Abstract
In agriculture, chitosan has become popular as a metabolic enhancer; however, no deep information has been obtained yet regarding its mechanisms on vegetative tissues. This work was conducted to test the impact of chitosan applied at different plant growth stages on plant development, physiology, and response to wounding as well as fruit shape and composition. Five concentrations of chitosan were tested on tomato. The most effective chitosan doses that increased leaf number, leaf area, plant biomass, and stomatal conductance were 0.75 and 1 mg mL−1. Chitosan (1 mg mL−1) applied as foliar spray increased the levels of jasmonoyl–isoleucine and abscisic acid in wounded roots. The application of this dose at vegetative and flowering stages increased chlorophyll fluorescence (Fv/Fm) values, whereas application at the fruit maturation stage reduced the Fv/Fm values. This decline was positively correlated with fruit shape and negatively correlated with the pH and the content of soluble sugars, lycopene, total flavonoids, and nitrogen in fruits. Moreover, the levels of primary metabolites derived from glycolysis, such as inositol phosphate, lactic acid, and ascorbic acid, increased in response to treatment of plants with 1 mg mL−1- chitosan. Thus, chitosan application affects various plant processes by influencing stomata aperture, cell division and expansion, fruit maturation, mineral assimilation, and defense responses.
1. Introduction
With the increase in the population around the world, the demand for marine resources has undergone a dramatic increase [1]. Thus, the post-catch seafood losses have seen a significant increase which, therefore, leads to economic and environmental issues around the world. These issues occur not only in the landing and distribution chains but also during the consumption of seafood. In order to reduce the impact of seafood use in the environment, the most effective strategies that are recommended are the appropriate use of captured biomass as well as the valorization of seafood waste by extracting valuable compounds, such as pigments (i.e., carotenoids), polymers (i.e., chitin, chitosan), oils (i.e., polyunsaturated fatty acids), proteins (i.e., collagen), and minerals (i.e., hydroxyapatite), for use in agriculture, the pharmaceutical industry, and other fields [2].
Tomato is one of the predominant crops in the world, serving as part of many dishes because of its richness in antioxidants (i.e., lycopene) and vitamins (i.e., vitamin C) [3]. Globally, it is the 12th most popular agricultural product with a cultivated area extended to about 5 Mio ha and an annual production of more than 180 Mio tonnes [4]. In the coming years, the demand for tomato will increase steadily with the concomitant increase in the world’s population reaching 9 billion by 2050 [5]. Thus, a big challenge of agricultural communities is to produce enough nutritious food with improved physico-chemical characteristics and without any alteration of its quality. Therefore, to increase the yield and to obtain high-quality tomato, many agricultural practices have been applied, such as using pesticides, genetic transformation, grafting, and application of nutrients and biostimulants [6,7,8,9,10,11,12]. Among them and regardless of their benefit, the application of biostimulants is the most suitable approach in terms of effectiveness, environmental protection, and easy use without being restricted by the necessity of genetic similarity. Among the biostimulants that are known for their efficiency, there is chitosan, an amino polysaccharide produced from insect cuticles, fungal cell walls, or seafood shells [13]. This polymer acts as an antimicrobial agent, antitranspirant, elicitor, and promoter of the growth of plants and beneficial microorganisms [14,15,16,17]. Regarding its effect on plant growth, it has been shown that it improves photosynthesis via the enlargement of chloroplasts and the stimulation of the biosynthesis of photosynthetic pigments (chlorophyll a, b, and carotenoids) [18]. It also affects plants in a manner similar to phytohormones, which are involved in the regulation of morphogenesis, growth, and development of plants. Application of chitosan increases the content of indole-3-acetic acid (IAA), which promotes cell division, and activates the expression of a PIN1 gene that regulates auxin translocation [19,20]. In some cases, chitosan application triggers a negative impact on the plant system. For instance, the application of high doses of chitosan (1 mg mL−1) with low molecular weight to arabidopsis, tomato, or barley roots arrested shoot development and altered root cell division, growth, and structure, all possibly due to the overproduction of auxin and the alteration of its transport and signaling [20]. This inhibition may be related not only to the high chitosan dose but also to its low molecular weight and the acid used for its solubilization.
Chitosan’s effects on plant systems, however, vary and depend on its physico-chemical characteristics, such as structure, solubility, composition, acetylation degree, and molecular weight, its dose, its application mode, such as foliar spray, coating seeds, amendment, and soil drenching, as well as on the plant species studied and environmental conditions applied [20,21,22,23,24,25,26,27]. Despite the previously mentioned experimental evidence on the positive effects of chitosan as a plant biostimulant, the impact of its application to shoots on plants’ physiology, fruit size, and fruit composition is still not fully understood. Furthermore, there is no report on whether chitosan application at different plant growth stages, such as vegetative growth, flowering, fruit development, and ripening, might have different effects on biological parameters.
Wounding caused by mechanical damage or herbivore attack can threaten plant survival. Therefore, plants have developed many rapid response mechanisms to relieve this stress, either by increasing the expression of defense-related genes, increasing callose deposition, releasing oligosaccharides, or by producing hydrolytic enzymes, most of them mediated by phytohormones, such as jasmonic acid (JA) and abscisic acid (ABA) [28]. The biosynthesis of JA and ABA is initiated by the release of linolenic acid and the cleavage of C40 carotenoid precursor, respectively [29,30]. These phytohormones accumulate in wounded tissues and also mediate systemic signals to increase the immune response in non-wounded tissues [31,32]. Previous studies reported that application of chitosan accelerates wound healing in potato tubers and rice leaves by increasing the thickness of periderm and inducing the levels of proteinase inhibitors and of JA [33,34]. It is not known, however, whether chitosan impacts only the locally treated tissue (local stimulation) or also the non-treated, distant tissues/organs (systemic stimulation).
This work aimed to elucidate the effects of chitosan application on tomato plants in terms of growth, yield, and fruit features using chitosan isolated from Parapenaeus longirostris shells. To avoid the negative effect of chitosan on plants, a low dose of acetic acid was used for chitosan solubilization. In addition, the levels of JA and ABA in shoots and roots have been analyzed to clarify alterations in the wound responses after chitosan application to shoots or roots.
2. Materials and Methods
2.1. Plant Material and Treatments
Chitosan (deacetylation degree 4–17%; molecular weight 318.53 kDa), produced from shrimp waste (Parapenaeus longirostris) according to the method described by El Amerany et al. [23], was dissolved in 0.05% (v/v) acetic acid to a final concentration of 1 mg mL−1. From this, three additional solutions of different concentrations (0.25, 0.50, and 0.75 mg mL−1) were prepared. Distilled water served as control and corresponded to 0 mg mL−1.
Tomato cv. Campbell 33 seeds were germinated in polystyrene trays containing peat substrate at 28 °C. After one week, seedlings were transplanted into plastic pots (4 kg) filled with sand and peat mixture (2:1 [v/v]). When plants reached the four-leaf stage, chitosan solutions were applied 6 times on the shoots of identically sized plants every two weeks.
Every week, leaf number (LN) was counted. At the harvest time (twelve weeks after transplantation), stem height (SH), root length (RL), leaf area (LA), fresh and dry weights of shoots and roots (FWS, DWS, FWR, and DWR), and total fresh weight of fruits were determined. SH and RL were measured from the soil contact point to the tip of shoots or roots, respectively. LA was determined using Mesurim software. Plant biomass (shoots, roots, and stems) was weighed before and after their dryness in an oven at 80 °C for 2 days. Additionally, the diameter of ripened fruits was determined using a vernier caliper. Fruits at the same maturity stage were stored at −80 °C or lyophilized for subsequent analysis.
