Protein Adsorption Performance of a Novel Functionalized Cellulose-Based Polymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
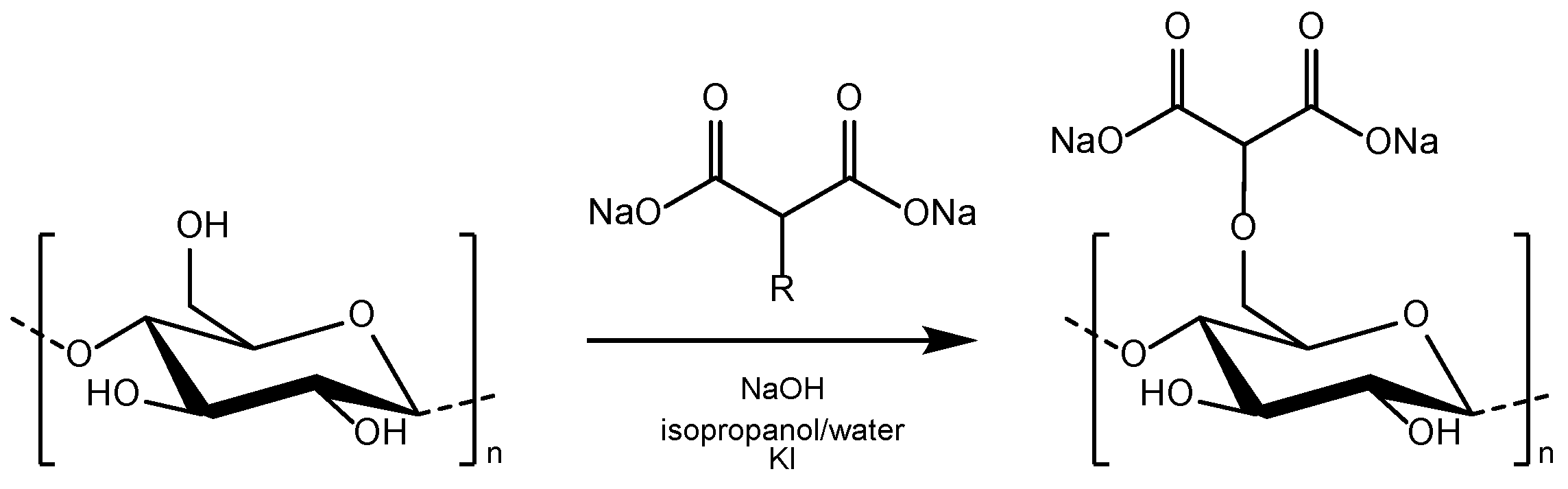
2.2. Preparation of Dicarboxymethyl Cellulose
2.3. Characterization of Dicarboxymethyl Cellulose
2.4. Protein Adsorption Studies
2.5. Reusability Study
3. Results and Discussion
3.1. Characterization of Dicarboxymethyl Cellulose
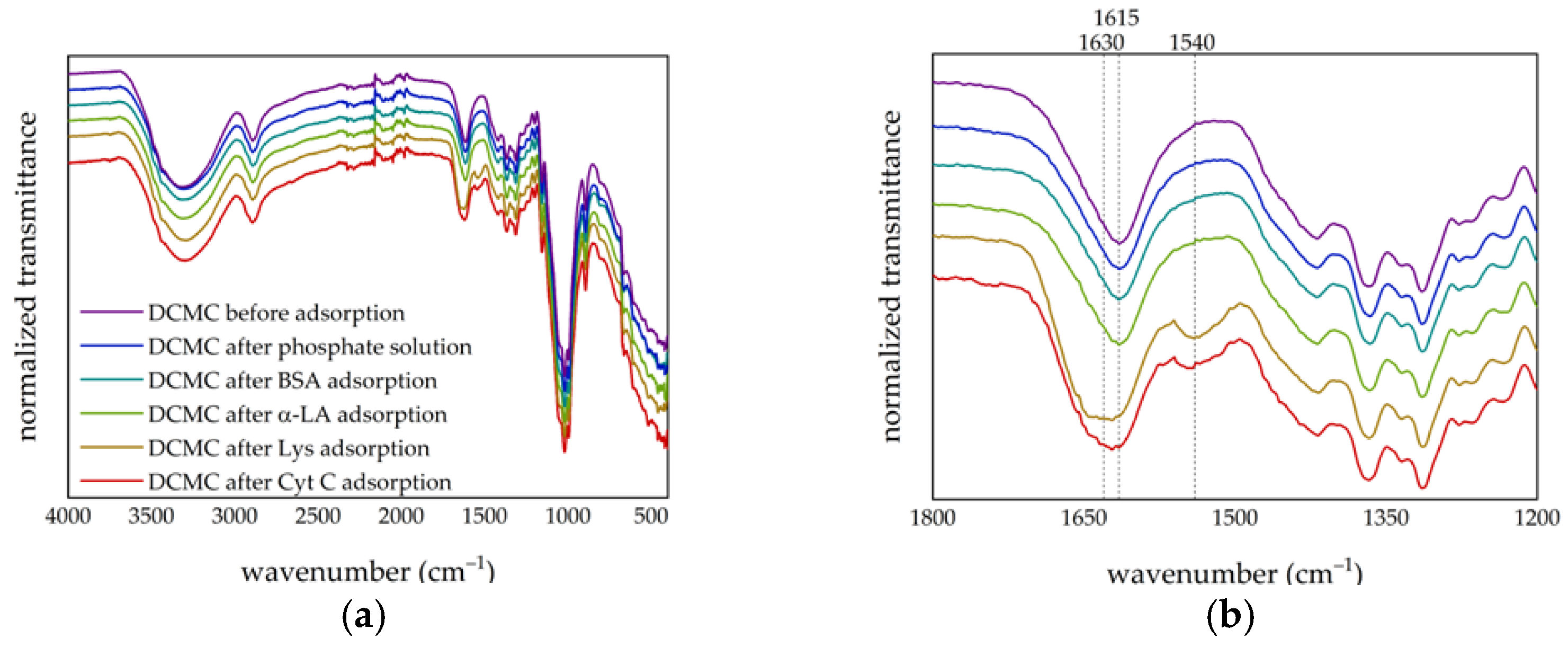
3.1.1. FT-IR Analysis
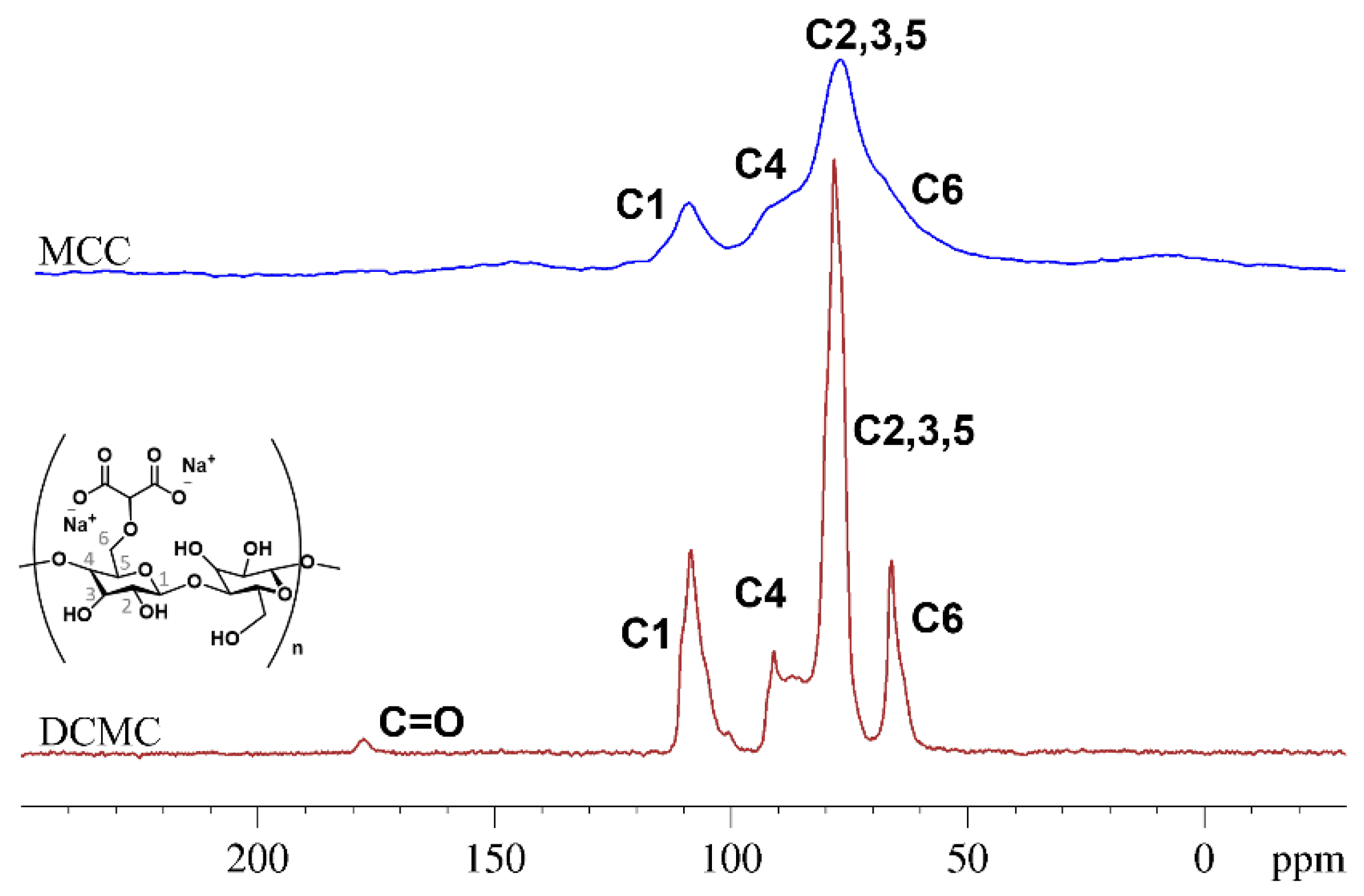
3.1.2. Solid-State 13C NMR Spectroscopy
3.1.3. Thermal Analysis
3.1.4. BET Surface Analysis
3.1.5. Degree of Substitution
3.2. Protein Adsorption Experiments Using Dicarboxymethyl Cellulose
Effect of Adsorbent Dosage
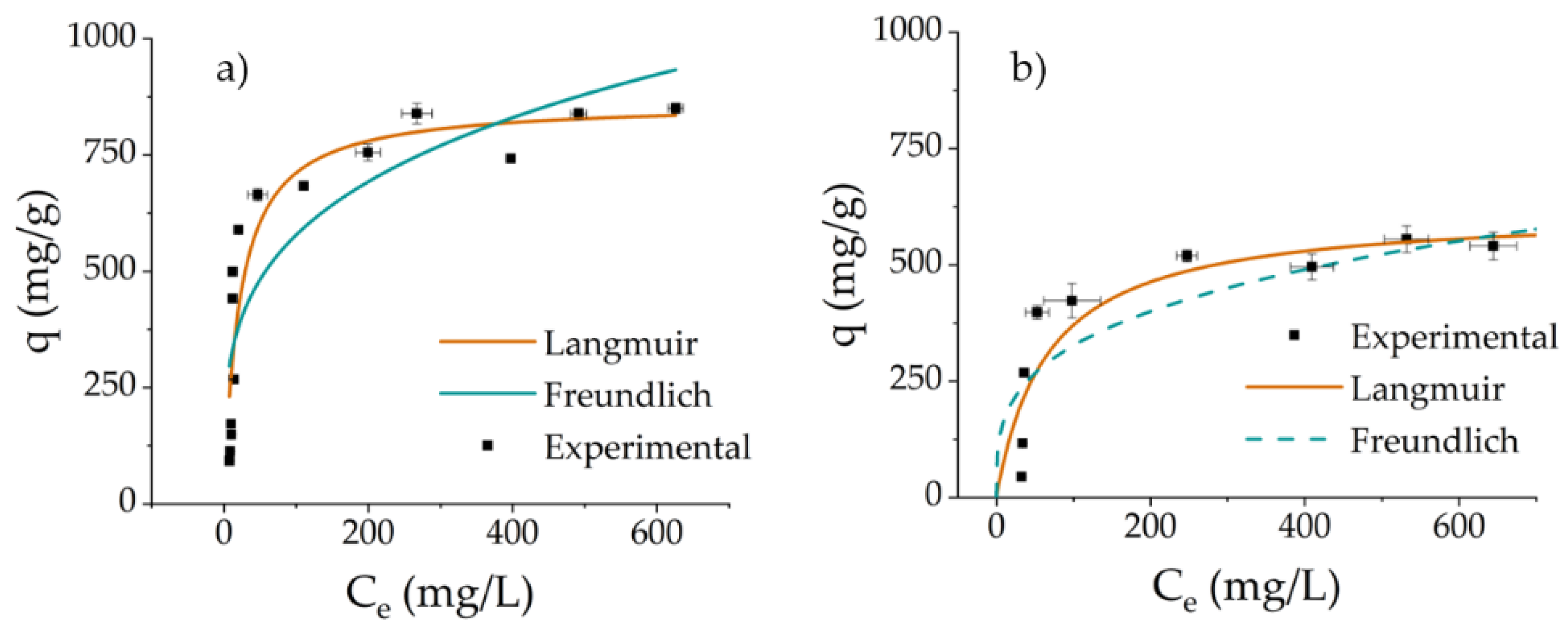
3.3. Adsorption Isotherms
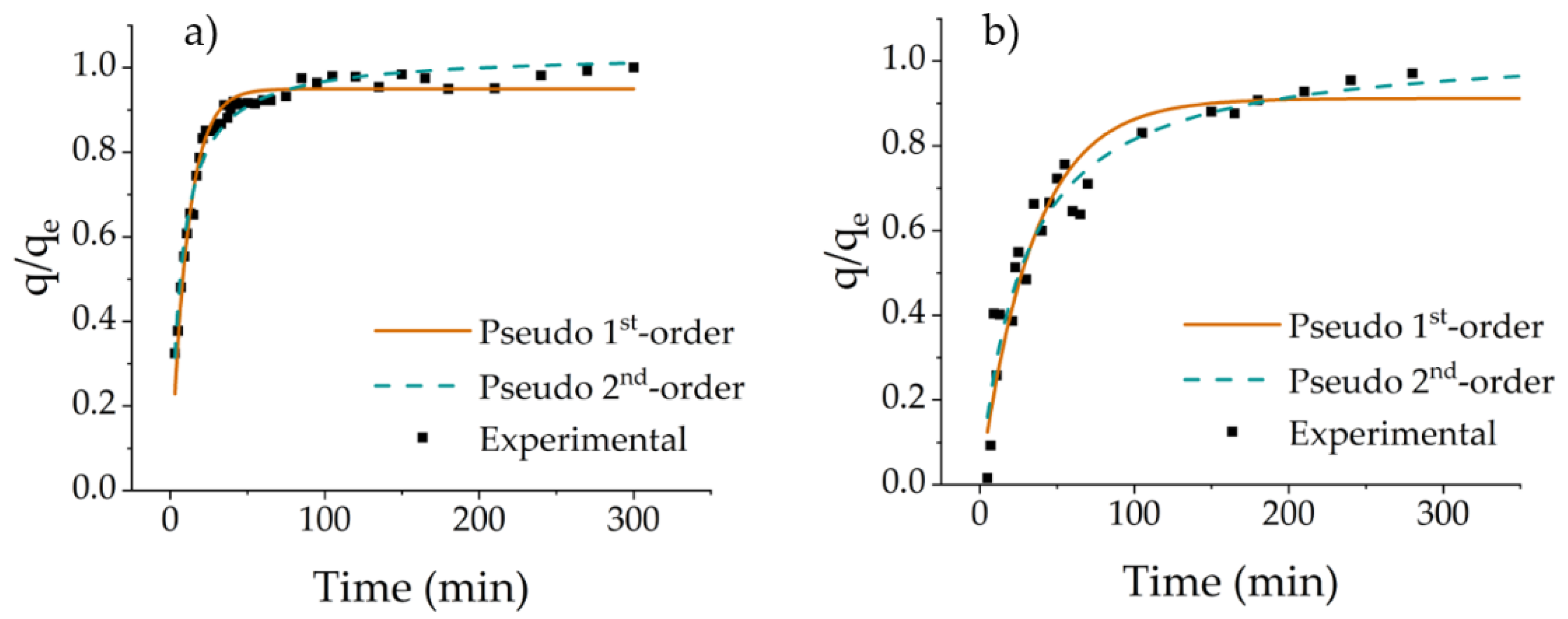
3.4. Adsorption Kinetics
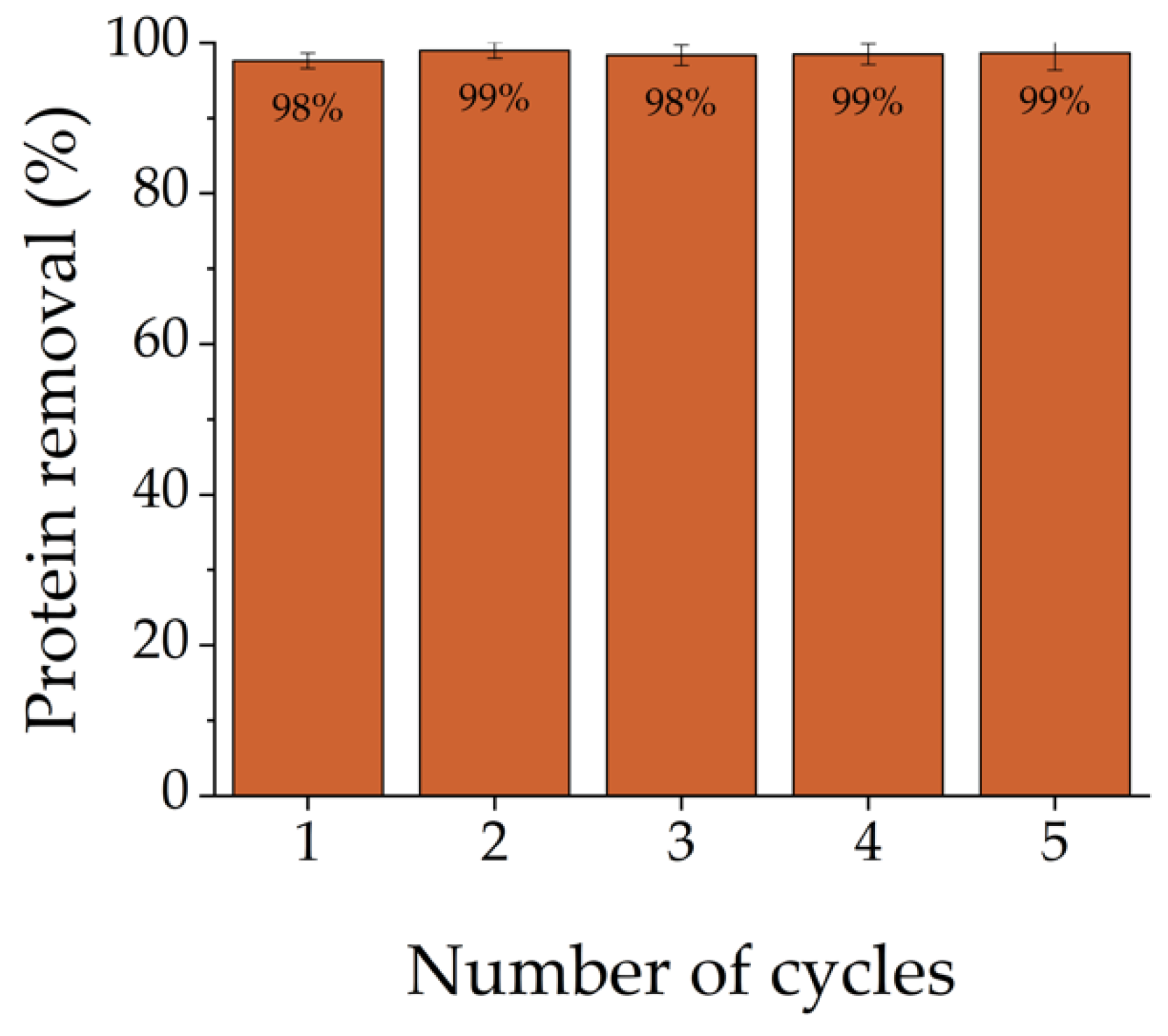
3.5. Reusability Study
3.6. Proposed Adsorption Mechanism of DCMC
3.7. Comparison with Other Adsorbents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiao, L.; Zhao, L.; Ai, H.; Li, Y.; Liu, Y.; Du, K. Diethylaminoethyl-Modified Magnetic Starlike Organic Spherical Adsorbent: Fabrication, Characterization, and Potential for Protein Adsorption. Ind. Eng. Chem. Res. 2019, 58, 4099–4107. [Google Scholar] [CrossRef]
- Mesgari-Shadi, A.; Sarrafzadeh, M.-H.; Divband, B.; Barar, J.; Omidi, Y. Batch adsorption/desorption for purification of scFv antibodies using nanozeolite microspheres. Microporous Mesoporous Mater. 2018, 264, 167–175. [Google Scholar] [CrossRef]
- Atyaksheva, L.F.; Kasyanov, I.A.; Ivanova, I.I. Adsorptive Immobilization of Proteins on Mesoporous Molecular Sieves and Zeolites. Pet. Chem. 2019, 59, 327–337. [Google Scholar] [CrossRef]
- Bhakta, S.A.; Evans, E.; Benavidez, T.E.; Garcia, C.D. Protein adsorption onto nanomaterials for the development of biosensors and analytical devices: A review. Anal. Chim. Acta 2015, 872, 7–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hlady, V.; Buijs, J. Protein adsorption on solid surfaces. Curr. Opin. Biotechnol. 1996, 7, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Talha, M.; Ma, Y.; Kumar, P.; Lin, Y.; Singh, A. Role of protein adsorption in the bio corrosion of metallic implants—A review. Colloids Surf. B Biointerfaces 2019, 176, 494–506. [Google Scholar] [CrossRef]
- Quan, X.; Liu, J.; Zhou, J. Multiscale modeling and simulations of protein adsorption: Progresses and perspectives. Curr. Opin. Colloid Interface Sci. 2019, 41, 74–85. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, Y.-T.; Zeng, K.; Heinze, T.; Groth, T.; Zhang, K. Recent Progress on Cellulose-Based Ionic Compounds for Biomaterials. Adv. Mater. 2021, 33, 2000717. [Google Scholar] [CrossRef] [Green Version]
