Moisture-Related Shrinkage Behavior of Wood at Macroscale and Cellular Level
Abstract
1. Introduction
2. Materials and Methods
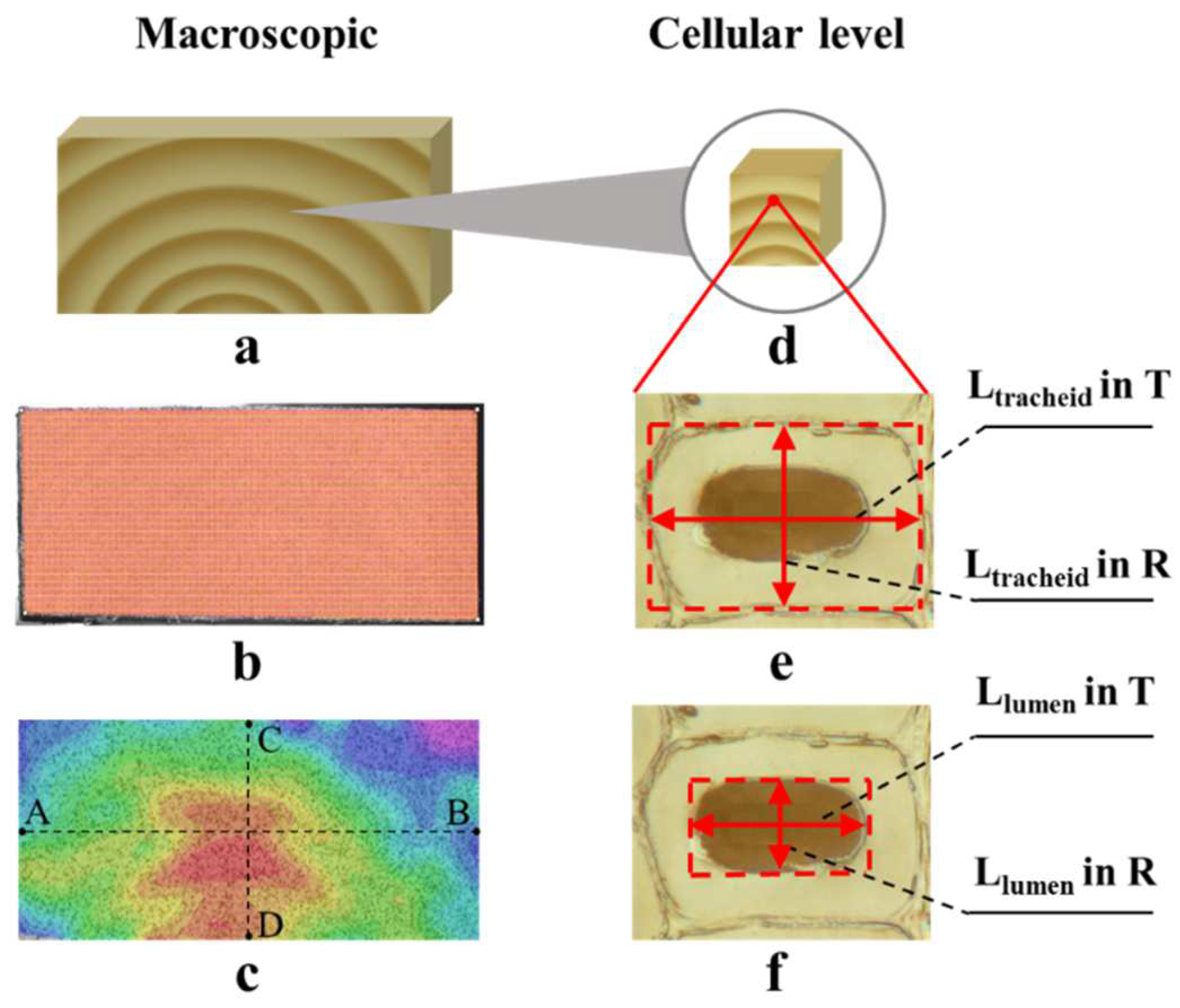
2.1. Specimen Preparation
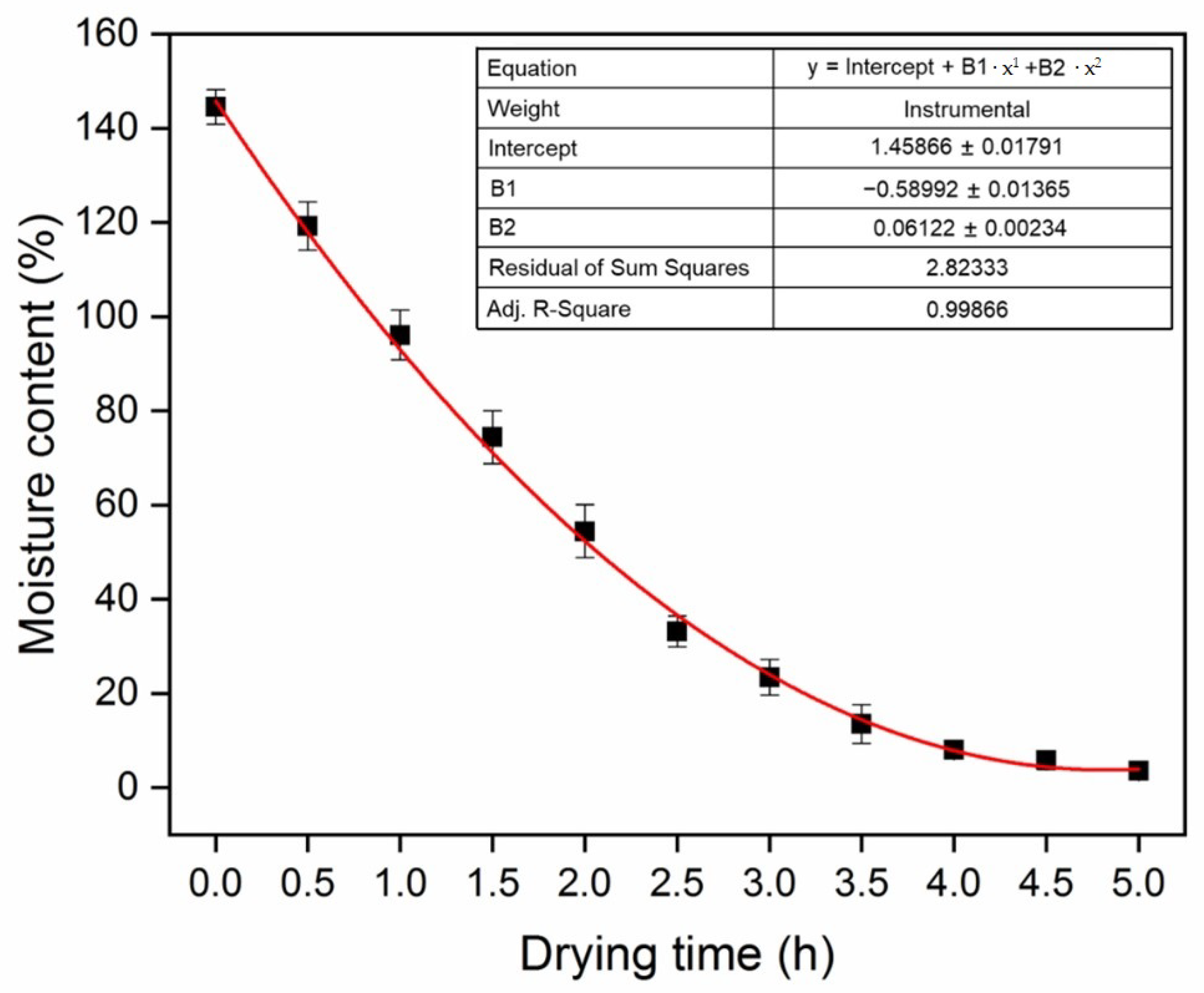
2.2. Macroscopic Shrinkage Measurements
2.3. Microscopic Observation
3. Results
3.1. Shrinkage Behavior at Macroscopic Level
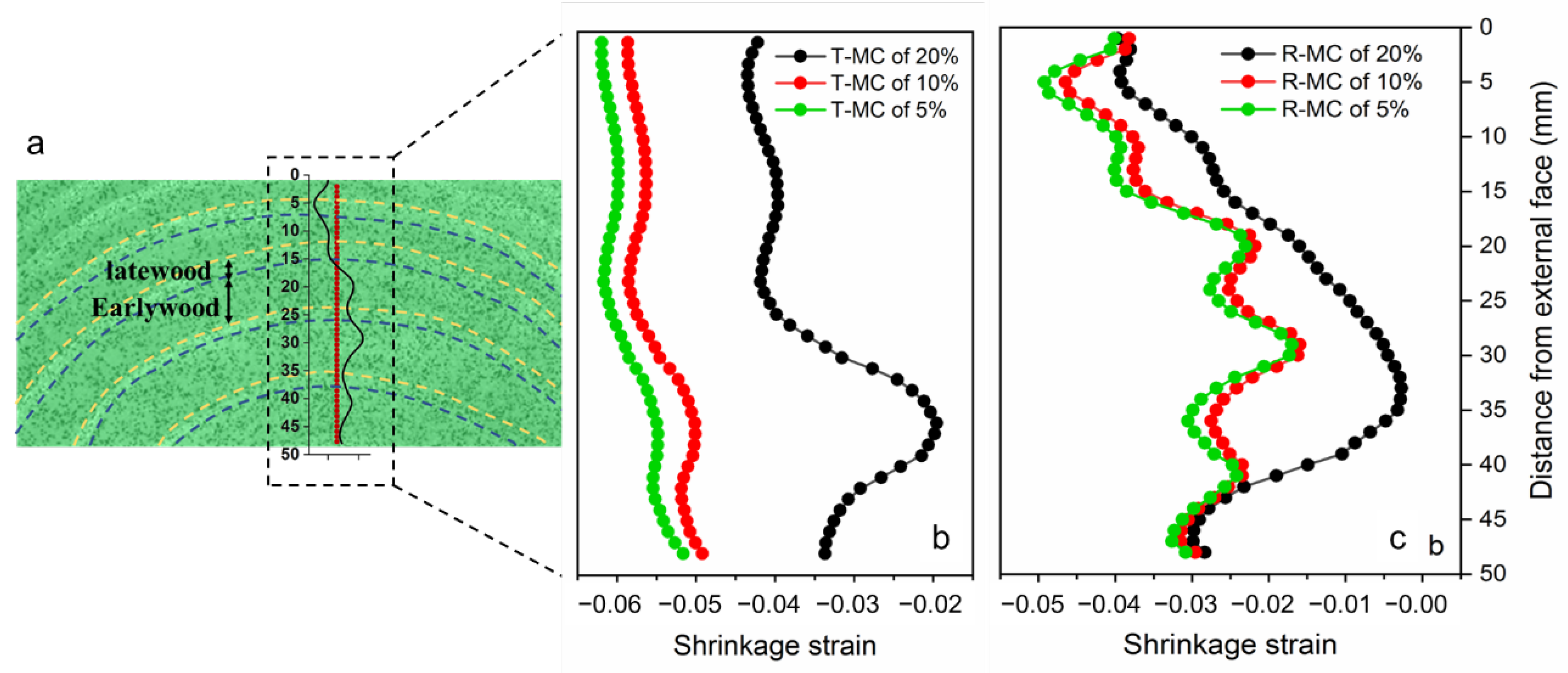
3.1.1. Full-Field Distribution of Shrinkage Strain
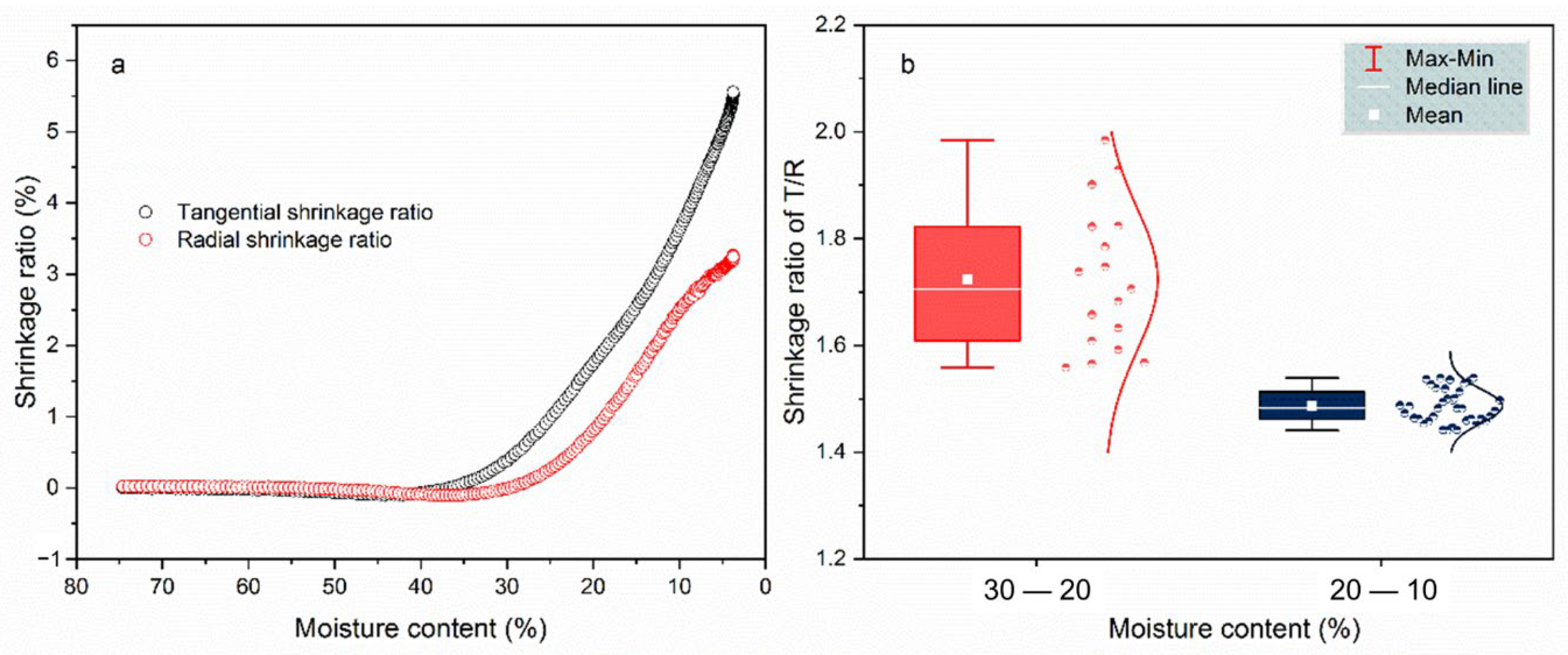
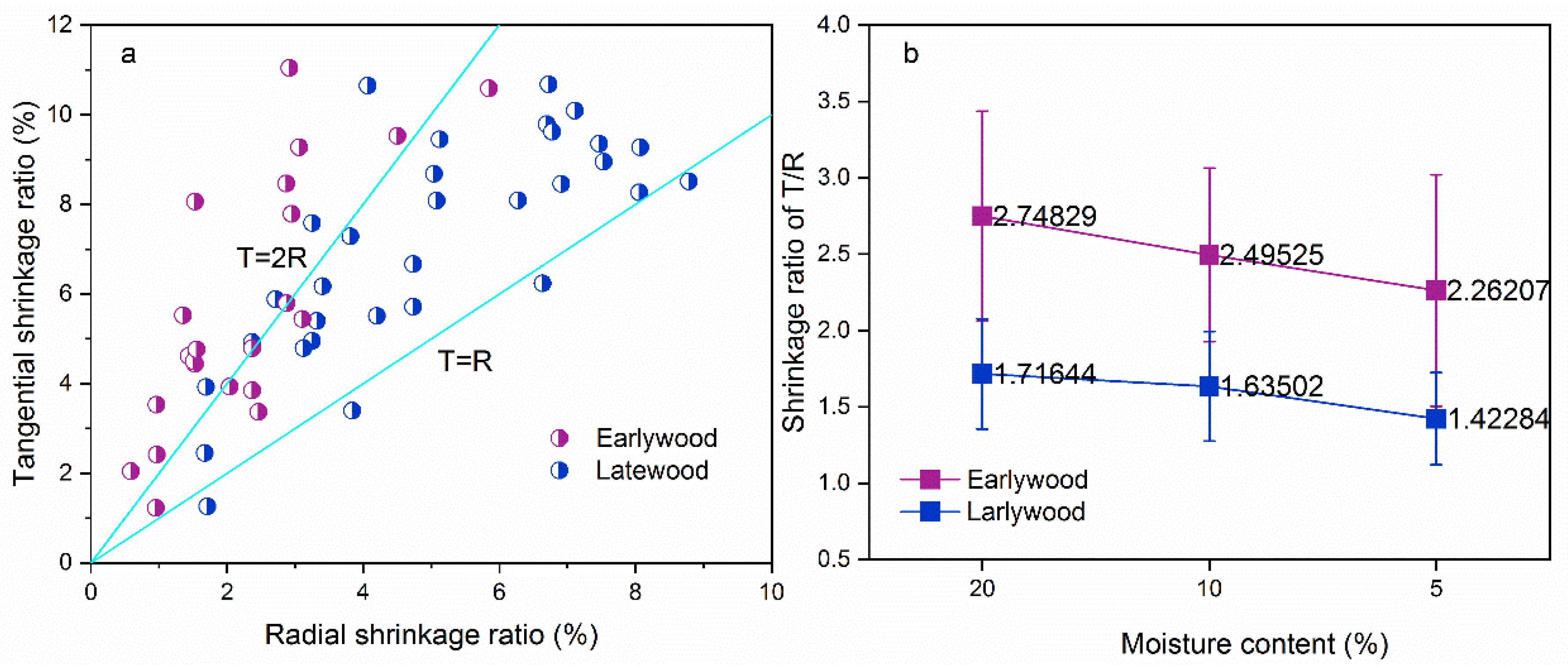
3.1.2. Wood Anisotropic Shrinkage
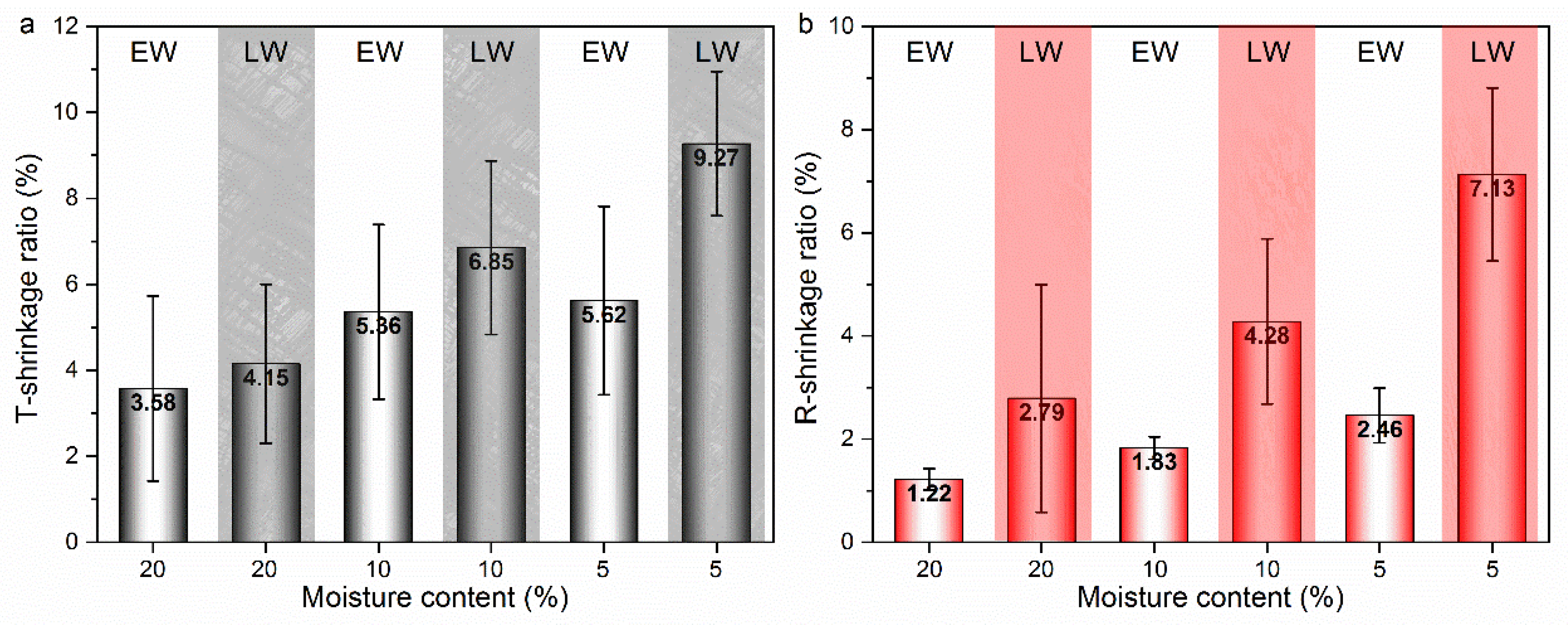
3.2. Shrinkage Behavior at Cellular Level
3.2.1. Shrinkage Ratio of Tracheid and Lumen
3.2.2. Shrinkage Ratio of Tracheid and Lumen
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, Z.; Zhou, Y.; Gao, X.; Liu, H.; Zhou, F. Changes of water related properties in radiata pine wood due to heat treatment. Constr. Build. Mater. 2019, 227, 116692. [Google Scholar] [CrossRef]