Tomato plants cv. MicroTom were grown in lecaton (particle size 2–5 mm, Original Lamstedt Ton; FiboExClay, Lamsted, Germany) and fertilized weekly with 10 mL Long Ashton solution. Three-week-old seedlings were divided into two groups: one for foliar spray application, while the other one was treated by soil drenching. As mentioned above, leaves or roots were treated with chitosan every two weeks until petals fell and the fruit stage began (in total for a period of 45 days). After 24 h of the latest treatment, the 4th fully developed leaves of each plant were collected and directly flash frozen in liquid nitrogen. The 5th leaves were wounded by squeezing with forceps. After 30 min, lecaton was removed from the roots, from which one half was directly flash frozen, while the other part was wounded by squeezing with forceps. After 30 min, the wounded samples were frozen and all the samples were stored at −80 °C for hormonal analysis and RNA isolation.
2.2. Plant Physiological Parameters
The 5th fully developed leaves (from the apex) of Campbell33 were used to estimate chlorophyll fluorescence (Fv/Fm ratio) and stomatal conductance (gs). The measurements were performed after the 2nd, 6th, and 12th week of chitosan application using a fluorometer (Opti-sciences OSI 30 p) and a leaf porometer (LP1989, Decagon Device, Inc., Washington, DC, USA) [35,36].
2.3. Phytohormone Analysis
12-oxo-phytodienoic acid (OPDA), JA, jasmonoyl–isoleucine (JA–Ile), and ABA were extracted according to Balcke et al. [37]. Homogenized leaves or roots (50 mg) were mixed with 200 μL methanol spiked with internal standards ([2H5]–OPDA, [2H6]–JA, [2H2]–JA–Ile, and [2H6]–ABA) and centrifuged at 12,000 rpm at 0 °C for 5 min. Afterwards, 200 μL of supernatant was collected and diluted 10 times by adding 2% formic acid. Phytohormones were pre-purified by solid-phase extraction (SPE) using a 96-well filtration microplate that contained an adsorbens with a high cation exchange capacity (Chromabond, HR-XC, Macherey, Nagel, Düren, Germany) and then analyzed using UHPLC–MS/MS [37].
2.4. Real-Time qPCR
Total RNA was isolated from roots using an RNeasy Plant Mini Kit (Qiagen, Hilden, Germany). DNA contamination was removed using a DNA-freeTm Kit (Thermo Fisher Scientific, Waltham, MA, USA) and cDNA was synthesized using a ProtoScript II First Strand cDNA Synthesis Kit (New England Biolabs GmbH, Frankfurt/M., Germany).
Real-time qPCR was carried out as described by El Amerany et al. [23] using the following primer sets: 5′-TTCTACTTCGGCGATTACGGTC-3′ and 5′-GGTTAAGTACGCTCCCTGAACG-3′ for Allene Oxide Cyclase (SlAOC), 5′-ACATAATAGGCAAAGTCTCA-3′ and 5′-GTTGAAGAAGAAGAGGAGTT-3′ for 9-cis-epoxicarotenoid dioxygenase 1 (SlNCED1), as well as 5′-ACCACGAAGCTCTCCAGGAG-3′ and 5′-CATTGAACCCAACATTGTCACC-3′ for elongation factor, which was used as a housekeeping gene for calculation of relative transcript levels.
2.5. Fruit Physico-Chemical Parameters
Moisture content (MC) was measured using the method described by Chafi et al. [38]. Briefly, ripened fruits were weighed to determine their fresh weight (FW). Then, they were placed in an oven (70 °C) for drying until their weight became constant and weighed to measure their dry weight (DW). MC was calculated using the following formula:
The hydrogen potential (pH) and the total soluble sugar (Brix) of tomato juice were determined using a pH meter (Hanna instrument) and a refractometer, respectively.
2.6. Metabolomics Analysis
Using the same procedure as described previously to evaluate fruit development and quality, we applied targeted metabolomics [39]. A total of 145 hydrophilic metabolites that cover essential pathways of the central carbon and energy metabolism were analyzed.
Briefly, 25 mg of freeze-dried fruit were homogenized with 0.9 mL of dichloromethane:ethanol (2:1 [v/v], −80 °C) and 0.2 mL trifluoroacetate and then centrifuged. Afterwards, the aqueous phase was transferred to a new tube and the remainder of the organic phase was re-extracted with 0.05 mL trifluoroacetate and centrifuged. After centrifugation, the obtained supernatant was combined with the previously collected one, frozen at −80 °C, and used for metabolomics analysis of hydrophilic metabolites later.
All metabolites were separated using a Waters ACQUITY ultra-high-performance liquid chromatography (UHPLC) system, equipped with a Nucleoshell RP18 column (2.1 × 150 mm, particle size 2.1 µm, Macherey and Nagel, GmbH, Düren, Germany), a binary solvent manager, and an ACQUITY sample manager (Waters GmbH, Eschborn, Germany).
Hydrophilic metabolites of the central carbon and energy metabolism were analyzed on a triple quadrupole (QqQ) mass spectrometer using Multiple Reaction Monitoring (MRM) (QTRAP 6500, AB Sciex GmbH, Darmstadt, Germany), equipped with electrospray ionization mass source (ESI) operating in negative ion mode.
Data processing and peak identification were achieved using MultiQuant™ (version 3.0), MasterView™ (version 1.2), and PeakView™ (version 2.0) software.
2.7. Sugar Content
To determine the levels of glucose, fructose, and sucrose, an enzymatic method was used as described previously [40]. An amount of 25 mg of lyophilized tomato fruits was mixed with 500 µL 80% (v/v) aqueous ethanol and incubated in a thermomixer at 1000 rpm at 80 °C for 1 h, followed by cooling on ice and centrifugation at 14,000× g at 4 °C for 5 min. The supernatant was collected and transferred to a new tube for evaporation to dryness (60–78 °C). The residue was dissolved in 250 µL water and subsequently used for sugar analysis in three technical replicates. Reactions were conducted in a 96-well plate with reaction mixtures each containing 100 µL diluted supernatant (7%) and 0.1 U glucose-6-phosphate dehydrogenase (Merck, Darmstadt, Germany) diluted in 100 µL buffer (100 mM Imidazole/HCl, pH 6.92, 10 mM MgCl2, 2 mM ATP, 4 mM NAD). The reaction was performed in a microplate reader (Sunrise) and recorded at 340 nm wavelength for 3 min. Subsequently, 0.1 U hexokinase (Merck) was added to determine glucose level. The contents of fructose and sucrose were determined in the same way using 0.2 U phosphoglucose isomerase (Merck) and 1 U invertase (Merck), respectively. Levels were calculated using glucose, fructose, and sucrose as standards [23].
2.8. α-Tocopherol Content
Three ripe fruits from the same plant were pooled as one replicate. A total of 25 mg of freeze-dried fruits were mixed with glass and metal beads and 600 µL methanol (+/−10 mg L−1 α-tocopherol as internal standard; Sigma-Aldrich, Burlington, MA, USA) containing ascorbic acid (0.2 mg mL−1) and homogenized three times in a bead mill. The homogenized extracts were centrifuged at 11,500× g for 3 min at 0 °C and then 500 μL supernatant was transferred to a new tube. Then, the remaining residue was extracted again with 200 μL of the same solvent and both supernatants were mixed and stored at −80 °C for subsequent analysis.
Separation of α-tocopherol was carried out using the UHPLC system as above. Solvents used were 10 mmol L−1 ammonium acetate (eluent A) and a mixture of 40% 2-propanol and 60% acetonitrile (eluent B). The flow rate and temperature of the column were 400 μL min−1 and 45 °C, respectively, and the following gradient applied: 2 min at 60% B, 19 min at 95% B, and 3 min at 60% B.
The analysis of α-tocopherol was performed by tandem quadrupole time-of-flight mass spectrometry (Q-TOF-MS) using atmospheric pressure chemical ionization (APCI) operating in positive ion mode, MRM detection, and authentic standards.
