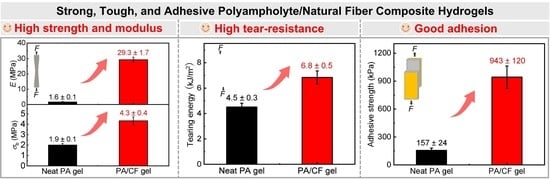
Strong, Tough, and Adhesive Polyampholyte/Natural Fiber Composite Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of CF Sheet
2.3. Fabrication of PA/CF Composite Hydrogels
2.4. Tensile Tests
2.5. Tearing Tests
2.6. Lap-Shear Tests
2.7. 90-Degree Peeling Tests
2.8. Water Content of Hydrogels
3. Results and Discussion
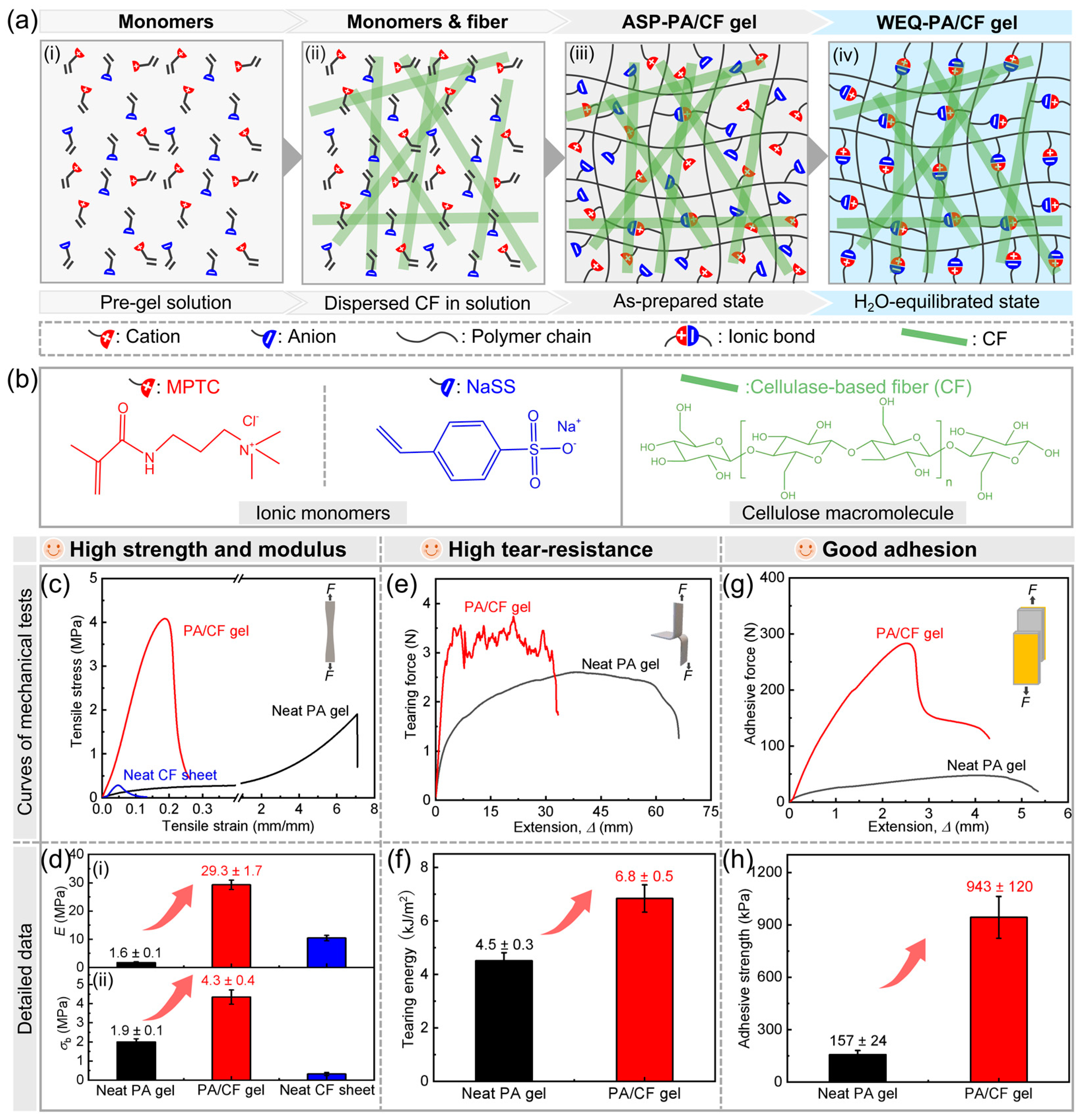
3.1. Fabrication and Characterizations of Hydrogels
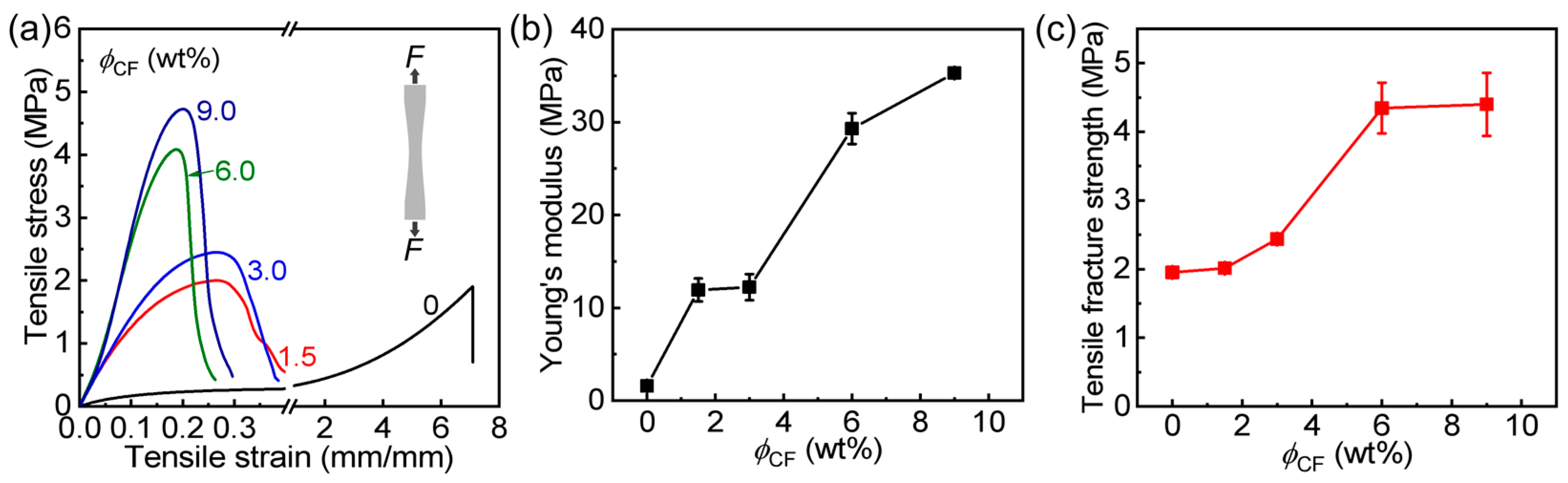
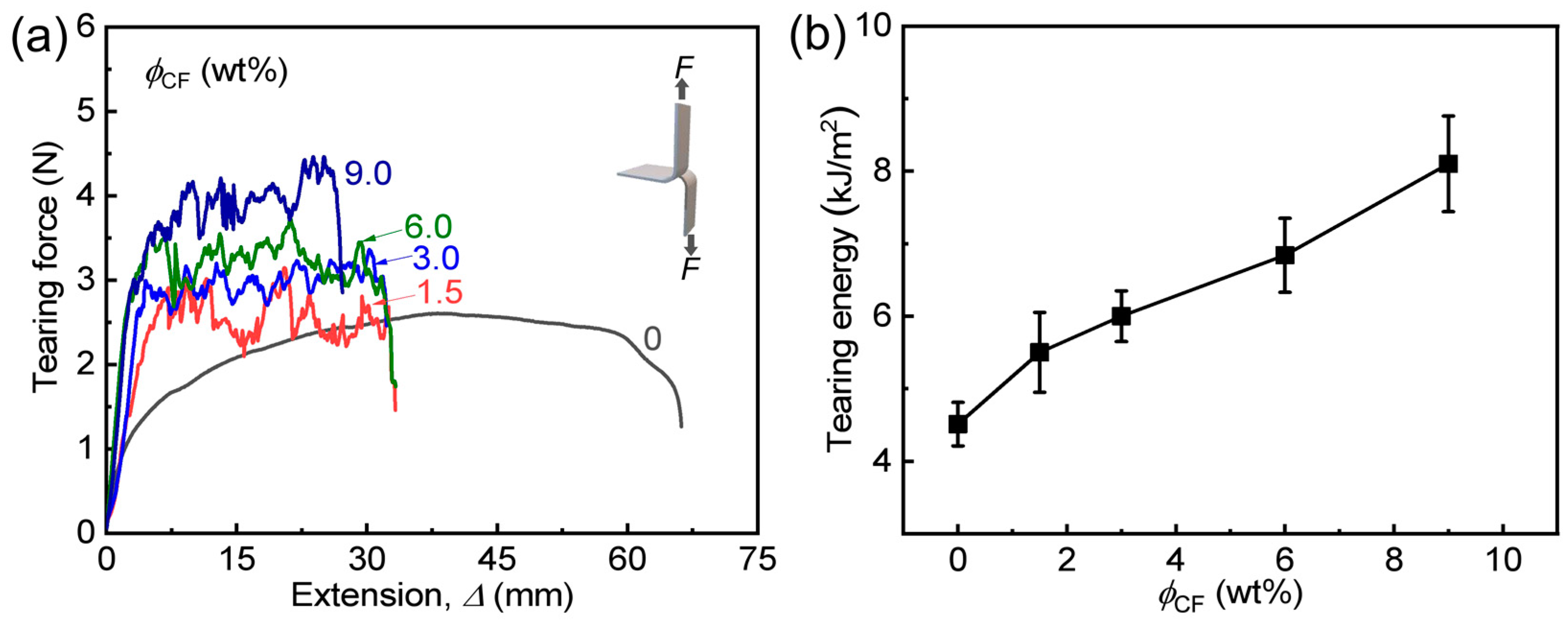
3.2. Tensile and Tearing Behaviors of Hydrogels
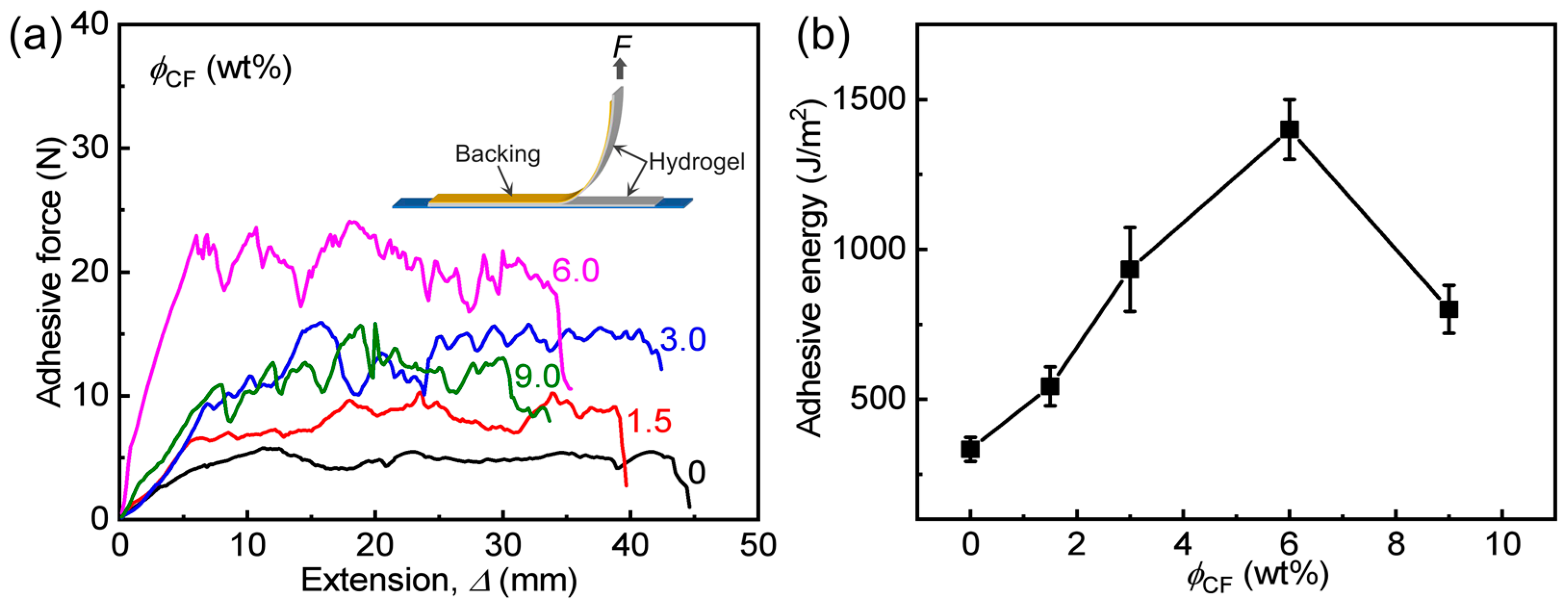
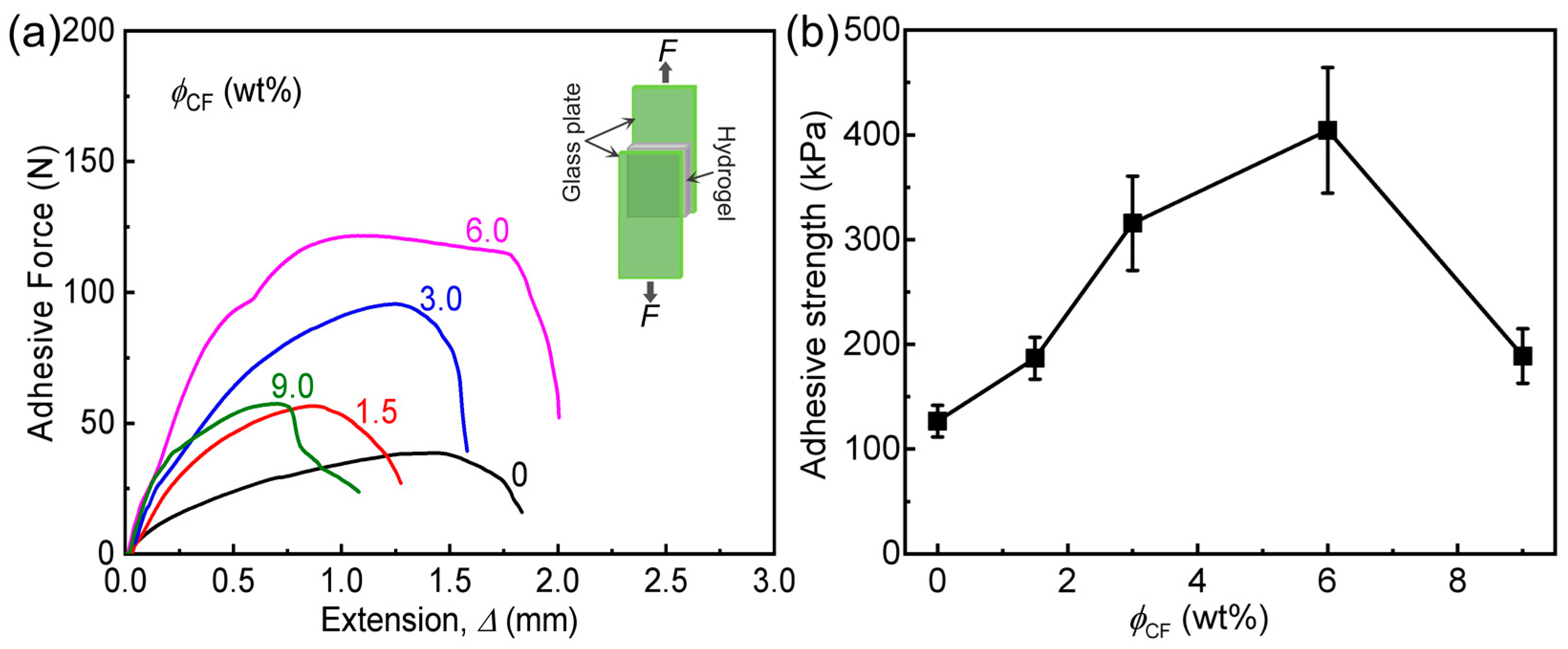
3.3. Adhesion Behaviors of Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.S.; Khademhosseini, A. Advances in Engineering Hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zeng, M.; Ren, J.; Wang, J.; Fan, L.; Xu, Q. Preparation and Swelling Properties of Graphene Oxide/Poly(Acrylic Acid-Co-Acrylamide) Super-Absorbent Hydrogel Nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2012, 401, 97–106. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Li, D.; Shu, M.; Shang, L.; Xia, M.; Huang, Y. High-Strength, Thermosensitive Double Network Hydrogels with Antibacterial Functionality. Soft Matter 2021, 17, 6688–6696. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Zhu, Q.L.; Li, C.Y.; Zheng, Q.; Wu, Z.L. Programmable Morphing Hydrogels for Soft Actuators and Robots: From Structure Designs to Active Functions. Acc. Chem. Res. 2022, 55, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, X.; Wang, L.; Makihata, M.; Liu, H.