Recent Advantages on Waste Management in Hydrogen Industry
Abstract
:1. Introduction
- Chemical industry—synthesis of ammonia, methanol, and hydrocarbons, as well as the recovery of metals from their oxide form [6].
- Energetics—an energy source for electric and thermal power engineering [9].
- Petrochemistry—oil refining (hydrogenation purification of petroleum products—hydrodesulfurization) [10].
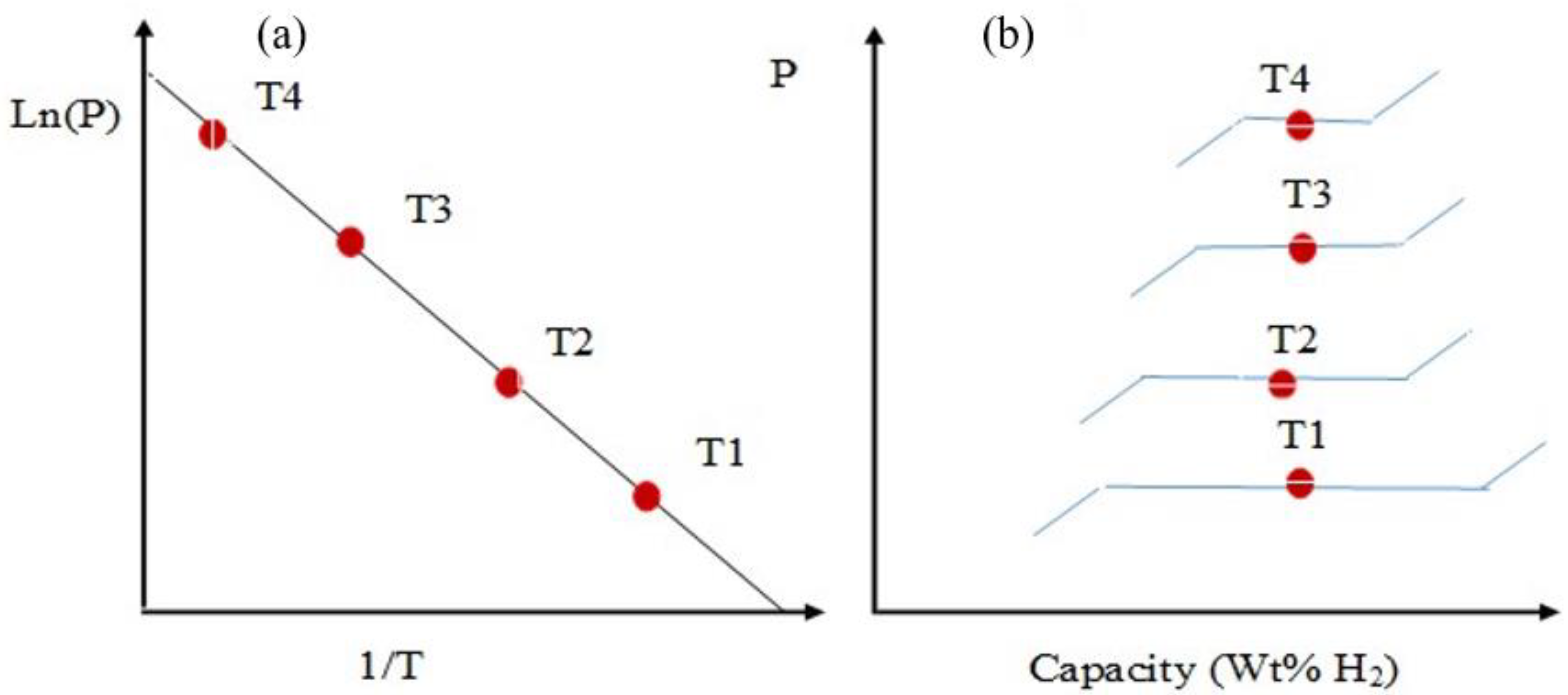
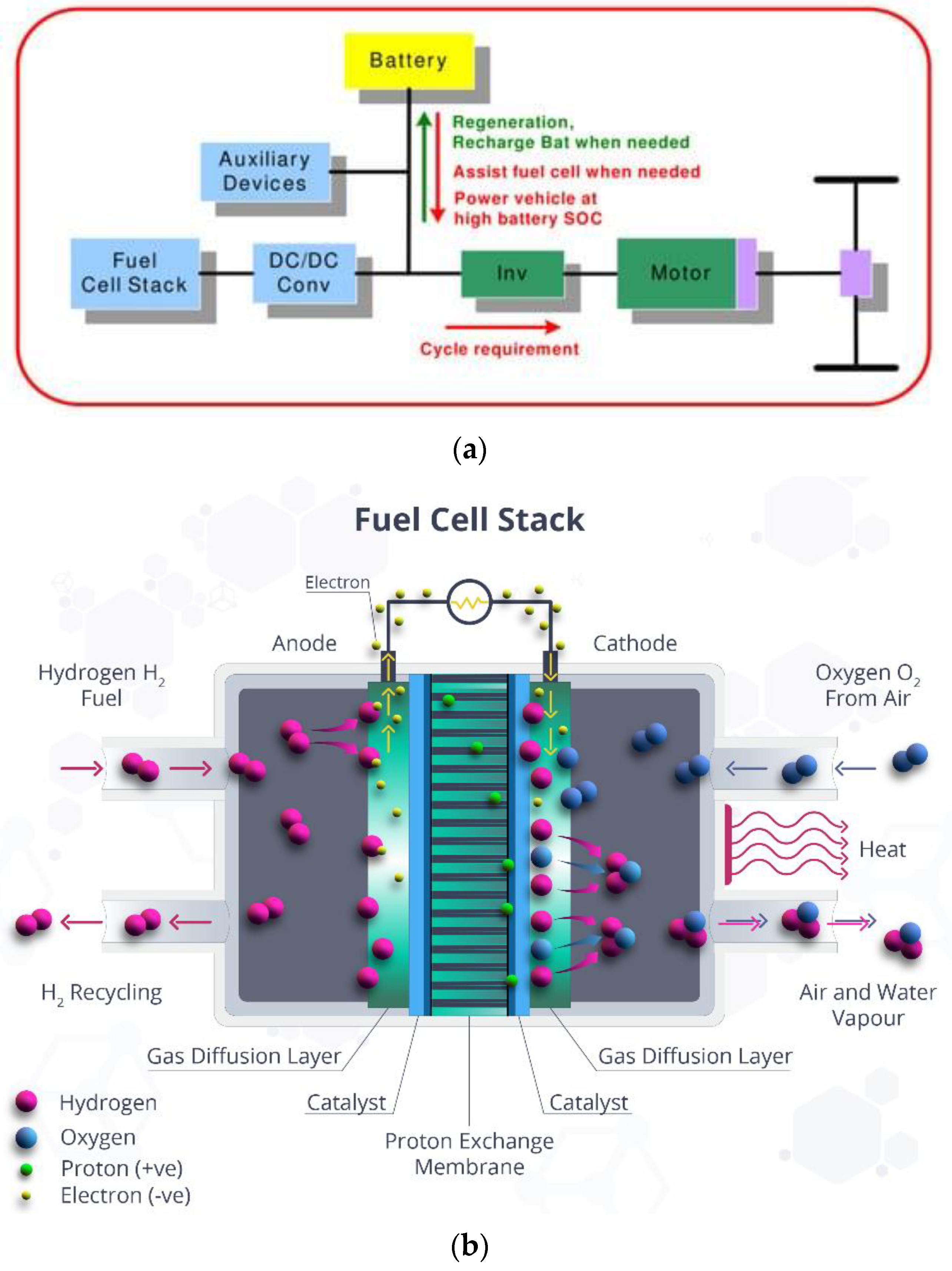
2. Design and Thermodynamics of HS Tank
3. Composite Materials for HS—Thermoset Composites
- Dissociative material in which molecular hydrogen is dissociated into hydrogen atoms occupying internodes;
- Materials with chemically bonded hydrogen;
- Materials that adsorb molecular hydrogen, in which molecular hydrogen attaches to the surface due to weak interactions, such as the Van der Waals force or physical sorption.
- MHs;
- Hydrides based on light metals;
- Chemical hydrides (complex hydrides);
- Nanostructured materials (adsorption of molecular hydrogen).