- Matsui, M.; Kiyozumi, Y.; Mizushina, Y.; Sakaguchi, K.; Mizukami, F. Adsorption and desorption behavior of basic proteins on zeolites. Sep. Purif. Technol. 2015, 149, 103–109. [Google Scholar] [CrossRef]
- Höhn, S.; Virtanen, S.; Boccaccini, A.R. Protein adsorption on magnesium and its alloys: A review. Appl. Surf. Sci. 2019, 464, 212–219. [Google Scholar] [CrossRef]
- Rahmati, M.; Mozafari, M. Protein adsorption on polymers. Mater. Today Commun. 2018, 17, 527–540. [Google Scholar] [CrossRef]
- Firkowska-Boden, I.; Zhang, X.; Jandt, K.D. Controlling Protein Adsorption through Nanostructured Polymeric Surfaces. Adv. Healthc. Mater. 2018, 7, 1700995. [Google Scholar] [CrossRef]
- Rana, A.K.; Frollini, E.; Thakur, V.K. Cellulose nanocrystals: Pretreatments, preparation strategies, and surface functionalization. Int. J. Biol. Macromol. 2021, 182, 1554–1581. [Google Scholar] [CrossRef]
- Muqeet, M.; Mahar, R.B.; Gadhi, T.A.; Ben Halima, N. Insight into cellulose-based-nanomaterials—A pursuit of environmental remedies. Int. J. Biol. Macromol. 2020, 163, 1480–1486. [Google Scholar] [CrossRef]
- Peng, B.; Yao, Z.; Wang, X.; Crombeen, M.; Sweeney, D.G.; Tam, K.C. Cellulose-based materials in wastewater treatment of petroleum industry. Green Energy Environ. 2020, 5, 37–49. [Google Scholar] [CrossRef]
- Wahib, S.A.; Da’na, D.A.; Ashfaq, M.Y.; Al-Ghouti, M.A. Functionalized cellulose nanocrystals as a novel adsorption material for removal of boron from water. Case Stud. Chem. Environ. Eng. 2021, 4, 100121. [Google Scholar] [CrossRef]
- Liu, C.; Yu, J.; You, J.; Wang, Z.; Zhang, M.; Shi, L.; Zhuang, X. Cellulose/Chitosan Composite Sponge for Efficient Protein Adsorption. Ind. Eng. Chem. Res. 2021, 60, 9159–9166. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Dalei, G.; Das, S.; Pradhan, M. Dialdehyde cellulose as a niche material for versatile applications: An overview. Cellulose 2022, 29, 5429–5461. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Elgarhy, G.S.; El-Subruiti, G.M.; Omer, A.M. Carboxymethyl cellulose/carboxylated graphene oxide composite microbeads for efficient adsorption of cationic methylene blue dye. Int. J. Biol. Macromol. 2020, 154, 307–318. [Google Scholar] [CrossRef]
- Tanzifi, M.; Tavakkoli Yaraki, M.; Beiramzadeh, Z.; Heidarpoor Saremi, L.; Najafifard, M.; Moradi, H.; Mansouri, M.; Karami, M.; Bazgir, H. Carboxymethyl cellulose improved adsorption capacity of polypyrrole/CMC composite nanoparticles for removal of reactive dyes: Experimental optimization and DFT calculation. Chemosphere 2020, 255, 127052. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, K.A.; Oyedotun, K.O.; Ighalo, J.O.; Amaku, J.F.; Olisah, C.; Adeola, A.O.; Iwuozor, K.O.; Akpomie, K.G.; Conradie, J. Cellulose derivatives and cellulose-metal-organic frameworks for CO2 adsorption and separation. J. CO2 Util. 2022, 64, 102163. [Google Scholar] [CrossRef]
