Effect of Graphene Oxide and Reduced Graphene Oxide on the Properties of Sunflower Oil-Based Polyurethane Films
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
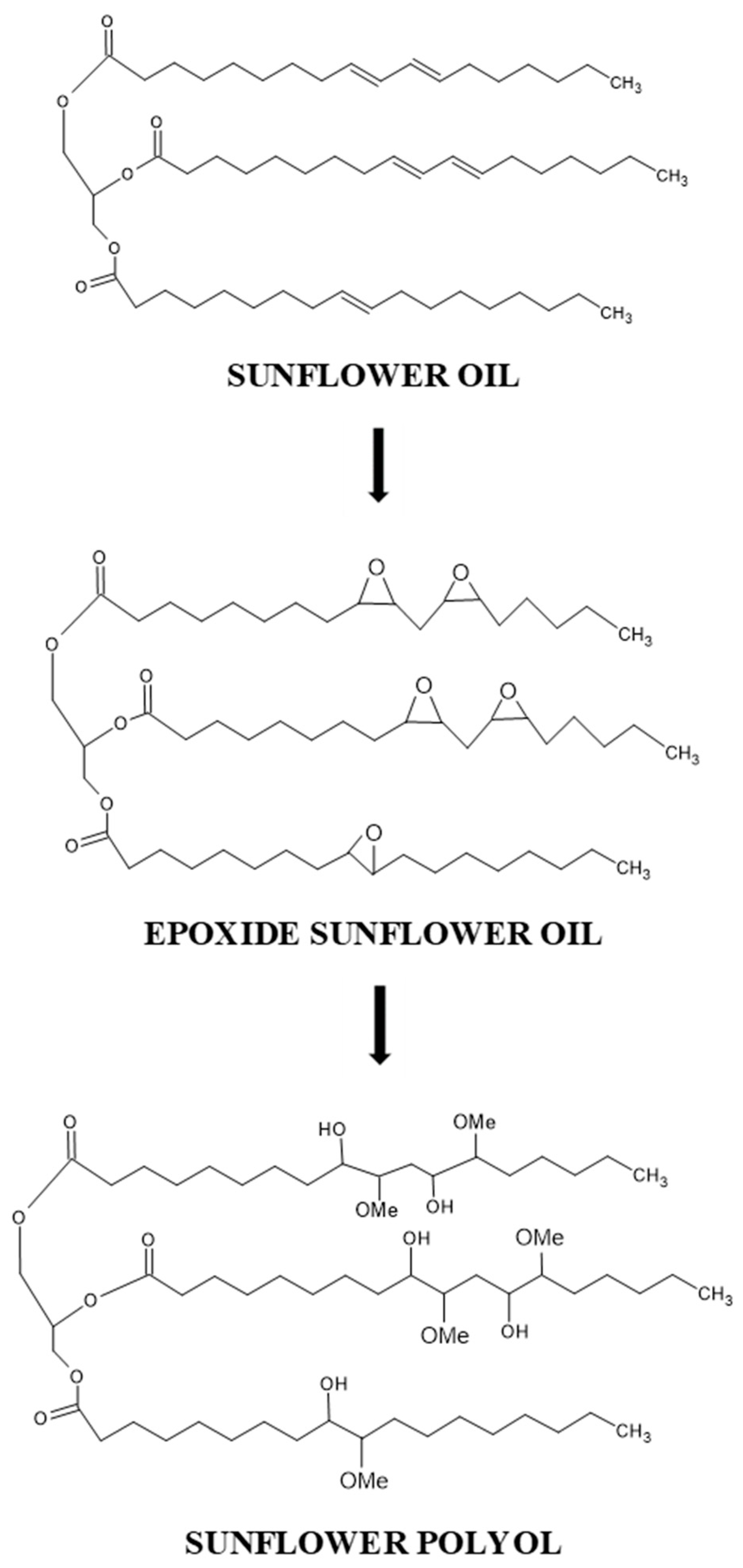
2.2. Synthesis of Epoxidized Sunflower Oil
2.3. Synthesis of SFO Polyol
2.4. Fabrication of Composite Films
2.5. Characterization of the Films
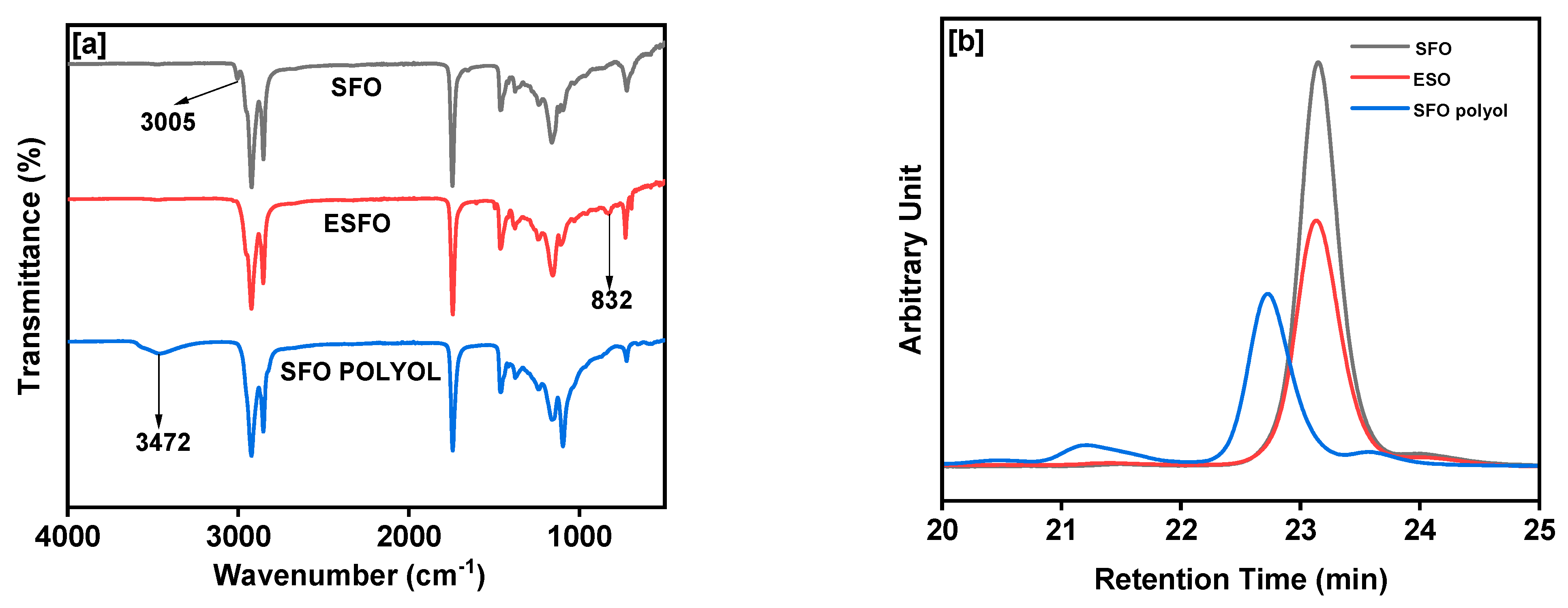
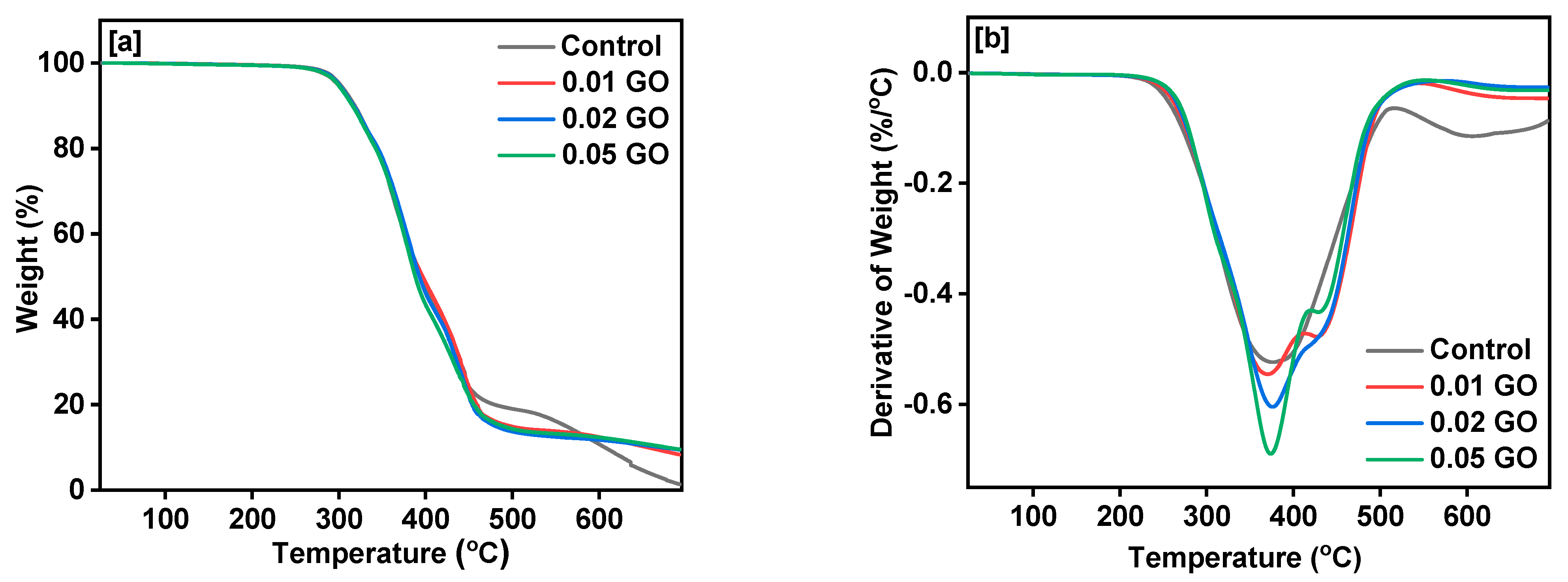
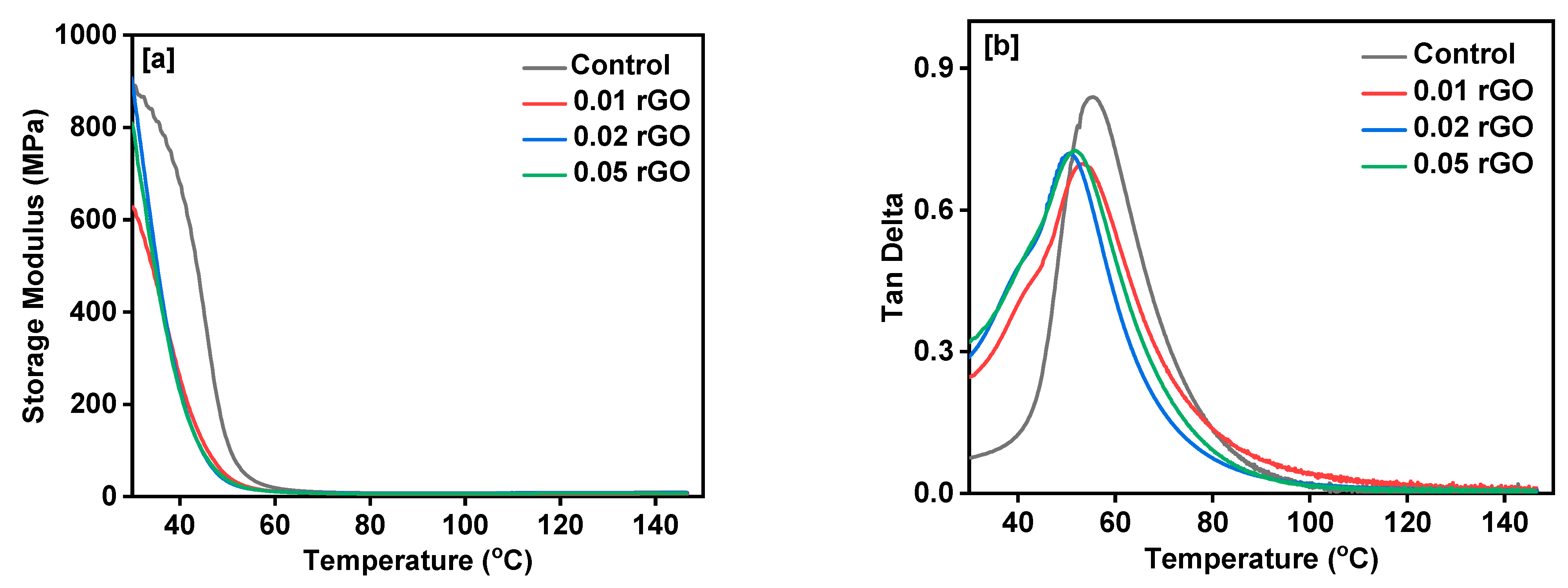
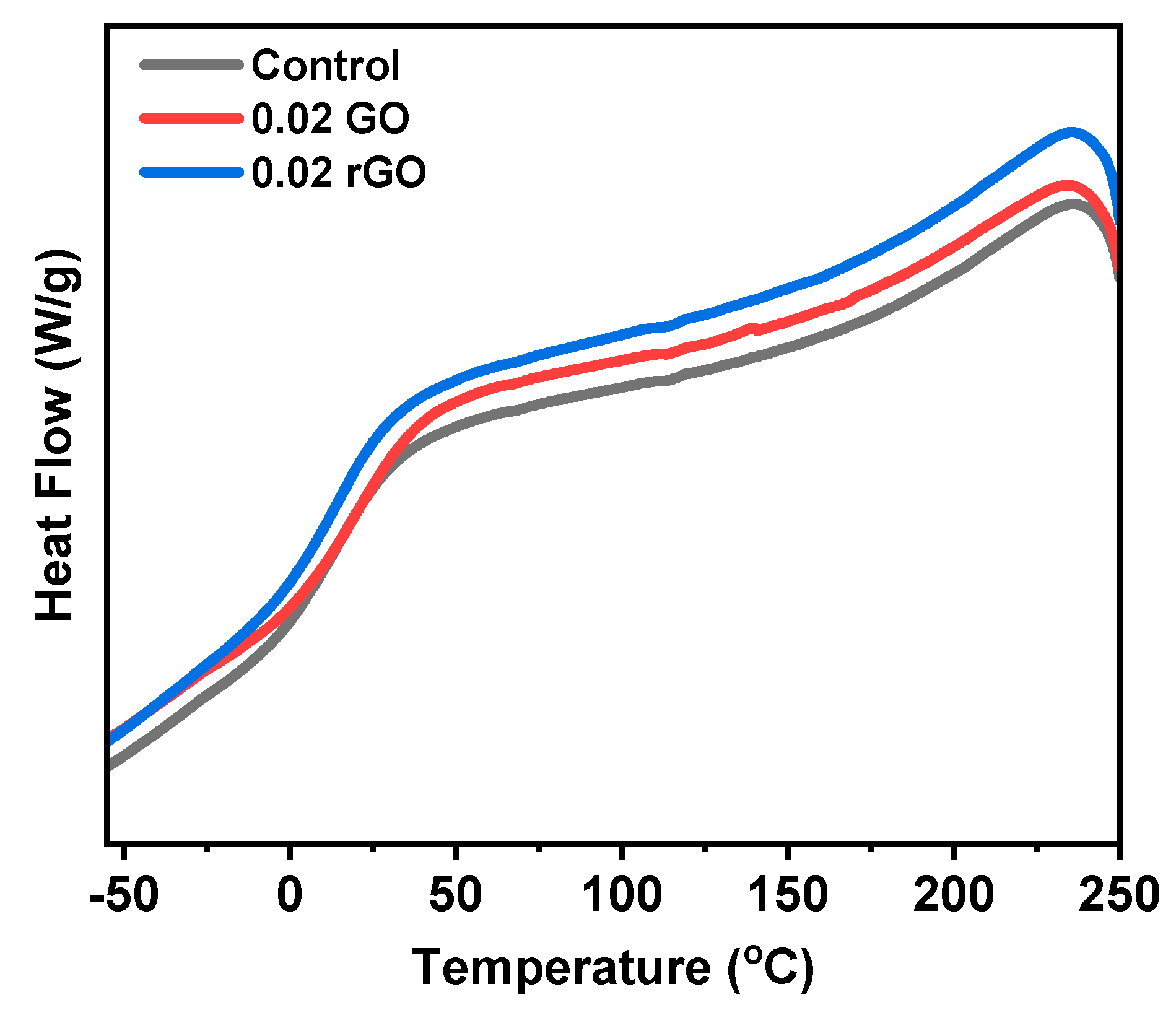
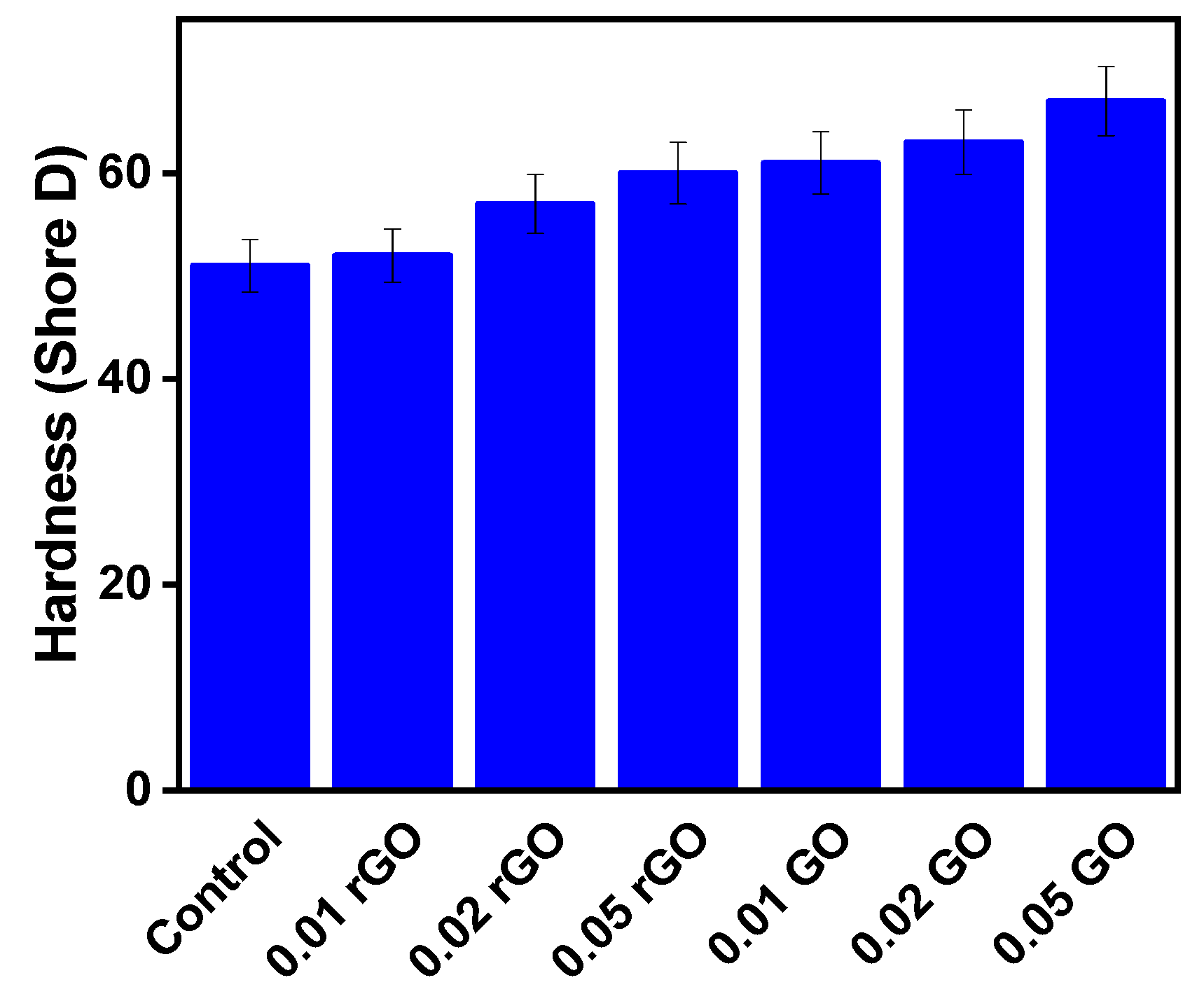
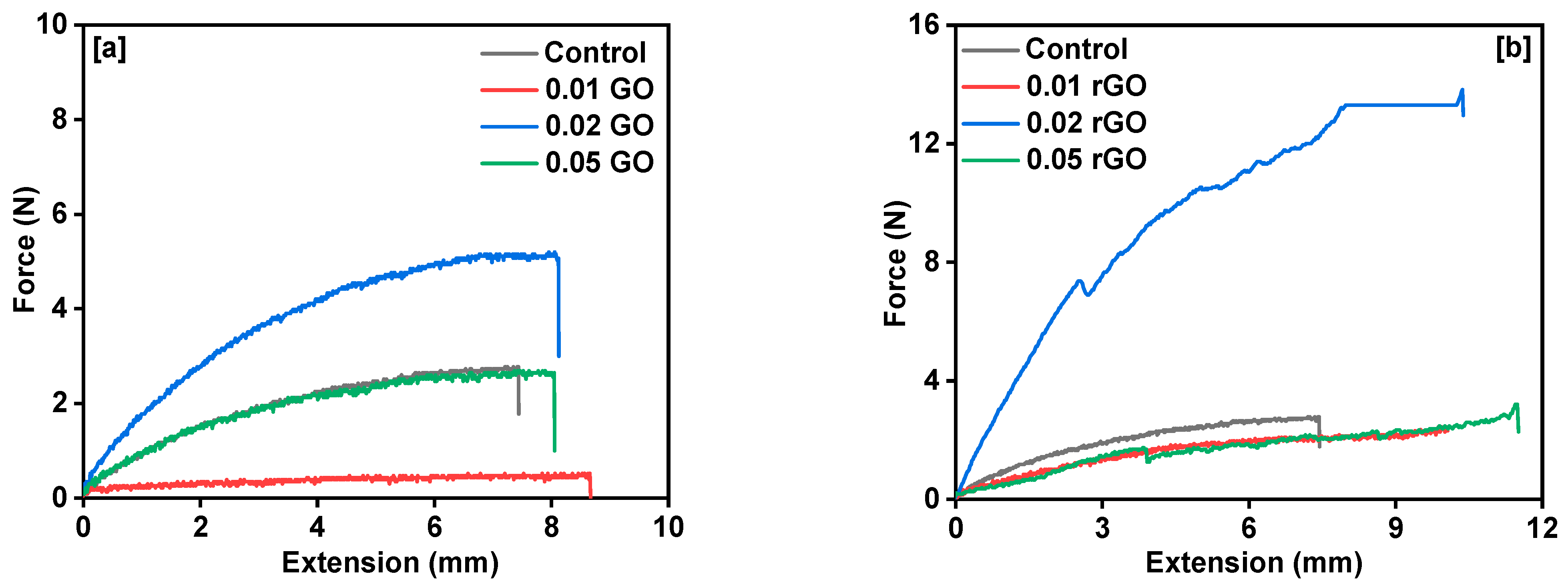
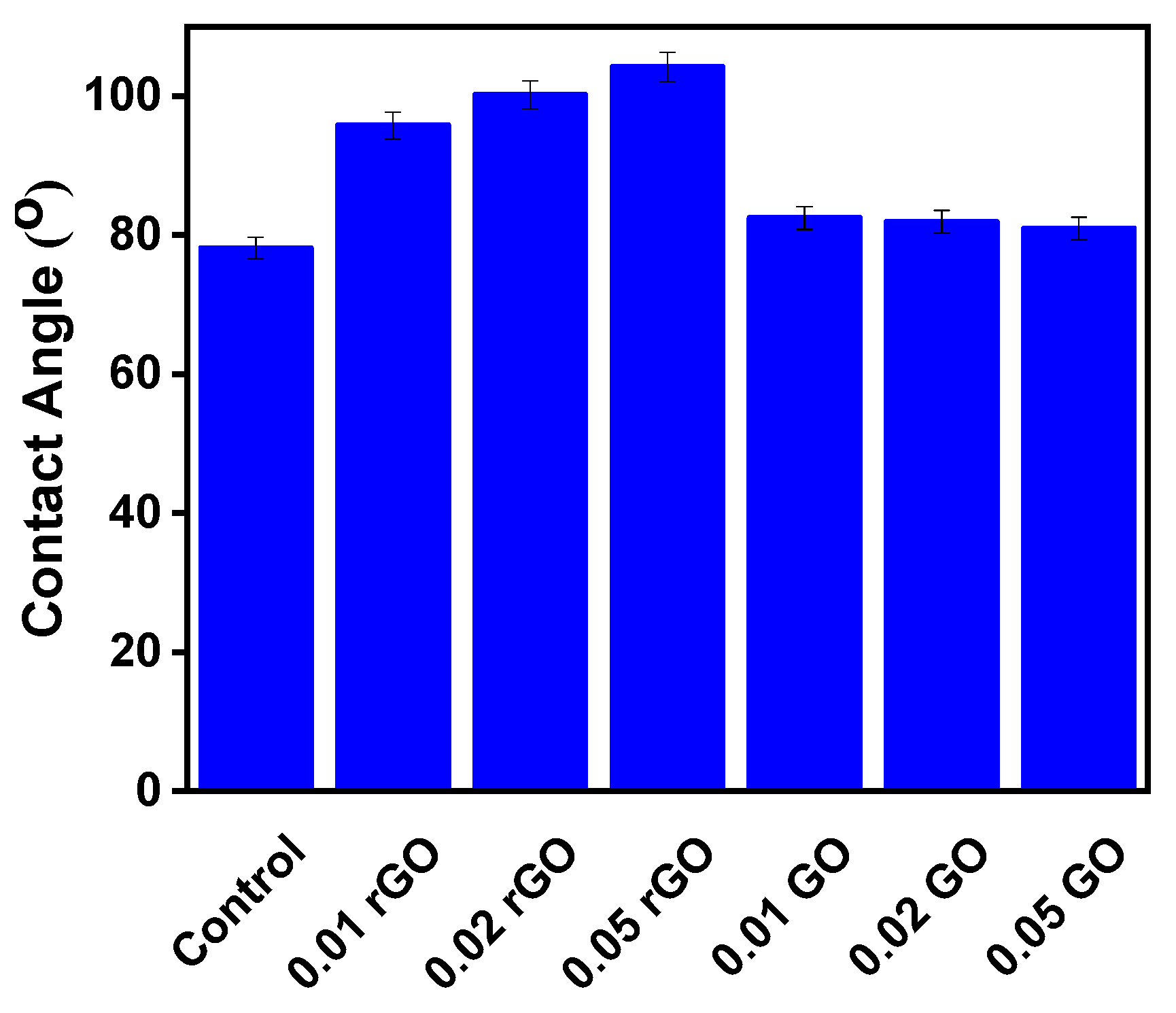
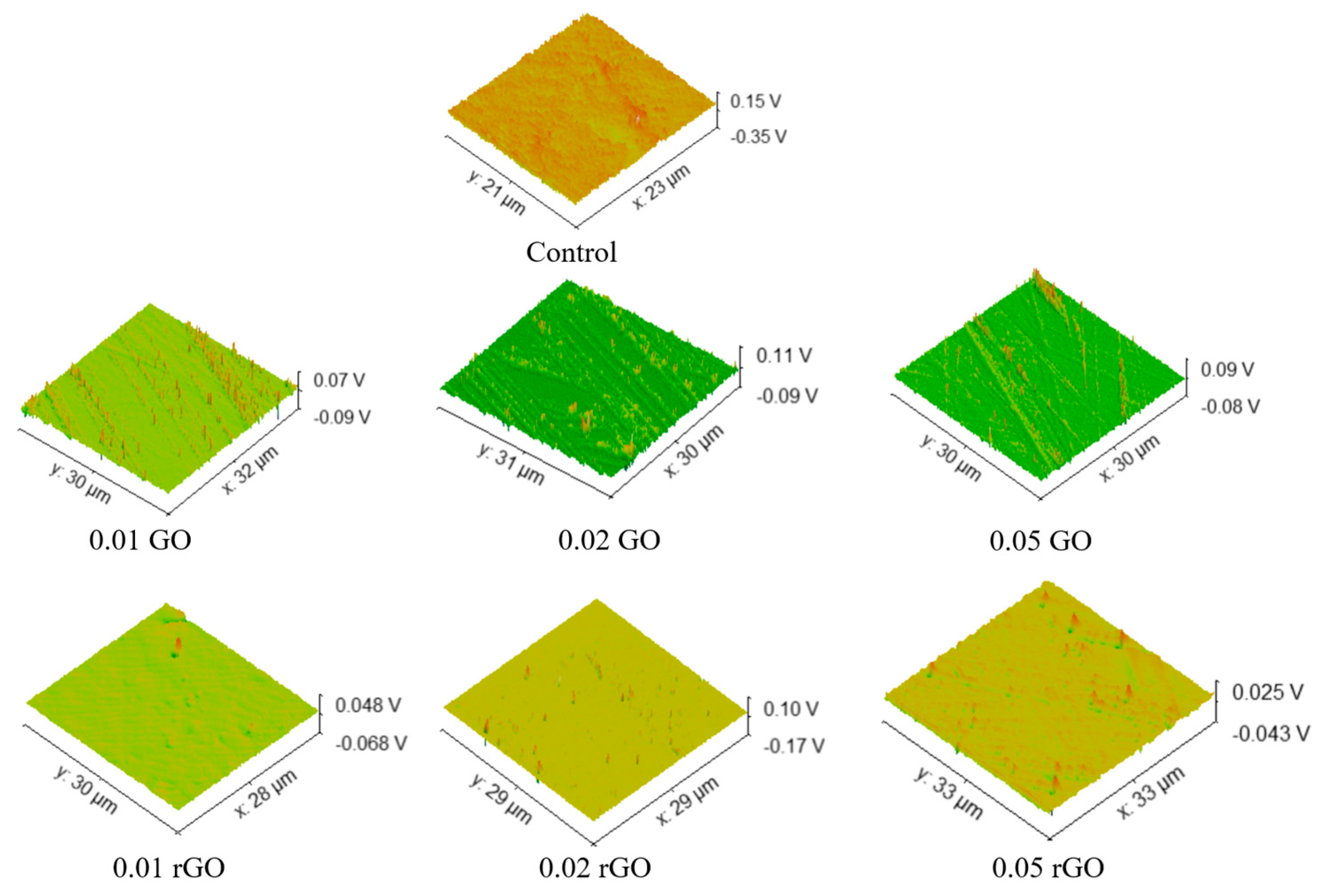
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethanes, 2nd ed.; Smithers Rapra Technology: Shrewsbury, UK, 2016; Volume 2. [Google Scholar]
- Dai, H.; Yang, L.; Lin, B.; Wang, C.; Shi, G. Synthesis and characterization of the different soy-based polyols by ring opening of epoxidized soybean oil with methanol, 1,2-ethanediol and 1,2-propanediol. JAOCS J. Am. Oil Chem. Soc. 2009, 86, 261–267. [Google Scholar] [CrossRef]
- Lu, Y.; Larock, R.C. New hybrid latexes from a soybean oil-based waterborne polyurethane and acrylics via emulsion polymerization. Biomacromolecules 2007, 8, 3108–3114. [Google Scholar] [CrossRef] [PubMed]
