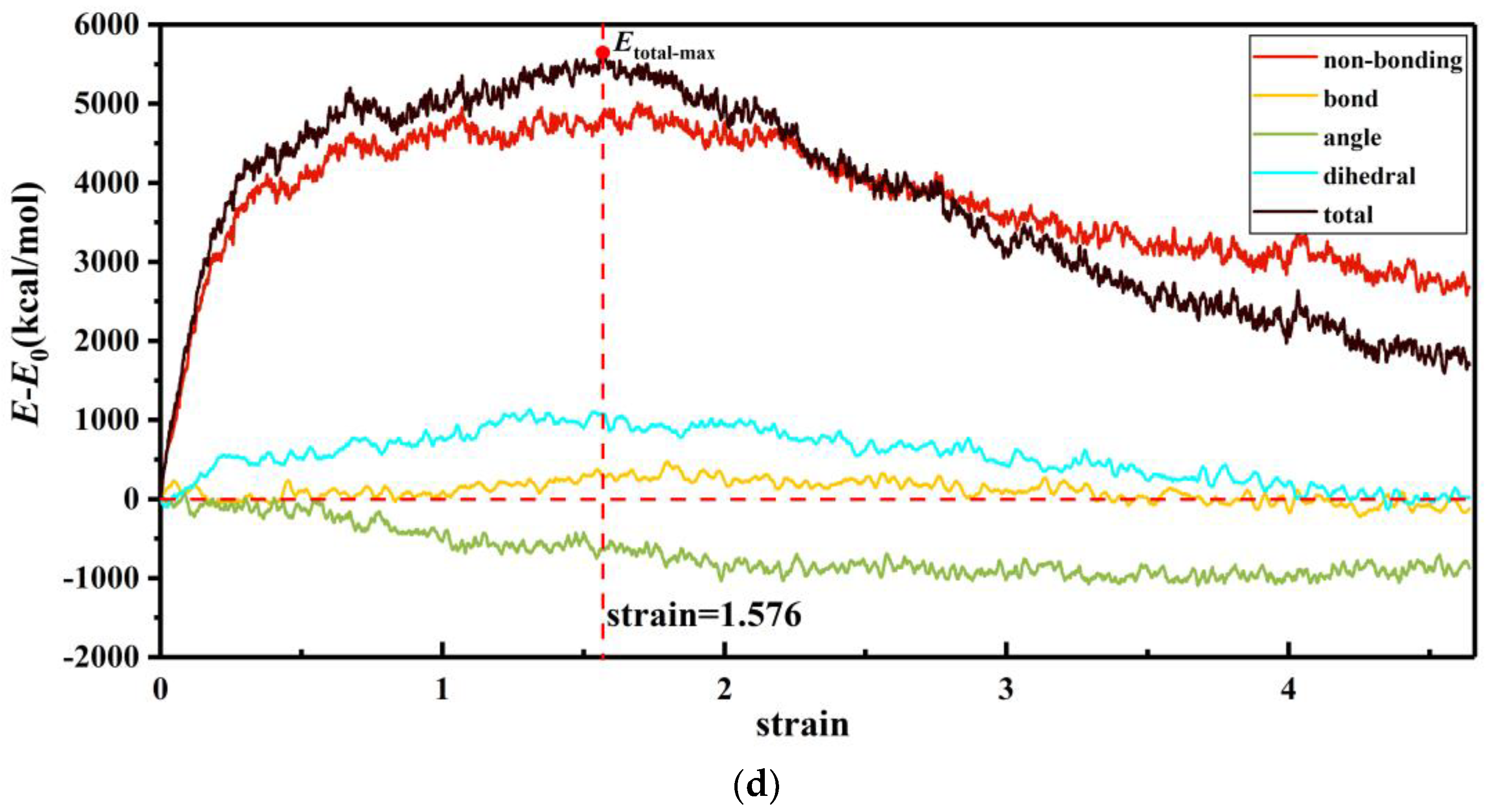
3.2. Internal Energy Analysis
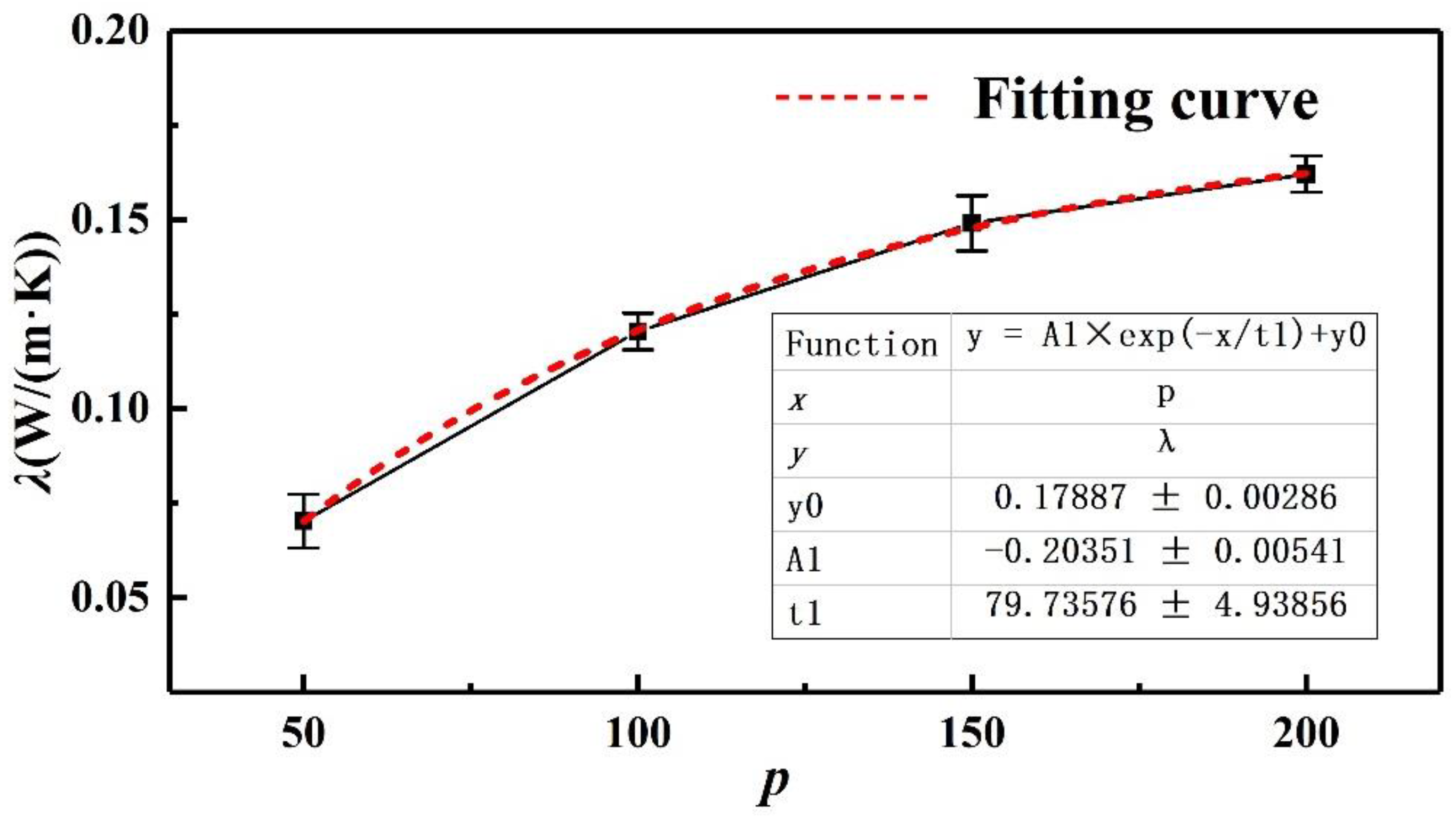
4. Results and Analysis of Thermal Conductivity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ouardad, S.; Bakleh, M.E.; Kostjuk, S.V.; Ganachaud, F.; Puskas, J.E.; Deffieux, A.; Peruch, F. Bio-inspired Cationic Polymerization of Isoprene and Analogues: State-of-the-Art. Polym. Int. 2012, 61, 149–156. [Google Scholar] [CrossRef]
- Gao, T.M.; Xie, R.H.; Zhang, L.H.; Gui, H.X.; Huang, M.F. Use of Rubber Process Analyzer for Characterizing the Molecular Weight Parameters of Natural Rubber. Int. J. Polym. Sci. 2015, 2015, 517260. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.; Othman, N.; Sreekantan, S.; Tan, K.S.; Nor, Z.M.; Ismail, H. Preparation and Characterization of Low-Molecular-Weight Natural Rubber Latex via Photodegradation Catalyzed by Nano TiO2. Polymers 2018, 10, 1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utara, S.; Boochathum, P. Effect of Molecular Weight of Natural Rubber on the Compatibility and Crystallization Behavior of LLDPE/NR Blends. Polym.-Plast. Technol. 2011, 50, 1019–1026. [Google Scholar] [CrossRef]
- Li, X.; Fang, Z.; Shen, X.; Yin, Q.; Chen, Z.; Tu, Q.; Pan, M. Study on Increasing the Binding Amount of Rubber and Reinforcing Filler by Adding Aromatic Solvent Oil. Polymers 2022, 14, 2745. [Google Scholar] [CrossRef]
- Chalid, M.; Husnil, Y.A.; Puspitasari, S.; Cifriadi, A. Experimental and Modelling Study of the Effect of Adding Starch-Modified Natural Rubber Hybrid to the Vulcanization of Sorghum Fibers-Filled Natural Rubber. Polymers 2020, 12, 3017. [Google Scholar] [CrossRef]
- Chollakup, R.; Suwanruji, P.; Tantatherdtam, R.; Smitthipong, W. New Approach on Structure-Property Relationships of Stabilized Natural Rubbers. J. Polym. Res. 2019, 26, 37. [Google Scholar] [CrossRef]