- Califano, A.; Baiesi, M.; Bertolin, C. Novel risk assessment tools for the climate-induced mechanical decay of wooden structures: Empirical and machine learning approaches. Forces Mech. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Arzola-Villegas, X.; Lakes, R.; Plaza, N.Z.; Jakes, J.E. Wood moisture-induced swelling at the cellular scale—Ab intra. Forests 2019, 10, 996–1015. [Google Scholar] [CrossRef]
- Skaar, C. Wood-Water Relationships; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Califano, A.; Foti, P.; Berto, F.; Baiesi, M.; Bertolin, C. Predicting damage evolution in panel paintings with machine learning. Procedia Struct. Integr. 2022, 41, 145–157. [Google Scholar] [CrossRef]
- Chiniforush, A.A.; Akbarnezhad, A.; Valipour, H.; Malekmohammadi, S. Moisture and temperature induced swelling/shrinkage of softwood and hardwood glulam and LVL: An experimental study. Constr. Build. Mater. 2019, 207, 70–83. [Google Scholar] [CrossRef]
- Leonardon, M.; Altaner, C.M.; Vihermaa, L.; Jarvis, M.C. Wood shrinkage: Influence of anatomy, cell wall architecture, chemical composition and cambial age. Eur. J. Wood Wood Prod. 2010, 68, 87–94. [Google Scholar] [CrossRef]
- Pang, S. Predicting anisotropic shringkage of softwood Part 1: Theories. Wood Sci. Technol. 2002, 36, 75–91. [Google Scholar] [CrossRef]
- Schulgasser, K.; Witztum, A. How the relationship between density and shrinkage of wood depends on its microstructure. Wood Sci. Technol. 2015, 49, 389–401. [Google Scholar] [CrossRef]
- Suchsland, O. The Swelling and Shrinking of Wood: A Practical Technology Primer; Forest Products Society: Madison, WI, USA, 2004. [Google Scholar]