- Seydibeyoǧlu, M.Ö.; Misra, M.; Mohanty, A.; Blaker, J.J.; Lee, K.Y.; Bismarck, A.; Kazemizadeh, M. Green polyurethane nanocomposites from soy polyol and bacterial cellulose. J. Mater. Sci. 2013, 48, 2167–2175. [Google Scholar] [CrossRef]
- Garrison, T.F.; Kessler, M.R.; Larock, R.C. Effects of unsaturation and different ring-opening methods on the properties of vegetable oil-based polyurethane coatings. Polymer 2014, 55, 1004–1011. Available online: http://www.sciencedirect.com/science/article/pii/S0032386114000299 (accessed on 2 November 2022). [CrossRef]
- Petrović, Z.S.; Zhang, W.; Javni, I. Structure and properties of polyurethanes prepared from triglyceride polyols by ozonolysis. Biomacromolecules 2005, 6, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Madbouly, S.A.; Kessler, M.R. Biobased polyurethanes prepared from different vegetable oils. ACS Appl. Mater. Interfaces 2015, 7, 1226–1233. [Google Scholar] [CrossRef]
- Hu, Y.; Tian, Y.; Cheng, J.; Zhang, J. Synthesis of Eugenol-Based Polyols via Thiol–Ene Click Reaction and High-Performance Thermosetting Polyurethane Therefrom. ACS Sustain. Chem. Eng. 2020, 8, 4158–4166. [Google Scholar] [CrossRef]