2.9. Lycopene Content
Lycopene levels were determined according to Sadler et al. [41]. A total of 500 mg of fresh tomato fruits was mixed with 12.5 mL ethanol:hexane solution (4:3, v/v) for 10 min. Then, 2 mL deionized water was added and the mixture was vortexed for 15 sec and left to stand on ice to phase separation. During the extraction processes, tubes were wrapped in aluminum foil to protect them from light.
The upper phase (hexane layer) was used to measure the absorbance at 502 nm in a UV/visible spectrophotometer (Specord 210 Plus, Analytik Jena, Jena, Germany). The lycopene content was determined using the formula described by Hilares et al. [42].
2.10. Total Flavonoid Content
The total flavonoids were determined using the method described by Fu et al. [43], with some modifications. Freeze-dried tomato fruits (500 mg) were mixed with 10 mL 80% (v/v) methanol, containing 1% formic acid and water at volume ratios of 1:10 to 1:15. The mixture was stirred at 150 rpm for 2 h in darkness and at room temperature. After standing for 5 min, the supernatant was filtered through Whatman No 1 filter paper and stored at −20 °C. Then, 500 µL extract was mixed with 300 µL of 5% (w/v) sodium nitrite. After 5 min of incubation at room temperature, 300 µL of 10% (w/v) aluminum nitrate was added. After 6 min of incubation, 2 mL of 1 M sodium hydroxide was added to stop the reaction. The absorbance was determined at 500 nm. Finally, the concentration of total flavonoids was estimated by using rutin as a standard.
2.11. Macronutrient Content
Macronutrients in tomato fruits were determined using the method described by Segarra et al. [44]. Briefly, 100 mg of freeze-dried fruits were digested with 2 mL nitric acid, 2 mL deionized water, and 1.2 mL hydrogen peroxide at 90 °C for 72 h. The resulting solution was filtrated and diluted with deionized water. The concentrations of N, P, and K were determined using ICP-AES (Varian-Vista, Mulgrave, Australia).
2.12. Statistical Analysis
All data except those of metabolomics were analyzed by CoStat 6.400 (CoHort software) and significant differences between treatments were determined using one-way or two-way ANOVA, followed by Duncan’s test at p ≤ 0.05. All biological replicates taken into account originated from one experimental set-up. However, all experiments were repeated and showed similar results. Principal component analyses (PCA) and partial least squares discriminant analysis (PLS-DA) were performed by web application metaboanalyst (https://www.metaboanalyst.ca/ (accessed on 20 September 2022)) to visualize correlations between treatments and variation in the targeted metabolomics study.
3. Results
3.1. Chitosan Effects on Plant Growth and Physiological Parameters
To study the potential effects of chitosan applied to shoots, the phenotype of tomato plants treated with chitosan was analyzed. For this, plants were treated every two weeks with different doses of chitosan ranging from 0.25 up to 1 mg mL−1, described as Ch0.25 up to Ch1. Treatments with water served as “non-treated” controls.
Determination of LN showed that treatment with Ch1, Ch0.75, and Ch0.50 increased it after the 2nd, 7th, and 10th week of treatment in comparison to non-treated plants (Figure 1), whereas there were no changes in LN after application of a low dose of chitosan (Ch0.25) (Figure 1).
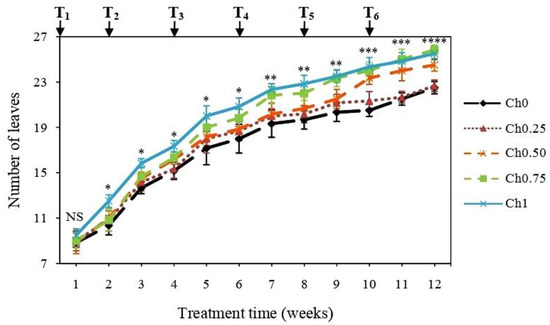
Figure 1.
Changes in leaf number of tomato plants receiving different doses of chitosan. Five treatments were applied until fruit ripening with 0 mg mL−1 (Ch0), 0.25 mg mL−1 (Ch0.25), 0.50 mg mL−1 (Ch0.50), 0.75 mg mL−1 (Ch0.75), and 1 mg mL−1 (Ch1) chitosan. T1, T2, T3, T4, T5, and T6 correspond to the 1st, 2nd, 3rd, 4th, 5th, and 6th application of chitosan. Results were expressed as the mean of 6 independent replicates ± SD. NS, no significant difference between treatments; *, a significant difference between “Ch1” and “Ch0”; **, a significant difference between “Ch1 and Ch0.75” and “Ch0”; ***, a significant difference between “Ch1, Ch0.75 and Ch0.50” and “Ch0”; ****, a significant difference between “Ch1 and Ch0.75”, “Ch0.50”, and “Ch0”.
Chitosan application to shoots did not affect SH (Figure 2a), but led to alterations in RL, which was only observed in plants treated with medium doses of chitosan in comparison to non-treated plants (Figure 2a). Foliar application of chitosan resulted in a slight increase in LA and fresh and dry weight of the aerial parts of plants (SFW and SDW). Here, the highest values were recorded in plants treated with Ch0.75, showing an increase of around 11%, 16%, and 13% for LA, SFW, and SDW, respectively, compared to control plants (Figure 2b–d). In contrast, chitosan application to shoots had no significant effect on RFW and RDW (Figure S1).
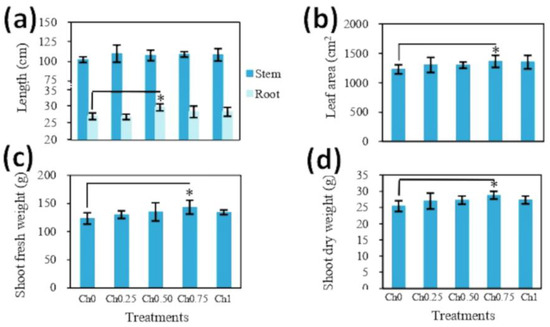
Figure 2.
Effect of foliar spray with chitosan on the length of shoots and roots (a), leaf area (b), and shoot biomass (c,d) of tomato plants. Five treatments were applied until fruit ripening with 0 mg mL−1 (Ch0), 0.25 mg mL−1 (Ch0.25), 0.50 mg mL−1 (Ch0.50), 0.75 mg mL−1 (Ch0.75), and 1 mg mL−1 (Ch1) chitosan, and four-month-old plants were analyzed. The results were expressed as the mean of 6 independent replicates ± SD. * indicates significant differences between treatments following one-way ANOVA (Duncan’s multiple range, p ≤ 0.05).
To shed light on how chitosan might positively affect shoot growth, two physiological parameters, Fv/Fm ratio and gs, were measured. After 2 weeks of treatment, a significant increase in the Fv/Fm ratio was noted only in plants treated with Ch1 (Figure 3a). The Fv/Fm ratio reached approximately 0.767 for control plants and was increased to 0.784 after treatment with Ch1. The increasing effect was more pronounced after 6 weeks of treatment, where the positive effect of other treatments (Ch0.50 and Ch0.75) was also visible. Here, the Fv/Fm ratio was still higher in plants treated with Ch1 (0.817) (Figure 3a). In plants harboring fruits (12 weeks of treatment), Fv/Fm ratios were, however, decreased after application of Ch0.75 and Ch1 (Figure 3a). Similarly, treatment with chitosan resulted in an increase in gs (Figure 3b). Mainly, the application of higher doses led to a significant increase in gs at all time points, although the general levels were decreasing over the time of the experiment.
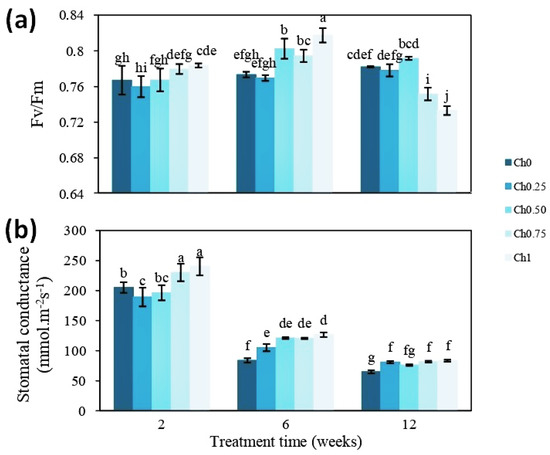
Figure 3.