4. Hydrogen Generation and Storage on the MoS2-Containing Materials
- Primary discharge stage (Vollmer reaction):
- H3O+ + e− → Hads + H2O
- Electrochemical desorption stage (Geyrovsky reaction):
- Hads + H3O+ + e− → H2 + H2O
- Recombination stage (Tafel reaction):
- Hads + Hads → H2
5. Processing of Composite Materials from the Hydrogen Industry
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Yang, Y.; Tong, L.; Yin, S.; Liu, Y.; Wang, L.; Qiu, Y.; Ding, Y. Status and challenges of applications and industry chain technologies of hydrogen in the context of carbon neutrality. J. Clean. Prod. 2022, 376, 134347. [Google Scholar] [CrossRef]
- Nimir, W.; Al-Othman, A.; Tawalbeh, M.; Al Makky, A.; Ali, A.; Karimi-Maleh, H.; Karimi, F.; Karaman, C. Approaches towards the development of heteropolyacid-based high temperature membranes for PEM fuel cells. Int. J. Hydrogen Energy, 2021; in press. [Google Scholar] [CrossRef]
- Modarres, M.; Kaminskiy, M.; Krivtsov, V.V. Reliability Engineering and Risk Analysis: A Practical Guide, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Olabi, A.G.; Abdelkareem, M.A.; Wilberforce, T.; Sayed, E.T. Application of graphene in energy storage device—A review. Renew. Sustain. Energy Rev. 2021, 135, 110026. [Google Scholar] [CrossRef]
- Shchegolkov, A.V.; Lipkin, M.S.; Shchegolkov, A.V. Preparation of WO3 Films on Titanium and Graphite Foil for Fuel Cell and Supercapacitor Applications by Electrochemical (Cathodic) Deposition Method. Russ. J. Gen. Chem. 2022, 92, 1161–1167. [Google Scholar] [CrossRef]
- Hu, Y.; Xue, N.; Zhang, Y.; Hu, P. An eco-friendly vanadium precipitation method through solution-phase hydrogen reduction with nickel catalysis. J. Taiwan Inst. Chem. Eng. 2022, 134, 104334. [Google Scholar] [CrossRef]
- Machado, G.; Coelho, C. Vertically-aligned carbon nanotube at low pressure by cold-wall thermal CVD using a two-phase deposition step. Carbon Trends 2021, 5, 100087. [Google Scholar] [CrossRef]
- Chan, K.; Maznam, N.; Hazan, M.; Ahmad, R.; Sa’Ari, A.; Azman, N.; Mamat, M.; Rahman, M.; Tanemura, M.; Yaakob, Y. Multi-walled carbon nanotubes growth by chemical vapour deposition: Effect of precursor flowing path and catalyst size. Carbon Trends 2022, 6, 100142. [Google Scholar] [CrossRef]
- Rejeb, O.; Alirahmi, S.M.; Assareh, E.; Assad, M.E.H.; Jemni, A.; Bettayeb, M.; Ghenai, C. Innovative integrated solar powered polygeneration system for green Hydrogen, Oxygen, electricity and heat production. Energy Convers. Manag. 2022, 269, 116073. [Google Scholar] [CrossRef]
- Speight, J.G. Effects in Refining. In High Acid Crudes; Gulf Professional Publishing: Houston, TX, USA, 2014; Chapter 4; pp. 77–109. [Google Scholar]
- Jafari, H.; Safarzadeh, S.; Azad-Farsani, E. Effects of governmental policies on energy-efficiency improvement of hydrogen fuel cell cars: A game-theoretic approach. Energy 2022, 254, 124394. [Google Scholar] [CrossRef]
- Ku, A.Y.; Reddi, K.; Elgowainy, A.; McRobie, J.; Li, J. Liquid pump-enabled hydrogen refueling system for medium and heavy duty fuel cell vehicles: Station design and technoeconomic assessment. Int. J. Hydrogen Energy 2022, 47, 25486–25498. [Google Scholar] [CrossRef]
- Boretti, A. Comparison of fuel economies of high efficiency diesel and hydrogen engines powering a compact car with a flywheel based kinetic energy recovery systems. Int. J. Hydrogen Energy 2010, 35, 8417–8424. [Google Scholar] [CrossRef]