- Peng, M.; Ho, Y.C.; Wang, W.C.; Chui, Y.H.; Gong, M. Measurement of wood shrinkage in jack pine using three dimesional digital image correlation (DIC). Holzforschung 2010, 66, 639–643. [Google Scholar] [CrossRef]
- Garcia, R.A.; Rosero-Alvarado, J.; Hernández, R.E. Swelling strain assessment of fiber and parenchyma tissues in the tropical hardwood Ormosia coccinea. Wood Sci. Technol. 2020, 54, 1447–1461. [Google Scholar] [CrossRef]
- Khoo, S.W.; Karuppanan, S.; Tan, C.S. A review of surface deformation and strain measurement using two-dimensional digital image correlation. Metrol. Meas. Syst. 2016, 23, 161–480. [Google Scholar] [CrossRef]
- Lanvermann, C.; Wittel, F.K.; Niemz, P. Full-field moisture induced deformation in Norway spruce: Intra-ring variation of transverse swelling. Eur. J. Wood Wood Prod. 2014, 72, 43–52. [Google Scholar] [CrossRef][Green Version]
- Sutton, M.A.; Orteu, J.J.; Schreier, H. Image Correlation for Shape, Motion and Deformation Measurements: Basic Concepts, Theory and Applications; Springer Science & Business Media, LLC: New York, NY, USA, 2009. [Google Scholar]
- Kang, H.Y.; Muszyński, L.; Milota, M.R. Optical measurement of deformations in drying lumber. Dry. Technol. 2011, 29, 127–134. [Google Scholar] [CrossRef]
- Peng, M.K.; Chui, Y.H.; Ho, Y.C.; Wang, W.C.; Zhou, Y.T. Investigation of shrinkage in softwood using digital image correlation method. Appl. Mech. Mater. 2011, 83, 157–161. [Google Scholar] [CrossRef]
- Fu, Z.; Weng, X.; Gao, Y.; Zhou, Y. Full-field tracking and analysis of shrinkage strain during moisture content loss in wood. Holzforschung 2021, 75, 436–443. [Google Scholar] [CrossRef]
- Almeida, G.; Huber, F.; Perré, P. Free shrinkage of wood determined at the cellular level using an environmental scanning electron microscope. Maderas. Cienc. Tecnol. 2014, 16, 187–198. [Google Scholar] [CrossRef]
