Cu-THQ-EFG Composite for Highly Selective Electrochemical CO2 Reduction to Formate at Low Overpotentials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Chemicals
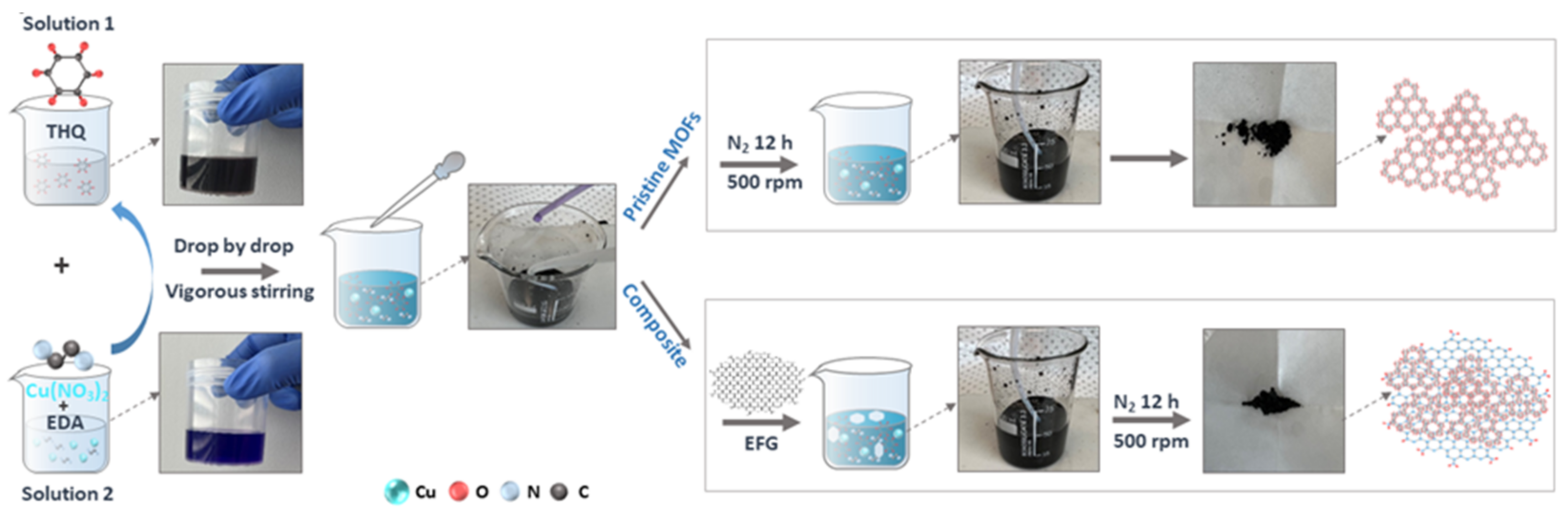
2.2. Synthesis of Cu-THQ-EFG Composite
2.3. Materials Characterizations
2.4. Electrocatalysis Characterizations
2.4.1. System Set-Up
2.4.2. Electrochemical Measurements
2.4.3. CO2 Reduction Product Analysis
3. Results and Discussion
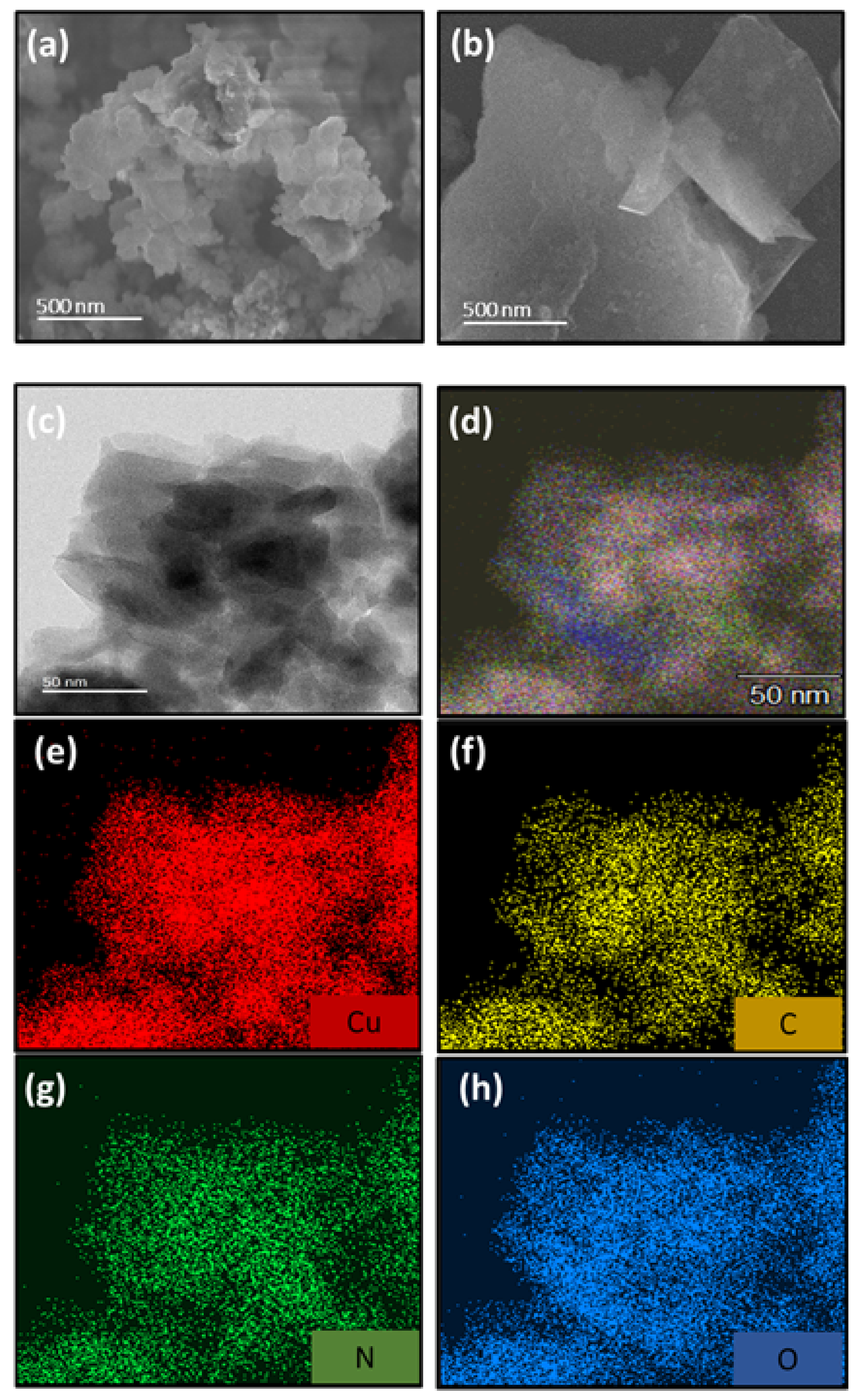
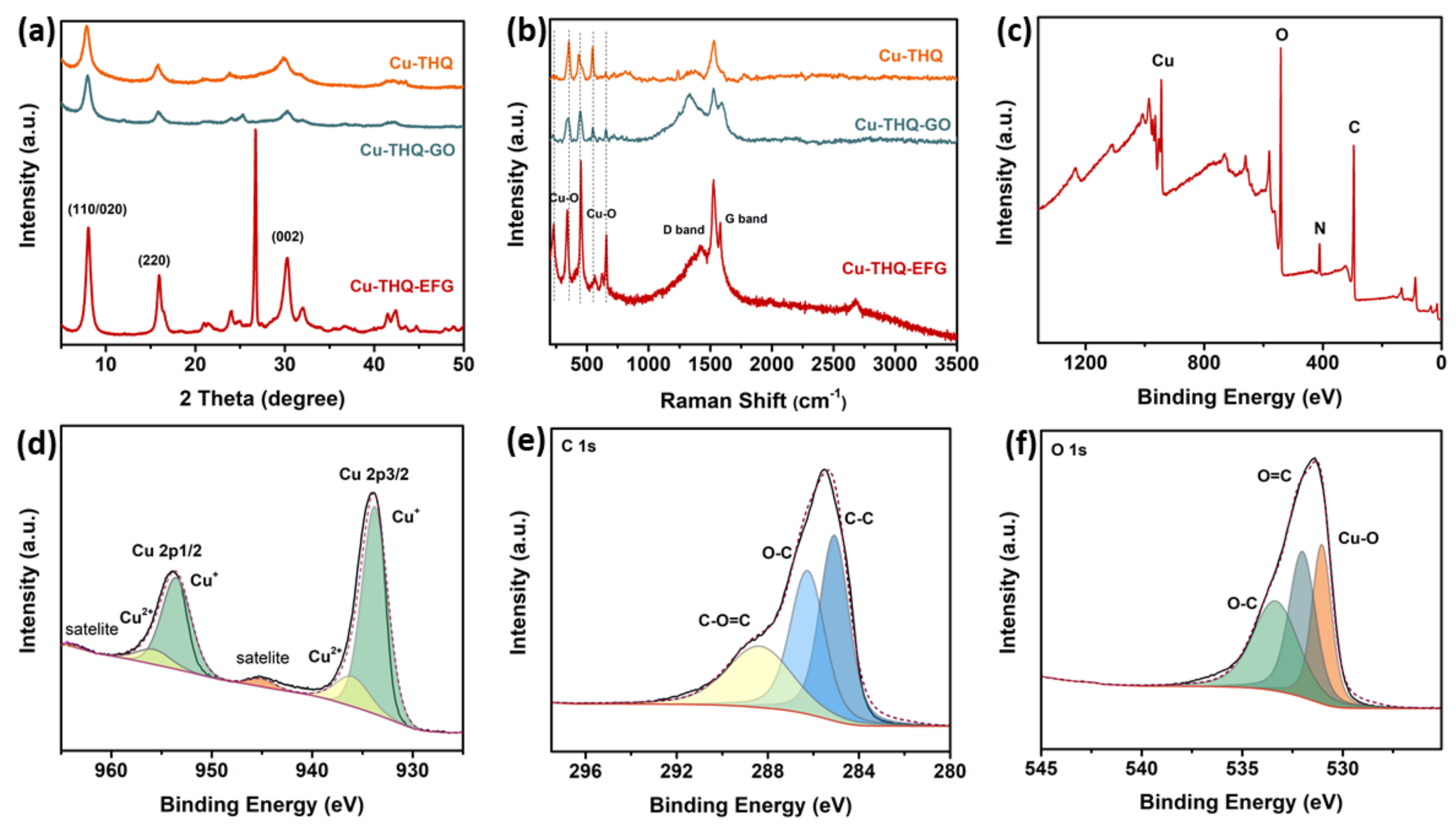
3.1. Structure Analysis
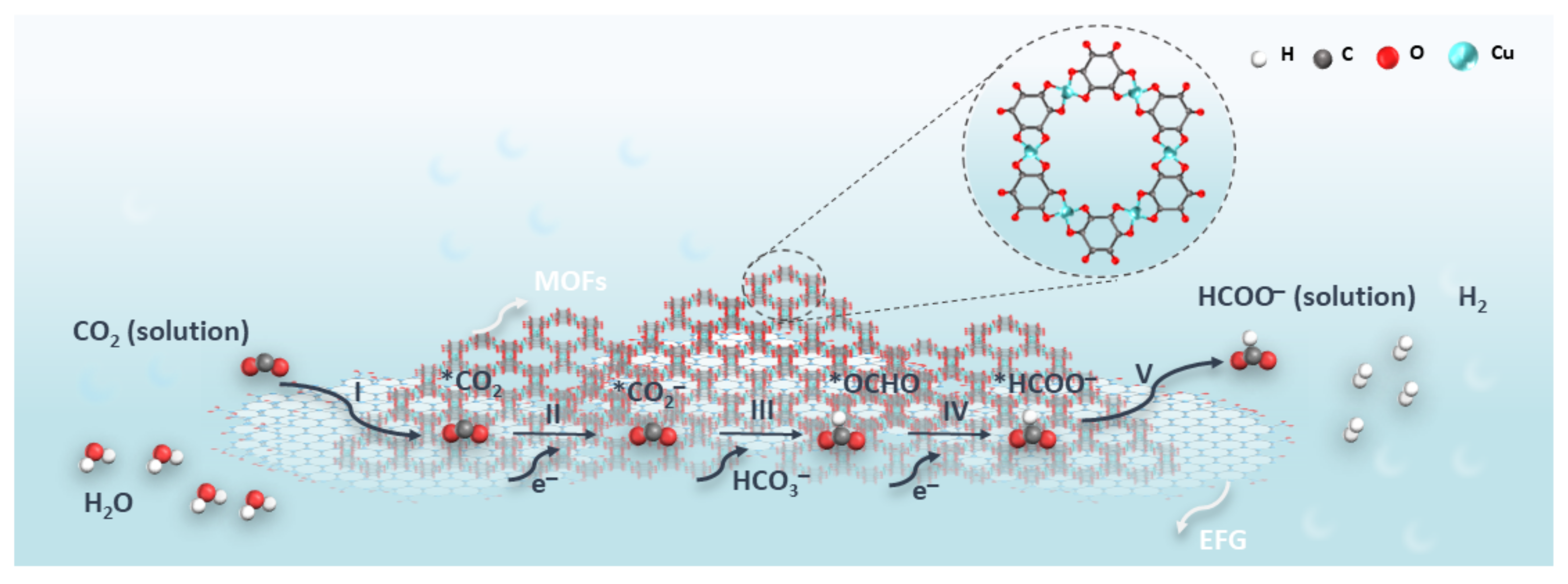
3.2. CO2RR Performance Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Q.; Rosen, J.; Zhou, Y.; Hutchings, G.S.; Kimmel, Y.C.; Chen, J.G.; Jiao, F. A selective and efficient electrocatalyst for carbon dioxide reduction. Nat. Commun. 2014, 5, 3242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Kapteijn, F.; Gascon, J. Engineering Metal–Organic Frameworks for the Electrochemical Reduction of CO2: A Minireview. Chem. Asian J. 2019, 14, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Xiao, M.; Miao, R.; Liu, Y.; Ren, M.; Jia, Z.; Han, D.; Yuan, Y.; Bai, Z.; Yang, L. Copper-Based Catalysts for Electrochemical Carbon Dioxide Reduction to Multicarbon Products. Electrochem. Energy Rev. 2022, 5, 4. [Google Scholar] [CrossRef]
- Patil, S.B.; Wang, D.-Y. Electrochemical Reactions Towards the Formation of Heteroatomic Bonds beyond CO2 and N2 Reduction. Sustain. Energy Fuels 2022, 6, 3283–3303. [Google Scholar] [CrossRef]
