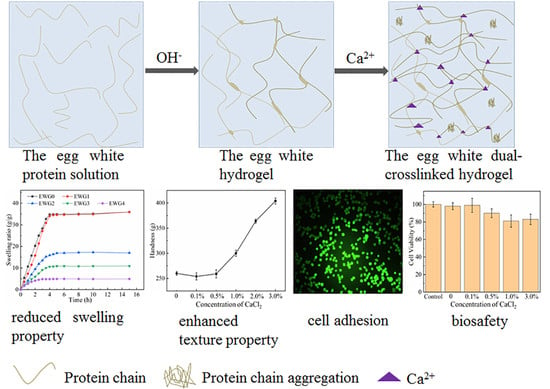
Preparation and Properties of Egg White Dual Cross-Linked Hydrogel with Potential Application for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Egg White Hydrogel
2.3. Characterization
2.4. Swelling Tests
2.5. Texture Tests
2.6. Cell Experiments
2.7. Statistical Analysis
3. Results and Discussion
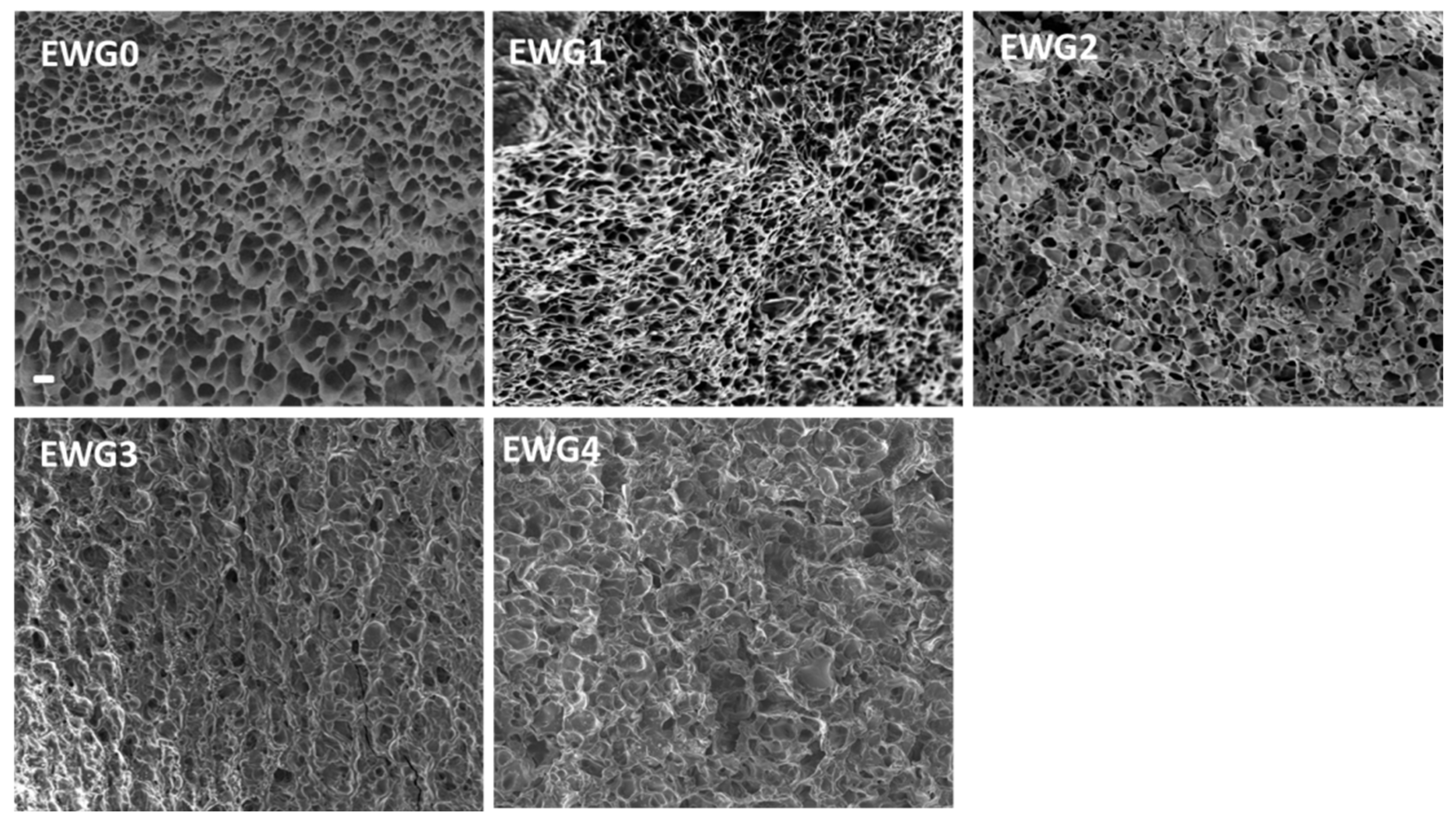
3.1. Preparation and Morphology Analysis of the EWG Hydrogel
3.2. FT−IR Analysis
3.3. XRD Analysis and Thermal Stability
3.4. Microscopic Examination
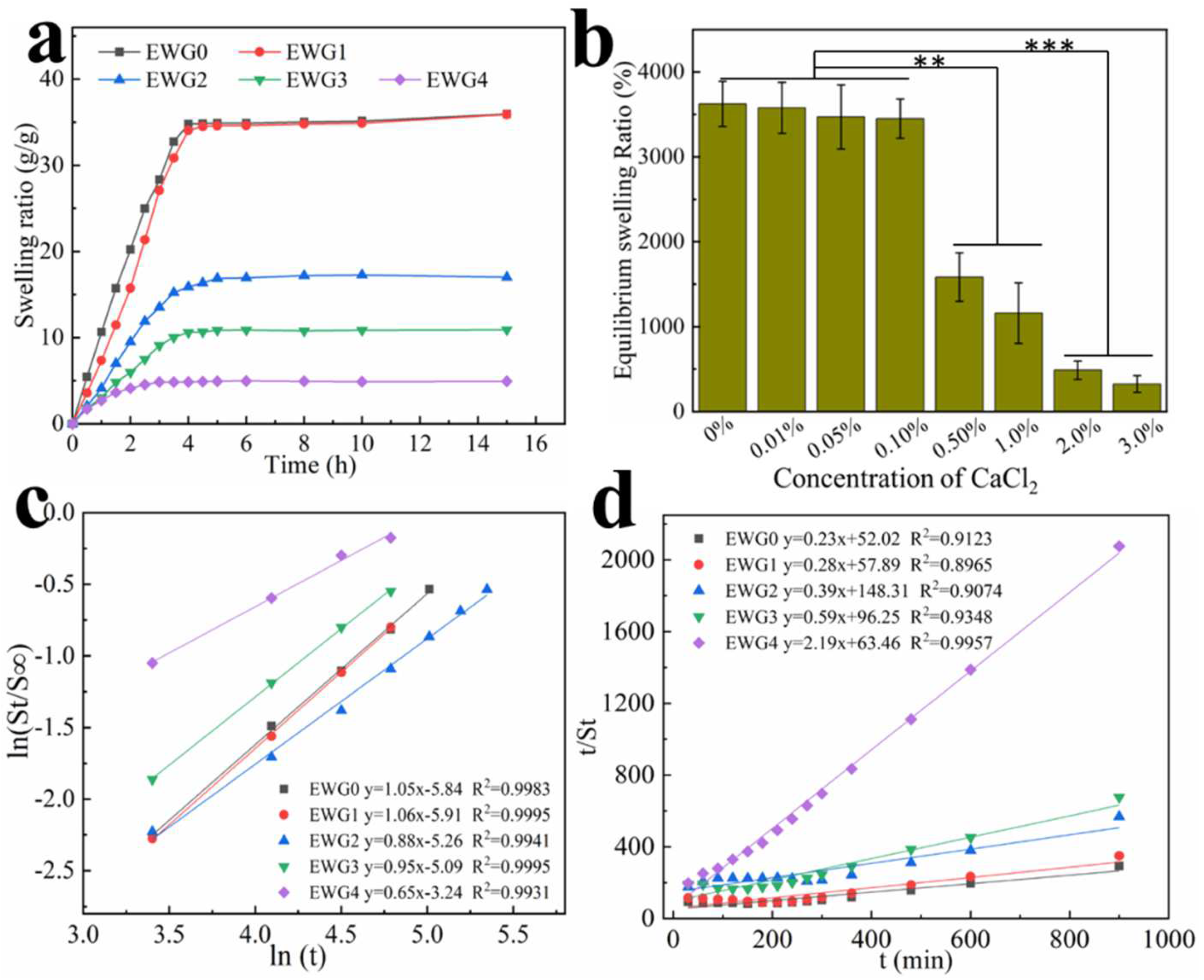
3.5. Effect of Calcium Ions on Hydrogel Swelling Performance
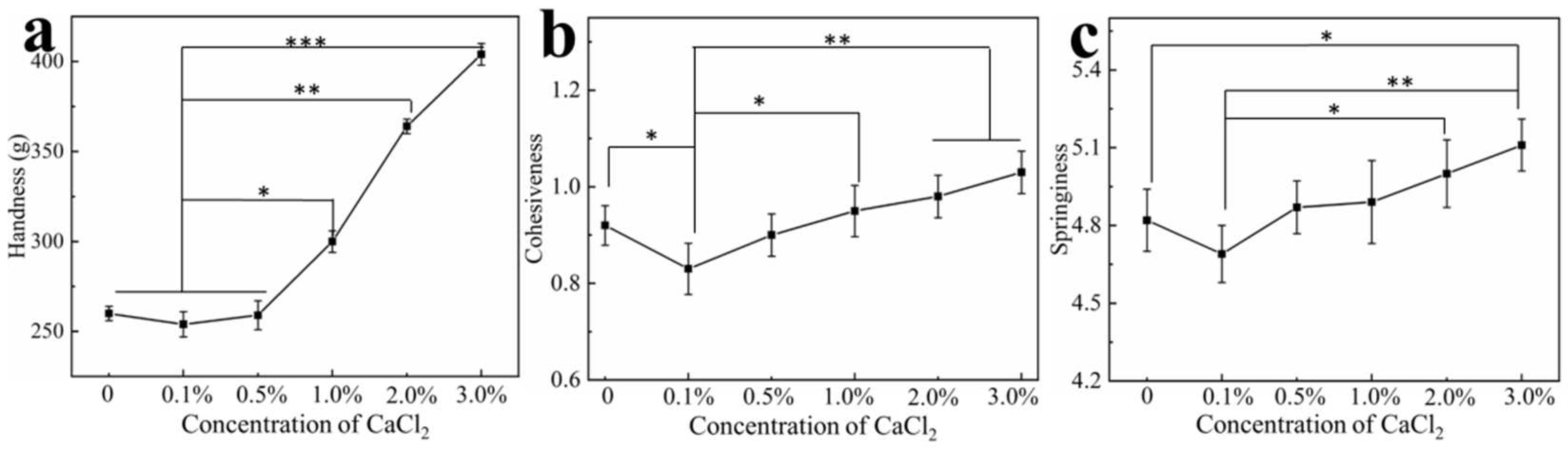
3.6. Effect of Calcium Ions on Hydrogel Texture
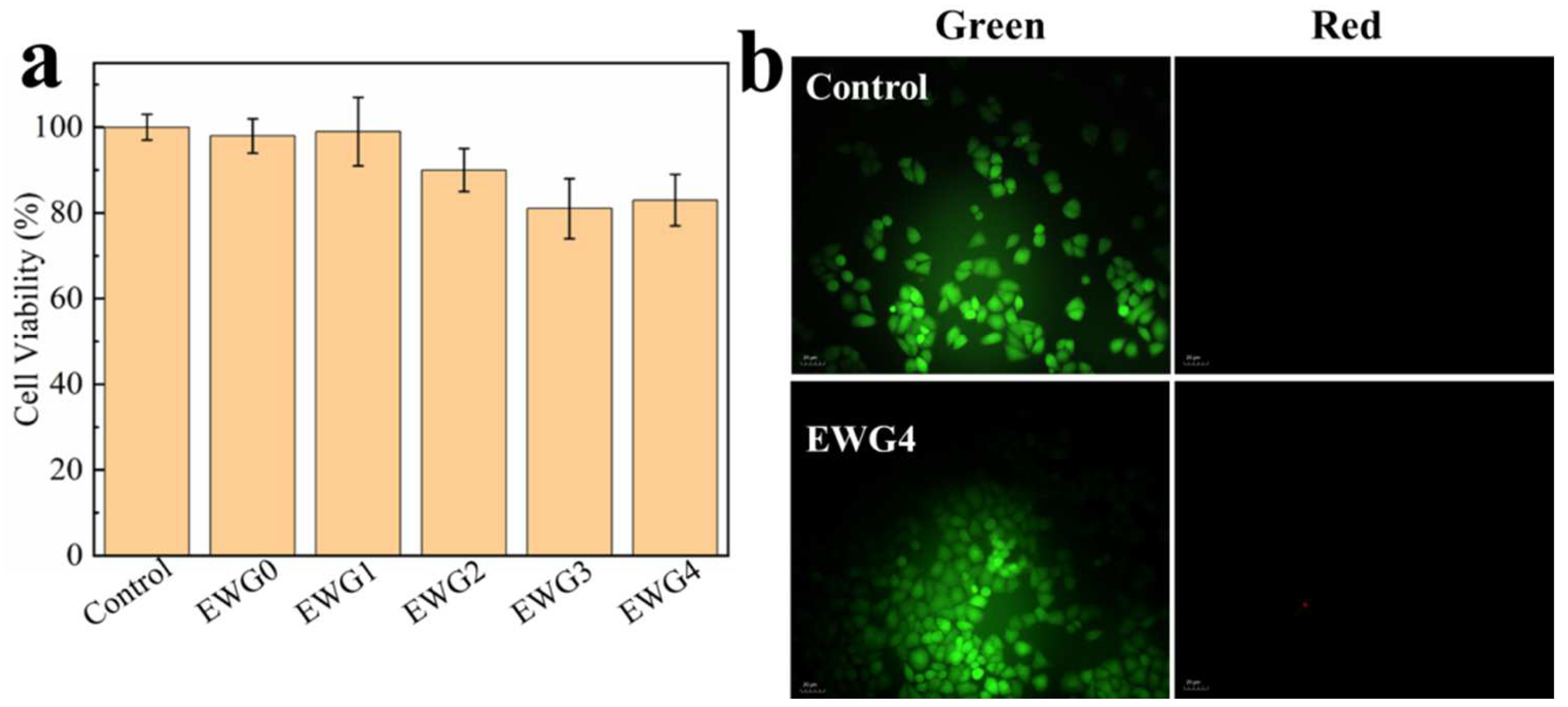
3.7. Cytocompatibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Banu, J.R.; Kavitha, S.; Kannah, R.Y.; Devi, T.P.; Gunasekaran, M.; Kim, S.H.; Kumar, G. A review on biopolymer production via lignin valorization. Bioresour. Technol. 2019, 290, 121790. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xie, L.; Wang, Z.; Mao, S.; Gong, Y.; Wang, Y. Biomass-derived ordered mesoporous carbon nano-ellipsoid encapsulated metal nanoparticles inside: Ideal nanoreactors for shape-selective catalysis. Chem. Commun. 2019, 56, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, D.; Luo, C.; Fu, Q.; Pan, C. Highly porous graphitic biomass carbon as advanced electrodematerials for supercapacitors. Green Chem. 2017, 19, 4132–4140. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. A review on the design and hydration properties of natural polymer-based hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef]