Evolution of chlorophyll fluorescence (a) and stomatal conductance (b) in plants treated and non-treated with chitosan. Five treatments were applied until fruit ripening with 0 mg mL−1 (Ch0), 0.25 mg mL−1 (Ch0.25), 0.50 mg mL−1 (Ch0.50), 0.75 mg mL−1 (Ch0.75), and 1 mg mL−1 (Ch1) chitosan. Data were obtained after the 2nd, 6th, and 12th week of treatment. Results were expressed as the mean of 4 independent replicates ± SD. Values followed by different lowercase letters are significantly different according to Duncan’s multiple range test (p ≤ 0.05).
3.2. Chitosan Effects on Plant’s Response to Wounding
To understand the effect of chitosan on plant defense mechanisms, the levels of JA, its precursor 12-oxo phytodienoic acid (OPDA), and its active form jasmonoyl–isoleucine (JA–Ile) as well as those of ABA, in responses to chitosan applied to shoots or roots as well as to wounding, were determined. In order to prevent accumulation of hormones due to the harvest, plants of the cultivar MicroTom were grown in expanded clay. This enabled the most rapid harvest of shoots and roots.
Plants treated with different doses of chitosan were either wounded on their leaves or, 30 min later on, roots and samples were taken from leaves and roots before and 30 min after wounding. Quantitative analysis revealed that OPDA, JA, and ABA levels in non-wounded leaves were similar, irrespective of treatment with chitosan (Figure 4a,b,d). The levels of JA–Ile in leaves treated with Ch0.25 and Ch0.50 were, however, approximately 52% higher than those of non-treated plants (Figure 4c). In all plants, wounding of leaves caused a tremendous increase in the levels of OPDA, JA, JA–Ile, and ABA within leaves (Figure 4a–d). Here, no differences were detectable in the hormone levels from leaves treated or non-treated with chitosan (Figure 4a–d).
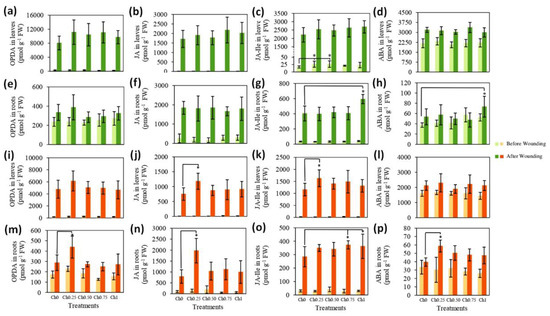
Figure 4.
Changes in the levels of plants’ defense hormones after chitosan application to shoots (a–h) and to roots (i–p) +/−wounding stress. Means ± SD, n = 4. * indicates significant differences between treatments following one-way or two-way ANOVA (Duncan’s multiple range, p ≤ 0.05).
To explore whether the translocation of phytohormones, produced during wounding of leaves, from the shoots to roots was the reason for their apparent stability in the above-ground part of the plants, we determined the levels of the same phytohormones in roots. As Figure 4e shows, foliar application of chitosan and wounding of roots did not affect the level of OPDA in roots compared to non-treated/non-wounded control. The contents of JA in wounded roots from chitosan-treated and non-treated plants were 540–610% higher than those of non-wounded roots (Figure 4f). The application of Ch1 to shoots resulted in a slight increase in the levels of JA–Ile (18%) in roots and these levels were also significantly higher (47%) after wounding of roots, in comparison to roots of non-treated plants (Figure 4g). As shown in Figure 4h, neither chitosan nor wounding affected the levels of ABA in roots; however, their levels in wounded roots from plants treated with Ch1 were significantly higher than those in control.
Regarding chitosan application to roots, Figure 4i–p shows no significant changes in phytohormone levels in non-wounded leaves or roots. The contents of OPDA, JA, JA–Ile, and ABA were increased in leaves and roots by wounding. The application of Ch0.25 to roots led, however, to a further increase in the levels of JA and JA–Ile in wounded leaves by 57 and 41%, respectively, and in the level of OPDA, JA, and ABA in wounded roots by 53%, 150%, and 50%, respectively. Additionally, the increase in the content of JA–Ile in wounded roots correlated with the concentration of applied chitosan (Figure 4o).
To confirm the data obtained from the application of chitosan to shoots, the transcript levels of two genes that encode key enzymes of the biosynthesis of OPDA and ABA were determined in roots. Allene oxide cyclase (AOC) and 9-cis-epoxycarotenoid dioxygenase (NCED1) catalyze the biosynthesis of the correct stereo-isomeric form of OPDA used for JA biosynthesis [45] and the key regulated step in ABA biosynthesis by cleaving xanthophylls into xanthoxin, a precursor of ABA [46], respectively. Table 1 shows that the transcript levels of the AOC gene were similar in all root samples, while NCED1 expression was significantly induced in wounded roots and more pronounced in those from plants treated with Ch1.

Table 1.
Changes in transcript level of Allene Oxide Cyclase and 9-Cis-Epoxycarotenoid Dioxygenase in roots after foliar application of chitosan and wounding for 30 min. Means ± SD, n = 4. NS means non-significant differences between treatments while different letters indicate significant differences between treatments following two-way ANOVA (Duncan’s multiple range, p ≤ 0.05).
3.3. Chitosan Effects on Tomato Fruits
Chitosan application significantly increased the total fruit weight by about 50%, 57%, and 36% in plants treated with Ch0.25, Ch0.50, and Ch0.75, respectively. However, there is no significant effect on the yield after the application of Ch1 (Table 2).

Table 2.
Impact of foliar spray with chitosan on yield, fruit size, water content, Brix index, and pH.
Fruit size analysis revealed that Campbell 33 fruits had a flattened shape because the transverse diameter was significantly larger than the longitudinal one and their SI was far from 1 (Table 2). Additionally, fruits from plants treated with chitosan were found to have a slightly flattened shape, except those from plants treated with Ch0.50 appearing in an almost rounded shape with an SI value close to 1 (Table 2). Moreover, the continuous application of Ch1 for 3 months decreased fruit size and water content and increased the total soluble sugars (Brix values, Table 2). Further, the pH values were significantly increased in fruits from plants treated with Ch0.75 and Ch1 compared to non-treated plants (Table 2).
To shed light on how chitosan affects the metabolic pathways of the primary metabolism, targeted profiling for polar metabolites was conducted and the data set was analyzed by multivariate methods.
Principal component analysis (PCA) showed that the first two principal components explain about the 40% of the variance in the data set (Figure 5a). Notably, the scores plot (Figure 5a) indicated that the first component, which represents 21.6% of the total variation, allowed us to distinguish the tomato groups according to chitosan concentration used for application. In addition, it showed that Ch0, Ch0.25, and Ch0.50 groups are clustered together, which indicates a similarity between them. However, the separation was only observed between the Ch0 group and either the Ch0.75 group or Ch1 group. As expected, this indicates that the high doses of chitosan applied to tomato plants have an effect on central carbon and energy metabolism in tomato fruits, in comparison to those of control.
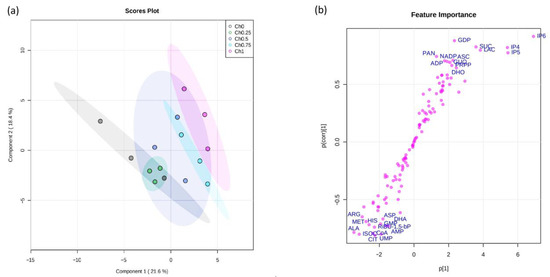
Figure 5.