-C.; Zhou, T.; Zhao, X. Bioadhesive Ultrasound for Long-Term Continuous Imaging of Diverse Organs. Science 2022, 377, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shang, L.; Li, D.; Wang, W.; Chen, S.; Zhong, H.; Huang, Y.; Long, S. High-Strength, Strong-Adhesion, and Antibacterial Polyelectrolyte Complex Hydrogel Films from Natural Polysaccharides. Polym. Test. 2022, 109, 107547. [Google Scholar] [CrossRef]
- Qiao, Z.; Parks, J.; Choi, P.; Ji, H.-F. Applications of Highly Stretchable and Tough Hydrogels. Polymers 2019, 11, 1773. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.P. Why Are Double Network Hydrogels so Tough? Soft Matter 2010, 6, 2583. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly Stretchable and Tough Hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.L.; Kurokawa, T.; Kuroda, S.; Bin Ihsan, A.; Akasaki, T.; Sato, K.; Haque, M.A.; Nakajima, T.; Gong, J.P. Physical Hydrogels Composed of Polyampholytes Demonstrate High Toughness and Viscoelasticity. Nat. Mater. 2013, 12, 932–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.J.; Sun, T.L.; Zhang, A.K.; Ikura, Y.; Nakajima, T.; Nonoyama, T.; Kurokawa, T.; Ito, O.; Ishitobi, H.; Gong, J.P. Tough Physical Double-Network Hydrogels Based on Amphiphilic Triblock Copolymers. Adv. Mater. 2016, 28, 4884–4890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, M.; Wu, S.; Ma, Y.; Zhao, Y.; Chen, Z.; Frenkel, I.; Strzalka, J.; Zhou, H.; Zhu, X.; He, X. Strong Tough Hydrogels via the Synergy of Freeze-Casting and Salting Out. Nature 2021, 590, 594–599. [Google Scholar] [CrossRef]