- Bethke, K.; Palantöken, S.; Andrei, V.; Roß, M.; Raghuwanshi, V.S.; Kettemann, F.; Greis, K.; Ingber, T.T.K.; Stückrath, J.B.; Valiyaveettil, S.; et al. Functionalized Cellulose for Water Purification, Antimicrobial Applications, and Sensors. Adv. Funct. Mater. 2018, 28, 1800409. [Google Scholar] [CrossRef]
- Xia, L.; Wang, A.; Zhang, C.; Liu, Y.; Guo, H.; Ding, C.; Wang, Y.; Xu, W. Environmentally friendly dyeing of cotton in an ethanol–water mixture with excellent exhaustion. Green Chem. 2018, 20, 4473–4483. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Hossain, M.S.; Kassim, M.H.M.; Balakrishnan, V.; Habila, M.A.; Zulkharnain, A.; Zulkifli, M.; Yahaya, A.N.A. Biosorption of Cr(VI) Using Cellulose Nanocrystals Isolated from the Waterless Pulping of Waste Cotton Cloths with Supercritical CO2: Isothermal, Kinetics, and Thermodynamics Studies. Polymers 2022, 14, 887. [Google Scholar] [CrossRef]
- Al-Gorair, A.S.; Sayed, A.; Mahmoud, G.A. Engineered Superabsorbent Nanocomposite Reinforced with Cellulose Nanocrystals for Remediation of Basic Dyes: Isotherm, Kinetic and Thermodynamic Studies. Polymers 2022, 14, 567. [Google Scholar] [CrossRef]
- Bertsch, P.; Fischer, P. Adsorption and interfacial structure of nanocelluloses at fluid interfaces. Adv. Colloid Interface Sci. 2020, 276, 102089. [Google Scholar] [CrossRef]
- Onwukamike, K.N.; Grelier, S.; Grau, E.; Cramail, H.; Meier, M.A.R. Critical Review on Sustainable Homogeneous Cellulose Modification: Why Renewability Is Not Enough. ACS Sustain. Chem. Eng. 2019, 7, 1826–1840. [Google Scholar] [CrossRef] [Green Version]
- Chagas, R.; Gericke, M.; Ferreira, R.B.; Heinze, T.; Ferreira, L.M. Synthesis and characterization of dicarboxymethyl cellulose. Cellulose 2020, 27, 1965–1974. [Google Scholar] [CrossRef] [Green Version]
- Gago, D.; Chagas, R.; Ferreira, L.M. The Effect of Dicarboxymethyl Cellulose on the Prevention of Protein Haze Formation on White Wine. Beverages 2021, 7, 57. [Google Scholar] [CrossRef]
- Gago, D.; Chagas, R.; Ferreira, L.M.; Velizarov, S.; Coelhoso, I. A Novel Cellulose-Based Polymer for Efficient Removal of Methylene Blue. Membranes 2020, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Saracino, F.; Brinco, J.; Gago, D.; da Silva, M.G.; Ferreira, R.B.; Ricardo-da-Silva, J.; Chagas, R.; Ferreira, L.M. DCMC as a Promising Alternative to Bentonite in White Wine Stabilization. Impact on Protein Stability and Wine Aromatic Fraction. Molecules 2021, 26, 6188. [Google Scholar] [CrossRef]
- Chen, J.; Xu, E.; Wei, Y.; Chen, M.; Wei, T.; Zheng, S. Graph Clustering Analyses of Discontinuous Molecular Dynamics Simulations: Study of Lysozyme Adsorption on a Graphene Surface. Langmuir 2022, 38, 10817–10825. [Google Scholar] [CrossRef]