- Sun, Z.; Song, C.; Wang, C.; Hu, Y.; Wu, J. Hydrogel-based controlled drug delivery for cancer treatment: A review. Mol. Pharm. 2019, 17, 373–391. [Google Scholar] [CrossRef]
- Tavakoli, J.; Tang, Y. Hydrogel based sensors for biomedical applications: An updated review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; He, J.; Guo, B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, X.; Dong, J. Porifera-inspired cost-effective and scalable “porous hydrogel sponge” for durable and highly efficient solar-driven desalination. Chem. Eng. J. 2022, 427, 130905. [Google Scholar] [CrossRef]
- Palanivelu, S.D.; Armir, N.A.Z.; Zulkifli, A.; Hair, A.H.A.; Salleh, K.M.; Lindsey, K.; Che-Othman, M.H.; Zakaria, S. Hydrogel Application in Urban Farming: Potentials and Limitations—A Review. Polymers 2022, 14, 2590. [Google Scholar] [CrossRef]
- Mu, X.; Yuen, J.S.K., Jr.; Choi, J.; Zhang, Y.; Cebe, P.; Jiang, X.; Zhang, Y.S.; Kaplan, D.L. Conformation-driven strategy for resilient and functional protein materials. Proc. Natl. Acad. Sci. USA 2022, 119, e2115523119. [Google Scholar] [CrossRef]
- Shen, Y.; Levin, A.; Kamada, A.; Toprakcioglu, Z.; Rodriguez-Garcia, M.; Xu, Y.; Tuomas, P.J.K. From protein building blocks to functional materials. ACS Nano 2021, 15, 5819–5837. [Google Scholar] [CrossRef] [PubMed]
- Zustiak, S.P.; Wei, Y.; Leach, J.B. Protein–hydrogel interactions in tissue engineering: Mechanisms and applications. Tissue Eng. Part B-Rev. 2013, 19, 160–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davari, N.; Bakhtiary, N.; Khajehmohammadi, M.; Sarkari, S.; Tolabi, H.; Ghorbani, F.; Ghalandari, B. Protein-Based Hydrogels: Promising Materials for Tissue Engineering. Polymers 2022, 14, 986. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, X.; Li, X.; Ma, C.; Chu, X.; Wang, L.; Xu, W. A review on recent advances of Protein-Polymer hydrogels. Eur. Polym. J. 2022, 162, 110881. [Google Scholar] [CrossRef]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; Gonzalez-Garcia, C.; Dalby, M.J.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater. Today Bio. 2021, 10, 100098. [Google Scholar] [CrossRef]
- Nelson, D.W.; Gilbert, R.J. Extracellular Matrix-Mimetic Hydrogels for Treating Neural Tissue Injury: A Focus on Fibrin, Hyaluronic Acid, and Elastin-Like Polypeptide Hydrogels. Adv. Healthc. Mater. 2021, 10, 2101329. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Mashimo, Y.; Mie, M.; Kobatake, E. Temperature-responsive multifunctional protein hydrogels with elastin-like polypeptides for 3-D angiogenesis. Biomacromolecules 2020, 21, 1126–1135. [Google Scholar] [CrossRef]
- Kapoor, S.; Kundu, S.C. Silk protein-based hydrogels: Promising advanced materials for biomedical applications. Acta Biomater. 2016, 31, 17–32. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Cabral JM, S.; da Silva, C.L.; Vashishth, D. Bone matrix non-collagenous proteins in tissue engineering: Creating new bone by mimicking the extracellular matrix. Polymers 2021, 13, 1095. [Google Scholar] [CrossRef]
- Yue, S.; He, H.; Li, B.; Hou, T. Hydrogel as a biomaterial for bone tissue engineering: A review. Nanomaterials 2020, 10, 1511. [Google Scholar] [CrossRef]
- Pham, H.M.; Zhang, Y.; Munguia-Lopez, J.G.; Tran, S.D. Egg White Alginate as a Novel Scaffold Biomaterial for 3D Salivary Cell Culturing. Biomimetics 2021, 7, 5. [Google Scholar] [CrossRef]