- Lian, W.Z.; Fan, Z.W.; Cui, K.; Yin, P.; Yang, J.; Jiang, H.; Tang, L.; Sun, T. Tough Hydrogels with Dynamic H-Bonds: Structural Heterogeneities and Mechanical Performances. Macromolecules 2021, 54, 8996–9006. [Google Scholar] [CrossRef]
- Du, C.; Zhang, X.N.; Sun, T.L.; Du, M.; Zheng, Q.; Wu, Z.L. Hydrogen-Bond Association-Mediated Dynamics and Viscoelastic Properties of Tough Supramolecular Hydrogels. Macromolecules 2021, 54, 4313–4325. [Google Scholar] [CrossRef]
- Zhang, X.N.; Du, C.; Wang, Y.J.; Hou, L.X.; Du, M.; Zheng, Q.; Wu, Z.L. Influence of the α-Methyl Group on Elastic-To-Glassy Transition of Supramolecular Hydrogels with Hydrogen-Bond Associations. Macromolecules 2022, 55, 7512–7525. [Google Scholar] [CrossRef]
- Cui, W.; Zheng, Y.; Zhu, R.; Mu, Q.; Wang, X.; Wang, Z.; Liu, S.; Li, M.; Ran, R. Strong Tough Conductive Hydrogels via the Synergy of Ion-Induced Cross-Linking and Salting-Out. Adv. Funct. Mater. 2022, 32, 2204823. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Z.; Zhao, Z.; Wang, M.; Dong, L.; Gao, H.; Liu, C.; Zhou, P.; Chen, L.; Shi, C.; et al. Anti-Swelling, Robust, and Adhesive Extracellular Matrix-Mimicking Hydrogel Used as Intraoral Dressing. Adv. Mater. 2022, 34, 2200115. [Google Scholar] [CrossRef]
- Chen, L.; Cui, Y.; Ren, W. Tough and Rapidly Stimuli-Responsive Luminescent Hydrogels for Multi-Dimensional Information Encryption and Storage. Polymer 2022, 243, 124621. [Google Scholar] [CrossRef]
