Development of Smart Bilayer Alginate/Agar Film Containing Anthocyanin and Catechin-Lysozyme
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Butterfly Pea Anthocyanin Extraction
2.3. Fabrication of Smart Bilayer Film
2.4. Smart Bilayer Film Characterization
2.4.1. Thickness
2.4.2. Appearance and Color
2.4.3. Light Transmission and Transparency
2.4.4. Morphology of Film
2.4.5. Mechanical Properties
2.4.6. Water Vapor Permeability (WVP)
2.4.7. Film Solubility
2.4.8. pH Sensitivity
2.4.9. Response to Volatile Ammonia and Acetic Acid
2.4.10. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.11. Thermal Stability
2.4.12. Bioactive Compounds and Antioxidant Properties
2.4.13. Antimicrobial Activity of the Film
2.5. Application of Smart Bilayer Film for Monitoring Shrimp Freshness
2.6. Statistical Analyses
3. Results and Discussion
3.1. Thickness
3.2. Mechanical Properties
3.3. Water Vapor Permeability (WVP)
3.4. Film Solubility
3.5. Film Appearance and Color
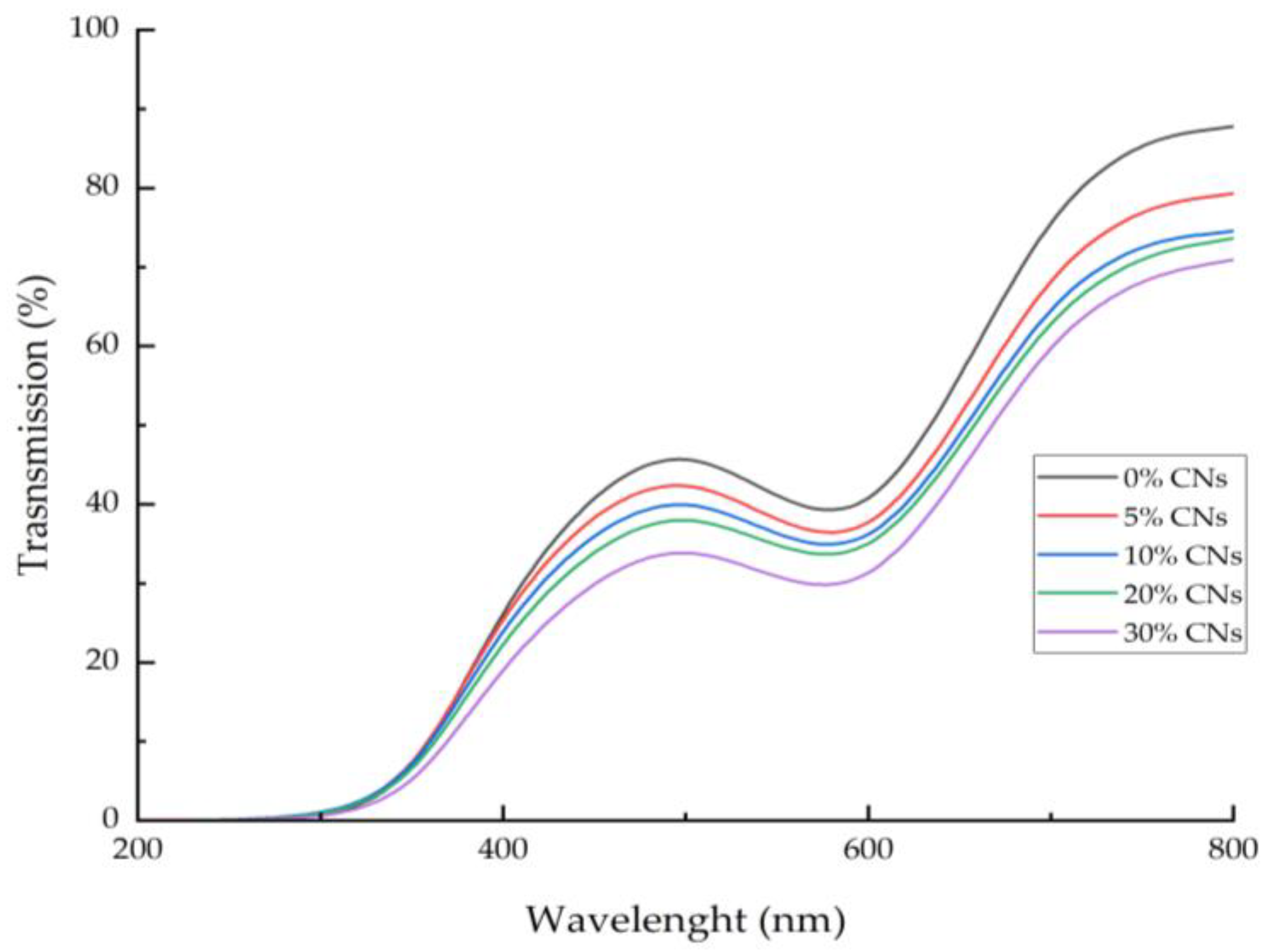
3.6. Light Transmission and Transparency
3.7. Morphology of Film
3.8. pH Sensitivity
3.9. Response to Volatile Ammonia and Acetic Acid
3.10. FTIR
3.11. Thermal Stability
3.12. Bioactive Compounds and Antioxidant Properties
3.13. Antimicrobial Activity
3.14. Application of Smart Bilayer Film for Monitoring Shrimp Freshness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghoshal, G. Recent Trends in Active, Smart, and Intelligent Packaging for Food Products; Food packaging and preservation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 343–374. [Google Scholar]
- Young, E.; Mirosa, M.; Bremer, P. A systematic review of consumer perceptions of smart packaging technologies for food. Front. Sustain. Food Syst. 2020, 4, 63. [Google Scholar] [CrossRef]
- Chen, S.; Brahma, S.; Mackay, J.; Cao, C.; Aliakbarian, B. The role of smart packaging system in food supply chain. J. Food Sci. 2020, 85, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi, B.; Nurfawaidi, A. On-package dual sensors label based on pH indicators for real-time monitoring of beef freshness. Food Control 2017, 82, 91–100. [Google Scholar] [CrossRef]