PCA and oPLS-DA score plots of tomato fruit metabolome from plants treated with different concentrations of chitosan (0, 0.25, 0.50, 0.75, and 1 mg mL−1). (a) PCA plots; (b) S-plot derived from oPLS-DA model between Ch0 and Ch1 groups.
To explain the variation between the Ch0 group and Ch1 group, the S-plot of the orthogonal partial least squares discriminant analysis (oPLS-DA) was generated (Figure 5b). The S-plot separates the variation in the data that arises from the chitosan treatment from other uncorrelated variation. Metabolites that scattered in the right-hand corner are positively correlated with the Ch1 group and include inositol phosphates (IP6, IP5, and IP4), sucrose (SUC), ascorbic acid (ASC), and lactic acid (LAC). By contrast, levels of TCA cycle substrates (i.e., citrate (CIT), isocitrate (ISOC)) and some of the amino acids (i.e., alanine (ALA), methionine (MET), arginine (ARG), histidine (HIS), aspartic acid (ASP)) appeared to be reduced in Ch1 treatment relative to the Ch0 control.
It is well-known that sugars, antioxidants, and minerals are essential in the human diet not only to avoid metabolic diseases but also to ensure metabolism regulation. Therefore, maintaining a high level of these components would be of great importance. The results of Table 3 show that Ch1 application to tomato shoots had a significant effect on the content of glucose, fructose, and sucrose, which were increased respectively by 27%, 28%, and 57%, compared to fruits from non-treated plants. These results confirm those found by metabolomics analysis. In addition, chitosan application did not show any effect on the content of α-tocopherol as well as on the levels of P and K; however, the level of N was increased by about 18%, 43%, and 38% in fruits from plants treated with Ch0.50, Ch0.75, and Ch1, respectively (Table 3).

Table 3.
Chitosan effects on the levels of sugars, vitamin E, lycopene, and flavonoids as well as the acquisition of macronutrients.
Regarding pigments, all treatments resulted in an increase (Table 3). The application of Ch0.75 and Ch1 generated high levels of lycopene (84–90%) and flavonoids (39–51%).
4. Discussion
Presently, there is a worldwide trend to explore new alternatives to applying chemical treatments that reduce the loss of agricultural products. In fact, priority is given to methods that have no harmful side effects on human health and the environment. Among these methods, there is the use of chitosan, which has become a promising biostimulant and an adequate treatment for better development of fruits and vegetables. The effects of chitosan depend on many factors: (i) its applied dose, (ii) its application mode, (iii) its intrinsic properties (acetylation degree, molecular weight, pH, nitrogen content), and (iv) the environmental conditions of the plant (soil composition, temperature, water availability, salinity) [47,48,49,50,51].
Our data showed that foliar spray of chitosan, especially 0.75 mg mL−1, can improve the growth of tomato plants. This treatment increased leaf number, leaf area, and fresh and dry weights of shoots, in comparison to non-treated plants. Its application had, however, no effect on root growth. The improvement of vegetative growth under chitosan treatment might be attributed to a better functioning of chloroplasts and the increase in O2 production and CO2 fixation [52,53]. Therefore, to verify the effect of chitosan on photosynthetic performance, Fv/Fm and gs values were determined. Our data showed that the values of gs were reduced when non-treated plants pass one growth stage to another. However, these plants always kept the same values of Fv/Fm. Thus, the reduction in gs values might be dependent on soil water level and the age of plants [54,55]. Chitosan application affected Fv/Fm values differently. The values of Fv/Fm were increased after 2 weeks and 6 weeks of chitosan application. This result is in agreement with those found by Ahmad et al. [53,56]. The effect of chitosan could be due to the increase in the energy transfer efficiency of the antenna molecules which are localized in the photosystem II reaction center (PSII) [56] or the release of nitrogen from chitosan for use in the synthesis of the photosynthetic system components [57]. In addition, the improvement in chlorophyll fluorescence appears to be in good agreement with stomatal conductance aperture after the second and sixth week of treatment. The opening of stomata under chitosan application ensures strong assimilation of CO2 which, therefore, induces the photosynthetic activity of the plant. These results appear to be similar to those found by Sharma et al. [58]. However, after twelve weeks of applying treatment, a decrease in Fv/Fm values was observed only in plants treated with high doses of chitosan (0.75 and 1 mg mL−1). Czékus et al. [59] revealed that the application of 10 mg mL−1 chitosan to tomato plants can increase the emission of phytohormones, such as ethylene, by leaves, leading to leaf abscission. This study suggests that the decrease in quantum yields in leaves might be attributed to the increase in the ethylene content when plants received 6 mg mL−1 chitosan. In addition, the reducing effect of 1 mg mL−1 chitosan on plant and fruit growth might be related to the high production of ethylene [60]. Additionally, application of high doses of chitosan might result in a high availability of nitrogen, which is among the most important mineral elements that affect genes involved in ethylene biosynthesis [61].
Leaf and root damage caused by mechanical wounding trigger the synthesis of OPDA, JA, JA–Ile, and ABA. These phytohormones are accumulated in neighboring cells after the perception of damage-associated molecular patterns released from the damaged cells [62]. To ensure plant survival and growth–defense balance, the levels of JA and its bioactive form JA–Ile, as well as ABA, increase to induce the expression of wound-inducible genes, such as those encoding proteinase inhibitor II (PI2) and enzymes involved in the production of defense compounds, such as flavonoids and terpenoid indole alkaloids [31,63,64]. Similar impacts have been found for roots of Arabidopsis thaliana grown in substrate supplemented with chitosan (1 mg mL−1), leading to a higher accumulation of JA in comparison to plants grown without chitosan [20]. When leaves of tomato plants were treated with chitosan, especially with low and medium doses, an increase in the level of JA–Ile was visible in shoots only. After application of high doses of chitosan, however, the levels of JA–Ile in shoots were found to be unaltered. This might be due to a translocation of phytohormones to adjacent leaves or to the stem to be transported later to roots via the phloem [65]. Indeed, the contents of JA–Ile and ABA were induced in wounded roots when both treatments (Ch1 + wounding) were applied to shoots, suggesting their contribution in triggering systemic wound signals. To sort out the most effective applied dose of chitosan to heal shoot and root damage as well as to clarify whether the establishment of a systemic reaction is related to phytohormone translocation or linked to other signals triggered by chitosan action, a complex hormone profiling over different time periods might provide detailed information.
Our study has shown that chitosan application to shoots not only increased the above-ground biomass of plants but also affected the physico-chemical characteristics of fruits. The application of a medium dose of chitosan (Ch0.50) resulted in a significant increase in fruit yield and their longitudinal diameter; their shapes have become rounded instead of flattened. The application of a high dose of chitosan (Ch1), however, decreased fruit size and their relative water content, and increased Brix values and sugars contents. Fruit development is generally stimulated by three types of phytohormones: auxins, gibberellins, and cytokinins [66,67,68]. These phytohormones are produced inside the ovary after fertilization to stimulate cell division and expansion. It is tempting to speculate that the increase in the fruit size from plants treated with Ch0.50 might be due to an increase in the content of phytohormones, such as IAA. In contrast, the decrease in the fruit size from plants treated with Ch1 might be attributable to an early synthesis of ethylene, which is known to cause sugar accumulation and acidity reduction [59]. In addition, the application of chitosan to the aerial part of plants (leaves, stems, and fruits) increased the content of nitrogen in fruits. This increment might be attributed to releasing nitrogen from chitosan (polysaccharides—NH2) under the action of hydrolytic enzymes or promoting of xylem vessels’ growth which, therefore, increases nutrient uptake and nutritional status, especially nitrogen, to synthesize amino acids [24,69].