- Wu, J.; Huang, Y.; Ye, W.; Li, Y. CO2 reduction: From the electrochemical to photochemical approach. Adv. Sci. 2017, 4, 1700194. [Google Scholar] [CrossRef]
- Kumar, B.; Atla, V.; Brian, J.P.; Kumari, S.; Nguyen, T.Q.; Sunkara, M.; Spurgeon, J.M. Reduced SnO2 Porous Nanowires with a High Density of Grain Boundaries as Catalysts for Efficient Electrochemical CO2-into-HCOOH Conversion. Angew. Chem. Int. Ed. 2017, 56, 3645–3649. [Google Scholar] [CrossRef]
- Ahmad, T.; Liu, S.; Sajid, M.; Li, K.; Ali, M.; Liu, L.; Chen, W. Electrochemical CO2 Reduction to C2+ Products Using Cu-Based Electrocatalysts: A Review. Nano Res. Energy 2022, 1, e9120021. [Google Scholar] [CrossRef]
- Lu, X.F.; Xia, B.Y.; Zang, S.Q.; Lou, X.W. Metal–organic frameworks based electrocatalysts for the oxygen reduction reaction. Angew. Chem. 2020, 132, 4662–4678. [Google Scholar] [CrossRef]
- Dong, B.-X.; Qian, S.-L.; Bu, F.-Y.; Wu, Y.-C.; Feng, L.-G.; Teng, Y.-L.; Liu, W.-L.; Li, Z.-W. Electrochemical reduction of CO2 to CO by a heterogeneous Catalyst of Fe–porphyrin-based metal–organic framework. ACS Appl. Energy Mater. 2018, 1, 4662–4669. [Google Scholar] [CrossRef]
- Hinogami, R.; Yotsuhashi, S.; Deguchi, M.; Zenitani, Y.; Hashiba, H.; Yamada, Y. Electrochemical reduction of carbon dioxide using a copper rubeanate metal organic framework. ECS Electrochem. Lett. 2012, 1, H17. [Google Scholar] [CrossRef]
- Jiang, X.; Li, H.; Xiao, J.; Gao, D.; Si, R.; Yang, F.; Li, Y.; Wang, G.; Bao, X. Carbon dioxide electroreduction over imidazolate ligands coordinated with Zn (II) center in ZIFs. Nano Energy 2018, 52, 345–350. [Google Scholar] [CrossRef]