- Akkaya, R.; Akkaya, B.; Çakıcı, G.T. Chitosan–poly(acrylamide-co-maleic acid) composite synthesis, characterization, and investigation of protein adsorption behavior. Polym. Bull. 2022, 1–16. [Google Scholar] [CrossRef]
- Layman, D.K.; Lönnerdal, B.; Fernstrom, J.D. Applications for α-lactalbumin in human nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, X.; Wang, M.; Zhang, D.; Yang, Q.; Liu, T.; Lei, R.; Zhu, S.; Zhao, Y.; Chen, C. Probing Adsorption Behaviors of BSA onto Chiral Surfaces of Nanoparticles. Small 2018, 14, 1703982. [Google Scholar] [CrossRef]
- Zhao, D.; Li, L.; Zhou, J. Simulation insight into the cytochrome c adsorption on graphene and graphene oxide surfaces. Appl. Surf. Sci. 2018, 428, 825–834. [Google Scholar] [CrossRef]
- Shang, W.; Nuffer, J.H.; Muñiz-Papandrea, V.A.; Colón, W.; Siegel, R.W.; Dordick, J.S. Cytochrome c on Silica Nanoparticles: Influence of Nanoparticle Size on Protein Structure, Stability, and Activity. Small 2009, 5, 470–476. [Google Scholar] [CrossRef]
- Perçin, I.; Karakoç, V.; Ergün, B.; Denizli, A. Metal-immobilized magnetic nanoparticles for cytochrome C purification from rat liver. Biotechnol. Appl. Biochem. 2016, 63, 31–40. [Google Scholar] [CrossRef]
- Li, Z.; Guan, P.; Hu, X.; Ding, S.; Tian, Y.; Xu, Y.; Qian, L. Preparation of Molecularly Imprinted Mesoporous Materials for Highly Enhancing Adsorption Performance of Cytochrome C. Polymers 2018, 10, 298. [Google Scholar] [CrossRef]
- Gai, K.; Kang, M.; Huang, Q.; Zheng, S.; Zhang, L.; Zhang, C.; Hao, L. A novel, green, and biocompatible graphene-based carbonaceous material for immobilization of cytochrome c. J. Mater. Res. 2018, 33, 4270–4277. [Google Scholar] [CrossRef]
- Simoes-Cardoso, J.C.; Kojo, H.; Yoshimoto, N.; Yamamoto, S. Microcalorimetric Analysis of the Adsorption of Lysozyme and Cytochrome c onto Cation-Exchange Chromatography Resins: Influence of Temperature on Retention. Langmuir 2020, 36, 3336–3345. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Senan, P. Adsorption characteristics of cytochrome C onto cationic Langmuir monolayers of sulfonated poly(glycidylmethacrylate)-grafted cellulose: Mass transfer analysis, isotherm modeling and thermodynamics. Chem. Eng. J. 2011, 168, 678–690. [Google Scholar] [CrossRef]
- Alveroglu, E.; İlker, N.; Shah, M.T.; Rajar, K.; Gokceoren, A.T.; Koc, K. Effects of gel morphology on the lysozyme adsorption and desorption kinetics of temperature sensitive magnetic gel composites. Colloids Surf. B Biointerfaces 2019, 181, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Odabaşi, M.; Say, R.; Denizli, A. Molecular imprinted particles for lysozyme purification. Mater. Sci. Eng. C 2007, 27, 90–99. [Google Scholar] [CrossRef]