Fruit ripening is a complex process which is mainly characterized by the increase in sugars and aroma levels, the hydrolysis of starch and gross molecules, as well as the decrease in photorespiration [67]. The analysis of features of central carbon and energy metabolism showed a metabolic shift in the intermediates of glycolysis, TCA cycle, and the levels of metabolites involved therein, nucleotides, inositol phosphate, amino acids, and vitamin pathways in fruits after chitosan application. Our data showed that chitosan application to shoots affected the fruit metabolome by increasing the levels of sucrose and reducing the levels of organic acids, especially citrate. The generation of sucrose under chitosan application to the aerial part of plant can be due to the activation of amylase or the degradation of chitosan [70]. However, the decrease in citrate might be due to the involvement of the carbon skeletons resulting from glycolysis in other pathways, especially those of ascorbic acid and inositol phosphate. This is in agreement with the results obtained by Sajid et al. [71]. In addition, chitosan application has increased the levels of natural antioxidants (i.e., ascorbic acid, phytic acid, pantothenic acid, lycopene, and flavonoids). This could be attributed to a large production of sugars which were then converted to either glucose-6-phosphate or to different cellular metabolites to cope with the oxidative stress induced by chitosan treatment [39,72,73,74,75,76]. Thus, the increased levels of antioxidants in fruits of plants treated with chitosan when compared to untreated plants might point to the ability of chitosan to prevent cell damage.
5. Conclusions
Foliar application of chitosan exerts a beneficial impact on the aerial part of tomato plants, which is more pronounced in fruits than in leaves or roots. The analysis of fruits’ metabolites revealed that application of chitosan stimulated a series of primary and secondary metabolic pathways of carbon and nitrogen metabolisms. For instance, it enhanced CO2 fixation, nitrogen, and phosphorus levels, resulting in a higher production of sucrose, thereby providing carbon skeletons for synthesis of other metabolites, such as phospholipids, and antioxidants. In addition, chitosan applied to shoots or to roots led to a stronger plant response to wounding, as visible by higher levels of JA–Ile and ABA in roots after wounding of shoots. With these data, we showed that chitosan might be a very useful biostimulant in tomato cultivation and production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym14225006/s1, Figure S1: Effect of foliar spray with chitosan on root biomass (a,b) of tomato plants. Five treatments were applied until fruit ripening with 0 mg mL−1 (Ch0), 0.25 mg mL−1 (Ch0.25), 0.50 mg mL−1 (Ch0.50), 0.75 mg mL−1 (Ch0.75), and 1 mg mL−1 (Ch1) chitosan, and four-month-old plants were analyzed. The results were expressed as the mean of 6 independent replicates ± SD. There were no significant differences between treatments following one-way ANOVA (Duncan’s multiple range, p ≤ 0.05).
Author Contributions
Conceptualization, F.E.A. and B.H.; methodology, F.E.A., M.R., G.B., S.W., A.M., M.T. and B.H.; software, F.E.A. and G.B.; validation, M.R., G.B., S.W., A.M., M.T. and B.H.; formal analysis, F.E.A. and G.B.; investigation, F.E.A.; resources, M.R., S.W., A.M., M.T. and B.H.; data curation, F.E.A., M.R., G.B., S.W., A.M., M.T. and B.H.; writing—original draft preparation, F.E.A.; writing—review and editing, F.E.A., M.R., G.B., S.W., A.M., M.T. and B.H.; supervision, B.H.; funding acquisition, F.E.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Centre National pour la Recherche Scientifique et Technique (CNRST) Maroc [33UCA2015, 2015]; and Deutscher Akademischer Austauschdienst (DAAD) [91645793, 2018].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data sets used in the current study are available from the corresponding author on request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ADP | adenosine diphosphate |
| ALA | alanine |
| AMP | adenosine monophosphate |
| ARG | arginine |
| ASC | ascorbate |
| ASP | aspartic acid |
| Ch | Chitosan |
| CIT | citrate |
| CoA | coenzyme A |
| DHA | dehydroascorbic acid |
| DHO | dihydroorotate |
| GDP | guanosine diphosphate |
| GMP | guanosine monophosphate |
| GUO | guanosine |
| HIS | histidine |
| IP4 | sum of inositol tetrakisphosphates |
| IP5 | sum of inositol pentakisphosphates |
| IP6 | inositol hexakisphosphate |
| ISOC | isocitrate |
| LAC | lactate |
| MET | methionine |
| NADP | nicotinamide adenine dinucleotide phosphate (oxidized) |
| PAN | pantothenate |
| PRPP | phosphoribosyl pyrophosphate |
| SUC | sucrose |
| RiBU-1,5-bP | ribulose1,5-bisphosphate |
| UMP | uridine monophosphate |
References
- Coppola, D.; Lauritano, C.; Palma Esposito, F.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish waste: From problem to valuable resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Negi, T.; Sagar, N.A.; Kumar, Y.; Tarafdar, A.; Sirohi, R.; Sindhu, R.; Pandey, A. Sustainable processes for treatment and management of seafood solid waste. Sci. Total Environ. 2022, 817, 152951. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, D.K.; Jadhav, S.J.; Yu, M.H. Quality and nutritional composition of tomato fruit as influenced by certain biochemical and physiological changes. Qual. Plant. 1974, 24, 85–113. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products. 2019. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 29 September 2021).
- Fróna, D.; Szenderák, J.; Harangi-Rákos, M. The challenge of feeding the world. Sustainability 2019, 11, 5816. [Google Scholar] [CrossRef]
- Goetz, M.; Hooper, L.C.; Johnson, S.D.; Rodrigues, J.C.M.; Vivian-Smith, A.; Koltunow, A.M. Expression of aberrant forms of AUXIN RESPONSE FACTOR8 stimulates parthenocarpy in Arabidopsis and tomato. Plant Physiol. 2007, 145, 351–366. [Google Scholar] [CrossRef]
- Huang, W.; Liao, S.; Lv, H.; Khaldun, A.B.M.; Wang, Y. Characterization of the growth and fruit quality of tomato grafted on a woody medicinal plant, Lyciumchinense. Sci. Hortic 2015, 197, 447–453. [Google Scholar] [CrossRef]
- Kariathi, V.; Kassim, N.; Kimanya, M. Pesticide exposure from fresh tomatoes and its relationship with pesticide application practices in Meru district. Cogent Food Agric. 2016, 2, 1196808. [Google Scholar] [CrossRef]
- Elmer, W.H.; White, J.C. The use of metallic oxide nanoparticles to enhance growth of tomatoes and eggplants in disease infested soil or soilless medium. Environ. Sci. Nano 2016, 3, 1072–1079. [Google Scholar] [CrossRef]
- Koleška, I.; Hasanagić, D.; Todorović, V.; Murtić, S.; Klokić, I.; Parađiković, N.; Kukavica, B. Biostimulant prevents yield loss and reduces oxidative damage in tomato plants grown on reduced NPK nutrition. J. Plant Interact. 2017, 12, 209–218. [Google Scholar] [CrossRef]