- Kung, C.-W.; Audu, C.O.; Peters, A.W.; Noh, H.; Farha, O.K.; Hupp, J.T. Copper nanoparticles installed in metal–organic framework thin films are electrocatalytically competent for CO2 reduction. ACS Energy Lett. 2017, 2, 2394–2401. [Google Scholar] [CrossRef]
- Wu, J.-X.; Hou, S.-Z.; Zhang, X.-D.; Xu, M.; Yang, H.-F.; Cao, P.-S.; Gu, Z.-Y. Cathodized copper porphyrin metal–organic framework nanosheets for selective formate and acetate production from CO2 electroreduction. Chem. Sci. 2019, 10, 2199–2205. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Liu, J.; Gao, Y.; Gong, C.; Addicoat, M.; Heine, T.; Wöll, C.; Sun, L. Highly oriented MOF thin film-based electrocatalytic device for the reduction of CO2 to CO exhibiting high faradaic efficiency. J. Mater. Chem. A 2016, 4, 15320–15326. [Google Scholar] [CrossRef]
- Kornienko, N.; Zhao, Y.; Kley, C.S.; Zhu, C.; Kim, D.; Lin, S.; Chang, C.J.; Yaghi, O.M.; Yang, P. Metal–organic frameworks for electrocatalytic reduction of carbon dioxide. J. Am. Chem. Soc. 2015, 137, 14129–14135. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.-J.; Cao, S.-M.; Feng, B.-Q.; Dong, B.-X.; Ding, Y.-X.; Zheng, Q.-H.; Teng, Y.-L.; Li, Z.-W.; Liu, W.-L.; Feng, L.-G. Revealing the structure–activity relationship of two Cu-porphyrin-based metal–organic frameworks for the electrochemical CO2-to-HCOOH transformation. Dalton Trans. 2020, 49, 14995–15001. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Jin, W.; Wu, Z. Recent development of two-dimensional metal–organic framework derived electrocatalysts for hydrogen and oxygen electrocatalysis. Nanoscale 2020, 12, 18497–18522. [Google Scholar] [CrossRef]
