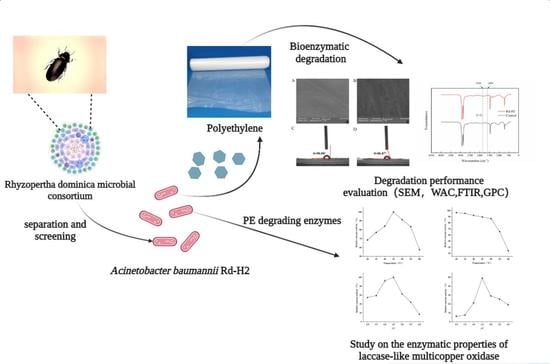
Screening of Polyethylene-Degrading Bacteria from Rhyzopertha Dominica and Evaluation of Its Key Enzymes Degrading Polyethylene
Abstract
:1. Introduction
2. Materials and Methods
2.1. PE Material and Culture Medium
2.2. Preliminary Screening of PE-Degrading Strains
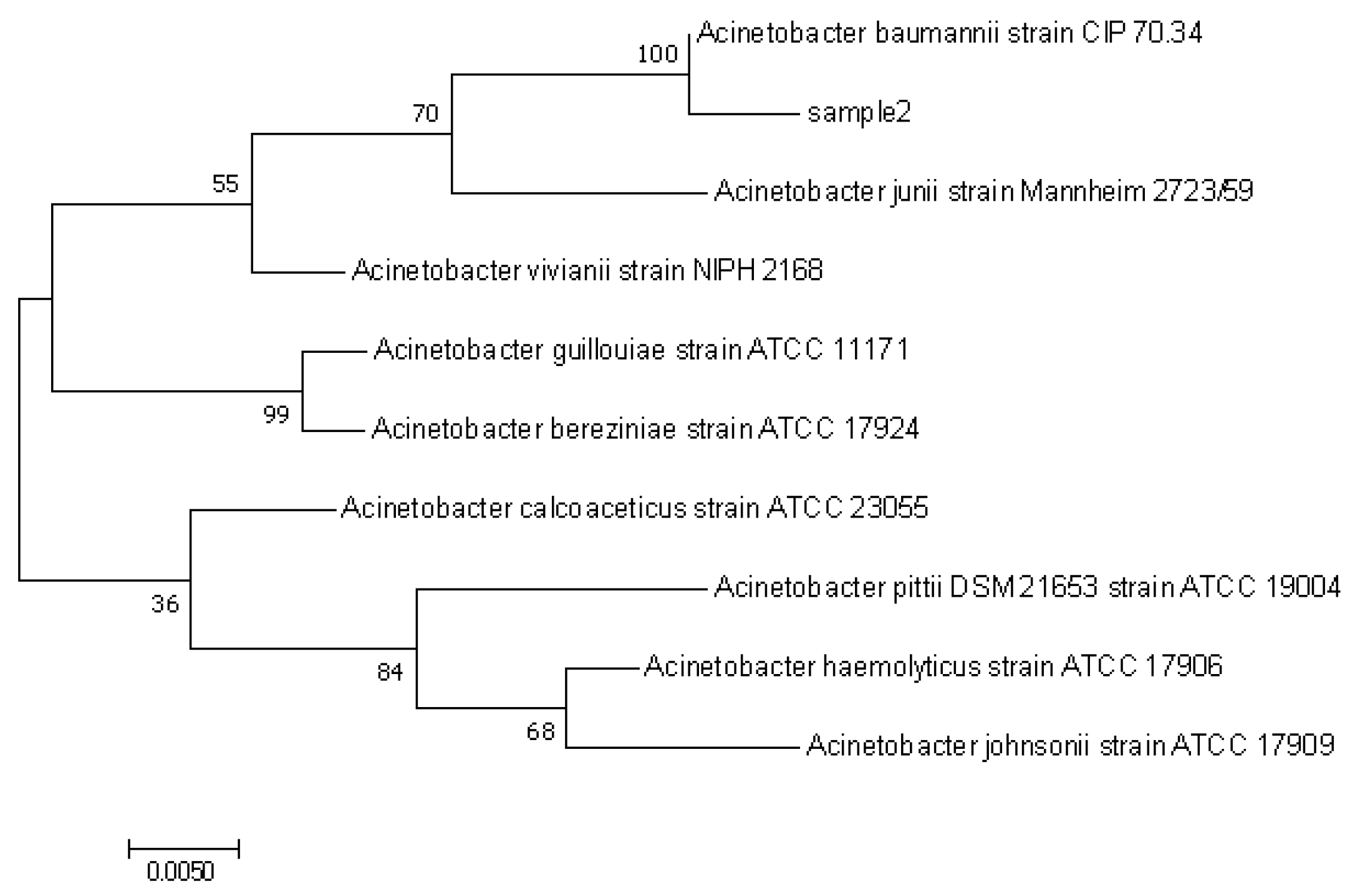
2.3. Identification of PE Degrading Strains
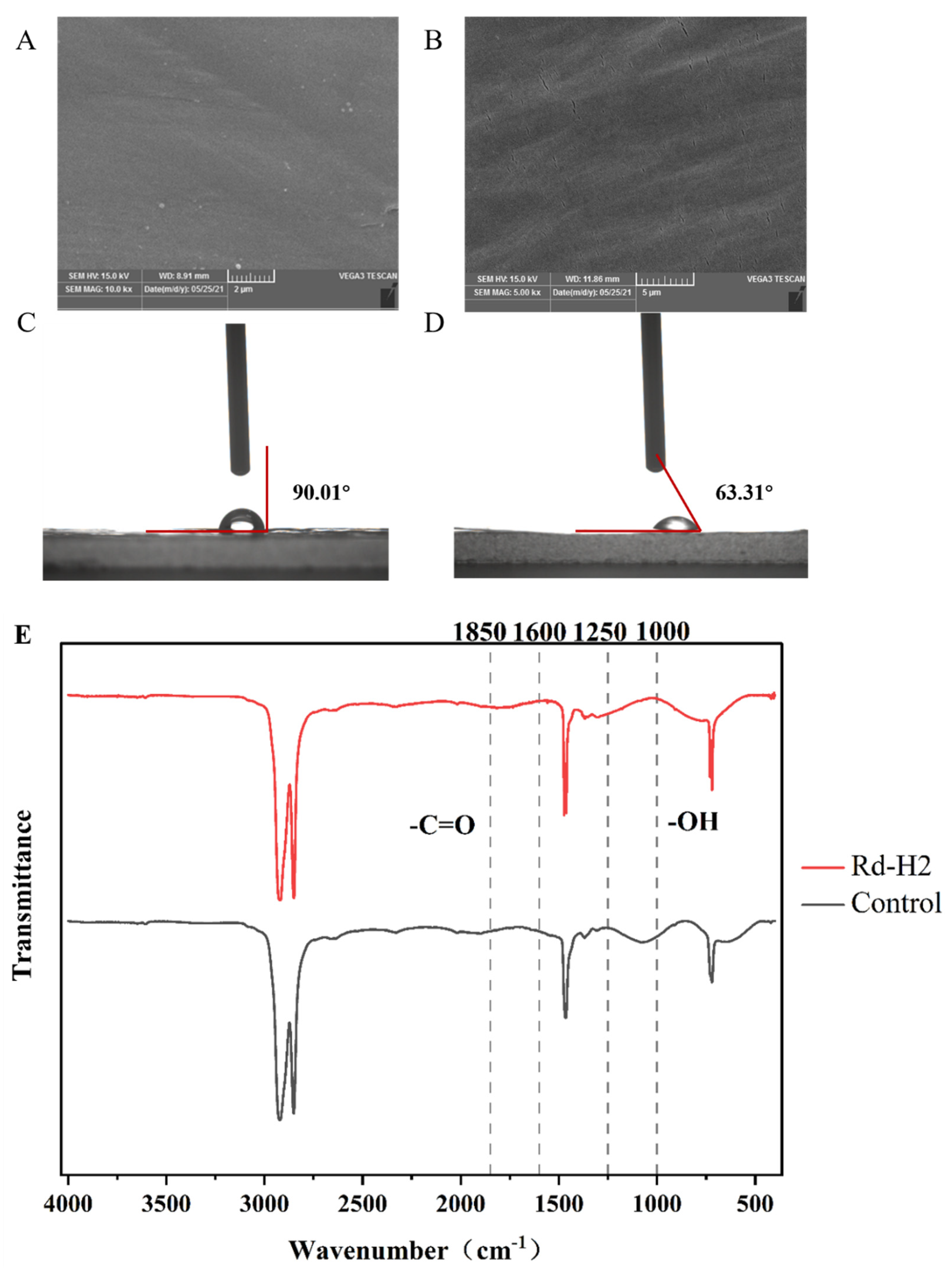
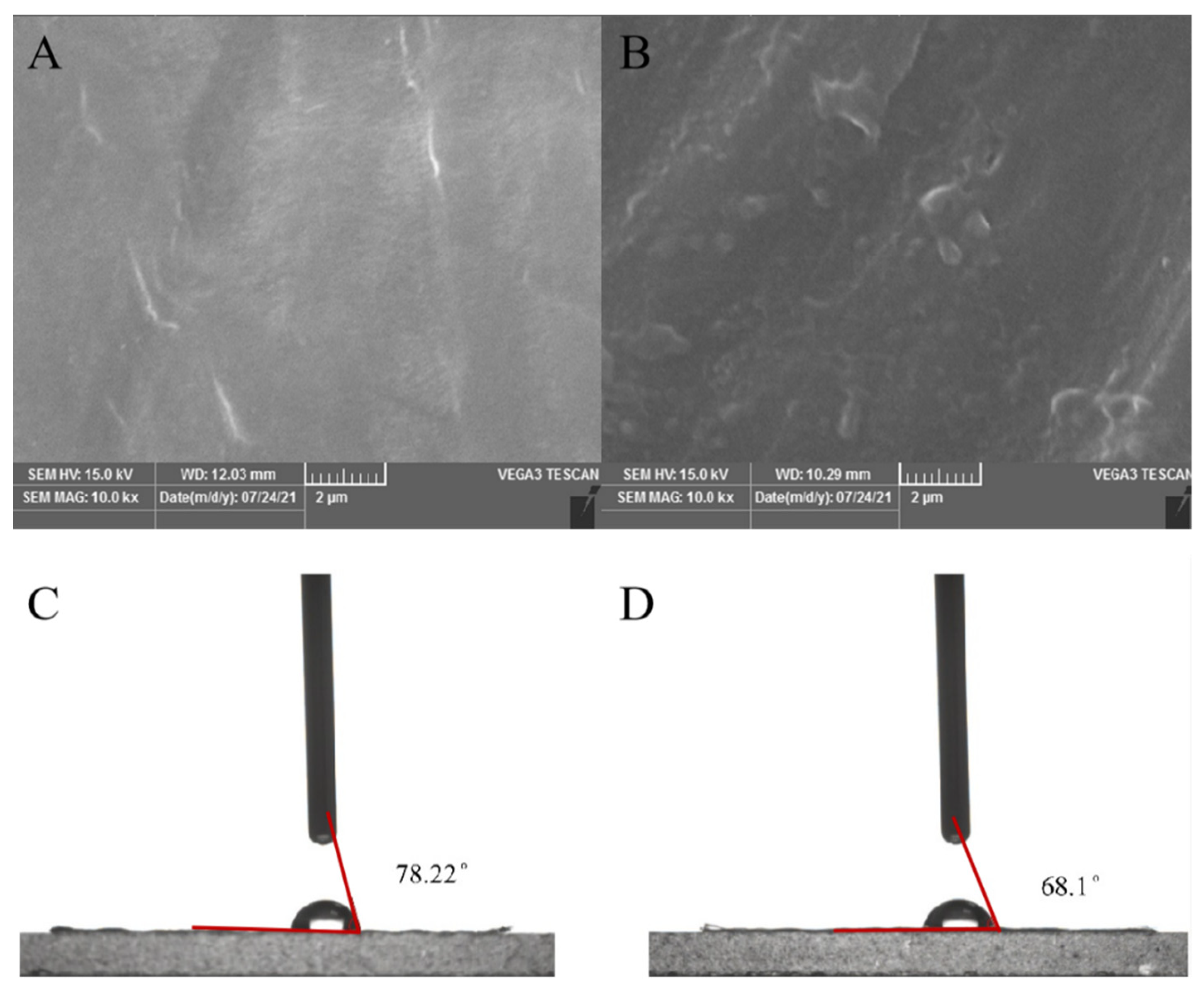
2.4. Determination of PE Degradation Properties
2.5. Heterologous Expression of the Laccase-like Multicopper Oxidase
2.6. Multi-Copper Oxidase Laccase Activity Assay
2.7. Effects of Temperature and pH on the Activity and Stability of Multicopper Oxidase
2.8. Effect of Metal Ions on Enzyme Activity
2.9. Characterization of PE Degradation by Laccase-like Multi-Copper Oxidase
3. Results and Discussion
3.1. Screening and Identification of PE-Degrading Strains
3.2. Degradation Performance of Rd-H2 on LDPE Films
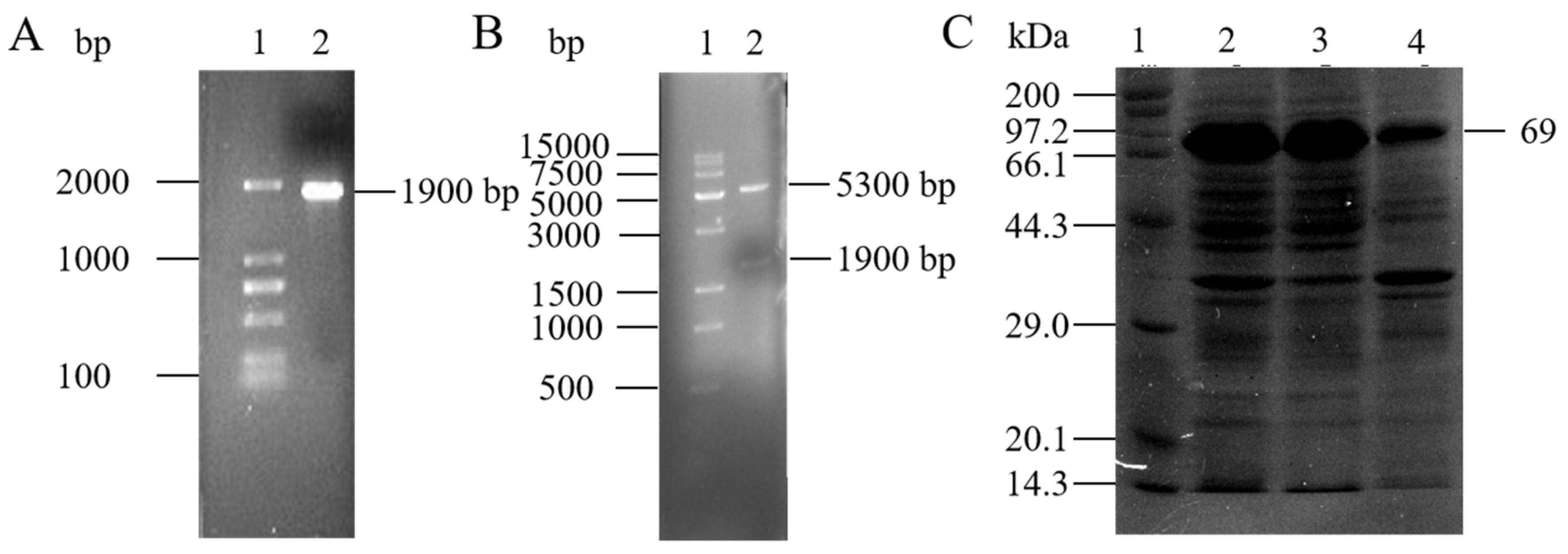
3.3. Cloning and Expression of AbMco Gene
3.4. Effects of Temperature and pH on the Enzymatic Activity and Stability of Multicopper Oxidase
3.5. The Effect of Metal Ions on the Activity of Multicopper Oxidase
3.6. Degradation Performance of Multi-Copper Oxidase on PE Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, Y.; Yu, J. Laccase-catalyzed decolorization of synthetic dyes. Water Res. 1999, 33, 3512–3520. [Google Scholar] [CrossRef]