- Coleman, D.; Kopp, M.; Wagner, T.; Scheppat, B. The value chain of green hydrogen for fuel cell buses—A case study for the Rhine-Main area in Germany. Int. J. Hydrogen Energy 2020, 45, 5122–5133. [Google Scholar] [CrossRef]
- Charters, D. A comparison of energy vectors in powering hybrid buses. Renew. Energy Focus 2016, 17, 73–74. [Google Scholar] [CrossRef]
- Emonts, B.; Schiebahn, S.; Görner, K.; Lindenberger, D.; Markewitz, P.; Merten, F.; Stolten, D. Re-energizing energy supply: Electrolytically-produced hydrogen as a flexible energy storage medium and fuel for road transport. J. Power Sources 2017, 342, 320–326. [Google Scholar] [CrossRef]
- Zhao, K.; Fang, X.; Cui, C.; Kang, S.; Zheng, A.; Zhao, Z. Co-production of syngas and H2 from chemical looping steam reforming of methane over anti-coking CeO2/La0.9Sr0.1Fe1−xNixO3 composite oxides. Fuel 2022, 317, 123455. [Google Scholar] [CrossRef]
- Peláez-Peláez, S.; Colmenar-Santos, A.; Pérez-Molina, C.; Rosales, A.-E.; Rosales-Asensio, E. Techno-economic analysis of a heat and power combination system based on hybrid photovoltaic-fuel cell systems using hydrogen as an energy vector. Energy 2021, 224, 120110. [Google Scholar] [CrossRef]
- Karimkashi, S.; Kahila, H.; Kaario, O.; Larmi, M.; Vuorinen, V. Numerical study on tri-fuel combustion: Ignition properties of hydrogen-enriched methane-diesel and methanol-diesel mixtures. Int. J. Hydrogen Energy 2020, 45, 4946–4962. [Google Scholar] [CrossRef]
- Wang, H.; Ji, C.; Shi, C.; Yang, J.; Ge, Y.; Wang, S.; Chang, K.; Meng, H.; Wang, X. Parametric modeling and optimization of the intake and exhaust phases of a hydrogen Wankel rotary engine using parallel computing optimization platform. Fuel 2022, 324, 124381. [Google Scholar] [CrossRef]
- Gomez, A.; Smith, H. Liquid hydrogen fuel tanks for commercial aviation: Structural sizing and stress analysis. Aerosp. Sci. Technol. 2019, 95, 105438. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, Z.; Ji, C.; Li, X.; Di, L.; Wu, Z. Potential improvement in combustion and pollutant emissions of a hydrogen-enriched rotary engine by using novel recess configuration. Chemosphere 2022, 299, 134491. [Google Scholar] [CrossRef]
- Collodi, G. Hydrogen Production via Steam Reforming with CO2 Capture. Chem. Eng. Trans. 2010, 19, 37–42. [Google Scholar]
- Santhanam, K.S.; Press, R.J.; Miri, M.J.; Bailey, A.V.; Takacs, G.A. Introduction to Hydrogen Technology; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Takeda, S.; Nam, H.; Chapman, A. Low-carbon energy transition with the sun and forest: Solar-driven hydrogen production from biomass. Int. J. Hydrogen Energy 2022, 47, 24651–24668. [Google Scholar] [CrossRef]
- Lakhlifi, A.; Dahoo, P.R.; Picaud, S.; Mousis, O. A simple van’t Hoff law for calculating Langmuir constants in clathrate hydrates. Chem. Phys. 2015, 448, 53–60. [Google Scholar] [CrossRef]
- Jiang, W.; Sun, P.; Li, P.; Zuo, Z.; Huang, Y. Transient thermal behavior of multi-layer insulation coupled with vapor cooled shield used for liquid hydrogen storage tank. Energy 2021, 231, 120859. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Kim, J.H.; Vo, T.T.N.; Kim, N.; Ahn, H.S. Design of portable hydrogen tank using adsorption material as storage media: An alternative to Type IV compressed tank. Appl. Energy 2022, 310, 118552. [Google Scholar] [CrossRef]
- Su, Y.; Lv, H.; Zhou, W.; Zhang, C. Review of the Hydrogen Permeability of the Liner Material of Type IV On-Board Hydrogen Storage Tank. World Electr. Veh. J. 2021, 12, 130. [Google Scholar] [CrossRef]