- Cao, J.; Li, J.; Chen, Y.; Zhang, L.; Zhou, J. Dual Physical Crosslinking Strategy to Construct Moldable Hydrogels with Ultrahigh Strength and Toughness. Adv. Funct. Mater. 2018, 28, 1800739. [Google Scholar] [CrossRef]
- Pei, X.; Zhang, H.; Zhou, Y.; Zhou, L.; Fu, J. Stretchable, Self-Healing and Tissue-Adhesive Zwitterionic Hydrogels as Strain Sensors for Wireless Monitoring of Organ Motions. Mater. Horiz. 2020, 7, 1872–1882. [Google Scholar] [CrossRef]
- Yu, H.C.; Zheng, S.Y.; Fang, L.; Ying, Z.; Du, M.; Wang, J.; Ren, K.F.; Wu, Z.L.; Zheng, Q. Reversibly Transforming a Highly Swollen Polyelectrolyte Hydrogel to an Extremely Tough One and Its Application as a Tubular Grasper. Adv. Mater. 2020, 32, 2005171. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Qiu, J.; Lu, C.; Jin, S.; Zhang, G.; Sakai, E. Multi-Sacrificial Bonds Enhanced Double Network Hydrogel with High Toughness, Resilience, Damping, and Notch-Insensitivity. Polymers 2020, 12, 2263. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, H.; Zhu, L.; Zheng, J. Fundamentals of Double Network Hydrogels. J. Mater. Chem. B 2015, 3, 3654–3676. [Google Scholar] [CrossRef]
- Yu, H.C.; Li, C.Y.; Du, M.; Song, Y.; Wu, Z.L.; Zheng, Q. Improved Toughness and Stability of κ-Carrageenan/Polyacrylamide Double-Network Hydrogels by Dual Cross-Linking of the First Network. Macromolecules 2019, 52, 629–638. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, L.; Zhou, J.; Li, X.; Liu, J.; Zeng, M. Mechanical Enhancement of Graphene Oxide-Filled Chitosan-Based Composite Hydrogels by Multiple Mechanisms. J. Mater. Sci. 2020, 55, 14690–14701. [Google Scholar] [CrossRef]