- Benaffari, W.; Boutasknit, A.; Anli, M.; Ait-El-Mokhtar, M.; Ait-Rahou, Y.; Ben-Laouane, R.; Ben Ahmed, H.; Mitsui, T.; Baslam, M.; Meddich, A. The native arbuscular mycorrhizal fungi and vermicompost-based organic amendments enhance soil fertility, growth performance, and the drought stress tolerance of quinoa. Plants 2022, 11, 393. [Google Scholar] [CrossRef]
- El Amerany, F.; Aboudamia, F.Z.; Janah, I.; Taourirte, M.; Rhazi, M. Polymer-Based Nanoparticles (NPs): A Promising Approach for Crop Productivity. In Plant and Nanoparticles, 1st ed.; Chen, J.T., Ed.; Springer: Singapore, 2022; pp. 119–154. [Google Scholar]
- Abo Elsoud, M.M.; El Kady, E.M. Current trends in fungal biosynthesis of chitin and chitosan. Bull. Natl. Res. Cent. 2019, 43, 59. [Google Scholar] [CrossRef]
- Iriti, M.; Picchi, V.; Rossoni, M.; Gomarasca, S.; Ludwig, N.; Gargano, M.; Faoro, F. Chitosan antitranspirant activity is due to abscisic acid-dependent stomatal closure. Environ. Exp. Bot. 2009, 66, 493–500. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitosan effects on plant systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef]
- Kipkoech, C.; Kinyuru, J.N.; Imathiu, S.; Meyer-Rochow, V.B.; Roos, N. In Vitro Study of Cricket Chitosan’s Potential as a Prebiotic and a Promoter of Probiotic Microorganisms to Control Pathogenic Bacteria in the Human Gut. Foods 2021, 10, 2310. [Google Scholar] [CrossRef]
- Chamnanmanoontham, N.; Pongprayoon, W.; Pichayangkura, R.; Roytrakul, S.; Chadchawan, S. Chitosan enhances rice seedling growth via gene expression network between nucleus and chloroplast. Plant Growth Regul. 2015, 75, 101–114. [Google Scholar] [CrossRef]
- Saharan, V.; Pal, A. Chitosan Based Nanomaterials in Plant Growth and Protection; Springer: New Delhi, India, 2016; pp. 33–41. [Google Scholar]
- Lopez-Moya, F.; Escudero, N.; Zavala-Gonzalez, E.A.; Esteve-Bruna, D.; Blázquez, M.A.; Alabadí, D.; Lopez-Llorca, L.V. Induction of auxin biosynthesis and WOX5 repression mediate changes in root development in Arabidopsis exposed to chitosan. Sci. Rep. 2017, 7, 16813. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef] [PubMed]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef] [PubMed]
- El Amerany, F.; Meddich, A.; Wahbi, S.; Porzel, A.; Taourirte, M.; Rhazi, M.; Hause, B. Foliar application of chitosan increases tomato growth and influences mycorrhization and expression of endochitinase-encoding genes. Int. J. Mol. Sci. 2020, 21, 535. [Google Scholar] [CrossRef] [PubMed]
- El Amerany, F.; Rhazi, M.; Wahbi, S.; Taourirte, M.; Meddich, A. The effect of chitosan, arbuscular mycorrhizal fungi, and compost applied individually or in combination on growth, nutrient uptake, and stem anatomy of tomato. Sci. Hortic. 2020, 261, 109015. [Google Scholar] [CrossRef]
- Colman, S.L.; Salcedo, M.F.; Mansilla, A.Y.; Iglesias, M.J.; Fiol, D.F.; Martín-Saldaña, S.; Alvarez, V.A.; Chevalier, A.A.; Casalongué, C.A. Chitosan microparticles improve tomato seedling biomass and modulate hormonal, redox and defense pathways. Plant Physiol. Biochem. 2019, 143, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Asgari-Targhi, G.; Iranbakhsh, A.; Ardebili, Z.O. Potential benefits and phytotoxicity of bulk and nano-chitosan on the growth, morphogenesis, physiology, and micropropagation of Capsicum annuum. Plant Physiol. Biochem. 2018, 127, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Pichyangkura, R.; Chadchawan, S. Biostimulant activity of chitosan in horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Savatin, D.V.; Gramegna, G.; Modesti, V.; Cervone, F. Wounding in the plant tissue: The defense of a dangerous passage. Front. Plant Sci. 2014, 5, 470. [Google Scholar] [CrossRef]
- Halitschke, R.; Schittko, U.; Pohnert, G.; Boland, W.; Baldwin, I.T. Molecular interactions between the specialist herbivore Manducasexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 2001, 125, 711–717. [Google Scholar] [CrossRef]
- Suttle, J.C.; Lulai, E.C.; Huckle, L.L.; Neubauer, J.D. Wounding of potato tubers induces increases in ABA biosynthesis and catabolism and alters expression of ABA metabolic genes. J. Plant Physiol. 2013, 170, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Peña-Cortés, H.; Fisahn, J.; Willmitzer, L. Signals involved in wound-induced proteinase inhibitor II gene expression in tomato and potato plants. Proc. Natl. Acad. Sci. USA 1995, 92, 4106–4113. [Google Scholar] [CrossRef]
- Koo, A.J.; Howe, G.A. The wound hormone jasmonate. Phytochemistry 2009, 70, 1571–1580. [Google Scholar] [CrossRef]
- Rakwal, R.; Tamogami, S.; Agrawal, G.K.; Iwahashi, H. Octadecanoid signaling component “burst” in rice (Oryza sativa L.) seedling leaves upon wounding by cut and treatment with fungal elicitor chitosan. Biochem. Biophys. Res. Commun. 2002, 295, 1041–1045. [Google Scholar] [CrossRef]
- Ozeretskovskaya, O.L.; Vasyukova, N.I.; Chalenko, G.I.; Gerasimova, N.G.; Revina, T.A.; Valueva, T.A. Wound healing and induced resistance in potato tubers. Appl. Biochem. Microbiol. 2009, 45, 199–203. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Sobeih, W.Y.; Dodd, I.C.; Bacon, M.A.; Grierson, D.; Davies, W.J. Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial root-zone drying. J. Exp. Bot. 2004, 55, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Balcke, G.U.; Handrick, V.; Bergau, N.; Fichtner, M.; Henning, A.; Stellmach, H.; Tissier, A.; Hause, B.; Frolov, A. An UPLC-MS/MS method for highly sensitive high-throughput analysis of phytohormones in plant tissues. Plant Methods 2012, 8, 47. [Google Scholar] [CrossRef]
- Chafi, A.; Benabbes, R.; Bouakka, M.; Hakkou, A.; Kouddane, N.; Berrichi, A. Pomological study of dates of some date palm varieties cultivated in Figuig oasis. J. Mater. Environ. Sci. 2015, 6, 1266–1275. [Google Scholar]
- El Amerany, F.; Taourirte, M.; Wahbi, S.; Meddich, A.; Rhazi, M. Use of metabolomics data analysis to identify fruit quality markers enhanced by the application of an aminopolysaccharide. RSC Adv. 2021, 11, 35514–35524. [Google Scholar] [CrossRef]
- Schaarschmidt, S.; Qu, N.; Strack, D.; Sonnewald, U.; Hause, B. Local induction of the alc gene switch in transgenic tobacco plants by acetaldehyde. Plant Cell Physiol. 2004, 45, 1566–1577. [Google Scholar] [CrossRef]
- Sadler, G.; Davis, J.; Dezman, D. Rapid extraction of lycopene and β-carotene from reconstituted tomato paste and pink grapefruit homogenates. J. Food Sci. 1990, 55, 1460–1461. [Google Scholar] [CrossRef]
- Hilares, R.T.; Dos Santos, J.G.; Shiguematsu, N.B.; Ahmed, M.A.; da Silva, S.S.; Santos, J.C. Low-pressure homogenization of tomato juice using hydrodynamic cavitation technology: Effects on physical properties and stability of bioactive compounds. Ultrason. Sonochem. 2019, 54, 192–197. [Google Scholar] [CrossRef]
- Fu, X.; Cheng, S.; Liao, Y.; Huang, B.; Du, B.; Zeng, W.; Jiang, Y.; Duan, X.; Yang, Z. Comparative analysis of pigments in red and yellow banana fruit. Food Chem. 2018, 239, 1009–1018. [Google Scholar] [CrossRef]
- Segarra, G.; Casanova, E.; Borrero, C.; Avilés, M.; Trillas, I. The suppressive effects of composts used as growth media against Botrytis cinerea in cucumber plants. Euro J. Plant Pathol. 2007, 117, 393–402. [Google Scholar] [CrossRef]