- Kong, D.; Wang, L.; Chen, X.; Xia, W.; Su, L.; Zuo, F.; Yan, Z.; Chen, S.; Wu, J. Chemical-biological degradation of polyethylene combining Baeyer–Villiger oxidation and hydrolysis reaction of cutinase. Green Chem. 2022, 24, 2203–2211. [Google Scholar] [CrossRef]
- Mahmood, Q.; Li, X.; Qin, L.; Wang, L.; Sun, W.-H. Structural evolution of iminopyridine support for nickel/palladium catalysts in ethylene (oligo)polymerization. Dalton Trans. 2022, 51, 14375–14407. [Google Scholar] [CrossRef]
- Moharir, R.V.; Kumar, S. Challenges associated with plastic waste disposal and allied microbial routes for its effective degradation: A comprehensive review. J. Clean. Prod. 2019, 208, 65–76. [Google Scholar] [CrossRef]
- Jiakang, R.; Yixin, H.; Yu, Y. Microbial Degradation and Valorization of Plastic Wastes. Front. Microbiol. 2020, 11, 442. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshehrei, F. Biodegradation of Synthetic and Natural Plastic by Microorganisms. J. Appl. Environ. Microbiol. 2017, 5, 8–19. [Google Scholar]
- Royer, S.J.; Ferrón, S.; Wilson, S.T.; Karl, D.M. Production of methane and ethylene from plastic in the environment. PLoS ONE 2018, 13, e0200574. [Google Scholar] [CrossRef]
- Webb, H.K.; Arnott, J.; Crawford, R.J.; Ivanova, E.P. Plastic Degradation and Its Environmental Implications with Special Reference to Poly(ethylene terephthalate). Polymers 2012, 5, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Tribedi, P.; Sil, A.K. Low-density polyethylene degradation by Pseudomonas sp. AKS2 biofilm. Environ. Sci. Pollut. Res. 2013, 20, 4146–4153. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, Y.; Wu, W.M.; Zhao, J.; Jiang, L. Evidence of Polyethylene Biodegradation by Bacterial Strains from the Guts of Plastic-Eating Waxworms. Environ. Sci. Technol. 2014, 48, 13776–13784. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yuan, Z.; Zhai, J.; Li, R.; Huo, Y.; Zhang, X.; Jing, W. Screening, identification and degradation characteristics of polyethylene degrading bacteria in soil. Acta Microbiol. Sin. 2020, 60, 2836–2843. [Google Scholar]
- Zhang, J.; Gao, D.; Li, Q.; Zhao, Y.; Li, L.; Lin, H.; Bi, Q.; Zhao, Y. Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci. Total Environ. 2020, 704, 135931. [Google Scholar] [CrossRef]
- Ameen, F.; Moslem, M.; Hadi, S.; Al-Sabri, A.E. Biodegradation of Low Density Polyethylene (Ldpe) by Mangrove Fungi from the Red Sea Coast. Prog. Rubber Plast. Recycl. Technol. 2015, 31, 125–143. [Google Scholar] [CrossRef]
- Fujisawa, M.; Hirai, H.; Nishida, T. Degradation of Polyethylene and Nylon-66 by the Laccase-Mediator System. J. Polym. Environ. 2001, 9, 103–108. [Google Scholar] [CrossRef]
- Santo, M.; Weitsman, R.; Sivan, A. The role of the copper-binding enzyme–laccase–in the biodegradation of polyethylene by the actinomycete Rhodococcus ruber. Int. Biodeterior. Biodegrad. 2013, 84, 204–210. [Google Scholar] [CrossRef]