- Kurek, M.; Hlupić, L.; Ščetar, M.; Bosiljkov, T.; Galić, K. Comparison of Two pH Responsive Color Changing Bio-Based Films Containing Wasted Fruit Pomace as a Source of Colorants. J. Food Sci. 2019, 84, 2490–2498. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.-W. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. Nutr. 2021, 61, 2297–2325. [Google Scholar] [CrossRef]
- Müller, P.; Schmid, M. Intelligent packaging in the food sector: A brief overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef]
- Merz, B.; Capello, C.; Leandro, G.C.; Moritz, D.E.; Monteiro, A.R.; Valencia, G.A. A novel colorimetric indicator film based on chitosan, polyvinyl alcohol and anthocyanins from jambolan (Syzygium cumini) fruit for monitoring shrimp freshness. Int. J. Biol. Macromol. 2020, 153, 625–632. [Google Scholar] [CrossRef]
- Liu, D.; Cui, Z.; Shang, M.; Zhong, Y. A colorimetric film based on polyvinyl alcohol/sodium carboxymethyl cellulose incorporated with red cabbage anthocyanin for monitoring pork freshness. Food Packag. Shelf Life 2021, 28, 100641. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, T.-S.; Yan, H.; Hu, X.; Ren, T. Novel pH-sensitive films based on starch/polyvinyl alcohol and food anthocyanins as a visual indicator of shrimp deterioration. Int. J. Biol. Macromol. 2020, 145, 768–776. [Google Scholar] [CrossRef]
- Luchese, C.L.; Abdalla, V.F.; Spada, J.C.; Tessaro, I.C. Evaluation of blueberry residue incorporated cassava starch film as pH indicator in different simulants and foodstuffs. Food Hydrocoll. 2018, 82, 209–218. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Faseha, A.; Benjakul, S.; Kaewprachu, P. Application of anthocyanin as a color indicator in gelatin films. Food Biosci. 2020, 36, 100603. [Google Scholar] [CrossRef]
- Thanh, V.; Tran, N.Y.; Linh, N.; Vy, T.A.; Truc, T.T. Application of anthocyanin natural colors from Butterfly Pea (Clitoria ternatea L.) extracts to cupcake. IOP Conf.Ser. Mater. Sci. Eng. 2020, 736, 062014. [Google Scholar] [CrossRef]
- Oguis, G.K.; Gilding, E.K.; Jackson, M.A.; Craik, D.J. Butterfly pea (Clitoria ternatea), a cyclotide-bearing plant with applications in agriculture and medicine. Front. Plant Sci. 2019, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.M.; Pearson, B.; Marble, S.C. Butterfly Pea (Clitoria ternatea) Flower Extract (BPFE) and Its Use as a pH-Dependent Natural Colorant: (ENH-1309/EP573, 4/2019). EDIS, 2019. Available online: https://edis.ifas.ufl.edu/publication/EP573 (accessed on 18 October 2022).