- Zhang, Y.; Pham, H.M.; Munguia-Lopez, J.G.; Kinsella, J.M.; Tran, S.D. The optimization of a novel hydrogel—Egg white-alginate for 2.5 D tissue engineering of salivary spheroid-like structure. Molecules 2020, 25, 5751. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, M.; Zhu, K.X.; Guo, X.N.; Peng, W.; Zhou, H.M. Heat-induced interaction between egg white protein and wheat gluten. Food Chem. 2016, 197, 699–708. [Google Scholar] [CrossRef]
- He, W.; Xiao, N.; Zhao, Y.; Yao, Y.; Xu, M.; Du, H.; Wu, N.; Tu, Y. Effect of polysaccharides on the functional properties of egg white protein: A review. J. Food Sci. 2021, 86, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Babaei, J.; Khodaiyan, F.; Mohammadian, M. Effects of enriching with gellan gum on the structural, functional, and degradation properties of egg white heat-induced hydrogels. Int. J. Biol. Macromol. 2019, 128, 94–100. [Google Scholar] [CrossRef]
- Uman, S.; Dhand, A.; Burdick, J.A. Recent advances in shear-thinning and self-healing hydrogels for biomedical applications. J. Appl. Polym. Sci. 2020, 137, 48668. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Huang, X.; Ma, B.; Chen, Y.; Batool, Z.; Fu, X.; Jin, Y. Modification methods and applications of egg protein gel properties: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2233–2252. [Google Scholar] [CrossRef] [PubMed]
- Stamboroski, S.; Boateng, K.; Lierath, J.; Kowalik, T.; Thiel, K.; Koppen, S.; Noeske, P.L.M.; Bruggemann, D. Influence of divalent metal ions on the precipitation of the plasma protein fibrinogen. Biomacromolecules 2021, 22, 4642–4658. [Google Scholar] [CrossRef]
- Deng, C.; Shao, Y.; Xu, M.; Yao, Y.; Wu, N.; Hu, H.; Zhao, Y.; Tu, Y. Effects of metal ions on the physico-chemical, microstructural and digestion characteristics of alkali-induced egg white gel. Food Hydrocoll. 2020, 107, 105956. [Google Scholar] [CrossRef]