- Sowmya, H.V.; Ramalingappa; Krishnappa, M.; Thippeswamy, B. Degradation of polyethylene by Trichoderma harzianum—SEM, FTIR, and NMR analyses. Environ. Monit. Assess. 2014, 186, 6577–6586. [Google Scholar] [CrossRef]
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: A never-ending story. Cell. Mol. Life Sci. 2010, 67, 369–385. [Google Scholar] [CrossRef]
- Claus, H. Laccases and their occurrence in prokaryotes. Arch. Microbiol. 2003, 179, 145–150. [Google Scholar] [CrossRef]
- Kai, S.; Shunyao, L.; Youbin, S.; Qingguo, H. Advances in laccase-triggered anabolism for biotechnology applications. Crit. Rev. Biotechnol. 2021, 41, 969–993. [Google Scholar]
- Zhang, A.; Hou, Y.; Wang, Q.; Wang, Y. Characteristics and polyethylene biodegradation function of a novel cold-adapted bacterial laccase from Antarctic sea ice psychrophile Psychrobacter sp. NJ228. J. Hazard. Mater. 2022, 439, 129656. [Google Scholar]
- Bourbonnais, R.; Paice, M.G. Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990, 267, 99–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sridharan, R.; Krishnaswamy Veena, G.; Senthil, K.P. Analysis and microbial degradation of Low-Density Polyethylene (LDPE) in Winogradsky column. Environ. Res. 2021, 201, 111646. [Google Scholar] [CrossRef]
- Ojha, N.; Pradhan, N.; Singh, S.; Barla, A.; Shrivastava, A.; Khatua, P.; Rai, V.; Bose, S. Evaluation of HDPE and LDPE degradation by fungus, implemented by statistical optimization. Sci. Rep. 2017, 7, 39515. [Google Scholar] [CrossRef]