- Roy, K.; Debnath, S.C.; Pongwisuthiruchte, A.; Potiyaraj, P. Recent Advances of Natural Fibers Based Green Rubber Composites: Properties, Current Status, and Future Perspectives. J. Appl. Polym. Sci. 2021, 138, e50866. [Google Scholar] [CrossRef]
- Tsai, D.H.; Macdonald, R.A. Molecular-Dynamical Study of 2nd Sound in Solid Excited by a Strong Heat Pulse. Phys. Rev. B. 1976, 14, 4714–4723. [Google Scholar] [CrossRef]
- Ladd, A.J.C.; Moran, B.; Hoover, W. Lattice Thermal-Conductivity—A Comparison of Molecular-Dynamics and Anharmonic Lattice-Dynamics. Phys. Rev. B 1986, 34, 5058–5064. [Google Scholar] [CrossRef]
- Vasilev, A.; Lorenz, T.; Breitkopf, C. Prediction of Thermal Conductivities of Rubbers by MD Simulations-New Insights. Polymers 2022, 14, 2046. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Xu, Z.J.; Zhang, D.H. Synthesis and application of epoxy-ended hyperbranched polymers. Chem. Eng. J. 2018, 343, 283–302. [Google Scholar] [CrossRef]
- Maurel, G.; Goujon, F.; Schnell, B.; Malfreyt, P. Prediction of Structural and Thermomechanical Properties of Polymers from Multiscale Simulations. RSC Adv. 2015, 5, 14065–14073. [Google Scholar] [CrossRef]
- Masumoto, Y.; Iida, Y. Investigation of the Microscopic Viscoelastic Property for Cross-linked Polymer Network by Molecular Dynamics Simulation. Tire Sci. Technol. 2011, 39, 44–58. [Google Scholar] [CrossRef]
- Uddin, M.S.; Ju, J. Multiscale Modeling of a Natural Rubber: Bridging a Coarse-grained Molecular Model to the Rubber Network Theory. Polymer 2016, 101, 34–47. [Google Scholar] [CrossRef]
- Kitamura, R.; Kageyama, T.; Koyanagi, J.; Ogihara, S. Estimation of Biaxial Tensile and Compression Behavior of Polypropylene Using Molecular Dynamics Simulation. Adv. Compos. Mater. 2019, 28, 135–146. [Google Scholar] [CrossRef]