- Wei, Z.Z.; Dong, X.; Zhang, Y.Q. A mechanically robust egg white hydrogel scaffold with excellent biocompatibility by three-step green processing. Sci. China Technol. Sci. 2022, 65, 1599–1612. [Google Scholar] [CrossRef]
- Huang, K.; Hou, J.; Gu, Z.; Wu, J. Egg-white-/eggshell-based biomimetic hybrid hydrogels for bone regeneration. ACS Biomater. Sci. Eng. 2019, 5, 5384–5391. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, M.; Zhang, Y.; Yin, J.; Pei, R. Nanocomposite hydrogels for tissue engineering applications. Nanoscale 2020, 12, 14976–14995. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Zhang, W.; Zhang, Z.; Jiang, X. Recent advances in scaffold design and material for vascularized tissue-engineered bone regeneration. Adv. Healthc. Mater. 2019, 8, 1801433. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Y.N.; Ma, B.; Shao, J.; Liu, H.; Ge, S. Gingipain-responsive thermosensitive hydrogel loaded with SDF-1 facilitates in situ periodontal tissue regeneration. ACS Appl. Mater. Inter. 2021, 13, 36880–36893. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Deng, X.; Attalla, R.; Sadowski, L.P.; Chen, M.; Majcher, M.J.; Urosev, I.; Yin, D.C.; Selvaganapathy, P.R.; Filipe, C.D.M.; Hoare, T. Autonomously Self-Adhesive Hydrogels as Building Blocks for Additive Manufacturing. Biomacromolecules 2018, 19, 62–70. [Google Scholar] [CrossRef]
- Gao, X.; Yao, Y.; Wu, N.; Xu, M.; Zhao, Y.; Tu, Y. The sol-gel-sol transformation behavior of egg white proteins induced by alkali. Int. J. Biol. Macromol. 2020, 155, 588–597. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, Q.; Ming, J.; Dou, H.; Zuo, B.; Qin, M.; Li, F.; Kaplan, D.L.; Zhang, X. Silk dissolution and regeneration at the nanofibril scale. J. Mater. Chem. B 2014, 2, 3879–3885. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, X.; Shi, S.Q.; Li, J. A tough and mildew-proof soybean-based adhesive inspired by mussel and algae. Polymers 2020, 12, 756. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.R.; Zhang, B.; Fan, J.L.; Yang, Q.; Chen, H.Q. Effects of sodium tripolyphosphate modification on the structural, functional, and rheological properties of rice glutelin. Food Chem. 2019, 281, 18–27. [Google Scholar] [CrossRef]
- Li, K.; Jin, S.; Wei, Y.; Li, X.; Li, J.; Shi, S.Q.; Li, J. Bioinspired hyperbranched protein adhesive based on boronic acid-functionalized cellulose nanofibril and water-soluble polyester. Compos. Part B-Eng. 2021, 219, 108943. [Google Scholar] [CrossRef]
- Farjami, T.; Babaei, J.; Nau, F.; Dupont, D.; Madadlou, A. Effects of thermal, non-thermal and emulsification processes on the gastrointestinal digestibility of egg white proteins. Trends Food Sci. Technol. 2021, 107, 45–56. [Google Scholar] [CrossRef]
- Van den Berg, L.; Rosenberg, Y.; Van Boekel, M.A.J.S.; Rosenberg, M.; Velde, F. Microstructural features of composite whey protein/polysaccharide gels characterized at different length scales. Food Hydrocoll. 2009, 23, 1288–1298. [Google Scholar] [CrossRef]
- Sheng, L.; Liu, Q.; Dong, W.; Cai, Z. Effect of high intensity ultrasound assisted glycosylation on the gel properties of ovalbumin: Texture, rheology, water state and microstructure. Food Chem. 2022, 372, 131215. [Google Scholar] [CrossRef]
- Guo, L.; Niu, X.; Chen, X.; Lu, F.; Gao, J.; Chang, Q. 3D direct writing egg white hydrogel promotes diabetic chronic wound healing via self-relied bioactive property. Biomaterials 2022, 282, 121406. [Google Scholar] [CrossRef]