- Show, P.L.; Ooi, C.W.; Lee, X.J.; Yang, C.-L.; Liu, B.-L.; Chang, Y.-K. Batch and dynamic adsorption of lysozyme from chicken egg white on dye-affinity nanofiber membranes modified by ethylene diamine and chitosan. Int. J. Biol. Macromol. 2020, 162, 1711–1724. [Google Scholar] [CrossRef]
- Pereira, R.G.; Veloso, C.M.; da Silva, N.M.; de Sousa, L.F.; Bonomo, R.C.F.; de Souza, A.O.; Souza, M.O.d.G.; Fontan, R.d.C.I. Preparation of activated carbons from cocoa shells and siriguela seeds using H3PO4 and ZnCL2 as activating agents for BSA and α-lactalbumin adsorption. Fuel Process. Technol. 2014, 126, 476–486. [Google Scholar] [CrossRef]
- Cabilio, N.R.; Omanovic, S.; Roscoe, S.G. Electrochemical Studies of the Effect of Temperature and pH on the Adsorption of α-Lactalbumin at Pt. Langmuir 2000, 16, 8480–8488. [Google Scholar] [CrossRef]
- Narambuena, C.F. On the reasons for α-lactalbumin adsorption on a charged surface: A study by Monte Carlo simulation. Colloids Surf. B Biointerfaces 2019, 174, 511–520. [Google Scholar] [CrossRef]
- Lim, G.W.; Lim, J.K.; Ahmad, A.L.; Chan, D.J.C. Influences of diatom frustule morphologies on protein adsorption behavior. J. Appl. Phycol. 2015, 27, 763–775. [Google Scholar] [CrossRef]
- Soleymani, M.; Akbari, A.; Mahdavinia, G.R. Magnetic PVA/laponite RD hydrogel nanocomposites for adsorption of model protein BSA. Polym. Bull. 2019, 76, 2321–2340. [Google Scholar] [CrossRef]
- Wang, J.; Huyan, Y.; Yang, Z.; Zhang, H.; Zhang, A.; Kou, X.; Zhang, Q.; Zhang, B. Preparation of surface protein imprinted thermosensitive polymer monolithic column and its specific adsorption for BSA. Talanta 2019, 200, 526–536. [Google Scholar] [CrossRef]
- Kubiak-Ossowska, K.; Jachimska, B.; Al Qaraghuli, M.; Mulheran, P.A. Protein interactions with negatively charged inorganic surfaces. Curr. Opin. Colloid Interface Sci. 2019, 41, 104–117. [Google Scholar] [CrossRef] [Green Version]
- Baghdady, Y.Z.; Schug, K.A. Online Comprehensive High pH Reversed Phase × Low pH Reversed Phase Approach for Two-Dimensional Separations of Intact Proteins in Top-Down Proteomics. Anal. Chem. 2019, 91, 11085–11091. [Google Scholar] [CrossRef]
- Lu, J.; Wan, Y.; Cui, Z. Fractionation of Lysozyme and Chicken Egg Albumin Using Ultrafiltration with 30-kDa Commercial Membranes. Ind. Eng. Chem. Res. 2005, 44, 7610–7616. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. A 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Lagergren, S. About the Theory of So-Called Adsorption of Soluble Substances. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Alnaief, M.; Sandouqa, A.; Altarawneh, I.; Al-Shannag, M.; Alkasrawi, M.; Al-hamamre, Z. Adsorption Characteristics and Potential of Olive Cake Alkali Residues for Biodiesel Purification. Energies 2021, 14, 16. [Google Scholar] [CrossRef]