- Choi, E.-K.; Jeon, I.-Y.; Bae, S.-Y.; Lee, H.-J.; Shin, H.S.; Dai, L.; Baek, J.-B. High-yield exfoliation of three-dimensional graphite into two-dimensional graphene-like sheets. Chem. Commun. 2010, 46, 6320–6322. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Z.; Dai, Q.; Chen, J.F.; Dai, L. Edge functionalization of graphene and two-dimensional covalent organic polymers for energy conversion and storage. Adv. Mater. 2016, 28, 6253–6261. [Google Scholar] [CrossRef]
- Sekiya, R.; Haino, T. Edge-Functionalized Nanographenes. Chem. Eur. J. 2021, 27, 187–199. [Google Scholar] [CrossRef]
- Jeon, I.Y.; Bae, S.Y.; Seo, J.M.; Baek, J.B. Scalable production of edge-functionalized graphene nanoplatelets via mechanochemical ball-milling. Adv. Funct. Mater. 2015, 25, 6961–6975. [Google Scholar] [CrossRef]
- Jia, L.; Wagner, P.; Chen, J. Electrocatalyst Derived from NiCu–MOF Arrays on Graphene Oxide Modified Carbon Cloth for Water Splitting. Inorganics 2022, 10, 53. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A.A. Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Wallace, G.G.; Officer, D.L.; Jalili, R.; Walker, A.J.; Ryder, G.M.; Faisal, S.N. Dispersible Edge Functionalised Graphene Platelets. U.S. Patent Application 17/283,705, 21 October 2021. [Google Scholar]