- Payal, R.S.; Fujimoto, K.; Jang, C.; Shinoda, W.; Takei, Y.; Shima, H.; Tsunoda, K.; Okazaki, S. Molecular Mechanism of Material Deformation and Failure in Butadiene Rubber: Insight from All-atom Molecular Dynamics Simulation Using a Bond Breaking Potential Model. Polymer 2019, 170, 113–119. [Google Scholar] [CrossRef]
- Engelmann, S.; Meyer, J.; Hentschke, R. Computer Simulation of Thermal Conductivity in Vulcanized Polyisoprene at Variable Strain and Temperature. Phys. Rev. B 2017, 96, 054110. [Google Scholar] [CrossRef]
- Li, S.H.; Yu, X.X.; Bao, H.; Yang, N. High Thermal Conductivity of Bulk Epoxy Resin by Bottom-Up Parallel-Linking and Strain: A Molecular Dynamics Study. J. Phys. Chem. C 2018, 122, 13140–13147. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Yang, M.; Liu, C.L.; Li, X.B.; Tang, D.W. Thermal Conductivity of Cross-linked Polyethylene from Molecular Dynamics Simulation. J. Appl. Phys. 2017, 122, 035104. [Google Scholar] [CrossRef]
- Zhao, J.H.; Jiang, J.W.; Wei, N.; Zhang, Y.C.; Rabczuk, T. Thermal Conductivity Dependence on Chain Length in Amorphous Polymers. J. Appl. Phys. 2013, 113, 184304. [Google Scholar] [CrossRef]
- Terao, T.; Lussetti, E.; Muller-Plathe, F. Nonequilibrium Molecular dynamics Methods for Computing the Thermal Conductivity: Application to Amorphous Polymers. Phys. Rev. E 2007, 75, 057701. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.P.; Zhang, M.Y.; Gao, Y.F.; Mei, L.Y.; Fu, Y.Z.; Liu, Y.Q. Effect of Stretching on the Thermal Conductivity of Single Polyethylene Chains by Molecular Dynamics Simulations. Acta Polym. Sin. 2014, 6, 789–793. [Google Scholar] [CrossRef]
- Pal, S.; Balasubramanian, G.; Puri, I.K. Modifying Thermal Transport in Electrically Conducting Polymers: Effects of Stretching and Combining Polymer Chains. J. Chem. Phys. 2012, 136, 044901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, T.F.; Esfarjani, K.; Shiomi, J.; Henry, A.; Chen, G. Molecular Dynamics Simulation of Thermal Energy Transport in Polydimethylsiloxane (PDMS). J. Appl. Phys. 2011, 109, 074321. [Google Scholar] [CrossRef] [Green Version]