- Molkov, V.; Dadashzadeh, M.; Kashkarov, S.; Makarov, D. Performance of hydrogen storage tank with TPRD in an engulfing fire. Int. J. Hydrogen Energy 2021, 46, 36581–36597. [Google Scholar] [CrossRef]
- Ahamed, M.I.; Shakeel, N.; Anwar, N.; Khan, A.; Asiri, A.M.; Dzudzevic-Cancar, H. 4—Graphene-based nanocomposite for hydrogen storage application. In Micro and Nano Technologies, Nanomaterials for Hydrogen Storage Applications; Sen, F., Khan, A., Asiri, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 57–78. ISBN 9780128194768. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, D.; Ding, W. High hydrogen storage ability of a decorated g-C3N4 monolayer decorated with both Mg and Li: A density functional theory (DFT) study. Int. J. Hydrogen Energy 2022, 47, 28548–28555. [Google Scholar] [CrossRef]
- Özdoğan, E.; Hüner, B.; Süzen, Y.O.; Eşiyok, T.; Uzgören, I.N.; Kıstı, M.; Uysal, S.; Selçuklu, S.B.; Demir, N.; Kaya, M.F. Effects of tank heating on hydrogen release from metal hydride system in VoltaFCEV Fuel Cell Electric Vehicle. Int. J. Hydrogen Energy, 2022; in press. [Google Scholar] [CrossRef]
- Manoharan, Y.; Hosseini, S.E.; Butler, B.; Alzhahrani, H.; Senior, B.T.F.; Ashuri, T.; Krohn, J. Hydrogen Fuel Cell Vehicles; Current Status and Future Prospect. Appl. Sci. 2019, 9, 2296. [Google Scholar] [CrossRef]
- Malleswararao, K.; Aswin, N.; Kumar, P.; Dutta, P.; Murthy, S.S. Experiments on a novel metal hydride cartridge for hydrogen storage and low temperature thermal storage. Int. J. Hydrogen Energy 2022, 47, 16144–16155. [Google Scholar] [CrossRef]
- Bai, X.-S.; Yang, W.-W.; Yang, Y.-J.; Zhang, K.-R.; Yang, F.-S. Multi-variable optimization of metal hydride hydrogen storage reactor with gradient porosity metal foam and evaluation of comprehensive performance. Int. J. Hydrogen Energy 2022, 47, 35340–35351. [Google Scholar] [CrossRef]
- Banerjee, S.; Mukhopadhyay, P. Interstitial Ordering. In Pergamon Materials Series; Elsevier: Pergamon, Turkey, 2007; Chapter 8; Volume 12, pp. 717–781. [Google Scholar]
- Matysina, Z.A.; Gavrylyuk, N.A.; Kartel, M.T.; Veziroglu, A.; Veziroglu, T.N.; Pomytkin, A.P.; Schur, D.V.; Ramazanov, T.S.; Gabdullin, M.T.; Zolotarenko, A.D.; et al. Hydrogen sorption properties of new magnesium intermetallic compounds with MgSnCu4 type structure. Int. J. Hydrogen Energy 2021, 46, 25520–25532. [Google Scholar] [CrossRef]
- Chang, S.; Young, K.-H.; Ouchi, T.; Meng, T.; Nei, J.; Wu, X. Studies on Incorporation of Mg in Zr-Based AB2 Metal Hydride Alloys. Batteries 2016, 2, 11. [Google Scholar] [CrossRef]
- Young, K.-H.; Nei, J.; Wan, C.; Denys, R.V.; Yartys, V.A. Comparison of C14- and C15-Predomiated AB2 Metal Hydride Alloys for Electrochemical Applications. Batteries 2017, 3, 22. [Google Scholar] [CrossRef]
- Eisapour, A.H.; Eisapour, M.; Talebizadehsardari, P.; Walker, G.S. An innovative multi-zone configuration to enhance the charging process of magnesium based metal hydride hydrogen storage tank. J. Energy Storage 2021, 36, 102443. [Google Scholar] [CrossRef]
- Anbarasu, S.; Muthukumar, P.; Mishra, S.C. Tests on LmNi4.91Sn0.15 based solid state hydrogen storage device with embedded cooling tubes—Part A: Absorption process. Int. J. Hydrogen Energy 2014, 39, 3342–3351. [Google Scholar] [CrossRef]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Li, G.; Chen, Z.; Li, Y.; Zhang, D.; Yang, W.; Liu, Y.; Cao, L. Engineering Substrate Interaction to Improve Hydrogen Evolution Catalysis of Monolayer MoS2 Films beyond Pt. ACS Nano 2020, 14, 1707–1714. [Google Scholar] [CrossRef]