- Xu, B.; Liu, Y.; Wang, L.; Ge, X.; Fu, M.; Wang, P.; Wang, Q. High-Strength Nanocomposite Hydrogels with Swelling-Resistant and Anti-Dehydration Properties. Polymers 2018, 10, 1025. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; King, D.R.; Sun, T.L.; Nonoyama, T.; Kurokawa, T.; Nakajima, T.; Gong, J.P. Energy-Dissipative Matrices Enable Synergistic Toughening in Fiber Reinforced Soft Composites. Adv. Funct. Mater. 2017, 27, 1605350. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; King, D.R.; Cui, W.; Sun, T.L.; Guo, H.; Kurokawa, T.; Brown, H.R.; Hui, C.-Y.; Gong, J.P. Superior Fracture Resistance of Fiber Reinforced Polyampholyte Hydrogels Achieved by Extraordinarily Large Energy-Dissipative Process Zones. J. Mater. Chem. A 2019, 7, 13431–13440. [Google Scholar] [CrossRef]
- Hui, C.-Y.; Liu, Z.; Phoenix, S.L.; King, D.R.; Cui, W.; Huang, Y.; Gong, J.P. Mechanical Behavior of Unidirectional Fiber Reinforced Soft Composites. Extreme Mech. Lett. 2020, 35, 100642. [Google Scholar] [CrossRef]
- Gong, J.P. Friction and Lubrication of Hydrogels—Its Richness and Complexity. Soft Matter 2006, 2, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Zhang, G.; Shi, M.; Suo, Z. Fracture, Fatigue, and Friction of Polymers in Which Entanglements Greatly Outnumber Cross-Links. Science 2021, 374, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Zhang, T.; Lin, S.; Parada, G.A.; Zhao, X. Tough Bonding of Hydrogels to Diverse Non-Porous Surfaces. Nat. Mater. 