- Jewett, A.I.; Stelter, D.; Lambert, J.; Saladi, S.M.; Roscioni, O.M.; Ricci, M.; Autin, L.; Maritan, M.; Bashusqeh, S.M.; Keyes, T.; et al. Moltemplate: A Tool for Coarse-Grained Modeling of Complex Biological Matter and Soft Condensed Matter Physics. J. Mol. Biol. 2021, 433, 166841. [Google Scholar] [CrossRef]
- Rackers, J.A.; Wang, Z.; Lu, C.; Laury, M.L.; Lagardere, L.; Schnieders, M.J.; Piquemal, J.P.; Ren, P.Y.; Ponder, J.W. Tinker 8: Software Tools for Molecular Design. J. Chem. Theory Comput. 2018, 14, 5273–5289. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Tirado-Rives, J. Potential Energy Functions for Atomic-level Simulations of Water and Organic and Biomolecular Systems. Proc. Natl. Acad. Sci. USA 2005, 102, 6665–6670. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, W.L.; Maxwell, D.S.; TiradoRives, J. Development and Testing of the OPLS all-atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Molinari, N.; Khawaja, M.; Sutton, A.P.; Mostofi, A.A. Molecular Model for HNBR with Tunable Cross-Link Density. J. Phys. Chem. B 2016, 120, 12700–12707. [Google Scholar] [CrossRef]
- Khawaja, M.; Sutton, A.P.; Mostofi, A.A. Molecular Simulation of Gas Solubility in Nitrile Butadiene Rubber. J. Phys. Chem. B 2017, 121, 287–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasilev, A.; Lorenz, T.; Breitkopf, C. Thermal Conductivity of Polyisoprene and Polybutadiene from Molecular Dynamics Simulations and Transient Measurements. Polymers 2020, 12, 1081. [Google Scholar] [CrossRef] [PubMed]
- Jewettaij Moltemplate GitHub. Available online: https://github.com/jewettaij/moltemplate/tree/master/moltemplate/force_fields (accessed on 4 November 2022).