- Qu, J.; Li, Y.; Li, F.; Li, T.; Wang, X.; Yin, Y.; Ma, L.; Schmidt, O.G.; Zhu, F. Direct Thermal Enhancement of Hydrogen Evolution Reaction of On-Chip Monolayer MoS2. ACS Nano 2022, 16, 2921–2927. [Google Scholar] [CrossRef]
- Tekalgne, M.A.; Van Nguyen, K.; Nguyen, D.L.T.; Nguyen, V.-H.; Nguyen, T.P.; Vo, D.-V.N.; Trinh, Q.T.; Hasani, A.; Do, H.H.; Lee, T.H.; et al. Hierarchical molybdenum disulfide on carbon nanotube-reduced graphene oxide composite paper as efficient catalysts for hydrogen evolution reaction. J. Alloys Compd. 2020, 823, 153897. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 Nanoparticles Grown on Graphene: An Advanced Catalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, M.; Yang, H.; Li, G.; Xing, S.; Li, M.; Xu, Y.; Zhang, Q.; Hu, S.; Liao, H.; et al. Creating Fluorine-Doped MoS2 Edge Electrodes with Enhanced Hydrogen Evolution Activity. Small Methods 2021, 5, 2100612. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Z.; Tsubaki, N. Activating and optimizing the MoS2@MoO3 S-scheme heterojunction catalyst through interface engineering to form a sulfur-rich surface for photocatalyst hydrogen evolution. Chem. Eng. J. 2022, 438, 135238. [Google Scholar] [CrossRef]
- Liao, T.; Sun, Z.; Sun, C.; Dou, S.X.; Searles, D.J. Electronic Coupling and Catalytic Effect on H2 Evolution of MoS2/Graphene Nanocatalyst. Sci. Rep. 2014, 4, 6256. [Google Scholar] [CrossRef]
- Tsai, C.; Li, H.; Park, S.; Park, J.; Han, H.S.; Nørskov, J.K.; Zheng, X.; Abild-Pedersen, F. Electrochemical generation of sulfur vacancies in the basal plane of MoS2 for hydrogen evolution. Nat. Commun. 2017, 8, 15113. [Google Scholar] [CrossRef]
- Khan, M.; Yousaf, A.B.; Chen, M.; Wei, C.; Wu, X.; Huang, N.; Qi, Z.; Li, L. Molybdenum sulfide/graphene-carbon nanotube nanocomposite material for electrocatalytic applications in hydrogen evolution reactions. Nano Res. 2016, 9, 837–848. [Google Scholar] [CrossRef]
- Wang, X.; Dai, J.; Xie, H.; Yang, C.; He, L.; Wu, T.; Liu, X.; Xu, Y.; Yuan, C.; Dai, L. In-situ construction of ultrathin MoP-MoS2 heterostructure on N, P and S co-doped hollow carbon spheres as nanoreactor for efficient hydrogen evolution. Chem. Eng. J. 2022, 438, 135544. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Tao, Z. Novel hydrogen storage properties of MoS2 nanotubes. J. Alloys Compd. 2003, 356–357, 413–417. [Google Scholar] [CrossRef]
- Passamonti, F.J.; Sedran, U. Recycling of waste plastics into fuels. LDPE conversion in FCC. Appl. Catal. B Environ. 2012, 125, 499–506. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Zhang, P.; Liang, C.; Wu, M.; Chen, X.; Liu, D.; Ma, J. High-efficient microwave plasma discharging initiated conversion of waste plastics into hydrogen and carbon nanotubes. Energy Convers. Manag. 2022, 268, 116017. [Google Scholar] [CrossRef]
- Jagodzińska, K.; Jönsson, P.G.; Yang, W. Pyrolysis and in-line catalytic decomposition of excavated landfill waste to produce carbon nanotubes and hydrogen over Fe- and Ni-based catalysts—Investigation of the catalyst type and process temperature. Chem. Eng. J. 2022, 446, 136808. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, Y.; Williams, P.T.; Yang, H.; Chen, H. Co-production of hydrogen and carbon nanotubes from real-world waste plastics: Influence of catalyst composition and operational parameters. Appl. Catal. B Environ. 2018, 221, 584–597. [Google Scholar] [CrossRef]