2015, 15, 190–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Nian, G.; Yang, C.; Qu, S.; Suo, Z. Bonding Dissimilar Polymer Networks in Various Manufacturing Processes. Nat. Commun. 2018, 9, 846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlett, M.D.; Croll, A.B.; Crosby, A.J. Designing Bio-Inspired Adhesives for Shear Loading: From Simple Structures to Complex Patterns. Adv. Funct. Mater. 2012, 22, 4985–4992. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, L.; Zhou, J.; Liu, T.; Yan, Y.; Long, S.; Li, X. Strong Tough Polyampholyte Hydrogels via the Synergistic Effect of Ionic and Metal–Ligand Bonds. Adv. Funct. Mater. 2021, 31, 2103917. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, Y.; Qian, S.; Zeng, P.; Long, S.; Li, X. Strengthening and Stiffening in Swollen Polyampholyte Hydrogels. Mater. Lett. 2022, 324, 132582. [Google Scholar] [CrossRef]
- Rao, P.; Sun, T.L.; Chen, L.; Takahashi, R.; Shinohara, G.; Guo, H.; King, D.R.; Kurokawa, T.; Gong, J.P. Tough Hydrogels with Fast, Strong, and Reversible Underwater Adhesion Based on a Multiscale Design. Adv. Mater. 2018, 30, 1801884. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yang, X.; Nian, G.; Suo, Z. Strength and Toughness of Adhesion of Soft Materials Measured in Lap Shear. J. Mech. Phys. Solids 2020, 143, 103988. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Huang, Y.; Long, S.; Li, H.; Li, X. Super Bulk and Interfacial Toughness of Amylopectin Reinforced PAAm/PVA Double-Network Hydrogels via Multiple Hydrogen Bonds. Macromol. Mater. Eng. 2020, 305, 1900450. [Google Scholar] [CrossRef]
- Liu, H.; Hu, X.; Li, W.; Zhu, M.; Tian, J.; Li, L.; Luo, B.; Zhou, C.; Lu, L. A Highly-Stretchable and Adhesive Hydrogel for Noninvasive Joint Wound Closure Driven by Hydrogen Bonds. Chem. Eng. J. 2023, 452, 139368. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, J.; Chen, X.; Zhang, J.; Chen, G.; Zhang, K.; Lin, J.; Guo, C.; Liu, J. Trigger-Detachable Hydrogel Adhesives for Bioelectronic Interfaces. Adv. Funct. Mater. 2021, 31, 2106446. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.; Liu, K.; Wang, K.; Fang, L.; Weng, L.-T.; Zhang, H.; Tang, Y.; Ren, F.; Zhao, C.; et al. Mussel-Inspired Adhesive and Tough Hydrogel Based on Nanoclay Confined Dopamine Polymerization. ACS Nano 2017, 11, 2561–2574. [Google Scholar] [CrossRef] [PubMed]
- Gan, D.; Xing, W.; Jiang, L.; Fang, J.; Zhao, C.; Ren, F.; Fang, L.; Wang, K.; Lu, X. Plant-Inspired Adhesive and Tough Hydrogel Based on Ag-Lignin Nanoparticles-Triggered Dynamic Redox Catechol Chemistry. Nat. Commun. 2019, 10, 1487. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Tang, Z.; Peng, S.; Zhang, P.; Sun, T.; Wei, W.; Zeng, L.; Guo, H.; Guo, H.; Meng, G. Modification of Hydrophobic Hydrogels into a Strongly Adhesive and Tough Hydrogel by Electrostatic Interaction. Macromolecules 2022, 55, 156–165. [Google Scholar] [CrossRef]