- Perré, P.; Huber, F. Measurement of free shrinkage at the tissue level using an optical microscope with an immersion objective: Results obtained for Douglas fir (Pseudotsuga menziesii) and spruce (Picea abies). Ann. Forest Sci. 2007, 64, 255–265. [Google Scholar] [CrossRef]
- Taguchi, A.; Murata, K.; Nakamura, M.; Nakano, T. Scale effect in the anisotropic deformation change of tracheid cells during water adsorption. Holzforschung 2011, 65, 253–256. [Google Scholar] [CrossRef][Green Version]
- Taguchi, A.; Murata, K.; Nakano, T. Observation of cell shapes in wood cross-sections during water adsorption by confocal laser-scanning microscopy (CLSM). Holzforschung 2010, 64, 627–631. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, Y.; Fu, Z. Interdependence of shrinkage behavior between wood macroscopic and cellular level during moisture content loss. Dry. Technol. 2021, 39, 1–8. [Google Scholar] [CrossRef]
- Glass, S.; Zelinka, S. Moisture Relations and Physical Properties of Wood. In Wood Handbook: Wood as an Engineering Material; United States Department of Agriculture Forest Service, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2021. [Google Scholar]
- Zhan, J.F.; Avramidis, S. Impact of conventional drying and thermal post-treatment on the residual stresses and shape deformations of larch lumber. Dry. Technol. 2017, 35, 15–24. [Google Scholar] [CrossRef]
- Gauvin, C.; Jullien, D.; Doumalin, P.; Dupré, J.C.; Gril, J. Image correlation to evaluate the influence of hygrothermal loading on wood. Strain 2014, 50, 428–435. [Google Scholar] [CrossRef]
- Siau, J.F. Wood: Influence of Moisture on Physical Properties; Department of Wood Science Forest Products, Virginia Polytechnic Institute and State University Blacksburg: Blacksburg, VA, USA, 1995.