- Chang, Q.; Darabi, M.A.; Liu, Y.; He, Y.; Zhong, W.; Mequanin, K.; Li, B.; Lu, F.; Xing, M.M.Q. Hydrogels from natural egg white with extraordinary stretchability, direct-writing 3D printability and self-healing for fabrication of electronic sensors and actuators. J. Mater. Chem. A 2019, 7, 24626–24640. [Google Scholar] [CrossRef]
- Ganji, F.; Vasheghani, F.S.; Vasheghani, F.E. Theoretical description of hydrogel swelling: A review. Iran. Polym. J. 2010, 19, 375–398. [Google Scholar]
- Olad, A.; Doustdar, F.; Gharekhani, H. Starch-based semi-IPN hydrogel nanocomposite integrated with clinoptilolite: Preparation and swelling kinetic study. Carbohyd. Polym. 2018, 200, 516–528. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, G.; Huang, Y. Prepration and characterization of silk fibroin-polyurethane composite hydrogels. Acta Polym. Sin. 2012, 12, 965–971. [Google Scholar] [CrossRef]
- Lau, M.H.; Tang, J.; Paulson, A.T. Texture profile and turbidity of gellan/gelatin mixed gels. Food Res. Int. 2000, 33, 665–671. [Google Scholar] [CrossRef]
- Chandra, M.V.; Shamasundar, B.A. Texture profile analysis and functional properties of gelatin from the skin of three species of fresh water fish. Int. J. Food Prop. 2015, 18, 572–584. [Google Scholar] [CrossRef]
- Chakraborty, A.; Basak, S. Interaction with Al and Zn induces structure formation and aggregation in natively unfolded caseins. J. Photochem. Photobiol. B 2008, 93, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Zhao, Y.; Xu, M.; Chen, Z.; Wang, S.; Tu, Y. Effects of copper ions on the charteristics of egg white gel induced by strong alkali. Poultry Sci. 2017, 96, 4116–4123. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Hu, Y.; Deng, Y.; Su, J. Recent advances in design of functional biocompatible hydrogels for bone tissue engineering. Adv. Funct. Mater. 2021, 31, 2009432. [Google Scholar] [CrossRef]