- Khandare, S.D.; Agrawal, D.; Mehru, N.; Chaudhary, D.R. Marine bacterial based enzymatic degradation of low-density polyethylene (LDPE) plastic. J. Environ. Chem. Eng. 2022, 10, 107437. [Google Scholar] [CrossRef]
- Gilan, I.; Hadar, Y.; Sivan, A. Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber. Appl. Microbiol. Biotechnol. 2004, 65, 97–104. [Google Scholar]
- Lou, H.; Fu, R.; Long, T.; Fan, B.; Guo, C.; Li, L.; Zhang, J.; Zhang, G. Biodegradation of polyethylene by Meyerozyma guilliermondii and Serratia marcescens isolated from the gut of waxworms (larvae of Plodia interpunctella). Sci. Total Environ. 2022, 853, 158604. [Google Scholar] [CrossRef]
- Copinet, A.; Bertrand, C.; Govindin, S.; Coma, V.; Couturier, Y. Effects of ultraviolet light (315 nm), temperature and relative humidity on the degradation of polylactic acid plastic films. Chemosphere 2004, 55, 763–773. [Google Scholar] [CrossRef]
- Gao, R.; Sun, C. A marine bacterial community capable of degrading poly(ethylene terephthalate) and polyethylene. J. Hazard. Mater. 2021, 416, 125928. [Google Scholar] [CrossRef]
- Sowmya, H.V.; Ramalingappa; Krishnappa, M.; Thippeswamy, B. Degradation of polyethylene by Penicillium simplicissimum isolated from local dumpsite of Shivamogga district. Environ. Dev. Sustain. 2015, 17, 731–745. [Google Scholar] [CrossRef]