- Tremblay-Parrado, K.-K.; García-Astrain, C.; Avérous, L. Click chemistry for the synthesis of biobased polymers and networks derived from vegetable oils. Green Chem. 2021, 23, 4296–4327. [Google Scholar] [CrossRef]
- Wilbon, P.A.; Chu, F.; Tang, C. Progress in renewable polymers from natural terpenes, terpenoids, and rosin. Macromol. Rapid Commun. 2013, 34, 8–37. [Google Scholar] [CrossRef]
- de Souza, F.M.; Choi, J.; Bhoyate, S.; Kahol, P.K.; Gupta, R.K. Expendable Graphite as an Efficient Flame-Retardant for Novel Partial Bio-Based Rigid Polyurethane Foams. C—J. Carbon Res. 2020, 6, 27. [Google Scholar] [CrossRef]
- Park, S.-H.; Alammar, A.; Fulop, Z.; Pulido, B.A.; Nunes, S.P.; Szekely, G. Hydrophobic thin film composite nanofiltration membranes derived solely from sustainable sources. Green Chem. 2021, 23, 1175–1184. [Google Scholar] [CrossRef]
- Zhang, C.; Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Gupta, R.K. Highly flame retardant and bio-based rigid polyurethane foams derived from orange peel oil. Polym. Eng. Sci. 2018, 58, 2078–2087. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Gupta, R.K. Sustainable flame-retardant polyurethanes using renewable resources. Ind. Crops Prod. 2018, 123, 480–488. [Google Scholar] [CrossRef]