- Stenzel, I.; Hause, B.; Maucher, H.; Pitzschke, A.; Miersch, O.; Ziegler, J.; Ryan, C.; Wasternack, C. Allene oxide cyclase dependence of the wound response and vascular bundle-specific generation of jasmonates in tomato—Amplification in wound signaling. Plant J. 2003, 33, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Taylor, I.B.; Burbidge, A.; Thompson, A.J. Control of abscisic acid synthesis. J. Exp. Bot. 2000, 51, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Drevinskas, T.; Naujokaitytė, G.; Maruška, A.; Kaya, M.; Sargin, I.; Daubaras, R.; Česonienė, L. Effect of molecular weight of chitosan on the shelf life and other quality parameters of three different cultivars of Actinidia kolomikta (kiwifruit). Carbohydr. Polym. 2017, 173, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Liu, S.; Xing, R.; Chen, X.; Li, P. Protective effect of chitosan on photosynthesis and antioxidative defense system in edible rape (Brassica rapa L.) in the presence of cadmium. Ecotoxicol. Environ. Saf. 2017, 138, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Al-Tawaha, A.R.; Turk, M.A.; Al-Tawaha, A.R.M.; Alu’datt, M.H.; Wedyan, M.; Al-Ramamneh, E.A.D.M.; Hoang, A.T. Using chitosan to improve growth of maize cultivars under salinity conditions. Bulg. J. Agric. Sci. 2018, 24, 437–442. [Google Scholar]
- Stasińska-Jakubas, M.; Hawrylak-Nowak, B. Protective, Biostimulating, and Eliciting Effects of Chitosan and Its Derivatives on Crop Plants. Molecules 2022, 27, 2801. [Google Scholar] [CrossRef]
- Barka, E.A.; Eullaffroy, P.; Clément, C.; Vernet, G. Chitosan improves development, and protects Vitis vinifera L. against Botrytis cinerea. Plant Cell Rep. 2004, 22, 608–614. [Google Scholar] [CrossRef]
- Ahmad, B.; Jaleel, H.; Shabbir, A.; Khan, M.M.A.; Sadiq, Y. Concomitant application of depolymerized chitosan and GA3 modulates photosynthesis, essential oil and menthol production in peppermint (Mentha piperita L.). Sci. Hortic. 2019, 246, 371–379. [Google Scholar] [CrossRef]
- Whitehead, D.; Barbour, M.M.; Griffin, K.L.; Turnbull, M.H.; Tissue, D.T. Effects of leaf age and tree size on stomatal and mesophyll limitations to photosynthesis in mountain beech (Nothofagus solandrii var. cliffortiodes). Tree Physiol. 2011, 31, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Peguero-Pina, J.J.; Mendoza-Herrer, Ó.; Gil-Pelegrín, E.; Sancho-Knapik, D. Cavitation limits the recovery of gas exchange after severe drought stress in holm oak (Quercus ilex L.). Forests 2018, 9, 443. [Google Scholar] [CrossRef]
- Ahmad, B.; Khan, M.M.A.; Jaleel, H.; Sadiq, Y.; Shabbir, A.; Uddin, M. Exogenously sourced γ-irradiated chitosan-mediated regulation of growth, physiology, quality attributes and yield in Mentha piperita L. Turk. J. Biol. 2017, 41, 388–401. [Google Scholar] [CrossRef]
- Shangguan, Z.; Shao, M.; Dyckmans, J. Effects of nitrogen nutrition and water deficit on net photosynthetic rate and chlorophyll fluorescence in winter wheat. J. Plant Physiol. 2000, 156, 46–51. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant 2015, 153, 284–298. [Google Scholar] [CrossRef]
- Czékus, Z.; Iqbal, N.; Pollák, B.; Martics, A.; Ördög, A.; Poór, P. Role of ethylene and light in chitosan-induced local and systemic defence responses of tomato plants. J. Plant Physiol. 2021, 263, 153461. [Google Scholar] [CrossRef]
- Vaseva, I.I.; Qudeimat, E.; Potuschak, T.; Du, Y.; Genschik, P.; Vandenbussche, F.; Van Der Straeten, D. The plant hormone ethylene restricts Arabidopsis growth via the epidermis. Proc. Natl. Acad. Sci. USA 2018, 115, E4130–E4139. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Trivellini, A.; Fatma, M.; Masood, A.; Francini, A.; Iqbal, N.; Ferrante, A.; Khan, N.A. Role of ethylene in responses of plants to nitrogen availability. Front. Plant Sci. 2015, 6, 927. [Google Scholar] [CrossRef]
- Vega-Muñoz, I.; Duran-Flores, D.; Fernández-Fernández, Á.D.; Heyman, J.; Ritter, A.; Stael, S. Breaking Bad News: Dynamic molecular mechanisms of wound response in plants. Front. Plant Sci. 2020, 11, 1959. [Google Scholar] [CrossRef]
- Dammann, C.; Rojo, E.; Sánchez-Serrano, J.J. Abscisic acid and jasmonic acid activate wound-inducible genes in potato through separate, organ-specific signal transduction pathways. Plant J. 1997, 11, 773–782. [Google Scholar] [CrossRef]
- Kang, J.N.; Lee, W.H.; Won, S.Y.; Chang, S.; Hong, J.P.; Oh, T.J.; Lee, S.M.; Kang, S.H. Systemic Expression of Genes Involved in the Plant Defense Response Induced by Wounding in Senna tora. Int. J. Mol. Sci. 2021, 22, 10073. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, Y.; Martinoia, E.; Geisler, M. Plant hormone transporters: What we know and what we would like to know. BMC Biol. 2017, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, F.G. Auxin distribution in fruits and its significance in fruit development. Am. J. Bot. 1939, 26, 189–194. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Nascimento, V.L.; Medeiros, D.B.; Nunes-Nesi, A.; Ribeiro, D.M.; Zsögön, A.; Araújo, W.L. Modifications in organic acid profiles during fruit development and ripening: Correlation or causation? Front. Plant Sci. 2018, 9, 1689. [Google Scholar] [CrossRef]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.P.; Lutts, S. Tomato fruit development and metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef]
- Farouk, S.; Mosa, A.A.; Taha, A.A.; El-Gahmery, A.M. Protective effect of humic acid and chitosan on radish (Raphanus sativus, L. var. sativus) plants subjected to cadmium stress. J. Stress Physiol. Biochem. 2011, 7, 99–116. [Google Scholar]
- Choudhary, R.C.; Joshi, A.; Kumari, S.; Kumaraswamy, R.V.; Saharan, V. Preparation of Cu-chitosan nanoparticle and its effect on growth and enzyme activity during seed germination in maize. J. Pharmacogn. Phytochem. 2017, 6, 669–673. [Google Scholar]
- Sajid, M.; Basit, A.; Ullah, Z.; Shah, S.T.; Ullah, I.; Mohamed, H.I.; Ullah, I. Chitosan-based foliar application modulated the yield and biochemical attributes of peach (Prunus persica L.) cv. Early grand. Bull. Natl. Res. Cent. 2020, 44, 150. [Google Scholar] [CrossRef]
- Raman, S.B.; Rathinasabapathi, B. Pantothenate synthesis in plants. Plant Sci. 2004, 167, 961–968. [Google Scholar] [CrossRef]
- Wang, S.Y.; Gao, H. Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (Fragaria x aranassa Duch.). LWT-Food Sci. Technol. 2013, 52, 71–79. [Google Scholar] [CrossRef]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of chitosan coating on the postharvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Mostofa, M.G.; Diaz-Vivancos, P.; Burritt, D.J.; Fujita, M.; Tran, L.S.P. Glutathione in Plant Growth, Development, and Stress Tolerance; Springer: Cham, Switzerland, 2017; pp. 1–421. [Google Scholar]
- Li, Z.; Zhang, Y.; Zhang, X.; Merewitz, E.; Peng, Y.; Ma, X.; Huang, L.; Yan, Y. Metabolic pathways regulated by chitosan contributing to drought resistance in white clover. J. Proteome Res. 2017, 16, 3039–3052. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).