| Concentrations of CaCl2 Solution | Hydrogel Samples | Relative Content (%) | |||
|---|---|---|---|---|---|
| β-Sheets | Random Coil | α-Helices | β-Turns | ||
| 0% | EWG0 | 23.56 | 14.93 | 14.56 | 46.96 |
| 0.1% | EWG1 | 34.54 | 12.35 | 11.41 | 41.71 |
| 0.5% | EWG2 | 33.21 | 13.55 | 13.34 | 39.91 |
| 1% | EWG3 | 36.38 | 14.54 | 15.11 | 33.98 |
| 2% | EWG4 | 21.98 | 31.12 | 15.81 | 31.08 |
| Sample | EWG0 | EWG1 | EWG2 | EWG3 | EWG4 |
|---|---|---|---|---|---|
| A | 52.02 | 57.89 | 148.32 | 96.25 | 63.46 |
| B | 0.23 | 0.28 | 0.39 | 0.59 | 2.19 |
| Ks × 10−3 (min−1) | 1.08 | 1.39 | 1.09 | 3.68 | 75.83 |
| S∞ (%) | 3969.3 | 3501.9 | 1675.5 | 1756.4 | 492.5 |
| ESR (%) | 5463.1 | 4786.1 | 2657.8 | 2219.7 | 518.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, B.; Yang, M.; Chao, Q.; Wang, L.; Zhang, L.; Gou, M.; Li, Y.; Liu, C.; Lu, K. Preparation and Properties of Egg White Dual Cross-Linked Hydrogel with Potential Application for Bone Tissue Engineering. Polymers 2022, 14, 5116. https://doi.org/10.3390/polym14235116
Duan B, Yang M, Chao Q, Wang L, Zhang L, Gou M, Li Y, Liu C, Lu K. Preparation and Properties of Egg White Dual Cross-Linked Hydrogel with Potential Application for Bone Tissue Engineering. Polymers. 2022; 14(23):5116. https://doi.org/10.3390/polym14235116
Chicago/Turabian StyleDuan, Bingchao, Minghui Yang, Quanchao Chao, Lan Wang, Lingli Zhang, Mengxing Gou, Yuling Li, Congjun Liu, and Kui Lu. 2022. "Preparation and Properties of Egg White Dual Cross-Linked Hydrogel with Potential Application for Bone Tissue Engineering" Polymers 14, no. 23: 5116. https://doi.org/10.3390/polym14235116