- Li, Z.; Gadipelli, S.; Li, H.; Howard, C.A.; Brett, D.J.; Shearing, P.R.; Guo, Z.; Parkin, I.P.; Li, F. Tuning the interlayer spacing of graphene laminate films for efficient pore utilization towards compact capacitive energy storage. Nat. Energy 2020, 5, 160–168. [Google Scholar] [CrossRef]
- Park, J.; Hinckley, A.C.; Huang, Z.; Feng, D.; Yakovenko, A.A.; Lee, M.; Chen, S.; Zou, X.; Bao, Z. Synthetic routes for a 2D semiconductive copper hexahydroxybenzene metal–organic framework. J. Am. Chem. Soc. 2018, 140, 14533–14537. [Google Scholar] [CrossRef]
- Luo, S.; Wang, Y.; Kan, X. Cu-THQ metal-organic frameworks: A kind of new inner reference for the reliable detection of dopamine base on ratiometric electrochemical sensing. Microchem. J. 2022, 172, 106903. [Google Scholar] [CrossRef]
- Jiang, Q.; Xiong, P.; Liu, J.; Xie, Z.; Wang, Q.; Yang, X.Q.; Hu, E.; Cao, Y.; Sun, J.; Xu, Y. A redox-active 2D metal–organic framework for efficient lithium storage with extraordinary high capacity. Angew. Chem. Int. Ed. 2020, 59, 5273–5277. [Google Scholar] [CrossRef]
- Guntern, Y.T.; Pankhurst, J.R.; Vávra, J.; Mensi, M.; Mantella, V.; Schouwink, P.; Buonsanti, R. Nanocrystal/metal–organic framework hybrids as electrocatalytic platforms for CO2 conversion. Angew. Chem. 2019, 131, 12762–12769. [Google Scholar] [CrossRef]
- Perfecto-Irigaray, M.; Albo, J.; Beobide, G.; Castillo, O.; Irabien, A.; Pérez-Yáñez, S. Synthesis of heterometallic metal–organic frameworks and their performance as electrocatalyst for CO2 reduction. RSC Adv. 2018, 8, 21092–21099. [Google Scholar] [CrossRef] [Green Version]