- Sabaghi, M.; Maghsoudlou, Y.; Khomeiri, M.; Ziaiifar, A.M. Active edible coating from chitosan incorporating green tea extract as an antioxidant and antifungal on fresh walnut kernel. Postharvest Biol. Technol. 2015, 110, 224–228. [Google Scholar] [CrossRef]
- Lesnierowski, G.; Stangierski, J. What’s new in chicken egg research and technology for human health promotion?-A review. Trends Food Sci. Technol. 2018, 71, 46–51. [Google Scholar] [CrossRef]
- Ehsani, A.; Hashemi, M.; Afshari, A.; Aminzare, M.; Raeisi, M.; Zeinali, T. Effect of different types of active biodegradable films containing lactoperoxidase system or sage essential oil on the shelf life of fish burger during refrigerated storage. LWT 2020, 117, 108633. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Suthiluk, P.; Kamhangwong, D.; Benjakul, S. Antimicrobial activity of some potential active compounds against food spoilage microorganisms. Afr. J. Biotechnol. 2012, 11, 13914–13921. [Google Scholar] [CrossRef]
- Zhao, G.; Lyu, X.; Lee, J.; Cui, X.; Chen, W.-N. Biodegradable and transparent cellulose film prepared eco-friendly from durian rind for packaging application. Food Packag. Shelf Life 2019, 21, 100345. [Google Scholar] [CrossRef]
- Zahan, K.A.; Azizul, N.M.; Mustapha, M.; Tong, W.Y.; Rahman, M.S.A. Application of bacterial cellulose film as a biodegradable and antimicrobial packaging material. Mater. Today Proc. 2020, 31, 83–88. [Google Scholar] [CrossRef]
- Rizwan, M.; Rubina Gilani, S.; Iqbal Durani, A.; Naseem, S. Materials diversity of hydrogel: Synthesis, polymerization process and soil conditioning properties in agricultural field. J. Adv. Res. 2021, 33, 15–40. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Gómez-Mascaraque, L.G.; Ballester, A.R.; Martínez-Abad, A.; Brodkorb, A.; López-Rubio, A. Production of unpurified agar-based extracts from red seaweed Gelidium sesquipedale by means of simplified extraction protocols. Algal Res. 2019, 38, 101420. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Madera-Santana, T.; Robledo, D.; Veleva, L.; Quintana, P.; Azamar, J. Degradation of agar films in a humid tropical climate: Thermal, mechanical, morphological and structural changes. Polym. Degrad. Stab. 2007, 92, 244–252. [Google Scholar] [CrossRef]
- Da Rocha, M.; Alemán, A.; Romani, V.P.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P.; Prentice, C. Effects of agar films incorporated with fish protein hydrolysate or clove essential oil on flounder (Paralichthys orbignyanus) fillets shelf-life. Food Hydrocoll. 2018, 81, 351–363. [Google Scholar] [CrossRef]
- Jang, S.A.; Lim, G.O.; Song, K.B. Use of nano-clay (Cloisite Na+) improves tensile strength and vapour permeability in agar rich red algae (Gelidium corneum)–gelatin composite films. Int. J. Food Sci. Technol. 2010, 45, 1883–1888. [Google Scholar] [CrossRef]
- Salama, H.E.; Aziz, M.S.A.; Sabaa, M.W. Novel biodegradable and antibacterial edible films based on alginate and chitosan biguanidine hydrochloride. Int. J. Biol. Macromol. 2018, 116, 443–450. [Google Scholar] [CrossRef]