- Li, Y.; Ragauskas, A.J. Kraft lignin-based rigid polyurethane foam. J. Wood Chem. Technol. 2012, 32, 210–224. [Google Scholar] [CrossRef]
- Alinejad, M.; Henry, C.; Nikafshar, S.; Gondaliya, A.; Bagheri, S.; Chen, N.; Singh, S.K.; Hodge, D.B.; Nejad, M. Lignin-based polyurethanes: Opportunities for bio-based foams, elastomers, coatings and adhesives. Polymers 2019, 11, 1202. [Google Scholar] [CrossRef] [PubMed]
- Seyed Shahabadi, S.I.; Kong, J.; Lu, X. Aqueous-Only, Green Route to Self-Healable, UV-Resistant, and Electrically Conductive Polyurethane/Graphene/Lignin Nanocomposite Coatings. ACS Sustain. Chem. Eng. 2017, 5, 3148–3157. [Google Scholar] [CrossRef]
- Quinsaat, J.E.Q.; Feghali, E.; van de Pas, D.J.; Vendamme, R.; Torr, K.M. Preparation of Mechanically Robust Bio-Based Polyurethane Foams Using Depolymerized Native Lignin. ACS Appl. Polym. Mater. 2021, 3, 5845–5856. [Google Scholar] [CrossRef]
- Guo, A.; Demydov, D.; Zhang, W.; Petrovic, Z.S. Polyols and polyurethanes from hydroformylation of soybean oil. J. Polym. Environ. 2002, 10, 49–52. [Google Scholar] [CrossRef]