- De Luna, P.; Liang, W.; Mallick, A.; Shekhah, O.; García de Arquer, F.P.; Proppe, A.H.; Todorović, P.; Kelley, S.O.; Sargent, E.H.; Eddaoudi, M. Metal–organic framework thin films on high-curvature nanostructures toward tandem electrocatalysis. ACS Appl. Mater. Interfaces 2018, 10, 31225–31232. [Google Scholar] [CrossRef]
- Choi, I.; Jung, Y.E.; Yoo, S.J.; Kim, J.Y.; Kim, H.-J.; Lee, C.Y.; Jang, J.H. Facile synthesis of M-MOF-74 (M = Co, Ni, Zn) and its application as an electrocatalyst for electrochemical CO2 conversion and H2 production. J. Electrochem. Sci. Technol. 2017, 8, 61–68. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, P.; Wang, Z.; Kang, P. Zinc imidazolate metal–organic frameworks (ZIF-8) for electrochemical reduction of CO2 to CO. ChemPhysChem 2017, 18, 3142–3147. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.S.; Kumar, S.S.; Kulandainathan, M.A. Highly selective electrochemical reduction of carbon dioxide using Cu based metal organic framework as an electrocatalyst. Electrochem. Commun. 2012, 25, 70–73. [Google Scholar] [CrossRef]
- Albo, J.; Vallejo, D.; Beobide, G.; Castillo, O.; Castaño, P.; Irabien, A. Copper-based metal–Organic porous materials for CO2 electrocatalytic reduction to alcohols. ChemSusChem 2017, 10, 1100–1109. [Google Scholar] [CrossRef]


| Catalyst | Electrolyte | Product | FE% | Potential (V vs. RHE) | Ref. |
|---|---|---|---|---|---|
| CR-MOF | 0.5 M KHCO3 | HCOOH | 63 | −0.76 | [10] |
| Cu-SIM NU-1000 | 0.1 M NaClO4 | HCOOH | 28 | −0.82 | [12] |
| Cu2(CuTCPP) NSs | 1 M H2O and 0.5 M EmimBF4 | HCOOH | 28.1 | −1.4 V vs. Ag/Ag+ | [13] |
| Cu-TCPP | 0.5 M KHCO3 | HCOOH | 28.3 | −0.7 | [16] |