- Eyni, H.; Tahermansouri, H.; Kiani, F.; Jahangiri, M. Kinetics, equilibrium and isotherms of Pb2+ adsorption from aqueous solutions on carbon nanotubes functionalized with 3-amino-5a,10a-dihydroxybenzo[b] indeno [2,l-d]furan-10-one. New Carbon Mater. 2019, 34, 512–523. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, Y.; Feng, C.; Zhang, Z.; Lei, Z.; Shimizu, K. Predicting equilibrium time by adsorption kinetic equations and modifying Langmuir isotherm by fractal-like approach. J. Mol. Liq. 2018, 268, 728–733. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, A. Development and validation of an adsorption kinetic model at solid-liquid interface using normalized Gudermannian function. J. Mol. Liq. 2019, 276, 67–77. [Google Scholar] [CrossRef]
- Gorgieva, S.; Vogrinčič, R.; Kokol, V. The Effect of Membrane Structure Prepared from Carboxymethyl Cellulose and Cellulose Nanofibrils for Cationic Dye Removal. J. Polym. Environ. 2019, 27, 318–332. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.S.; Nasir, M.F.; Murtaza, A. Study of carboxymethyl cellulose (CMC) coated manganite as potential candidate for magnetic hyperthermia applications. Mater. Chem. Phys. 2022, 286, 126198. [Google Scholar] [CrossRef]
- Liu, C.; Omer, A.M.; Ouyang, X.-K. Adsorptive removal of cationic methylene blue dye using carboxymethyl cellulose/k-carrageenan/activated montmorillonite composite beads: Isotherm and kinetic studies. Int. J. Biol. Macromol. 2018, 106, 823–833. [Google Scholar] [CrossRef]
- Ayouch, I.; Kassem, I.; Kassab, Z.; Barrak, I.; Barhoun, A.; Jacquemin, J.; Draoui, K.; Achaby, M.E. Crosslinked carboxymethyl cellulose-hydroxyethyl cellulose hydrogel films for adsorption of cadmium and methylene blue from aqueous solutions. Surf. Interfaces 2021, 24, 101124. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Rayung, M.; Norrrahim, M.N.F.; Shazleen, S.S.; Rani, M.S.A.; Shafi, A.R.; Aisyah, H.A.; Radzi, M.H.M.; Sabaruddin, F.A.; et al. Thermogravimetric Analysis Properties of Cellulosic Natural Fiber Polymer Composites: A Review on Influence of Chemical Treatments. Polymers 2021, 13, 2710. [Google Scholar] [CrossRef]
- Obele, C.M.; Ibenta, M.E.; Chukwuneke, J.L.; Nwanonenyi, S.C. Carboxymethyl cellulose and cellulose nanocrystals from cassava stem as thickeners in reactive printing of cotton. Cellulose 2021, 28, 2615–2633. [Google Scholar] [CrossRef]
- Wasupon, W. Fast and practical synthesis of carboxymethyl cellulose from office paper waste by ultrasonic-assisted technique at ambient temperature. Polym. Degrad. Stab. 2021, 184, 109473. [Google Scholar] [CrossRef]