- Fu, C.; Zheng, Z.; Yang, Z.; Chen, Y.; Shen, L. A fully bio-based waterborne polyurethane dispersion from vegetable oils: From synthesis of precursors by thiol-ene reaction to study of final material. Prog. Org. Coat. 2014, 77, 53–60. Available online: https://www.sciencedirect.com/science/article/pii/S0300944013002129 (accessed on 2 November 2022). [CrossRef]
- de Souza, F.M.; Kahol, P.K.; Gupta, R.K. Introduction to Polyurethane Chemistry. In Polyurethane Chemistry: Renewable Polyols and Isocyanates; Gupta, R.K., Kahol, P.K., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; Volume 1380, p. 1. [Google Scholar]
- Ranaweera, C.K.; Ionescu, M.; Bilic, N.; Wan, X.; Kahol, P.K.; Gupta, R.K. Biobased Polyols Using Thiol-Ene Chemistry for Rigid Polyurethane Foams with Enhanced Flame-Retardant Properties. J. Renew. Mater. 2017, 5 (Suppl. S1), 1–12. Available online: https://www.ingentaconnect.com/content/tsp/jrm/2017/00000005/a00101s1/art00001 (accessed on 2 November 2022). [CrossRef]
- Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Gupta, R.K. Castor-oil derived nonhalogenated reactive flame-retardant-based polyurethane foams with significant reduced heat release rate. J. Appl. Polym. Sci. 2019, 136, 47276. [Google Scholar] [CrossRef]