| Ag@Al-PMOF | 0.1 M KHCO3 | HCOOH | 3.0 | −1.1 | [29] |
| HKUST-1 | 0.5 M KHCO3 | C2H5OH/CH3OH | 15.9 | −0.37 | [30] |
| RE-ndc-fcu-MOF@AuNMEs | 0.1 M KHCO3 | CH4 | 0.5 ± 0.1 | −0.5 | [31] |
| Zn-MOF-74 | 0.5 M KHCO3 | CO | 21.72 | −0.91 | [32] |
| ZIF-8 | 0.5 M NaHCO3 | CO | 65 | −1.62 | [33] |
| Cu3(BTC)2 | 0.01 M MTBAEFB/DMF | C2H2O4 | 51 | −1.12 V vs. Ag/AgCl | [34] |
| CuAdeAce | 0.5 M KHCO3 | CH3OH/C2H5OH | 1.2 | −1.75 | [35] |
| Cu-THQ-EFG | 0.1 M NaHCO3 | HCOOH | 31.7 | −0.25 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, L.; Wagner, K.; Smyth, J.; Officer, D.; Chen, J.; Wagner, P. Cu-THQ-EFG Composite for Highly Selective Electrochemical CO2 Reduction to Formate at Low Overpotentials. Polymers 2022, 14, 5112. https://doi.org/10.3390/polym14235112
Jia L, Wagner K, Smyth J, Officer D, Chen J, Wagner P. Cu-THQ-EFG Composite for Highly Selective Electrochemical CO2 Reduction to Formate at Low Overpotentials. Polymers. 2022; 14(23):5112. https://doi.org/10.3390/polym14235112
Chicago/Turabian StyleJia, Lisha, Klaudia Wagner, Jamie Smyth, David Officer, Jun Chen, and Pawel Wagner. 2022. "Cu-THQ-EFG Composite for Highly Selective Electrochemical CO2 Reduction to Formate at Low Overpotentials" Polymers 14, no. 23: 5112. https://doi.org/10.3390/polym14235112
APA StyleJia, L., Wagner, K., Smyth, J., Officer, D., Chen, J., & Wagner, P. (2022). Cu-THQ-EFG Composite for Highly Selective Electrochemical CO2 Reduction to Formate at Low Overpotentials. Polymers, 14(23), 5112. https://doi.org/10.3390/polym14235112