- Clark, L.W. The decarboxylation of malonic acid in acid media. J. Phys. Chem. 1960, 64, 41–43. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Andrade Siqueira, T.C.; Zanette da Silva, I.; Rubio, A.J.; Bergamasco, R.; Gasparotto, F.; Aparecida de Souza Paccola, E.; Ueda Yamaguchi, N. Sugarcane Bagasse as an Efficient Biosorbent for Methylene Blue Removal: Kinetics, Isotherms and Thermodynamics. Int. J. Environ. Res. Public Health 2020, 17, 526. [Google Scholar] [CrossRef] [Green Version]
- Suhas; Gupta, V.K.; Carrott, P.J.M.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 2016, 216, 1066–1076. [Google Scholar] [CrossRef]
- Joseph, L.; Sylas, V.P.; Cyril, N.; Sanu, K.S.; Jose, S.; Anila, B.N.; Jose, J.M. Removal of endrin from aqueous medium using Accacia wood biochar: Kinetics and thermodynamic studies. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Rasoulpoor, K.; Poursattar Marjani, A.; Nozad, E. Competitive chemisorption and physisorption processes of a walnut shell based semi-IPN bio-composite adsorbent for lead ion removal from water: Equilibrium, Kinetic and Thermodynamic studies. Environ. Technol. Innov. 2020, 20, 101133. [Google Scholar] [CrossRef]
- Komorek, P.; Martin, E.; Jachimska, B. Adsorption and Conformation Behavior of Lysozyme on a Gold Surface Determined by QCM-D, MP-SPR, and FTIR. Int. J. Mol. Sci. 2021, 22, 1322. [Google Scholar] [CrossRef]
- van de Weert, M.; van ’t Hof, R.; van der Weerd, J.; Heeren, R.M.A.; Posthuma, G.; Hennink, W.E.; Crommelin, D.J.A. Lysozyme distribution and conformation in a biodegradable polymer matrix as determined by FTIR techniques. J. Control. Release 2000, 68, 31–40. [Google Scholar] [CrossRef]
- Edwards, J.V.; Prevost, N.T.; Condon, B.; French, A. Covalent attachment of lysozyme to cotton/cellulose materials: Protein verses solid support activation. Cellulose 2011, 18, 1239–1249. [Google Scholar] [CrossRef]
- Tamahkar, E.; Kutsal, T.; Denizli, A. Surface imprinted bacterial cellulose nanofibers for cytochrome c purification. Process Biochem. 2015, 50, 2289–2297. [Google Scholar] [CrossRef]
- Bushnell, G.W.; Louie, G.V.; Brayer, G.D. High-resolution three-dimensional structure of horse heart cytochrome c. J. Mol. Biol. 1990, 214, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Diamond, R. Real-space refinement of the structure of hen egg-white lysozyme. J. Mol. Biol. 1974, 82, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Chrysina, E.D.; Brew, K.; Acharya, K.R. Crystal Structures of Apo- and Holo-bovine α-Lactalbumin at 2.2-Å Resolution Reveal an Effect of Calcium on Inter-lobe Interactions. J. Biol. Chem. 2000, 275, 37021–37029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bujacz, A. Structures of bovine, equine and leporine serum albumin. Acta Crystallogr. Sect. D 2012, 68, 1278–1289. [Google Scholar] [CrossRef]
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef] [Green Version]
- Schrödinger, L.; DeLano, W. PyMOL, 2.4.0. 2020. Available online: http://www.pymol.org/pymol (accessed on 16 October 2022).