- Liu, J.; Yang, R.G. Length-dependent thermal conductivity of single extended polymer chains. Phys. Rev. B 2012, 86, 104307. [Google Scholar] [CrossRef] [Green Version]
- An, D.; Duan, X.Y.; Cheng, S.S.; Zhang, Z.Y.; Yang, B.; Lian, Q.S.; Li, J.X.; Sun, Z.J.; Liu, Y.Q.; Wong, C.P. Enhanced thermal conductivity of natural rubber based thermal interfacial materials by constructing covalent bonds and three-dimensional networks. Compos. Part A-Appl. Sci. Manuf. 2020, 135, 105928. [Google Scholar] [CrossRef]
- Li, J.C.; Zhao, X.Y.; Zhang, Z.X.; Xian, Y.; Lin, Y.T.; Ji, X.W.; Lu, Y.L.; Zhang, L.Q. Construction of interconnected Al2O3 doped rGO network in natural rubber nanocomposites to achieve significant thermal conductivity and mechanical strength enhancement. Compos. Sci. Technol. 2020, 186, 107930. [Google Scholar] [CrossRef]
- Schelling, P.K.; Phillpot, S.R.; Keblinski, P. Phonon Wave-packet Dynamics at Semiconductor Interfaces by Molecular-dynamics Simulation. Appl. Phys. Lett. 2002, 80, 2484. [Google Scholar] [CrossRef]





| Parameters | p = 50 (Mw = 3304) | p = 100 (Mw = 6604) | p = 150 (Mw = 9904) | p = 200 (Mw = 13,204) |
|---|---|---|---|---|
| Maximum strength (GPa) | 0.164 | 0.195 | 0.251 | 0.271 |
| Elasticity modulus (GPa) | 0.085 | 0.118 | 0.134 | 0.180 |
| Elongation at σmax | 146.2% | 188.0% | 174.1% | 153.3% |
| Elongation at break | 256.5% | 347.6% | 379.4% | 466.3% |
| Parameters | p = 50 (Mw = 3304) | p = 100 (Mw = 6604) | p = 150 (Mw = 9904) | p = 200 (Mw = 13,204) |
|---|---|---|---|---|
| strain | 1.507 | 1.864 | 1.777 | 1.576 |
| bond | 2.57% | 13.29% | 8.26% | 4.95% |
| angle | −11.93% | −11.58% | −10.39% | −11.29% |
| dihedral | 22.25% | 30.09% | 21.32% | 18.82% |
| non-bonding | 87.11% | 68.21% | 80.81% | 87.52% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Tu, Q.; Fang, Z.; Shen, X.; Yin, Q.; Zhang, X.; Pan, M. Molecular Dynamics Studies of the Mechanical Behaviors and Thermal Conductivity of Polyisoprene with Different Degrees of Polymerization. Polymers 2022, 14, 4950. https://doi.org/10.3390/polym14224950
Chen Z, Tu Q, Fang Z, Shen X, Yin Q, Zhang X, Pan M. Molecular Dynamics Studies of the Mechanical Behaviors and Thermal Conductivity of Polyisoprene with Different Degrees of Polymerization. Polymers. 2022; 14(22):4950. https://doi.org/10.3390/polym14224950
Chicago/Turabian StyleChen, Zhiyuan, Qunzhang Tu, Zhonghang Fang, Xinmin Shen, Qin Yin, Xiangpo Zhang, and Ming Pan. 2022. "Molecular Dynamics Studies of the Mechanical Behaviors and Thermal Conductivity of Polyisoprene with Different Degrees of Polymerization" Polymers 14, no. 22: 4950. https://doi.org/10.3390/polym14224950