- Ramanujam, S.; Zequine, C.; Bhoyate, S.; Neria, B.; Kahol, P.; Gupta, R. Novel Biobased Polyol Using Corn Oil for Highly Flame-Retardant Polyurethane Foams. C–J. Carbon Res. 2019, 5, 13. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galiá, M.; Cádiz, V. Plant oils as platform chemicals for polyurethane synthesis: Current state-of-the-art. Biomacromolecules 2010, 11, 2825–2835. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications-a review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Lu, Y.; Larock, R.C. Soybean-Oil-Based Waterborne Polyurethane Dispersions: Effects of Polyol Functionality and Hard Segment Content on Properties. Biomacromolecules 2008, 9, 3332–3340. [Google Scholar] [CrossRef] [PubMed]
- Irinislimane, R.; Belhaneche-Bensemra, N. Optimisation of Operatory Conditions for Synthesis of Sunflower Oil Biobased Polyols Using Design of Experiments and Spectroscopic Methods. J. Polym. Environ. 2021, 29, 851–858. [Google Scholar] [CrossRef]
- Production Volume of Sunflowerseed oil Worldwide from 2012/13 to 2022/22. Statista 2022. Available online: https://www.statista.com/statistics/613490/sunflowerseed-oil-production-volume-worldwide/#:~:text=Sunflowerseed%20oil%3A%20global%20production%20volume%202012%2F13-2021%2F22&text=In%202021%2F22%2C%20the%20global,approximately%2022.07%20million%20metric%20tons (accessed on 2 November 2022).