| Sample code (ϕ) (a) | E (MPa) | σb (MPa) | Wb (MJ m−3) | T (kJ m−2) | ωw (wt%) |
|---|---|---|---|---|---|
| PA | 1.6 ± 0.1 | 1.9 ± 0.1 | 5.9 ± 0.1 | 4.5 ± 0.3 | 53.5 ± 1.9 |
| PA/CF-1.5 | 11.9 ± 1.2 | 2.0 ± 0.1 | 0.50 ± 0.05 | 5.5 ± 0.6 | 57.1 ± 0.1 |
| PA/CF-3 | 12.2 ± 1.4 | 2.4 ± 0.1 | 0.70 ± 0.04 | 6.0 ± 0.4 | 65.6 ± 1.3 |
| PA/CF-6 | 29.3 ± 1.7 | 4.3 ± 0.4 | 0.60 ± 0.15 | 6.8 ± 0.5 | 62.9 ± 1.0 |
| PA/CF-9 | 35.3 ± 0.1 | 4.3 ± 0.5 | 0.80 ± 0.03 | 8.1 ± 0.7 | 60.6 ± 0.4 |
| Sample Code | (kPa) (a) | Γ (J m−2) | (kPa) (b) |
|---|---|---|---|
| PA | 157 ± 24 | 333 ± 40 | 127 ± 15 |
| PA/CF-1.5 | 360 ± 54 | 543 ± 65 | 187 ± 20 |
| PA/CF-3 | 667 ± 80 | 933 ± 140 | 316 ± 45 |
| PA/CF-6 | 943 ± 120 | 1400 ± 100 | 404 ± 60 |
| PA/CF-9 | 520 ± 90 | 800 ± 80 | 189 ± 26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Xiao, L.; Teng, Q.; Jiang, Y.; Deng, Q.; Li, X.; Huang, Y. Strong, Tough, and Adhesive Polyampholyte/Natural Fiber Composite Hydrogels. Polymers 2022, 14, 4984. https://doi.org/10.3390/polym14224984
Yan Y, Xiao L, Teng Q, Jiang Y, Deng Q, Li X, Huang Y. Strong, Tough, and Adhesive Polyampholyte/Natural Fiber Composite Hydrogels. Polymers. 2022; 14(22):4984. https://doi.org/10.3390/polym14224984
Chicago/Turabian StyleYan, Yongqi, Longya Xiao, Qin Teng, Yuanyuan Jiang, Qin Deng, Xuefeng Li, and Yiwan Huang. 2022. "Strong, Tough, and Adhesive Polyampholyte/Natural Fiber Composite Hydrogels" Polymers 14, no. 22: 4984. https://doi.org/10.3390/polym14224984