- Wang, H.; Huang, L.; Li, Y.; Ma, J.; Wang, S.; Zhang, Y.; Ge, X.; Wang, N.; Lu., F.; Liu, Y. Characterization and application of a novel laccase derived from Bacillus amyloliquefaciens. Int. J. Biol. Macromol. 2020, 150, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Telke, A.A.; Ghodake, G.S.; Kalyani, D.C.; Dhanve, R.S.; Govindwar, S.P. Biochemical characteristics of a textile dye degrading extracellular laccase from a Bacillus sp. ADR. Bioresour. Technol. 2011, 1020, 1752–1756. [Google Scholar] [CrossRef]
- Neelkant, K.S.; Shankar, K.; Jayalakshmi, S.K.; Sreeramulu, K. Purification, biochemical characterization, and facile immobilization of laccase from Sphingobacterium ksn-11 and its application in transformation of diclofenac. Appl. Biochem. Biotechnol. 2020, 192, 845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, S.; Diao, H.; Zhao, H.; Zhu, X.; Lu, F.; Zhaoxin, L. Purification and characterization of a temperature- and pH-stable laccase from the spores of Bacillus vallismortis fmb-103 and its application in the degradation of malachite green. J. Agric. Food Chem. 2013, 61, 5468–5473. [Google Scholar] [CrossRef] [PubMed]
- Javadzadeh, S.-G.; Asoodeh, A. A novel textile dye degrading extracellular laccase from symbiotic bacterium of Bacillus sp. CF96 isolated from gut termite (Anacanthotermes). Int. J. Biol. Macromol. 2020, 145, 355–363. [Google Scholar] [CrossRef]

| Serial Number | Weight Loss Rate/% |
|---|---|