- Kurek, M.; Galus, S.; Debeaufort, F. Surface, mechanical and barrier properties of bio-based composite films based on chitosan and whey protein. Food Packag. Shelf Life 2014, 1, 56–67. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lee, S.Y.; Rhee, C. Edible oxygen barrier bilayer film pouches from corn zein and soy protein isolate for olive oil packaging. LWT-Food Sci. Technol. 2010, 43, 1234–1239. [Google Scholar] [CrossRef]
- Valencia-Sullca, C.; Vargas, M.; Atarés, L.; Chiralt, A. Thermoplastic cassava starch-chitosan bilayer films containing essential oils. Food Hydrocoll. 2018, 75, 107–115. [Google Scholar] [CrossRef]
- Bertuzzi, M.A.; Slavutsky, A.M. Standard and New Processing Techniques Used in the Preparation of Films and Coatings at the Lab Level and Scale-Up; Edible Films and Coatings; CRC Press: Boca Raton, FL, USA, 2016; pp. 21–42. [Google Scholar]
- Peng, B.L.; Dhar, N.; Liu, H.; Tam, K. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. Can. J. Chem. Eng. 2011, 89, 1191–1206. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Q. Recent development in applications of cellulose nanocrystals for advanced polymer-based nanocomposites by novel fabrication strategies. In Nanocrystals—Synthesis, Characterization and Applications; IntechOpen: London, UK, 2012; pp. 103–120. [Google Scholar]
- Wang, L.-F.; Shankar, S.; Rhim, J.-W. Properties of alginate-based films reinforced with cellulose fibers and cellulose nanowhiskers isolated from mulberry pulp. Food Hydrocoll. 2017, 63, 201–208. [Google Scholar] [CrossRef]
- Roy, S.; Kim, H.-J.; Rhim, J.-W. Synthesis of Carboxymethyl Cellulose and Agar-Based Multifunctional Films Reinforced with Cellulose Nanocrystals and Shikonin. ACS Appl. Polym. Mater. 2021, 3, 1060–1069. [Google Scholar] [CrossRef]
- Salim, M.H.; Abdellaoui, Y.; Ait Benhamou, A.; Ablouh, E.-H.; El Achaby, M.; Kassab, Z. Influence of cellulose nanocrystals from pea pod waste on mechanical, thermal, biodegradability, and barrier properties of chitosan-based films. Cellulose 2022, 29, 5117–5135. [Google Scholar] [CrossRef]
- Tian, W.; Gao, X.; Zhang, J.; Yu, J.; Zhang, J. Cellulose nanosphere: Preparation and applications of the novel nanocellulose. Carbohydr. Polym. 2022, 277, 118863. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-T.; Chen, X.-Q.; Shen, W.-H.; Li, Z. Spherical vs rod-like cellulose nanocrystals from enzymolysis: A comparative study as reinforcing agents on polyvinyl alcohol. Carbohydr. Polym. 2021, 256, 117493. [Google Scholar] [CrossRef] [PubMed]
- Romruen, O.; Kaewprachu, P.; Karbowiak, T.; Rawdkuen, S. Isolation and Characterization Cellulose Nanosphere from Different Agricultural By-Products. Polymers 2022, 14, 2534. [Google Scholar] [CrossRef] [PubMed]
- Sai-Ut, S.; Suthiluk, P.; Tongdeesoontorn, W.; Rawdkuen, S.; Kaewprachu, P.; Karbowiak, T.; Debeaufort, F.; Degraeve, P. Using Anthocyanin Extracts from Butterfly Pea as pH Indicator for Intelligent Gelatin Film and Methylcellulose Film. Curr. Appl. Sci. Technol 2021, 21, 652–661. [Google Scholar]
- Rawdkuen, S.; Suthiluk, P.; Kamhangwong, D.; Benjakul, S. Mechanical, physico-chemical, and antimicrobial properties of gelatin-based film incorporated with catechin-lysozyme. Chem. Cent. J. 2012, 6, 131. [Google Scholar] [CrossRef]