- Li, J.; Cui, J.; Yang, J.; Li, Y.; Qiu, H.; Yang, J. Reinforcement of graphene and its derivatives on the anticorrosive properties of waterborne polyurethane coatings. Compos. Sci. Technol. 2016, 129, 30–37. Available online: http://www.sciencedirect.com/science/article/pii/S0266353816301567 (accessed on 2 November 2022). [CrossRef]
- Gupta, T.K.; Singh, B.P.; Tripathi, R.K.; Dhakate, S.R.; Singh, V.N.; Panwar, O.S.; Mathur, R.B. Superior nano-mechanical properties of reduced graphene oxide reinforced polyurethane composites. RSC Adv. 2015, 5, 16921–16930. [Google Scholar] [CrossRef]
- Fayyad, E.M.; Sadasivuni, K.K.; Ponnamma, D.; Al-Maadeed, M.A.A. Oleic acid-grafted chitosan/graphene oxide composite coating for corrosion protection of carbon steel. Carbohydr. Polym. 2016, 151, 871–878. [Google Scholar] [CrossRef]
- Balakrishnan, T.; Sathiyanarayanan, S.; Mayavan, S. Advanced Anticorrosion Coating Materials Derived from Sunflower Oil with Bifunctional Properties. ACS Appl. Mater. Interfaces 2015, 7, 19781–19788. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, J.; Zhang, S.; Liu, C.; Yu, X.; Chen, L.; Song, Y.; Han, S.; Xi, M.; Zhang, C.; et al. Flexible 3D porous graphene film decorated with nickel nanoparticles for absorption-dominated electromagnetic interference shielding. Chem. Eng. J. 2021, 421, 129763. Available online: https://www.sciencedirect.com/science/article/pii/S1385894721013486 (accessed on 2 November 2022). [CrossRef]
- Balaji, K.R.; Hardian, R.; Kumar, V.G.D.; Viswanatha, R.; Kumar, S.; Kumar, S.; Singh, A.; Santosh, M.S.; Szekely, G. Composite nanofiltration membrane comprising one-dimensional erdite, two-dimensional reduced graphene oxide, and silkworm pupae binder. Mater. Today Chem. 2021, 22, 100602. Available online: https://www.sciencedirect.com/science/article/pii/S2468519421001828 (accessed on 2 November 2022). [CrossRef]
- Gao, X.; Zhang, L.; Wang, S.; Yang, T.; Li, H. Soft Untethered Robots and Grippers Based on Humidity-Gated Magnetic-Responsive Film Actuators. ACS Appl. Polym. Mater. 2021, 3, 4726–4734. [Google Scholar] [CrossRef]
- Deka, H.; Karak, N.; Kalita, R.D.; Buragohain, A.K. Biocompatible hyperbranched polyurethane/multi-walled carbon nanotube composites as shape memory materials. Carbon 2010, 48, 2013–2022. [Google Scholar] [CrossRef]
- Chen, B.; Evans, J.R.G.; Greenwell, H.C.; Boulet, P.; Coveney, P.V.; Bowden, A.A.; Whiting, A. A critical appraisal of polymer–clay nanocomposites. Chem. Soc. Rev. 2008, 37, 568–594. [Google Scholar] [CrossRef]
- Qian, D.; Dickey, E.C.; Andrews, R.; Rantell, T. Load transfer and deformation mechanisms in carbon nanotube-polystyrene composites. Appl. Phys. Lett. 2000, 76, 2868–2870. [Google Scholar] [CrossRef]
- Mo, M.; Zhao, W.; Chen, Z.; Yu, Q.; Zeng, Z.; Wu, X.; Xue, Q. Excellent tribological and anti-corrosion performance of polyurethane composite coatings reinforced with functionalized graphene and graphene oxide nanosheets. RSC Adv. 2015, 5, 56486–56497. [Google Scholar] [CrossRef]
- Cui, J.; Xu, J.; Li, J.; Qiu, H.; Zheng, S.; Yang, J. A crosslinkable graphene oxide in waterborne polyurethane anticorrosive coatings: Experiments and simulation. Compos. Part B Eng. 2020, 188, 107889. Available online: http://www.sciencedirect.com/science/article/pii/S135983681935752X (accessed on 2 November 2022). [CrossRef]
- Yan, D.; Xu, L.; Chen, C.; Tang, J.; Ji, X.; Li, Z. Enhanced mechanical and thermal properties of rigid polyurethane foam composites containing graphene nanosheets and carbon nanotubes. Polym. Int. 2012, 61, 1107–1114. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Haghdadeh, P.; Ghaffari, M.; Ramezanzadeh, B.; Bahlakeh, G.; Saeb, M.R. The role of functionalized graphene oxide on the mechanical and anti-corrosion properties of polyurethane coating. J. Taiwan Inst. Chem. Eng. 2018, 86, 199–212. Available online: https://www.sciencedirect.com/science/article/pii/S1876107018300932 (accessed on 2 November 2022). [CrossRef]
- Liu, Y.; Ma, J.; Wu, T.; Wang, X.; Huang, G.; Liu, Y.; Qiu, H.; Li, Y.; Wang, W.; Gao, J. Cost-Effective Reduced Graphene Oxide-Coated Polyurethane Sponge As a Highly Efficient and Reusable Oil-Absorbent. ACS Appl. Mater. Interfaces 2013, 5, 10018–10026. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Monteavaro, L.L.; da Silva, E.O.; Costa, A.P.O.; Samios, D.; Gerbase, A.E.; Petzhold, C.L. Polyurethane networks from formiated soy polyols: Synthesis and mechanical characterization. J. Am. Oil Chem. Soc. 2005, 82, 365–371. [Google Scholar] [CrossRef]
- Cai, C.; Dai, H.; Chen, R.; Su, C.; Xu, X.; Zhang, S.; Yang, L. Studies on the kinetics of in situ epoxidation of vegetable oils. Eur. J. Lipid Sci. Technol. 2008, 110, 341–346. [Google Scholar] [CrossRef]
- Kahlerras, Z.; Irinislimane, R.; Bruzaud, S.; Belhaneche-Bensemra, N. Elaboration and Characterization of Polyurethane Foams Based on Renewably Sourced Polyols. J. Polym. Environ. 2020, 28, 3003–3018. [Google Scholar] [CrossRef]
- Hidayah, N.M.S.; Liu, W.W.; Lai, C.W.; Noriman, N.Z.; Khe, C.S.; Hashim, U.; Lee, H.C. Comparison on graphite, graphene oxide and reduced graphene oxide: Synthesis and characterization. AIP Conf. Proc. 2017, 1892, 150002. [Google Scholar]