- Assefi, M.; Mofarah, S.S.; Maroufi, S.; Nekouei, R.K.; Wang, W.; Kert, E.; Sahajwalla, V. Regeneration of hydrogen through thermal micronisation of end-of-life polymers for sustainable reduction of iron oxide. Fuel Process. Technol. 2022, 226, 107038. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Williams, P.T. Fe–Ni–MCM-41 Catalysts for Hydrogen-Rich Syngas Production from Waste Plastics by Pyrolysis–Catalytic Steam Reforming. Energy Fuels 2017, 31, 8497–8504. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Nahil, M.A.; Williams, P.T.; Wu, C. Development of Ni- and Fe-based catalysts with different metal particle sizes for the production of carbon nanotubes and hydrogen from thermo-chemical conversion of waste plastics. J. Anal. Appl. Pyrolysis 2017, 125, 32–39. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Hydrogen from waste plastics by way of pyrolysis–gasification. Proc. Inst. Civ. Eng.-Waste Resour. Manag. 2014, 167, 35–46. [Google Scholar] [CrossRef]
- Aminu, I.; Nahil, M.A.; Williams, P.T. High-yield hydrogen from thermal processing of waste plastics. Proc. Inst. Civ. Eng.-Waste Resour. Manag. 2022, 175, 3–13. [Google Scholar] [CrossRef]
- Barthelemy, H.; Weber, M.; Barbier, F. Hydrogen storage: Recent improvements and industrial perspectives. Int. J. Hydrogen Energy 2017, 42, 7254–7262. [Google Scholar] [CrossRef]
- Yusaf, T.; Fernandes, L.; Abu Talib, A.R.; Altarazi, Y.S.; Alrefae, W.; Kadirgama, K.; Ramasamy, D.; Jayasuriya, A.; Brown, G.; Mamat, R.; et al. Sustainable Aviation—Hydrogen Is the Future. Sustainability 2022, 14, 548. [Google Scholar] [CrossRef]
- Razi, F.; Dincer, I. Challenges, opportunities and future directions in hydrogen sector development in Canada. Int. J. Hydrogen Energy 2022, 47, 9083–9102. [Google Scholar] [CrossRef]



| Process of Hydrogen Generation | Specific Heat Consumption for Endothermic Reactions, qx (kJ/kg H2) | Specific Consumption of Reference Fuel (rf) to Provide Endothermic Reactions, b (kg rf/kgH2) | Specific rf Consumption for Production of kg H2, b* (kg rf/kgH2) | The Ratio of the Calorific Value of the Total Amount of Fuel to H2 on kg H2 Δ (kJ/kJ) |
|---|---|---|---|---|
| Conversion of Methane with Steam in Reactors with a Fluidized Bed of a Dispersed Catalyst CH4 + 2H2O→ CO2 + 4H2 (by-product CO2) | 34,987 | 1.32 | 4.74 | 1.12 |
| Carbon gasification of solid fuel with water vapor C + 2H2O→ CO2 + 2H2 (by-product CO2) | 67,958 | 2.89 | 6.24 | 1.37 |
| CH4 pyrolysis at 1350 °C CH4→2H2 + C (by-product C) | 18,922 | 0.72 | 7.56 | 1.815 |
| Water electrolysis 2H2O→ H2 + 0.5O2 + H2O (by-product O2) | 214,268.4 | 27.77 | 27.77 | 1.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shchegolkov, A.V.; Shchegolkov, A.V.; Zemtsova, N.V.; Stanishevskiy, Y.M.; Vetcher, A.A. Recent Advantages on Waste Management in Hydrogen Industry. Polymers 2022, 14, 4992. https://doi.org/10.3390/polym14224992
Shchegolkov AV, Shchegolkov AV, Zemtsova NV, Stanishevskiy YM, Vetcher AA. Recent Advantages on Waste Management in Hydrogen Industry. Polymers. 2022; 14(22):4992. https://doi.org/10.3390/polym14224992
Chicago/Turabian StyleShchegolkov, Alexander V., Aleksei V. Shchegolkov, Natalia V. Zemtsova, Yaroslav M. Stanishevskiy, and Alexandre A. Vetcher. 2022. "Recent Advantages on Waste Management in Hydrogen Industry" Polymers 14, no. 22: 4992. https://doi.org/10.3390/polym14224992
APA StyleShchegolkov, A. V., Shchegolkov, A. V., Zemtsova, N. V., Stanishevskiy, Y. M., & Vetcher, A. A. (2022). Recent Advantages on Waste Management in Hydrogen Industry. Polymers, 14(22), 4992. https://doi.org/10.3390/polym14224992