- Rao, M.; Kanatt, S.; Chawla, S.; Sharma, A. Chitosan and guar gum composite films: Preparation, physical, mechanical and antimicrobial properties. Carbohydr. Polym. 2010, 82, 1243–1247. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Effects of plasticizers on the properties of edible films from skin gelatin of bigeye snapper and brownstripe red snapper. Eur. Food Res. Technol. 2006, 222, 229–235. [Google Scholar] [CrossRef]
- ASTM. Standard test method for tensile properties of thin plastic sheeting-D882–02. In Annual Book of American Standard Testing Methods; ASTM International: West Conshohocken, PA, USA, 2002. [Google Scholar]
- ASTM. Standard test methods for water vapor transmission of materials. In Annual Book of ASTM Standards. Designation E96-E80; ASTM International: West Conshohocken, PA, USA, 1989. [Google Scholar]
- McHugh, T.H.; Avena-Bustillos, R.; Krochta, J. Hydrophilic edible films: Modified procedure for water vapor permeability and explanation of thickness effects. J. Food Sci. 1993, 58, 899–903. [Google Scholar] [CrossRef]
- Gennadios, A.; Handa, A.; Froning, G.W.; Weller, C.L.; Hanna, M.A. Physical properties of egg white− dialdehyde starch films. J. Agric. Food Chem. 1998, 46, 1297–1302. [Google Scholar] [CrossRef]
- Pereira Jr, V.A.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/PVA films with anthocyanins from Brassica oleraceae (Red Cabbage) as Time–Temperature Indicators for application in intelligent food packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Pereira de Abreu, D.; Cruz, J.M.; Paseiro Losada, P. Active and intelligent packaging for the food industry. Food Rev. Int. 2012, 28, 146–187. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Osako, K.; Rungraeng, N.; Rawdkuen, S. Characterization of fish myofibrillar protein film incorporated with catechin-Kradon extract. Int. J. Biol. Macromol. 2018, 107, 1463–1473. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Andrade-Mahecha, M.M.; Sobral, P.J.d.A.; Menegalli, F.C. Isolation and characterization of the flour and starch of plantain bananas (Musa paradisiaca). Starch-Stärke 2012, 64, 382–391. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chem. 2012, 134, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, M.M.; Moradi, M.; Tajik, H.; Forough, M.; Ezati, P.; Kuswandi, B. Development of an easy-to-use colorimetric pH label with starch and carrot anthocyanins for milk shelf life assessment. Int. J. Biol. Macromol. 2020, 153, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Akyuz, L.; Koc, B.; Kaya, M.; Ilk, S.; Cansaran-Duman, D.; Martinez, A.S.; Cakmak, Y.S.; Labidi, J.; Boufi, S. Novel, multifunctional mucilage composite films incorporated with cellulose nanofibers. Food Hydrocoll. 2019, 89, 20–28. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Preparation of nanocellulose from micro-crystalline cellulose: The effect on the performance and properties of agar-based composite films. Carbohydr. Polym. 2016, 135, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Ye, D.; Yang, W.; Zhang, S.; Chen, H.; Chang, C.; Zhang, L. Construction of transparent cellulose-based nanocomposite papers and potential application in flexible solar cells. ACS Sustain. Chem. Eng. 2018, 6, 8040–8047. [Google Scholar] [CrossRef]