- Yang, W.; Xiang, W.; Bao, Z.; Huang, C.; Ma, M.; Lu, X.; Yao, L.; Wang, Y. Phosphorus sorption capacity of various iron-organic matter associations in peat soils. Environ. Sci. Pollut. Res. 2022, 29, 77580–77592. [Google Scholar] [CrossRef]




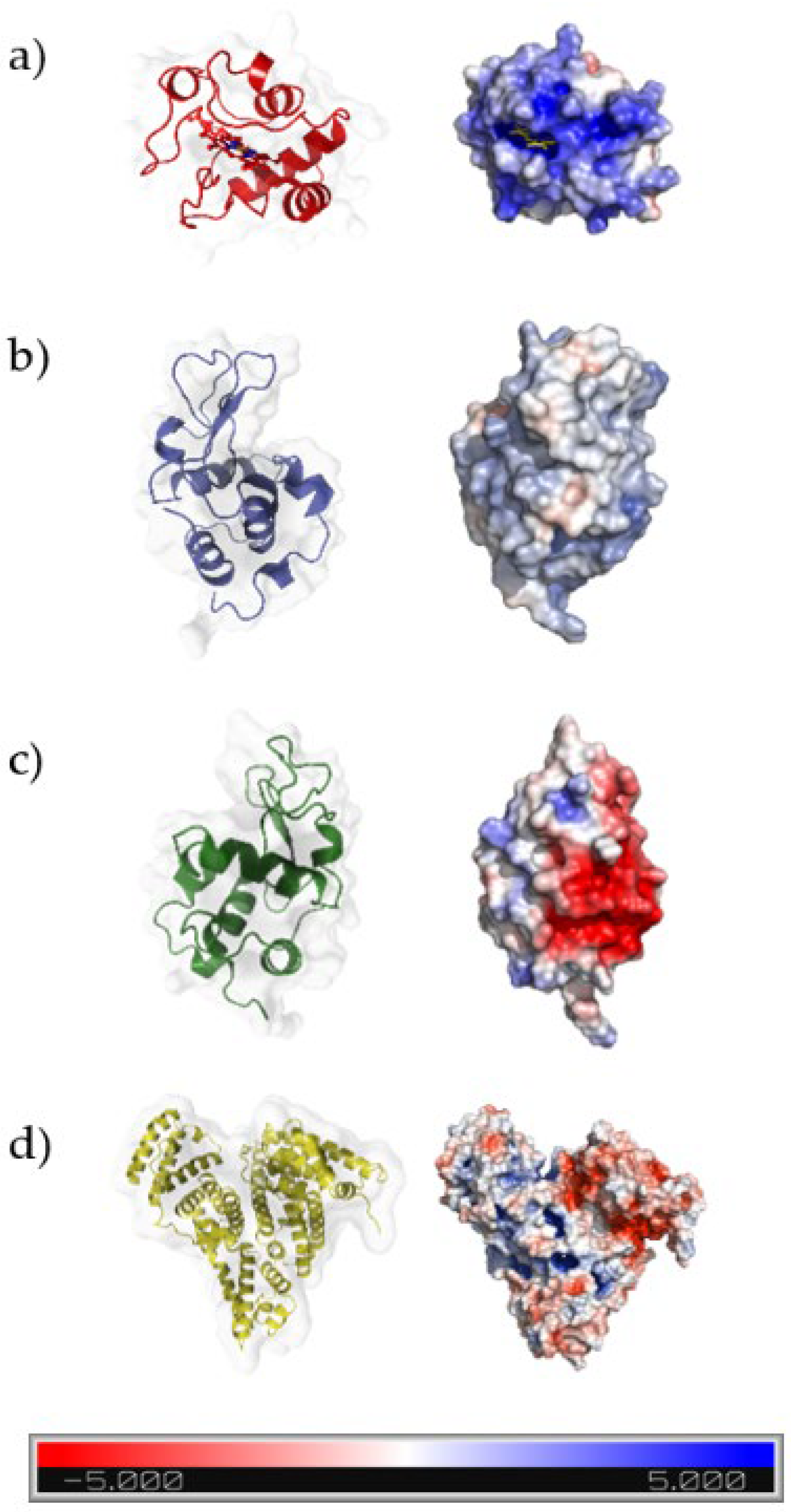
| Protein | Isoelectric Point | Molecular Weight (kDa) | λmax (nm) |
|---|---|---|---|
| Cyt C | 10–10.5 [54] | 12 [54] | 410 |
| Lys | 10.7 [55] | 14 [55] | 280 |
| α-LA | 4.5 [54] | 14 [54] | 280 |
| BSA | 4.9 [40] | 67 [40] | 280 |
| Protein | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|
| qm (mg g−1) | KL (L mg−1) | R2 | χ2 | n | KF (L mg−1) | R2 | χ2 | |
| Cyt C | 863.8 ± 57.3 | 0.047 ± 0.011 | 0.845 | 523 | 3.8 ± 0.7 | 173.3 ± 44.7 | 0.728 | 792 |
| Lys | 617.9 ± 58.7 | 0.015 ± 0.005 | 0.800 | 248 | 3.4 ± 0.9 | 85.2 ± 38.9 | 0.680 | 337 |
| Protein | Pseudo First-Order | Pseudo Second-Order | ||||||
|---|---|---|---|---|---|---|---|---|
| K1 (min−1) | qm (mg g−1) | R2 | χ2 | K2 (mg g−1 min−1), 10−3 | qm (mg g−1) | R2 | χ2 | |
| Cyt C | 0.092 ± 0.003 | 84.1 ± 0.6 | 0.969 | 6 | 1.600 ± 0.093 | 91.5 ± 0.8 | 0.969 | 4 |
| Lys | 0.029 ± 0.003 | 56.5 ± 1.8 | 0.914 | 29 | 0.563 ± 0.082 | 64.5 ± 2.2 | 0.937 | 23 |
| Protein | Adsorbent | qm (mg g−1) | KL (L mg−1) | pH | Temperature (°C) | Reference |
|---|---|---|---|---|---|---|
| Cyt C | DCMC | 850.5 | 0.047 ± 0.011 | 7 | 25 | This work |
| NIMS a | 38.61 | 0.001 | 7.4 | 25 | [40] | |
| MIMs b | 156.05 | 0.001 | 7.4 | 25 | [40] | |
| PGMA-g-Cell-SO3H c | 148.58 | 2.47 | 9 | 20 | [43] | |
| PGMA-g-Cell-SO3H c | 157.13 | 2.96 | 9 | 30 | [43] | |
| Lys | DCMC | 571.2 | 0.015 ± 0.005 | 7 | 25 | This work |
| Navicula sp. | 175.44 | 0.567 | 7 | 30 | [50] | |
| T. weissflogii | 185.19 | 0.900 | 7 | 30 | [50] | |
| P-EDA-Dye d | 588.24 | 0.006 | 7 | 25 | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gago, D.; Corvo, M.C.; Chagas, R.; Ferreira, L.M.; Coelhoso, I. Protein Adsorption Performance of a Novel Functionalized Cellulose-Based Polymer. Polymers 2022, 14, 5122. https://doi.org/10.3390/polym14235122
Gago D, Corvo MC, Chagas R, Ferreira LM, Coelhoso I. Protein Adsorption Performance of a Novel Functionalized Cellulose-Based Polymer. Polymers. 2022; 14(23):5122. https://doi.org/10.3390/polym14235122
Chicago/Turabian StyleGago, Diana, Marta C. Corvo, Ricardo Chagas, Luísa M. Ferreira, and Isabel Coelhoso. 2022. "Protein Adsorption Performance of a Novel Functionalized Cellulose-Based Polymer" Polymers 14, no. 23: 5122. https://doi.org/10.3390/polym14235122
APA StyleGago, D., Corvo, M. C., Chagas, R., Ferreira, L. M., & Coelhoso, I. (2022). Protein Adsorption Performance of a Novel Functionalized Cellulose-Based Polymer. Polymers, 14(23), 5122. https://doi.org/10.3390/polym14235122