- Almeida, G.; Hernández, R.E. Changes in physical properties of tropical and temperate hardwoods below and above the fiber saturation point. Wood Sci. Technol. 2006, 40, 599–613. [Google Scholar] [CrossRef]
- Lazarescu, C.; Avramidis, S. Drying related strain development in restrained wood. Dry. Technol. 2008, 26, 544–551. [Google Scholar] [CrossRef]
- Hanhijärvi, A.; Wahl, P.; Räsänen, J.; Silvennoinen, R. Observation of development of microcracks on wood surface caused by drying stresses. Holzforschung 2003, 57, 561–565. [Google Scholar] [CrossRef]
- Pang, S.; Herritsch, A. Physical properties of earlywood and latewood of Pinus radiata D. Don: Anisotropic shrinkage, equilibrium moisture content and fibre saturation point. Holzforschung 2005, 59, 654–661. [Google Scholar] [CrossRef]
- Patera, A.; Van den Bulcke, J.; Boone, M.N.; Derome, D.; Carmeliet, J. Swelling interactions of earlywood and latewood across a growth ring: Global and local deformations. Wood Sci. Technol. 2018, 52, 91–114. [Google Scholar] [CrossRef]
- Kifetew, G. Application of the deformation field measurement method to wood during drying. Wood Sci. Technol. 1996, 30, 455–462. [Google Scholar] [CrossRef]
- Kifetew, G.; Lindberg, H.; Wiklund, M. Tangential and radial deformation field measurements on wood during drying. Wood Sci. Technol. 1997, 3, 35–44. [Google Scholar] [CrossRef]
- Taylor, A.; Plank, B.; Standfest, G.; Petutschnigg, A. Beech wood shrinkage observed at the micro-scale by a time series of X-ray computed tomographs (μXCT). Holzforschung 2013, 67, 201–205. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Hayashi, K.; Liu, Y.; Cai, Y.; Sugimori, M. Relationships of anatomical characteristics versus shrinkage and collapse properties in plantation-grown eucalypt wood from China. J. Wood Sci. 2006, 52, 187–194. [Google Scholar] [CrossRef]
- Xue, Q.; Sun, W.; Fagerstedt, K.; Guo, X.; Dong, M.; Wang, W.; Cao, H. Effects of wood rays on the shrinkage of wood during the drying process. BioResources 2018, 13, 7086–7095. [Google Scholar] [CrossRef]
- Patera, A.; Derome, D.; Griffa, M.; Carmeliet, J. Hysteresis in swelling and in sorption of wood tissue. J. Struct. Biol. 2013, 182, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Derome, D.; Griffa, M.; Koebel, M.; Carmeliet, J. Hysteretic swelling of wood at cellular scale probed by phase-contrast X-ray tomography. J. Struct. Biol. 2011, 173, 180–190. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Fu, Z.; Zhou, Y.; Gao, X.; Zhou, F.; Cao, H. Moisture-Related Shrinkage Behavior of Wood at Macroscale and Cellular Level. Polymers 2022, 14, 5045. https://doi.org/10.3390/polym14225045
Gao Y, Fu Z, Zhou Y, Gao X, Zhou F, Cao H. Moisture-Related Shrinkage Behavior of Wood at Macroscale and Cellular Level. Polymers. 2022; 14(22):5045. https://doi.org/10.3390/polym14225045
Chicago/Turabian StyleGao, Yufa, Zongying Fu, Yongdong Zhou, Xin Gao, Fan Zhou, and Huimin Cao. 2022. "Moisture-Related Shrinkage Behavior of Wood at Macroscale and Cellular Level" Polymers 14, no. 22: 5045. https://doi.org/10.3390/polym14225045
APA StyleGao, Y., Fu, Z., Zhou, Y., Gao, X., Zhou, F., & Cao, H. (2022). Moisture-Related Shrinkage Behavior of Wood at Macroscale and Cellular Level. Polymers, 14(22), 5045. https://doi.org/10.3390/polym14225045