- Wang, Z.; Qiao, X.; Sun, K. Rice straw cellulose nanofibrils reinforced poly (vinyl alcohol) composite films. Carbohydr. Polym. 2018, 197, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Salari, M.; Khiabani, M.S.; Mokarram, R.R.; Ghanbarzadeh, B.; Kafil, H.S. Use of gamma irradiation technology for modification of bacterial cellulose nanocrystals/chitosan nanocomposite film. Carbohydr. Polym. 2021, 253, 117144. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Peng, W.; Zare, Y.; Rhee, K.Y. Effects of Size and Aggregation/Agglomeration of Nanoparticles on the Interfacial/Interphase Properties and Tensile Strength of Polymer Nanocomposites. Nanoscale Res. Lett. 2018, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Oun, A.A.; Rhim, J.-W. Preparation and characterization of sodium carboxymethyl cellulose/cotton linter cellulose nanofibril composite films. Carbohydr. Polym. 2015, 127, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Su, J.-F.; Huang, Z.; Yuan, X.-Y.; Wang, X.-Y.; Li, M. Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohydr. Polym. 2010, 79, 145–153. [Google Scholar] [CrossRef]
- Paralikar, S.A.; Simonsen, J.; Lombardi, J. Poly (vinyl alcohol)/cellulose nanocrystal barrier membranes. J. Membr. Sci. 2008, 320, 248–258. [Google Scholar] [CrossRef]
- Mehmood, A.; Ishaq, M.; Zhao, L.; Yaqoob, S.; Safdar, B.; Nadeem, M.; Munir, M.; Wang, C. Impact of ultrasound and conventional extraction techniques on bioactive compounds and biological activities of blue butterfly pea flower (Clitoria ternatea L.). Ultrason. Sonochem. 2019, 51, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yong, H.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Jiang, W. Development of antioxidant chitosan film with banana peels extract and its application as coating in maintaining the storage quality of apple. Int. J. Biol. Macromol. 2020, 154, 1205–1214. [Google Scholar] [CrossRef]
- Agustin, M.B.; Ahmmad, B.; De Leon, E.R.P.; Buenaobra, J.L.; Salazar, J.R.; Hirose, F. Starch-based biocomposite films reinforced with cellulose nanocrystals from garlic stalks. Polym. Compos. 2013, 34, 1325–1332. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues–wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Guenier, A.-S.; Salmieri, S.; Lacroix, M. Alginate and chitosan functionalization for micronutrient encapsulation. J. Agric. Food Chem. 2008, 56, 2528–2535. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Khadem, S.; Cakmak, Y.S.; Mujtaba, M.; Ilk, S.; Akyuz, L.; Salaberria, A.M.; Labidi, J.; Abdulqadir, A.H.; Deligöz, E. Antioxidative and antimicrobial edible chitosan films blended with stem, leaf and seed extracts of Pistacia terebinthus for active food packaging. RSC Adv. 2018, 8, 3941–3950. [Google Scholar] [CrossRef]