| Rd-H1 | 0.16 ± 0.08 |
| Rd-H2 | 0.62 ± 0.062 |
| Rd-H3 | 0.49 ± 0.020 |
| Rd-H4 | 0.20 ± 0.12 |
| Rd-H5 | 0.50 ± 0.002 |
| Rd-H6 | 0.21 ± 0.014 |
| Rd-H7 | 0.19 ± 0.012 |
| Rd-H8 | 0.21 ± 0.012 |
| Name | Control | Rd-H2 (30 d) |
|---|---|---|
| Mn | 19,979 | 18,158 |
| Mw | 81,315 | 79,244 |
| PD | 4.04 | 4.37 |
| Metal Ion (5 mmol/L) | Relative Activity/(%) |
|---|---|
| Control | 100 ± 0.73 |
| K+ | 88.26 ± 1.40 |
| Mg2+ | 62.46 ± 0.55 |
| Fe2+ | 92.63 ± 0.92 |
| Mn2+ | 95.80 ± 0.057 |
| Cu2+ | 102.14 ± 0.75 |
| Name | Control | abMCO |
|---|---|---|
| Mn | 34,197 | 16,347 |
| Mw | 1,355,592 | 134,314 |
| PD | 3.97 | 8.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Lin, Y.; Gou, H.; Feng, X.; Zhang, X.; Yang, L. Screening of Polyethylene-Degrading Bacteria from Rhyzopertha Dominica and Evaluation of Its Key Enzymes Degrading Polyethylene. Polymers 2022, 14, 5127. https://doi.org/10.3390/polym14235127
Zhang Y, Lin Y, Gou H, Feng X, Zhang X, Yang L. Screening of Polyethylene-Degrading Bacteria from Rhyzopertha Dominica and Evaluation of Its Key Enzymes Degrading Polyethylene. Polymers. 2022; 14(23):5127. https://doi.org/10.3390/polym14235127
Chicago/Turabian StyleZhang, Yao, Yuan Lin, Hongmei Gou, Xu Feng, Xian Zhang, and Lijuan Yang. 2022. "Screening of Polyethylene-Degrading Bacteria from Rhyzopertha Dominica and Evaluation of Its Key Enzymes Degrading Polyethylene" Polymers 14, no. 23: 5127. https://doi.org/10.3390/polym14235127