- Cui, P.; Lee, J.; Hwang, E.; Lee, H. One-pot reduction of graphene oxide at subzero temperatures. Chem. Commun. 2011, 47, 12370–12372. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. Green reduction of graphene oxide by aqueous phytoextracts. Carbon 2012, 50, 5331–5339. Available online: https://www.sciencedirect.com/science/article/pii/S0008622312006070 (accessed on 2 November 2022). [CrossRef]
- Low, F.W.; Lai, C.W.; Abd Hamid, S.B. Surface modification of reduced graphene oxide film by Ti ion implantation technique for high dye-sensitized solar cells performance. Ceram. Int. 2017, 43, 625–633. Available online: https://www.sciencedirect.com/science/article/pii/S0272884216317369 (accessed on 2 November 2022). [CrossRef]
- Kuila, T.; Bose, S.; Hong, C.E.; Uddin, M.E.; Khanra, P.; Kim, N.H.; Lee, J.H. Preparation of functionalized graphene/linear low density polyethylene composites by a solution mixing method. Carbon 2011, 49, 1033–1037. Available online: https://www.sciencedirect.com/science/article/pii/S0008622310007578 (accessed on 2 November 2022). [CrossRef]
- Chen, B.; Ma, N.; Bai, X.; Zhang, H.; Zhang, Y. Effects of graphene oxide on surface energy, mechanical, damping and thermal properties of ethylene-propylene-diene rubber/petroleum resin blends. RSC Adv. 2012, 2, 4683–4689. [Google Scholar] [CrossRef]
- Li, Y.; Pan, D.; Chen, S.; Wang, Q.; Pan, G.; Wang, T. In situ polymerization and mechanical, thermal properties of polyurethane/graphene oxide/epoxy nanocomposites. Mater. Des. 2013, 47, 850–856. Available online: https://www.sciencedirect.com/science/article/pii/S0261306913000083 (accessed on 2 November 2022). [CrossRef]
- Strankowski, M.; Korzeniewski, P.; Strankowska, J.A.S.A.; Thomas, S. Morphology, Mechanical and Thermal Properties of Thermoplastic Polyurethane Containing Reduced Graphene Oxide and Graphene Nanoplatelets. Materials 2018, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. Available online: http://www.sciencedirect.com/science/article/pii/S0079670009000550 (accessed on 2 November 2022). [CrossRef]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Madbouly, S.A. In situ polymerization of bio-based thermosetting polyurethane/graphene oxide nanocomposites. J. Appl. Polym. Sci. 2015, 132, 41751. [Google Scholar]
- Kim, J.; Cote, L.J.; Huang, J. Two Dimensional Soft Material: New Faces of Graphene Oxide. Acc. Chem. Res. 2012, 45, 1356–1364. [Google Scholar] [CrossRef]
- Sofla, R.L.M.; Rezaei, M.; Babaie, A. Investigation of the effect of graphene oxide functionalization on the physical, mechanical and shape memory properties of polyurethane/reduced graphene oxide nanocomposites. Diam. Relat. Mater. 2019, 95, 195–205. Available online: https://www.sciencedirect.com/science/article/pii/S0925963518308550 (accessed on 2 November 2022). [CrossRef]
- Lee, Y.R.; Raghu, A.V.; Jeong, H.M.; Kim, B.K. Properties of Waterborne Polyurethane/Functionalized Graphene Sheet Nanocomposites Prepared by an in situ Method. Macromol. Chem. Phys. 2009, 210, 1247–1254. [Google Scholar] [CrossRef]
- Pokharel, P.; Lee, D.S. Thermal and mechanical properties of reduced graphene oxide/polyurethane nanocomposite. J. Nanosci. Nanotechnol. 2014, 14, 5718–5721. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, R.; Koosha, M.; Mahyari, M. Improving the mechanical, thermal and electrical properties of polyurethane-graphene oxide nanocomposites synthesized by in-situ polymerization of ester-based polyol with hexamethylene diisocyanate. J. Polym. Res. 2016, 23, 1–11. [Google Scholar] [CrossRef]





| Polyol | Isocyanate | GO | rGO | Ʃ | |
|---|---|---|---|---|---|
| Control | 60.120 | 39.880 | 100.000 | ||
| 0.01 GO | 60.113 | 39.877 | 0.01 | 100.000 | |
| 0.02 GO | 60.107 | 39.873 | 0.02 | 100.000 | |
| 0.05 GO | 60.088 | 39.862 | 0.05 | 100.000 | |
| 0.01 rGO | 60.113 | 39.877 | 0.01 | 100.000 | |
| 0.02 rGO | 60.107 | 39.873 | 0.02 | 100.000 | |
| 0.05 rGO | 60.088 | 39.862 | 0.05 | 100.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suthar, V.; Asare, M.A.; de Souza, F.M.; Gupta, R.K. Effect of Graphene Oxide and Reduced Graphene Oxide on the Properties of Sunflower Oil-Based Polyurethane Films. Polymers 2022, 14, 4974. https://doi.org/10.3390/polym14224974
Suthar V, Asare MA, de Souza FM, Gupta RK. Effect of Graphene Oxide and Reduced Graphene Oxide on the Properties of Sunflower Oil-Based Polyurethane Films. Polymers. 2022; 14(22):4974. https://doi.org/10.3390/polym14224974
Chicago/Turabian StyleSuthar, Vishwa, Magdalene A. Asare, Felipe M. de Souza, and Ram K. Gupta. 2022. "Effect of Graphene Oxide and Reduced Graphene Oxide on the Properties of Sunflower Oil-Based Polyurethane Films" Polymers 14, no. 22: 4974. https://doi.org/10.3390/polym14224974
APA StyleSuthar, V., Asare, M. A., de Souza, F. M., & Gupta, R. K. (2022). Effect of Graphene Oxide and Reduced Graphene Oxide on the Properties of Sunflower Oil-Based Polyurethane Films. Polymers, 14(22), 4974. https://doi.org/10.3390/polym14224974