- Merci, A.; Urbano, A.; Grossmann, M.V.E.; Tischer, C.A.; Mali, S. Properties of microcrystalline cellulose extracted from soybean hulls by reactive extrusion. Food Res. Int. 2015, 73, 38–43. [Google Scholar] [CrossRef]
- Reddy, J.P.; Rhim, J.-W. Characterization of bionanocomposite films prepared with agar and paper-mulberry pulp nanocellulose. Carbohydr. Polym. 2014, 110, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Asma, C.; Meriem, E.; Mahmoud, B.; Djaafer, B. Physicochemical characterization of gelatin-cmc composite edibles films from polyion-complex hydrogels. J. Chil. Chem. Soc. 2014, 59, 2279–2283. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, C.; Chang, P.R.; Anderson, D.P.; Huneault, M.A. Pea starch-based composite films with pea hull fibers and pea hull fiber-derived nanowhiskers. Polym. Eng. Sci. 2009, 49, 369–378. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Cao, J.; Jiang, W. Improving the performance of edible food packaging films by using nanocellulose as an additive. Int. J. Biol. Macromol. 2021, 166, 288–296. [Google Scholar] [CrossRef]
- Perez Espitia, P.J.; de Fátima Ferreira Soares, N.; dos Reis Coimbra, J.S.; de Andrade, N.J.; Souza Cruz, R.; Alves Medeiros, E.A. Bioactive peptides: Synthesis, properties, and applications in the packaging and preservation of food. Compr. Rev. Food Sci. Food Saf. 2012, 11, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Jebali, A.; Hekmatimoghaddam, S.; Behzadi, A.; Rezapor, I.; Mohammadi, B.H.; Jasemizad, T.; Yasini, S.A.; Javadzadeh, M.; Amiri, A.; Soltani, M. Antimicrobial activity of nanocellulose conjugated with allicin and lysozyme. Cellulose 2013, 20, 2897–2907. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Puligundla, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review. Food Hydrocoll. 2017, 69, 286–292. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Krzyściak, P.; Talaga-Ćwiertnia, K.; Juszczak, L. Intelligent and active furcellaran-gelatin films containing green or pu-erh tea extracts: Characterization, antioxidant and antimicrobial potential. Int. J. Biol. Macromol. 2019, 122, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-T.; Tsai, I.-L.; Ho, Y.-C.; Hang, Y.-H.; Lin, C.; Tsai, M.-L.; Mi, F.-L. Active and intelligent gellan gum-based packaging films for controlling anthocyanins release and monitoring food freshness. Carbohydr. Polym. 2021, 254, 117410. [Google Scholar] [CrossRef]


| CNs Concentration in Film (%) | Thickness (mm) | TS * (MPa) | EAB * (%) | WVP (×10−10 g m/m2 s Pa) | Film Solubility (%) |
|---|---|---|---|---|---|
| 0 | 0.082 ± 0.002 d | 16.11 ± 1.32 c | 61.63 ± 3.30 a | 2.27 ± 0.06 a | 58.65 ± 1.06 b |
| 5 | 0.084 ± 0.002 d | 18.26 ± 2.01 b | 50.39 ± 7.19 b | 2.11 ± 0.10 a | 56.52 ± 1.32 bc |
| 10 | 0.091 ± 0.001 c | 18.63 ± 1.53 b | 46.27 ± 4.97 bc | 2.22 ± 0.05 a | 53.87 ± 2.43 c |
| 20 | 0.097 ± 0.001 b | 19.02 ± 1.20 ab | 41.86 ± 5.69 cd | 2.34 ± 0.11 a | 61.93 ± 1.72 a |
| 30 | 0.108 ± 0.002 a | 20.51 ± 2.06 a | 40.44 ± 4.17 d | 2.68 ± 0.08 a | 63.43 ± 0.97 a |
| CNs Concentration in Film (%) | Appearance | L* | a* | b* | Transparency (%) |
|---|---|---|---|---|---|
| 0 |  | 44.97 ± 1.19 c | −10.33 ± 0.63 c | −34.17 ± 0.66 b | 2.50 ± 0.01 a |
| 5 |  | 48.28 ± 0.23 b | −9.28 ± 0.45 b | −30.02 ± 0.64 a | 2.44 ± 0.02 b |
| 10 |  | 49.01 ± 1.06 ab | −8.67 ± 0.05 b | −29.89 ± 0.59 a | 2.41 ± 0.05 bc |
| 20 |  | 49.65 ± 0.81 ab | −8.59 ± 0.19 b | −29.65 ± 0.26 a | 2.39 ± 0.01 c |
| 30 |  | 50.07 ± 0.17 a | −7.57 ± 0.24 a | −29.51 ± 0.41 a | 2.27 ± 0.01 d |
| CNs Concentration in Film (%) | Upper Surface | Cross-Section |
|---|---|---|
| 0 |  |  |
| 5 |  |  |
| 10 |  |  |
| 20 |  |  |
| 30 |  |  |
| pH | BAE Solution (0.05% w/v) | Concentration (%) of Cellulose Nanosphere (CNs) | ||||
|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 20 | 30 | ||
| Original |  |  |  |  |  |  |
| 2 |  |  |  |  |  |  |
| 3 |  |  |  |  |  |  |
| 4 |  |  |  |  |  |  |
| 5 |  |  |  |  |  |  |
| 6 |  |  |  |  |  |  |
| 7 |  |  |  |  |  |  |
| 8 |  |  |  |  |  |  |
| 9 |  |  |  |  |  |  |
| 10 |  |  |  |  |  |  |
| 11 |  |  |  |  |  |  |
| 12 |  |  |  |  |  |  |
| CNs Concentration in Film (%) | Exposure Time (min) in 50% Acetic Acid | Initial Film | Exposure Time (min) in 0.1 M Ammonia | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 90 | 60 | 30 | 15 | 15 | 30 | 60 | 90 | ||
| 0 |  |  |  |  |  |  |  |  |  |
| 5 |  |  |  |  |  |  |  |  |  |
| 10 |  |  |  |  |  |  |  |  |  |
| 20 |  |  |  |  |  |  |  |  |  |
| 30 |  |  |  |  |  |  |  |  |  |
| CNs Concentration in Film (%) | Bioactive Compounds Antioxidant Properties | Antimicrobial Activity (mm) | ||||
|---|---|---|---|---|---|---|
| Anthocyanin (mg/100 g) | TPC (mg GAE/g) | FRAP (mM Fe (II)/g) | DPPH (mM Trolox/g) | E. coli | S. aureus | |
| 0 | 2.30 ± 0.24 a | 46.58 ± 1.14 a | 575.09 ± 1.18 a | 628.25 ± 8.50 c | 11.67 ± 0.58 a | 11.67 ± 0.58 a |
| 5 | 2.33 ± 0.23 a | 46.54 ± 1.08 a | 551.67 ± 13.97 ab | 911.54 ± 51.68 b | 10.67 ± 0.58 a | 10.83 ± 0.76 a |
| 10 | 2.19 ± 0.12 a | 45.66 ± 1.12 a | 525.96 ± 17.23 bc | 943.04 ± 35.17 b | 11.17 ± 0.29 a | 11.67 ± 0.29 a |
| 20 | 2.42 ± 0.08 a | 45.29 ± 0.16 a | 522.83 ± 26.47 bc | 1020.98 ± 15.39 a | 10.67 ± 0.58 a | 10.67 ± 0.58 a |
| 30 | 2.38 ± 0.21 a | 45.36 ± 0.08 a | 518.79 ± 2.52 c | 1033.87 ±9.31 a | 10.83 ± 0.29 a | 10.00 ± 0.00 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romruen, O.; Kaewprachu, P.; Karbowiak, T.; Rawdkuen, S. Development of Smart Bilayer Alginate/Agar Film Containing Anthocyanin and Catechin-Lysozyme. Polymers 2022, 14, 5042. https://doi.org/10.3390/polym14225042
Romruen O, Kaewprachu P, Karbowiak T, Rawdkuen S. Development of Smart Bilayer Alginate/Agar Film Containing Anthocyanin and Catechin-Lysozyme. Polymers. 2022; 14(22):5042. https://doi.org/10.3390/polym14225042
Chicago/Turabian StyleRomruen, Orapan, Pimonpan Kaewprachu, Thomas Karbowiak, and Saroat Rawdkuen. 2022. "Development of Smart Bilayer Alginate/Agar Film Containing Anthocyanin and Catechin-Lysozyme" Polymers 14, no. 22: 5042. https://doi.org/10.3390/polym14225042
APA StyleRomruen, O., Kaewprachu, P., Karbowiak, T., & Rawdkuen, S. (2022). Development of Smart Bilayer Alginate/Agar Film Containing Anthocyanin and Catechin-Lysozyme. Polymers, 14(22), 5042. https://doi.org/10.3390/polym14225042







